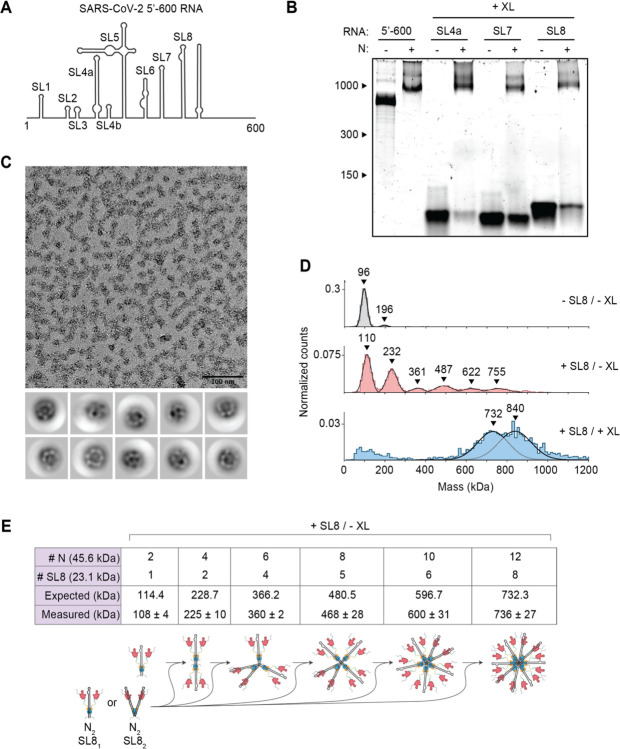
Figure 2. Stem-loop RNA, in complex with N protein, drives ribonucleosome formation.
(A) Schematic of RNA secondary structure in the 5’−600 RNA (45). (B) Native gel analysis of 15 μM N protein mixed with 256 ng/μl of the indicated RNAs. Samples containing stem-loop RNA were crosslinked to stabilize the resulting complex, while the 5’−600 RNA sample was left un-crosslinked. Corresponding denaturing gel analysis shown in fig. S1A. (C) Fractions 7 and 8 of GraFix-purified SL8 assembled vRNPs were combined and analyzed by negative stain electron microscopy and two-dimensional classification. (D) Mass photometry analysis of indicated N protein-RNA mixtures. Top: N protein alone; middle: N protein mixed with SL8, un-crosslinked; bottom: crosslinked complexes of N protein bound to SL8 (data reproduced from fig. S1B for ease of comparison). Representative of two independent experiments (table S1). (E) Predictions of N protein and RNA stoichiometry, based on measured masses of N protein in complex with SL8 RNA without crosslinker (D, middle panel). Measured masses are means ± standard deviation in two independent experiments (table S1). Below the table is a schematic of a proposed assembly mechanism in which N protein dimers, bound to one or two stem-loop RNAs, iteratively assemble to the full vRNP.