Abstract
Craniometaphyseal dysplasia (CMD), a rare genetic bone disorder, is characterized by life-long progressive thickening of craniofacial bones and metaphyseal flaring of long bones. The autosomal dominant form of CMD is caused by mutations in the progressive ankylosis gene ANKH (mouse ortholog Ank), encoding a pyrophosphate (PPi) transporter. We previously reported reduced formation and function of osteoblasts and osteoclasts in a knockin (KI) mouse model for CMD (AnkKI/KI) and in CMD patients. We also showed rapid protein degradation of mutant ANK/ANKH. Mutant ANK protein displays reduced PPi transport, which may alter the inorganic phosphate (Pi) and PPi ratio, an important regulatory mechanism for bone mineralization. Here we investigate whether reducing dietary Pi intake can ameliorate the CMD-like skeletal phenotype by comparing male and female Ank+/+ and AnkKI/KI mice exposed to low (0.3%) and normal (0.7%) Pi diet for 13 weeks from birth. Serum Pi and calcium (Ca) levels were not significantly changed by diet, whereas PTH and 25-hydroxy vitamin D (25-OHD) were decreased by low Pi diet but only in male Ank+/+ mice. Importantly, the 0.3% Pi diet significantly ameliorated mandibular hyperostosis in both sexes of AnkKI/KI mice. A tendency of decreased femoral trabeculation was observed in male and female Ank+/+ mice as well as in male AnkKI/KI mice fed with 0.3% Pi diet. In contrast, in female AnkKI/KI mice the 0.3% Pi diet resulted in increased metaphyseal trabeculation. This was also the only group that showed increased bone formation rate. Low Pi diet led to increased osteoclast numbers and increased bone resorption in all mice. We conclude that lowering but not depleting dietary Pi delays the development of craniofacial hyperostosis in CMD mice without severely compromising serum levels of Pi, Ca, PTH and 25-OHD. These findings may have implications for better clinical care of patients with CMD.
Keywords: Bone histomorphometry, bone QCT/μCT, genetic animal models, osteoblasts, osteoclasts
INTRODUCTION
Craniometaphyseal dysplasia (CMD) is a rare genetic bone disorder characterized by hyperostotic craniofacial bones and flaring metaphyses of long bones. CMD can be diagnosed early in infants and progresses throughout life (1,2). Patients with CMD often suffer from severe headache, hearing loss, visual impairment and facial palsy because hyperostosis of craniofacial bones frequently leads to obstruction of cranial foramina and compression of facial nerves. Genetic variants in the form of point mutations, one-amino-acid insertions or deletions that cluster mostly in the C terminus of the progressive ankylosis gene (ANKH) are responsible for the autosomal dominant form of CMD (3,4). ANK is a transmembrane protein localized on plasma membranes, endoplasmic reticulum, and Golgi apparatus (5,6). ANKH/ANK is highly conserved within vertebrates and ubiquitously expressed in skeletal and non-skeletal tissues to regulate or prevent tissue mineralization by transporting intracellular pyrophosphate (PPi) to the extracellular matrix(3,7). In vitro experiments in oocytes showed that CMD mutant ANK transports less PPi(7). Inhibition of ANK in primary articular chondrocytes results in decreased extracellular ATP (eATP) levels in a PPi-independent manner.(8). In addition to be a PPi transporter ANK likely has other unknown functions.
To study the pathogenesis of CMD, we have generated a knockin (KI) mouse model carrying an in-frame deletion of phenylalanine 377 (Phe377del) in ANK. AnkKI/KI mice replicate many features of human CMD, including hyperostotic craniofacial bones, narrowing of the cranial foramina, reduced sinus spaces, fusion of middle ear bones, and abnormal femoral shape with decreased metaphyseal but extensive diaphyseal trabeculation(9). We showed that mutant ANK/ANKH protein levels were significantly reduced due to rapid protein degradation while the expression of mutant Ank/ANKH mRNA transcripts was comparable to wild-type Ank/ANKH(5). Although AnkKI/KI mice showed a tendency of decreased serum Pi, the difference was not statistically significant in comparison to Ank+/+ mice(10). Other Pi regulating hormones such as PTH, 25-OHD, intact and cleaved FGF23, were unexpectedly normal in the CMD mouse model as well as in patients with CMD(11). AnkKI/KI mice have reduced osteoblastogenesis. Osteoclasts in mutant mice and CMD patients are reduced in numbers, have defective actin rings, and decreased resorptive activity, which greatly contributes to the skeletal phenotype of CMD(11,12). Despite advances in CMD research, the pathogenesis remains not fully elucidated. Treatment is still limited to surgical decompression of obstructed foramina and to plastic surgery for correcting craniofacial structures.
The importance of inorganic phosphate (Pi) in bone metabolism has long been recognized. Phosphate is a major component of hydroxyapatite (HA; Ca10(PO4)6(OH)2) and acts as a signaling molecule to regulate the differentiation of osteoblasts, osteoclasts, and chondrocytes(13,14). Imbalance of the extracellular Pi/PPi ratio can cause mineral-related pathological conditions(15–18). Reduced PPi or increased Pi levels can result in pathological calcification due to excessive HA deposition. Extracellular PPi (ePPi) is known as a potent inhibitor of mineralization. The homeostasis of Pi and PPi in the skeleton is mainly controlled by three proteins, ectonucleotide pyrophosphatase / phosphodiesterase-1 (ENPP1), which generates PPi from ATP, both extracellularly and intracellularly; tissue-nonspecific alkaline phosphatase (ALPL), which hydrolyzes ePPi to generate Pi; and PPi transporter ANK(19). ENPP1 and ATP-binding cassette subfamily C member 6 (ABCC6), which is primarily expressed in liver, are two major contributors to plasma PPi levels(20,21). Plasma PPi levels in patients with pseudoxanthoma elasticum due to inactivating ABCC6 mutations and ABCC6 knockout mice are reduced by 60–70%(20). Enpp1−/− mice also have very low plasma PPi levels(21). Given that Ank is ubiquitously expressed, it is likely that the ANK protein contributes to PPi levels locally and systemically. Plasma PPi levels in AnkKI/KI mice have not been reported.
Studies testing the effects of Pi depletion on bone by feeding animals with very low Pi diet (0.04 – 0.1%) have clearly described its detrimental effects on the skeleton within a short period of time (2 weeks), largely due to increased osteoclast activity and reduced bone mineralization(22). Here, we examine the effects of moderate dietary Pi reduction (0.3%) on the skeletal phenotype of AnkKI/KI mice over a longer period of time (from birth to adult age). These findings may have clinical implications for treatment of patients with CMD.
MATERIALS AND METHODS
Mouse model and tissue collection
A mouse model for CMD had previously been generated by introducing a TTC1130–1132 (phenylalanine 377) deletion in exon 9 in the Ank gene(9). Mice were bred from 129/Sv into a C57Bl/J6 background (N7). Low Pi diet containing 0.3% Pi (0.9% Ca and 2200 IU/kg vitamin D; Harlan Teklad) was provided to mothers with litters after birth and continued after weaning for 10 weeks. Control groups of mice were fed with normal mouse chow for a total of 13 weeks. Normal mouse chow contained 0.7% Pi (1% Ca, 1500 IU/kg vitamin D; Teklad Diet; Envigo). Mouse genotyping was confirmed at the time of weaning (3 weeks after birth) and repeated at the time of sacrificing animals. The animal protocol (101425-0819) was approved by the Institutional Animal Care and Use Committee (IACUC) of University of Connecticut Health and all work was performed in an AAALAC accredited facility.
For skeletal analysis, we collected 8 male and 8 female Ank+/+ and AnkKI/KI littermates fed with normal chow or with low phosphate diet. We injected mice intraperitoneally with calcein (6 mg/kg body weight) and alizarin complexone (30 mg/kg body weight) at an interval of 7 days. Two days after the second injection, mice were sacrificed at 13 weeks of age and body weight was measured. Femoral and mandibular bones from the same mouse were fixed in 10% formalin and subjected to histomorphometry and computed microtomography (μCT).
Plasma PPi measurement
Fasting blood was collected from the submandibular vein using an animal lancet (Goldenrod; Braintree Scientific) from 13-week-old Ank+/+ and AnkKI/KI mice fed with normal chow. Mouse blood collected in CTAD tubes (BD) was centrifuged at 1000 g for 10 minutes at 4 °C. Platelets were removed from plasma using a Centrisart I, 300,000 MWCO PES membrane filters (Sartorius) and was stored at −80°C until further processing.
Plasma PPi concentrations were determined by modifying a published protocol(20). Briefly, ATP sulfurylase was used to convert PPi into ATP in the presence of excess adenosine 5’-phosphosulfate (APS). For each 10 μl of plasma, 70 μl of a mixture containing 32 mU ATP sulfurylase (M0394; NEB), 16 μmol/L APS (A5508; Sigma), 80 μmol/L MgCl2 and 50 mmol/L HEPES (pH 7.4) were added. The mixture was incubated for 30 minutes at 37 °C, after which ATP sulfurylase was inactivated by incubation at 90 °C for 10 minutes. Generated ATP was quantified using the ATP-monitoring reagent BacTiter-Glo (G8230; Promega). Equal volume of BacTiter-Glo reagent was added to samples. Bioluminescence was subsequently determined in a microplate reader (EnSpire Multimode Reader; PerkinElmer). Each sample was measured in triplicate. The minimum plasma volume required for each assay was 40 μL.
Biochemical analysis
Fasting sera were collected in microtainer tubes (Becton Dickinson) from 13-week-old Ank+/+ and AnkKI/KI mice. Total serum calcium and phosphate were determined using a calcium reagent kit (Eagle Diagnostics) and a phosphorus reagent set (Eagle Diagnostics), respectively. Concentrations of mouse 25-OHD (25-OHD kit; Eagle Biosciences), and mouse intact PTH (mouse intact PTH ELISA kit; Immutopics International) were determined according to manufacturers’ instructions.
μCT analysis
Mandibles and femurs from 13-week-old Ank+/+ and AnkKI/KI male and female mice fed with normal chow or with low Pi diet (n=8 per group) were analyzed using μCT in the MicroCT facility at UConn Health (mCT20; ScanCo Medical AG, Bassersdorf, Switzerland). Total volume (TV) and bone volume (BV) of mandibles were obtained by scanning mandibles from mesially of 1st molars to distally of 3rd molars and measuring 80 slices of vertical sections starting from the full opening of the pulp in the mesial roots of 1st molars. For TV measurements of mandibles, the contour was placed tightly on bone surfaces. Molars and incisors were excluded for BV determination in mandibles. Volumetric regions were rendered as 3-dimensional arrays with an isometric voxel dimension of 12 μm. Trabecular measurements and cortical bone parameters were obtained in femurs as previously described(23).
Bone histomorphometry
Formalin-fixed femurs were frozen-embedded in OCT medium (Richard-Allan Scientific) and blocks were sectioned to the midpoint using a cryotome (CM3050S; Leica). Three sections (7 μm thickness) surrounding the central vein of the femur at 50μm intervals were collected using an adhesive tape transfer method (Cryofilm type IIC; Section-Lab Co, Ltd., Japan) as previously described(24). The mineralization status shown by calcein (green) and alizarin complexone (red) was recorded by fluorescent microscopy (Mirax Midi automated image acquisition system; Zeiss). Following the rules of traditional bone histomorphometry, parameters of bone forming activity were measured in the Computer Science Department at the University of Connecticut as previously described(23,24).
Staining for osteoblasts and osteoclasts on frozen-embedded bone sections
After scanning for mineralization images, the same sections were processed for cellular staining. For osteoclasts, tartrate-resistant acid phosphatase (TRAP) staining was performed using a fluorescent substrate (ELF-97; Invitrogen)(25). The acidic condition during TRAP staining removes the fluorescent mineralization labels. ELF-97 substrate stained for TRAP activity in mononuclear and multinuclear cells. Bone surfaces that had both, mineralization labeling and TRAP signals, were considered remodeling surfaces whereas surfaces with TRAP-positive cells but no mineralization labeling were considered resorbing surfaces. To detect osteoblasts, alkaline phosphatase (AP) was stained by the fluorescent substrate fast red(26). AP-positive cells located on calcein or AC labeled bone surfaces were considered active osteoblastic cells whereas AP-positive cells on non-labeled bone surfaces were considered inactive bone lining cells(23,24). The TRAP and AP signals were captured using filters optimized for tetracycline and retramethyl rhodamine iso-thiocyanate (TRITC), respectively.
Mouse bone marrow stromal cell cultures
Mouse bone marrow stromal cell cultures (BMSCs) were prepared as previously described(27). Briefly, bone marrow was flushed out from the shafts of femurs, tibia and humeri of 7- to 9-week-old mice. Cells were cultured at a density of 3×106 cells/well in 6-well culture plates in α-MEM (Invitrogen-Gibco) containing 10% fetal bovine serum (Hyclone), 100 IU/ml penicillin, and 100 μg/ml streptomycin. At day 3, half of the α-MEM medium was replaced with phosphate-free DMEM (Invitrogen-Gibco) containing 0.25 mM Pi and 1 mM Pi, respectively, by adding sodium phosphate (composed of sodium phosphate dibasic anhydrous and sodium dihydrogen phosphate monohydrate). 1 mM Pi is considered normal Pi concentration in many culture media, such as DMEM or α-MEM. 0.25 mM Pi is considered low phosphate. On day 7, cells were switched to 100% osteoblast differentiation medium containing 0.25 mM Pi or 1 mM Pi DMEM, 50 μg/ml ascorbic acid and 8 mM β-glycerophosphate (β -GP). Medium was changed every 2–3 days.
Mouse bone marrow-derived macrophage (BMM) cultures
BMM cultures were obtained from bone marrow flushed out from femora and tibia of 7- to 9-week-old mice and cultured for 18 to 24 hours in α-MEM containing 10 % FBS (Hyclone), 100 IU/ml penicillin, 100 μg/ml streptomycin (Invitrogen - Gibco). Non-adherent cells were collected and purified by Ficoll separation (Lymphoprep; Axis Shield, Oslo, Norway). Cells were cultured in 0.25 mM and 1 mM Pi in phosphate-free DMEM medium supplemented with M-CSF (30 ng/ml; Peprotech) for 2 days followed by M-CSF and RANKL (30 ng/ml; Peprotech) treatment to stimulate osteoclast differentiation.
In vitro osteoblast and osteoclast assays
For osteoblast cultures, we determined matrix expression by AP staining which was performed using a commercially available alkaline phosphatase kit (Sigma) according to manufacturer’s instructions. For osteoclast cultures, we analyzed osteoclast formation by TRAP staining as previously described(11). Briefly, BMMs cultured for 5 days in M-CSF and RANKL were fixed with 2.5 % glutaraldehyde and TRAP stained with a commercially available kit (Sigma). Images were taken by a Z1 Observer microscope (Zeiss). Experiments were performed in triplicate.
Quantitative real-time PCR (qPCR)
Total RNA from mouse BMSC and BMM cultures was isolated using TRIzol (Thermo Fisher Scientific) followed by Direct-zol RNA extraction (Zymo Research) according to manufacturers’ instructions. RNA was treated with DNase I and cDNA was synthesized by Superscript II reverse transcriptase (Invitrogen). qPCR was performed as previously described(11). Relative quantification of gene expression was determined by the ΔΔCt method. Data were normalized to mouse 18S gene expression. Primer sequences are listed in Supplemental Table 1.
Statistical Analysis
Statistical analysis was performed by one-way or two-way ANOVA followed by Tukey’s test, using Prism 8 software (GraphPad Software).
RESULTS
Blood biochemistry in AnkKI/KI mice fed with 0.3% or 0.7% Pi diet
Very low concentration of Pi diet decreases serum Pi, increases Ca, and affects other Pi regulated/regulating hormones, such as PTH, vitamin D, and FGF23(28). We determined the serum levels of Pi, Ca, PTH and 25-OHD in Ank+/+ and AnkKI/KI mice exposed to normal chow (0.7% Pi) or 0.3% Pi diet for 13 weeks since birth. Serum Pi and Ca levels were not significantly changed by 0.3% or the 0.7% Pi diets and were not different between Ank+/+ and AnkKI/KI mice (Table 1). In male Ank+/+ mice, PTH and 25-OHD were significantly decreased by the 0.3% Pi diet compared to male Ank+/+ mice fed with 0.7 Pi diet (Table 1). Values in female Ank+/+ mice did not differ significantly. We measured the plasma PPi levels in Ank+/+ and AnkKI/KI mice fed with 0.7% Pi diet. PPi levels were not measured in animals fed with 0.3% Pi diet due to insufficient plasma sample volumes. Our data showed that the PPi level was significantly decreased in AnkKI/KI mice fed with 0.7% Pi diet compared to Ank+/+ mice (Table 1). These data suggest an imbalance in the Pi/PPi ratio in AnkKI/KI mice. Taken together, our regimen of dietary Pi restriction starting from birth to adulthood (13 weeks) did not severely compromise the blood biochemistry involved in Pi metabolism in AnkKI/KI mice.
Table 1:
Biochemical analysis of plasma/serum from Ank+/+ and AnkKI/KI mice
| Tests | Samples | 0.3% Pi diet | 0.7% Pi diet | ||
|---|---|---|---|---|---|
| Ank +/+ | Ank KI/KI | Ank +/+ | Ank KI/KI | ||
| PPi | plasma | N/A | N/A | 1.52±0.22 (n=10) | 1.02±0.26* (n=12) |
| Pi | serum | 6.19±1.34 (n=25) | 6.05±0.84 (n=20) | 6.40±0.51 (n=15) | 6.20±1.04 (n=13) |
| Ca | serum | 9.40±0.43 (n=25) | 9.20±0.40 (n=20) | 9.77±0.69 (n=15) | 9.21±0.95 (n=13) |
| PTH | serum | 91.28±52.65# (male, n=14) | 150.7±121.10 (male, n=12) | 301.94±216.65 (male, n=12) | 259.69±122.82 (male, n=10) |
| 25OHD | serum | 41.95±7.76# (male, n=10) | 36.68±12.12 (male, n=10) | 55.52±12.88 (male, n=10) | 46.25±15.05 (male, n=4) |
PPi (μM); Pi (mg/dl); Ca (mg/dl); Intact PTH (pg/ml); 25OHD: 25-hydroxy vitamin D (ng/ml); Data are mean ± SD. p < 0.05 is indicated by:
significant difference between Ank+/+ and AnkKI/KI mice fed with the same diet;
significant difference between normal and Pi diet in mice with the same genotype. Data for serum PPi, Pi, Ca were combined from male and female mice. Male and female mice showed the same pattern of PPi, Pi and Ca levels.
Effects of low-Pi diet on skeletal phenotype of AnkKI/KI mice
Low Pi diet did not affect the general appearance and body weight of Ank+/+ and AnkKI/KI mice. Male AnkKI/KI mice weighed significantly less than male Ank+/+ littermates fed with normal mouse chow (0.7%) or low Pi (0.3%) diet (0.7% Pi diet: Ank+/+ mice = 28.65±2.65 g, AnkKI/KI mice = 23.66±2.03 g, p* = 0.005; 0.3% Pi diet: Ank+/+ mice = 28.72±5.05 g, AnkKI/KI mice = 22.71±1.48 g, p* = 0.008, data = mean±SD, n=8 per group). Interestingly, the female AnkKI/KI mice fed with 0.3% Pi diet showed a tendency of increased weight compared to AnkKI/KI females fed with normal chow (p = 0.058). The weight difference between female Ank+/+ and AnkKI/KI mice was significant when fed with the normal chow (0.7% Pi diet: Ank+/+ mice = 21.15±2.61 g, AnkKI/KI mice = 17.08±1.41 g, p* = 0.004, data = mean±SD, n=8 per group) and became insignificant when fed with the 0.3% Pi diet (0.3% Pi diet: Ank+/+ mice = 20.22±1.39 g, AnkKI/KI mice = 18.74±1.54 g, p = 0.072, data = mean±SD, n=8 per group).
Restriction of dietary phosphate partially rescued the CMD-like hyperostotic mandible phenotype of AnkKI/KI mice. The 0.3% Pi diet significantly decreased the mandibular bone volume (BV) and total volume (TV) in male and female AnkKI/KI mice while the bone volume fraction (BVF; BV/TV) remained unaltered (Figure 1A & 1B).
FIG. 1.
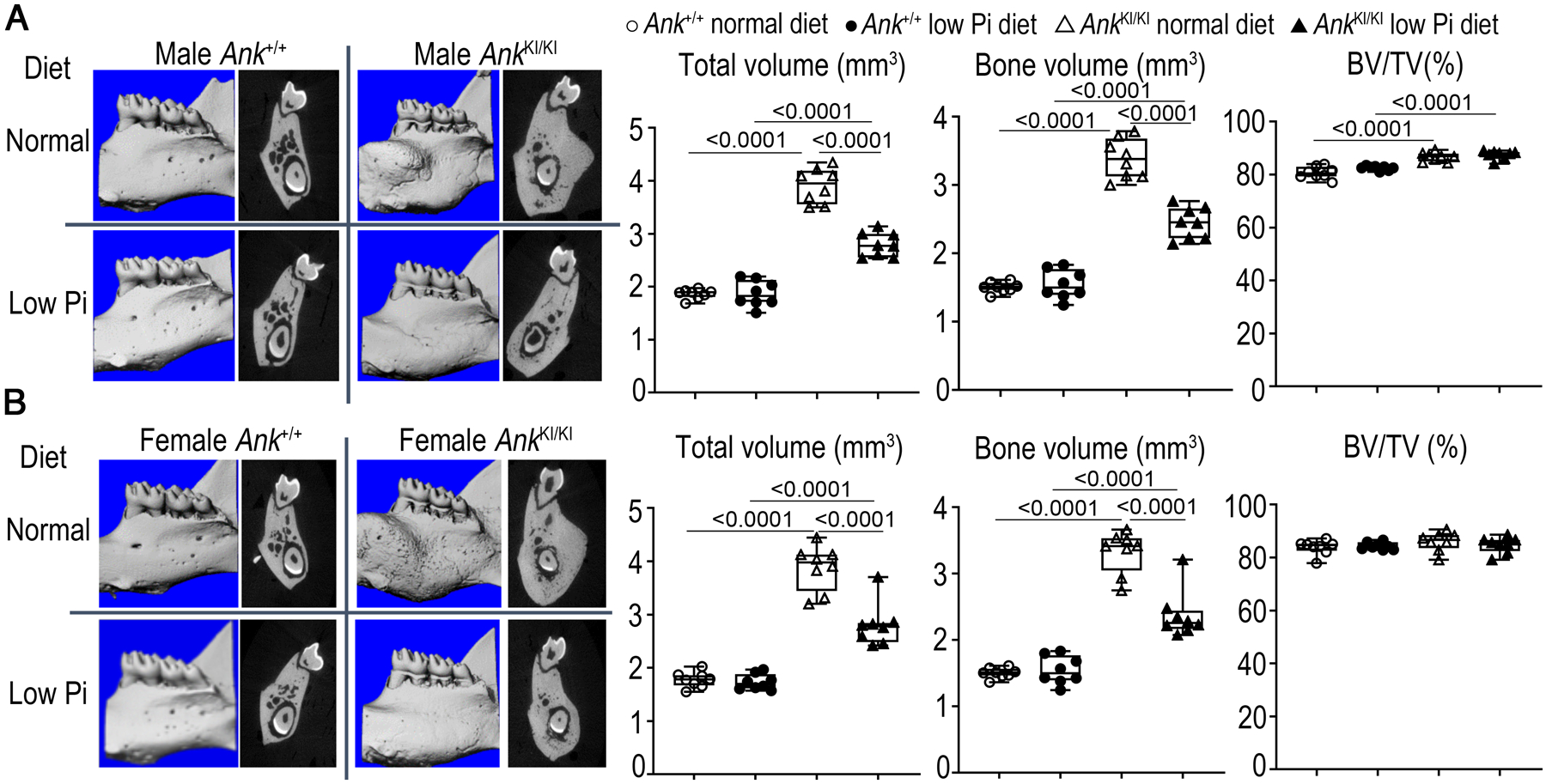
Restriction of dietary Pi ameliorates hyperostotic mandibles in AnkKI/KI mice. μCT images of 3D reconstruction of mandibles and sagittal plane through furcation of first mandibular molar in A) male Ank+/+ and AnkKI/KI mice; B) female Ank+/+ and AnkKI/KI mice. Histograms showing total volume (TV), bone volume (BV) and bone volume fraction (BV/TV). n=8 per group. p<0.05 indicates statistical significance by one-way ANOVA with Tukey’s test. p-values are indicated above the horizontal bars up to a value of 0.1.
In femurs, low Pi diet resulted in a tendency of decreased metaphyseal trabecular BVF, increased trabecular spacing, and decreased trabecular number in male and female Ank+/+ mice and in male AnkKI/KI mice (Figure 2, Supplemental Figure 1). Interestingly, this trend was reversed in female AnkKI/KI mice where feeding with low Pi diet resulted in increased BV and BVF (Figure 2B & 2D, Supplemental Figure 1).
FIG. 2.

Effects of dietary Pi on femoral trabecular and cortical bones in Ank+/+ and AnkKI/KI mice. Representative μCT images of internal view of Ank+/+ and AnkKI/KI femurs, 3D reconstruction of trabeculation in metaphysis and cross-sectional slice of cortical bone in diaphysis in A) male Ank+/+ and AnkKI/KI mice; B) female Ank+/+ and AnkKI/KI mice. Histograms show quantitative μCT data of C) male Ank+/+ and AnkKI/KI mice; D) female Ank+/+ and AnkKI/KI mice. n=8 per group. p<0.05 indicates statistical significance by one-way ANOVA with Tukey’s test. p-values are indicated above the horizontal bars up to a value of 0.1.
Male and female AnkKI/KI mice showed significantly increased cortical porosity compared to Ank+/+ mice with cortical porosity being more prominent in male mice (Figure 2). Low Pi diet significantly reduced the cortical porosity in male AnkKI/KI mice compared to male AnkKI/KI mice fed with normal diet (Figure 2A & 2C). Cortical porosity in female AnkKI/KI was not affected by low Pi diet (Figure 2B & 2D). Low Pi diet does not change the club-shaped phenotype of AnkKI/KI femurs as shown by enlarged periosteal perimeter and endosteal perimeter compared to Ank+/+ mice (Figure 2C & 2D). However, the periosteal perimeter in low phosphate-fed male AnkKI/KI mice was significantly reduced compared to their male AnkKI/KI littermates fed with normal mouse chow (Figure 2C).
Effects of low Pi diet on bone formation of Ank+/+ and AnkKI/KI mice in vivo
To determine the effects of low Pi diet on bone formation, we performed dynamic histomorphometry on the metaphyseal region of femurs from male and female Ank+/+ and AnkKI/KI mice. Bone formation rate (BFR) is a measure of mineral apposition rate (MAR) multiplied with the fraction of bone surface that is labeled (MS_BS). 0.3% Pi diet resulted in a significant reduction of BFR in male and female Ank+/+ mice (Figure 3). This reduction is mainly due to reduced MS/BS instead of MAR. Low Pi diet resulted in a tendency of BFR reduction in male AnkKI/KI mice, which was in contrast to the unexpected increase in BFR in female AnkKI/KI mice (Figure 3C & 3D).
FIG. 3.

Dynamic histomorphometry of Ank+/+ and AnkKI/KI mice exposed to normal and low Pi diet. Representative images of femurs with calcein (green) and alizarin complexone (red) double labeling in A) male Ank+/+ and AnkKI/KI mice; B) female Ank+/+ and AnkKI/KI mice. Quantitative measurement of histomorphometry of C) male Ank+/+ and AnkKI/KI mice; D) female Ank+/+ and AnkKI/KI mice. MS_BS: mineralizing surface per bone surface; MAR: mineral apposition rate; BFR: bone formation rate; AP_BS: alkaline phosphatase-positive labeled surface per bone surface; bone forming surface (AP_L/BS): proportion of AP signal on labeled bone surface; bone lining surface (AP_NL/BS): proportion of AP signal on non-labeled bone surface. p<0.05 indicates statistical significance by one-way ANOVA with Tukey’s test. p- values are indicated above or below the horizontal bars up to a value of 0.1.
Osteoblasts, whether actively forming bone or serving as bone lining cells capable of being activated to produce new matrix, are alkaline phosphatase- (AP−) positive. AP_BS measures the proportion of the total trabecular bone surface that is AP-positive. Low Pi diet affects AP_BS by significantly increasing AP_BS in female Ank+/+ and AnkKI/KI mice but not in male mice (Figure 3C & 3D).
We next examined the active bone forming surface and inactive bone lining surface by measuring the proportion of total surface where the AP signal overlapped with active mineralizing bone surface (AP_L/BS) and the AP signal on non-labeled bone surface (AP_NL/BS), respectively(23). Low Pi diet significantly increased the bone lining surface in female Ank+/+ and AnkKI/KI mice (Figure 3D). This effect of low Pi diet is less prominent in male mice. Low Pi diet tends to have no effect or a decrease of bone forming surfaces in male Ank+/+ and AnkKI/KI mice as well as in female Ank+/+ mice. Unexpectedly, the bone-forming surface was significantly increased in female AnkKI/KI mice fed with reduced Pi diet (Figure 3B & 3D). Taken together, in male mice low Pi diet negatively affects bone forming activity of osteoblasts and increases the surface of bone lining cells. The sex dimorphism detected for low Pi diet in AnkKI/KI mice was unexpected with female AnkKI/KI mice responding to low Pi diet with enhanced bone forming activity in femoral bones, which resulted in increased metaphyseal trabeculation.
Effects of low Pi diet on bone resorption of Ank+/+ and AnkKI/KI mice in vivo
To determine the effects of low Pi on bone resorption, femoral sections were stained for TRAP. TRAP signals identify cells in the osteoclast lineage but do not differentiate between mono- and multinucleated cells. The proportion of total TRAP-positive bone surface is expressed as TRAP_BS, which can be detected over either labeled (mineralizing) or non-labeled (nonmineralizing) surface on dynamic histomorphometry sections. Low Pi diet increased TRAP_BS significantly in male Ank+/+ mice and resulted in an increased tendency in male AnkKI/KI mice whereas this effect was not seen in female Ank+/+ and AnkKI/KI mice (Figure 4A–4D).
FIG. 4.

Effects of dietary Pi on bone resorbing activity/surface in Ank+/+ and AnkKI/KI mice. Representative images of femurs with TRAP staining to indicate osteoclast-like cells in A) male Ank+/+ and AnkKI/KI mice; B) female Ank+/+ and AnkKI/KI mice. Quantitative histomorphometry measurements of C) male Ank+/+ and AnkKI/KI mice; D) female Ank+/+ and AnkKI/KI mice. TRAP_BS: fraction of bone surface with TRAP label; TRAP_L/BS: proportion of mineralizing surface that is TRAP-labeled; TRAP_NL/BS: proportion of non-mineralizing surface that is TRAP-labeled. p<0.05 indicates statistical significance by one-way ANOVA with Tukey’s test. p-values are indicated above the horizontal bars up to a value of 0.1. Enlarged images of white square boxes in panels A) and B) showing TRAP labeled surfaces of cortical bones in E) male Ank+/+ and AnkKI/KI mice; F) female Ank+/+ and AnkKI/KI mice. “x” indicates periosteal surface; “*” indicates endosteal surface.
We next examined the effects of low Pi diet on the bone resorbing surface and bone remodeling surface. Bone resorbing surface is the proportion of total TRAP positive bone surface overlapping with non-labeled surface (TRAP_NL/TRAP) while the bone remodeling surface is the proportion of total TRAP activity that overlap with labeled surface (TRAP_L/TRAP)(23). Low Pi diet increased bone resorbing surface (TRAP_NL/TRAP) and decreased bone remodeling surface (TRAP_L/TRAP) in both male and female Ank+/+ and AnkKI/KI mice (Figure 4C & 4D). AnkKI/KI mice fed with normal mouse chow or 0.3% Pi diet showed clusters of TRAP-positive cells along endosteal surface areas, which may in part explain the club-shaped femurs (Figure 4E & 4F).
Effects of low Pi diet on osteoblasts and osteoclasts from Ank+/+ and AnkKI/KI mice in vitro
We next examined low Pi effects on mouse osteoblasts and osteoclasts in vitro. We did not observe any sex differences in data from BMSC and BMM cultures, therefore we only show data from female mice. We first performed Ank+/+ and AnkKI/KI BMSC cultures maintained in medium containing 0.25 mM and 1 mM Pi. To induce osteoblast differentiation, BMSCs were cultured in ascorbic acid and 8 mM β-glycerophosphate after plating for 7 days. AnkKI/KI BMSCs showed reduced AP staining compared to BMSCs from Ank+/+ mice. Reduced Pi concentration (0.25 mM) resulted in increased AP staining in Ank+/+ and AnkKI/KI BMSCs (Figure 5A). Although the difference in Pi concentration in our culture system may appear small due to the addition of 8 mM β-glycerophosphate, we still detected increased levels of Alpl, Phex, Dmp1 in BMSCs cultured in 0.25 mM Pi medium for 21 days (Figure 5B). Other genes regulating Pi or being regulated by Pi including Spp1, Ank, Enpp1 were not significantly affected (Figure 5B and data not shown). Fgf23 levels were too low to be detected (Ct value > 34, data not shown).
FIG. 5.

Effects of reduced Pi concentration on BMSC and BMM cultures derived from Ank+/+ and AnkKI/KI mice. A) AP staining of Ank+/+ and AnkKI/KI BMSCs cultured in osteoblast differentiating medium for 0, 7, 14 and 21 days. AnkKI/KI cultures show reduced AP staining. BMSCs cultured in 0.25 mM Pi showed more AP staining than those cultured in 1 mM Pi medium; B) representative fold-changes of Alpl, Phex, Dmp1 and Spp1 expression in Ank+/+ and AnkKI/KI BMSCs cultured in differentiation medium for 21 days and normalized to expression levels of Ank+/+ BMSCs at day 0, normalized to 18S mRNA from one experiment (n=3). C) Ank+/+ and AnkKI/KI BMMs stained for TRAP in osteoclast differentiating medium for 5 days. Histogram shows numbers of TRAP-stained multinucleated (nuclei ≥ 3) per well; D) measurement of osteoclast surface in pixel diagram. More than 300 osteoclasts per group were measured. Pooled data from three independent experiments. p<0.05 indicates statistical significance by two-way ANOVA with Tukey’s test. p-values are indicated above or below the horizontal bars up to a value of 0.1.
To examine how low Pi affects osteoclastogenesis, we performed BMM cultures from Ank+/+ and AnkKI/KI mice maintained in medium supplemented with 0.25 mM and 1 mM Pi. Consistent with our in vivo data, reduced Pi concentration resulted in increased numbers of osteoclasts (TRAP-positive cells with nuclei ≥ 3) in both Ank+/+ and AnkKI/KI BMM cultures (Figure 5C). Reduced Pi concentration led to a tendency of increased surface area of osteoclasts in both Ank+/+ and AnkKI/KI BMMs, which was more prominent in Ank+/+ cultures (Figure 5D).
DISCUSSION
Patients with CMD suffer from lifelong progressive thickening of craniofacial bones, often leading to neurological symptoms. Slowing the development of excessive bone mass accumulation in these patients may lessen the severity of facial paralysis, blindness, deafness, headaches, and reduce the need for recurrent surgeries. Dietary Pi restriction exerts beneficial effects in several disease mouse models and is used clinically in patients with renal disease(28–33). Male Klotho mutant mice (absence of Klotho) fed with low Pi (0.4%) diet for 26 weeks had increased lifespan and body weight(30). Very low Pi (0.02%) diet increased the survival of osteopetrosis (op/op) mice but resulted in a rickets-like phenotype(29). Restriction of Pi (0.1%) in diet normalized biochemical and skeletal phenotypes of a mouse model for tumoral calcinosis(28). Here, we investigated how the restriction of Pi intake impacts the CMD-related skeletal phenotype in AnkKI/KI mice. Our data show that low Pi diet alleviated the hyperostosis of mandibles in male and female AnkKI/KI mice. Low Pi diet also affected metaphyseal trabeculation and the cortical bone porosity/perimeter in mid-femur via a sex-specific response. Sex dimorphism affecting mandibles, cartilage and Klotho protein expression after feeding low Pi diet has also been reported in other mouse models but whether sex hormones are responsible for the sex-specific phenotype is unknown(30,34,35). Sex steroids, mainly estrogen and testosterone, have been associated with bone health via inhibitory effects on bone resorption or stimulatory effects on bone formation in a compartment-specific manner (cortical vs. cancellous bone)(36,37). Whether Pi intake influences sex hormone levels and whether Pi interacts with sex hormones to regulate bone metabolism has not been fully elucidated. AnkKI/KI mice may serve as a mouse model to study the involvement of sex hormones in bone remodeling and in a future study we will investigate whether sex hormones are differentially expressed in CMD patients. Our present study is the first report showing that moderate reduction of dietary Pi ameliorates the CMD-like mandibular phenotype but does not correct the club-shaped femurs in mice. Female AnkKI/KI mice may have benefited more from a reduced Pi diet than male AnkKI/KI mice because female AnkKI/KI mice showed an increase in metaphyseal trabeculation, when compared to AnkKI/KI mice fed with normal mouse chow and to Ank+/+ mice fed with normal and low Pi diet. Added trabeculation could potentially increase bone strength and resistance to fractures.
Low Pi diet has been shown to affect osteoblasts, osteoclasts and chondrocytes(28,38–40). Moderate hypophosphatemia significantly increases osteoclastic bone resorption, whereas rats with severe hypophosphatemia showed remarkable stimulation of bone resorption as well as inhibition of bone mineralization(41). Depletion of dietary Pi (0.04%–0.1%) in rats resulted in a significant decrease in body weight, cortical thickness of tibial diaphyses and Ca deposition in bone, as well as a decrease of the osteoid maturation rate in periosteal surfaces and an increase in the number and resorptive activity of osteoclasts in endosteal surfaces(22,38). Very low Pi diet also resulted in decreased apoptosis of hypertrophic chondrocytes leading to rachitic expansion of the growth plate(39). Moderate restriction of Pi improved the CMD-related skeletal phenotype in AnkKI/KI mice, which may be likely due to: 1) increase in bone resorption; 2) reduction of bone formation; 3) improving the Pi and PPi ratio. Plasma PPi levels in AnkKI/KI mice fed with normal diet are approximately 30% lower than in Ank+/+ littermates. This reduction is similar to findings in Ank knockout mice but less severe than in Enpp1−/− mice (data not shown). Our data show that ANK contributes to plasma PPi levels but to a lesser extent than has been shown for ABCC6 and ENPP1. The impact of low Pi diet on skeletal (local) and systemic PPi levels in AnkKI/KI mice needs to be further studied.
Mandibles and long bones in female AnkKI/KI mice respond to low Pi diet differently. We suspect that this phenomenon may result from sex-specific response to downstream signals elicited by mutant ANK and the inherent differences between mandibular and long bone. Mandibles are derived from neural crest cells of the neuroectoderm germ layer and undergo intramembranous ossification while femurs are derived from paraxial mesoderm and undergo endochondral ossification(42–44). Differential gene expression profiles in mandibular-derived bone cells and long bone have been reported(45). VEGF as a key regulator of angiogenesis and skeletogenesis shows increased expression in pre-osteoblasts and osteoblasts from long bones compared to preosteoblasts and osteoblasts from mandibles and therefore may have a stronger impact on long bones(46). We could not compare the impact of low Pi on osteoblasts and osteoclasts in mandibles and in long bones because dynamic histomorphometry in mandibles was not performed in this study. A limitation was that sectioning of undecalcified mandibles is challenging and the quantitative analysis of mandibular histomorphometry is not standardized.
We attempted to study the effects of low Pi on osteoblast activity by utilizing BMSC cultures, which routinely differentiate into mature osteoblasts in α-MEM supplemented with 25 mg/ml ascorbic acid and 8 mM β-GP. We used commercially available Pi-free DMEM since Pi-free α-MEM was not available. β-GP is an essential ingredient to promote mineralization of cultured osteoblasts by providing inorganic Pi, acting as a blocker of phosphatase activity and possibly altering matrix protein phosphorylation(47). We were aware that the use of β-GP may create a conundrum for our reduced Pi studies because almost 80% of 10 mM β-GP can be hydrolyzed to Pi in cell cultures within 24 hours(48). It has been recommended that β-GP should not exceed 2 mM in medium to avoid non-physiological mineral deposition in cultures(48). Without β-GP, we were unable to induce mineral nodule formation in BMM cultures (data not shown). Even with 8 mM β-GP, we found that the expression of Fgf23, a marker of mature osteoblasts/ osteocytes, was not adequately induced (qPCR: Ct value > 34) in BMSC cultures (data not shown). Despite this caveat, we made several interesting findings: 1) AP staining and Alpl gene expression were increased in cultures with 0.25 mM Pi, consistent with earlier studies of AP inhibition by Pi(49); 2) Phex is especially sensitive to a change of Pi concentration and may play an important role in the adaptive response to Pi as the Phex gene is highly induced in 0.25 mM Pi cultures, especially in the Ank+/+ culture; 3) with this mild difference in Pi concentration, Spp1, Ank, and Enpp1, were not significantly changed (data not shown). We did not detect sex-specific differences in BMSC or BMM cultures derived from male and female mice. This is most likely owed to the fact that cell culture systems only contain selective cell types, which do not fully reflect the systemic or paracrine effects of growth factors/ cytokines/ hormones etc. in an in vivo environment.
Acute or chronic deprivation of Pi, especially in growing animals and humans, can negatively impact bone health, thus should be practiced with caution(22). Restriction of Pi intake needs to be applied cautiously to avoid undesirable side effects. When young and growing animals were fed with very low concentrations of Pi (0.03% Pi in mice for 3–5 days; 0.04% or 0.2% in rats for 10–14 days), mRNA expression levels of certain genes expressed in kidney led to significant changes in the serum biochemistry including decreased Pi, increased Ca, decreased PTH, and increased vitamin D(22,38,50). In this study, we exposed growing Ank+/+ and AnkKI/KI mice with a moderately restricted Pi diet (0.3%) for a relatively long period of time (13 weeks). Unlike previous experiments with very low Pi diet, serum levels of Pi and Ca were not significantly affected at the time points that we measured. This is consistent with a study by Ichikawa et al., where they compared mice fed with 0.3% and 0.6% Pi diet for 12 weeks and found no changes in serum levels for Pi, Ca, creatinine, alkaline phosphatase, osteocalcin, TRAP, and FGF23(28). No to moderate changes in serum biochemistry may be explained by 1) compensatory effects after long-term supplement of low Pi diet; 2) large variability in serum samples; 3) adaptive mechanisms that make AnkKI/KI mice more resistant to reduced Pi concentrations.
Phosphorus binders, classified into calcium/aluminum free, calcium-based, magnesium-based, and aluminum-based, have been used to lower Pi in humans(31–33). Aluminum-based phosphorus binders may have toxic effects and induce bone disease(51). The use of calcium-based binders requires careful monitoring of elevated serum calcium levels. Dietary control of Pi intake is considered more physiological and the first phase of therapeutic control of hyperphosphatemia in patients with chronic kidney disease although it relies heavily on patient compliance. A very-low-protein diet can result in significant reduction of serum Pi levels and renal Pi filtration without the use of phosphorus binders(32). A reduced Pi diet can be achieved by omitting processed foods that usually contain phosphate additives(52,53) and consuming fresh vegetables and meats (non-processed food) which contains significantly less phosphorous(54). Another significant hidden source of phosphate is from beverages such as red/white wine, cola, and beer(55). Results from our animal study suggest that dietary Pi represents a modifiable target to reduce or ameliorate the CMD-like skeletal phenotype in mice, especially mandibular hyperostosis. This dietary strategy may also help patients with CMD to slow down progressive mandibular or cranial bone deposition. Given the common use of Pi in Western diet, more studies are needed to define the health impact of reduced Pi diet and the effectiveness in controlling progression of bone deposition in CMD patients.
Supplementary Material
ACKNOWLEDGMENTS
We wish to thank the μCT core and the histomorphometry core directed by Dr. David Rowe for their support. The project was supported by NIH/NIDCR grants DE025664 to IPC and DE019458 to EJR.
Grant Support:
DE025664 (IPC); DE019458 (EJR)
Footnotes
Conflict of Interest:
All authors have no conflict of interest.
REFERENCES
- 1.Elcioglu N, Hall CM. Temporal aspects in craniometaphyseal dysplasia: autosomal recessive type. American journal of medical genetics. Mar 19 1998;76(3):245–51. [DOI] [PubMed] [Google Scholar]
- 2.Ramseyer LT, Leonard JC, Stacy TM. - Bone scan findings in craniometaphyseal dysplasia. - Clinical Nuclear Medicine. 1993;18(2):137–9. [DOI] [PubMed] [Google Scholar]
- 3.Nurnberg P, Thiele H, Chandler D, Hohne W, Cunningham ML, Ritter H, et al. Heterozygous mutations in ANKH, the human ortholog of the mouse progressive ankylosis gene, result in craniometaphyseal dysplasia. Nature genetics. May 2001;28(1):37–41. [DOI] [PubMed] [Google Scholar]
- 4.Reichenberger E, Tiziani V, Watanabe S, Park L, Ueki Y, Santanna C, et al. Autosomal dominant craniometaphyseal dysplasia is caused by mutations in the transmembrane protein ANK. American journal of human genetics. Jun 2001;68(6):1321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanaujiya J, Bastow E, Luxmi R, Hao Z, Zattas D, Hochstrasser M, et al. Rapid degradation of progressive ankylosis protein (ANKH) in craniometaphyseal dysplasia. Sci Rep. Oct 24 2018;8(1):15710. Epub 2018/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seifert W, Posor Y, Schu P, Stenbeck G, Mundlos S, Klaassen S, et al. The progressive ankylosis protein ANK facilitates clathrin- and adaptor-mediated membrane traffic at the trans-Golgi network-to-endosome interface. Human molecular genetics. Sep 01 2016;25(17):3836–48. [DOI] [PubMed] [Google Scholar]
- 7.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. Jul 14 2000;289(5477):265–70. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal AK, Gohr CM, Mitton-Fitzgerald E, Lutz MK, Dubyak GR, Ryan LM. The progressive ankylosis gene product ANK regulates extracellular ATP levels in primary articular chondrocytes. Arthritis Res Ther. Oct 17 2013;15(5):R154. Epub 2013/11/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen IP, Wang CJ, Strecker S, Koczon-Jaremko B, Boskey A, Reichenberger EJ. Introduction of a Phe377del mutation in ANK creates a mouse model for craniometaphyseal dysplasia. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. Jul 2009;24(7):1206–15. Epub 2009/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Dutra EH, Reichenberger EJ, Chen IP. Dietary phosphate supplement does not rescue skeletal phenotype in a mouse model for craniometaphyseal dysplasia. Journal of negative results in biomedicine. Oct 26 2016;15(1):18. Epub 2016/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen IP, Wang L, Jiang X, Aguila HL, Reichenberger EJ. A Phe377del mutation in ANK leads to impaired osteoblastogenesis and osteoclastogenesis in a mouse model for craniometaphyseal dysplasia (CMD). Human molecular genetics. Mar 1 2011;20(5):948–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen IP, Luxmi R, Kanaujiya J, Hao Z, Reichenberger EJ. Craniometaphyseal Dysplasia Mutations in ANKH Negatively Affect Human Induced Pluripotent Stem Cell Differentiation into Osteoclasts. Stem cell reports. Nov 14 2017;9(5):1369–76. Epub 2017/10/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck GR Jr. Inorganic phosphate as a signaling molecule in osteoblast differentiation. J Cell Biochem. Oct 1 2003;90(2):234–43. Epub 2003/09/25. [DOI] [PubMed] [Google Scholar]
- 14.Beck GR Jr., Moran E, Knecht N. Inorganic phosphate regulates multiple genes during osteoblast differentiation, including Nrf2. Exp Cell Res. Aug 15 2003;288(2):288–300. Epub 2003/08/14. [DOI] [PubMed] [Google Scholar]
- 15.Fedde KN, Blair L, Silverstein J, Coburn SP, Ryan LM, Weinstein RS, et al. Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. Dec 1999;14(12):2015–26. Epub 2000/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurley KA, Chen H, Guenther C, Nguyen ET, Rountree RB, Schoor M, et al. Mineral formation in joints caused by complete or joint-specific loss of ANK function. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. Aug 2006;21(8):1238–47. [DOI] [PubMed] [Google Scholar]
- 17.Okawa A, Nakamura I, Goto S, Moriya H, Nakamura Y, Ikegawa S. Mutation in Npps in a mouse model of ossification of the posterior longitudinal ligament of the spine. Nature genetics. Jul 1998;19(3):271–3. Epub 1998/07/14. [DOI] [PubMed] [Google Scholar]
- 18.Sweet HO, Green MC. Progressive ankylosis, a new skeletal mutation in the mouse. J Hered. Mar-Apr 1981;72(2):87–93. Epub 1981/03/01. [DOI] [PubMed] [Google Scholar]
- 19.Orriss IR. Extracellular pyrophosphate: The body’s “water softener”. Bone. Jan 16 2020:115243. Epub 2020/01/20. [DOI] [PubMed] [Google Scholar]
- 20.Jansen RS, Duijst S, Mahakena S, Sommer D, Szeri F, Varadi A, et al. ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler Thromb Vasc Biol. Sep 2014;34(9):1985–9. Epub 2014/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lomashvili KA, Narisawa S, Millan JL, O’Neill WC. Vascular calcification is dependent on plasma levels of pyrophosphate. Kidney international. Jun 2014;85(6):1351–6. Epub 2014/04/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivey JL, Morey ER, Baylink DJ. The effects of phosphate depletion on bone. Adv Exp Med Biol. 1978;103:373–80. Epub 1978/01/01. [DOI] [PubMed] [Google Scholar]
- 23.Rowe DW, Adams DJ, Hong SH, Zhang C, Shin DG, Renata Rydzik C, et al. Screening Gene Knockout Mice for Variation in Bone Mass: Analysis by muCT and Histomorphometry. Curr Osteoporos Rep. Apr 2018;16(2):77–94. Epub 2018/03/07. [DOI] [PubMed] [Google Scholar]
- 24.Hong SH, Jiang X, Chen L, Josh P, Shin DG, Rowe D. Computer-Automated Static, Dynamic and Cellular Bone Histomorphometry. J Tissue Sci Eng. Dec 24 2012;Suppl 1:004. Epub 2012/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filgueira L Fluorescence-based staining for tartrate-resistant acidic phosphatase (TRAP) in osteoclasts combined with other fluorescent dyes and protocols. J Histochem Cytochem. Mar 2004;52(3):411–4. Epub 2004/02/18. [DOI] [PubMed] [Google Scholar]
- 26.Cox WG, Singer VL. A high-resolution, fluorescence-based method for localization of endogenous alkaline phosphatase activity. J Histochem Cytochem. Nov 1999;47(11):1443–56. Epub 1999/11/02. [DOI] [PubMed] [Google Scholar]
- 27.Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC, et al. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. Jan 2002;17(1):15–25. Epub 2002/01/05. [DOI] [PubMed] [Google Scholar]
- 28.Ichikawa S, Austin AM, Gray AK, Allen MR, Econs MJ. Dietary phosphate restriction normalizes biochemical and skeletal abnormalities in a murine model of tumoral calcinosis. Endocrinology. Dec 2011;152(12):4504–13. Epub 2011/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCary LC, Smith CM, DeLuca HF. Hypophosphatemia and the development of rickets in osteopetrotic (op/op) mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. Nov 1997;12(11):1944–51. Epub 1998/02/07. [DOI] [PubMed] [Google Scholar]
- 30.Morishita K, Shirai A, Kubota M, Katakura Y, Nabeshima Y, Takeshige K, et al. The progression of aging in klotho mutant mice can be modified by dietary phosphorus and zinc. J Nutr. Dec 2001;131(12):3182–8. Epub 2001/12/12. [DOI] [PubMed] [Google Scholar]
- 31.Isakova T, Gutierrez OM, Smith K, Epstein M, Keating LK, Juppner H, et al. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant. Feb 2011;26(2):584–91. Epub 2010/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Iorio B, Di Micco L, Torraca S, Sirico ML, Russo L, Pota A, et al. Acute effects of very-low-protein diet on FGF23 levels: a randomized study. Clin J Am Soc Nephrol. Apr 2012;7(4):581–7. Epub 2012/03/01. [DOI] [PubMed] [Google Scholar]
- 33.Gasu V, Ashong M, Seferi A, Fitzpatrick A. Effectiveness of phosphate binders in adult patients with end stage renal disease receiving hemodialysis: a systematic review. JBI Database System Rev Implement Rep. Jan 2019;17(1):49–73. Epub 2018/09/12. [DOI] [PubMed] [Google Scholar]
- 34.Hassan MG, Vargas R, Zaher AR, Ismail HA, Lee C, Cox TC, et al. Altering calcium and phosphorus levels in utero affects adult mouse mandibular morphology. Orthod Craniofac Res. May 2019;22 Suppl 1:113–9. Epub 2019/05/11. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura A, Miyado K, Nasu M, Kono T, Umezawa A. Phosphorus-insufficient maternal milk is associated with ectopic expression of collagen I and female-specific bony changes in infant mouse cartilages. Regen Ther. Jun 2015;1:5–10. Epub 2015/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ALBRIGHT F, SMITH PH, RICHARDSON AM. POSTMENOPAUSAL OSTEOPOROSIS: ITS CLINICAL FEATURES. Journal of the American Medical Association. 1941;116(22):2465–74. [Google Scholar]
- 37.Khosla S, Monroe DG. Regulation of Bone Metabolism by Sex Steroids. Cold Spring Harbor perspectives in medicine. Jan 2 2018;8(1). Epub 2017/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baylink D, Wergedal J, Stauffer M. Formation, mineralization, and resorption of bone in hypophosphatemic rats. J Clin Invest. Dec 1971;50(12):2519–30. Epub 1971/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu ES, Zalutskaya A, Chae BT, Zhu ED, Gori F, Demay MB. Phosphate interacts with PTHrP to regulate endochondral bone formation. Endocrinology. Oct 2014;155(10):3750–6. Epub 2014/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raisz LG. The pharmacology of bone. Ration Drug Ther. Jun 1971;5(6):1–7. Epub 1971/06/01. [PubMed] [Google Scholar]
- 41.Raisz LG, Niemann I. Effect of phosphate, calcium and magnesium on bone resorption and hormonal responses in tissue culture. Endocrinology. Sep 1969;85(3):446–52. Epub 1969/09/01. [DOI] [PubMed] [Google Scholar]
- 42.Chung UI, Kawaguchi H, Takato T, Nakamura K. Distinct osteogenic mechanisms of bones of distinct origins. J Orthop Sci. 2004;9(4):410–4. Epub 2004/07/28. [DOI] [PubMed] [Google Scholar]
- 43.Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220. Epub 2000/10/14. [DOI] [PubMed] [Google Scholar]
- 44.Reichert JC, Gohlke J, Friis TE, Quent VM, Hutmacher DW. Mesodermal and neural crest derived ovine tibial and mandibular osteoblasts display distinct molecular differences. Gene. Aug 1 2013;525(1):99–106. Epub 2013/05/02. [DOI] [PubMed] [Google Scholar]
- 45.Lee JT, Choi SY, Kim HL, Kim JY, Lee HJ, Kwon TG. Comparison of gene expression between mandibular and iliac bone-derived cells. Clin Oral Investig. Jul 2015;19(6):1223–33. Epub 2014/11/05. [DOI] [PubMed] [Google Scholar]
- 46.Marini M, Bertolai R, Ambrosini S, Sarchielli E, Vannelli GB, Sgambati E. Differential expression of vascular endothelial growth factor in human fetal skeletal site-specific tissues: Mandible versus femur. Acta Histochem. Apr 2015;117(3):228–34. Epub 2015/03/15. [DOI] [PubMed] [Google Scholar]
- 47.Boskey AL, Guidon P, Doty SB, Stiner D, Leboy P, Binderman I. The mechanism of beta-glycerophosphate action in mineralizing chick limb-bud mesenchymal cell cultures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. Nov 1996;11(11):1694–702. Epub 1996/11/01. [DOI] [PubMed] [Google Scholar]
- 48.Chung CH, Golub EE, Forbes E, Tokuoka T, Shapiro IM. Mechanism of action of beta-glycerophosphate on bone cell mineralization. Calcif Tissue Int. Oct 1992;51(4):305–11. Epub 1992/10/01. [DOI] [PubMed] [Google Scholar]
- 49.Jung K, Pergande M. Influence of inorganic phosphate on the activity determination of isoenzymes of alkaline phosphatase in various buffer systems. Clin Chim Acta. Mar 28 1980;102(2–3):215–9. Epub 1980/03/28. [DOI] [PubMed] [Google Scholar]
- 50.Meyer MH, Dulde E, Meyer RA Jr. The genomic response of the mouse kidney to low-phosphate diet is altered in X-linked hypophosphatemia. Physiol Genomics. Jun 17 2004;18(1):4–11. Epub 2004/04/01. [DOI] [PubMed] [Google Scholar]
- 51.Jimenez PM, Douthat W, Orias M. Can aluminum-based binders be used safely? Semin Dial. Jul-Aug 2011;24(4):430–1. Epub 2011/08/02. [DOI] [PubMed] [Google Scholar]
- 52.Calvo MS, Uribarri J. Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. Am J Clin Nutr. Jul 2013;98(1):6–15. Epub 2013/05/31. [DOI] [PubMed] [Google Scholar]
- 53.Uribarri J, Calvo MS. Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin Dial. May-Jun 2003;16(3):186–8. Epub 2003/05/20. [DOI] [PubMed] [Google Scholar]
- 54.Gutierrez OM, Luzuriaga-McPherson A, Lin Y, Gilbert LC, Ha SW, Beck GR Jr. Impact of Phosphorus-Based Food Additives on Bone and Mineral Metabolism. J Clin Endocrinol Metab. Nov 2015;100(11):4264–71. Epub 2015/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Savica V, Calo LA, Monardo P, Caldarera R, Cavaleri A, Santoro D, et al. High phosphate content beverages in dialysis patients: relevance for hyperphosphatemia and cardiovascular risk. Nutr Metab Cardiovasc Dis. Oct 2008;18(8):e39–40. Epub 2008/06/24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


