Abstract
Since the US Food and Drug Administration (FDA) began monitoring the quality of pharmaceutical manufacturing by enforcing current good manufacturing practices roughly 60 years ago, forces related to the global economy have changed, rendering the task of monitoring quality more difficult. Alternative strategies by groups like Valisure, LLC, and the University of Kentucky Drug Quality Study to monitor the quality of the currently circulated US drug supply through end-product testing and screening have resulted in several concerning findings. Given the successful approaches of identifying quality defects in pharmaceuticals by non-regulatory bodies, and considering the changing landscape and pressures on manufacturing, the FDA, large buying groups, and the US Department of Defense should consider these alternative strategies as a means to augment current regulatory activities.
Keywords: Drug quality, FDA, Globalization
Introduction
With a long history of quality regulation in the pharmaceutical industry, the US Food and Drug Administration (FDA) is considered a global leader among similar regulatory bodies in other countries. The FDA approach to the monitoring of a drug manufacturer’s quality through process validation was adopted in the 1960s [1]. Almost 60 years later, long after the vicissitudes of manufacturer globalization, unanticipated economic forces, unprecedented drug shortages, and the advent of a global pandemic, introspection by FDA regarding the best strategy for monitoring quality is warranted. The authors will review this regulatory history and the forces shaping drug quality challenges and summarize recent efforts to assess drug quality that are alternative to traditional regulatory methods.
Regulatory History and Directional Decision Regarding Quality
The FDA is considered, globally, to be the preeminent regulator of pharmaceutical quality. With its early beginnings under the US Department of Agriculture’s Bureau of Chemistry, the passage of the 1906 Pure Food and Drug Act set the FDA on a trajectory to continually improve the pharmaceutical industry for consumers [2].
After addressing adulteration, misbranding, and eventually the actual proven safety of drugs for use in humans, the FDA required that drugs be proven effective before use with the passage of the 1962 Kefauver Harris Amendment to the Federal Food, Drug, and Cosmetic Act. A key piece of information included in this amendment was a new definition of adulteration. Products manufactured in facilities where the process did not conform to current good manufacturing practices (cGMP) would be deemed tainted moving forward [1]. Although this was the first mention of cGMP in the Federal Register, the practice of monitoring the manufacturing process and the associated supporting data would further develop and be referred to as process validation. Essentially data associated with the manufacturing process needed to conform to the acronym ALCOA in that data needed to be attributable, legible, contemporaneously recorded, original or a true copy, and accurate [3].
While developing the cGMP approach, the FDA oversaw the testing of 4600 pharmaceuticals that were approved and in use between 1938 and 1962. This exhaustive work took years and found that roughly 8% of the products tested to be either supra- or subtherapeutic [4]. The labor-intensive work of this study coupled with the maturing framework of cGMP compliance efforts led the FDA to pursue an approach that focused on manufacturing process more than the product. Understandably, the prospect of end-product testing in the 1970s presented challenges with regard to labor, expertise, and technology. With cGMPs now representing the sine qua non for drug quality, the FDA set out to monitor quality through monitoring manufacturing processes by visiting the plants and observing processes, practices, and documentation.
The decision to pursue quality through monitoring for cGMPs was made at a time when most of the drugs consumed in the USA had the majority of their active pharmaceutical ingredients (API) manufactured and final product manufactured in the USA. Equipped with cGMPs, as outlined in Title 21 of the Code of Federal Regulations (CFR), FDA inspectors could then perform unannounced site visits to facilities, remain on site as long as needed, and hold manufacturers accountable for their actions. It should be noted that manufacturing in the USA is no guarantee of quality, however. A number of US manufacturers produce drugs under consent decrees, and most of the so-called “16 Black Holes” (manufacturers with the largest long-term unresolved cGMP violations) are in the USA [5].
Economic Pressures on Drug Prices Lack a Counterbalance
Since Sen. Estes Kefauver held the first congressional hearing on rising drug prices in 1959, congress has held hearings on drug prices every decade since [6]. The ritual of elected US officials revisiting drug prices continues to build pressure among the general public given that most countries with similar sizes and economies have successfully put some level of price controls in place [7]. Although the historical attempts of addressing drug prices in these political arenas have largely been grandstanding, the effect has saturated the media such that concerns over drug quality have rarely surfaced.
The 1984 Hatch–Waxman Act created an avenue for generic manufacturers to enter the market much faster and an incentive to be first to market [8]. Hatch–Waxman led to considerable expansion of generic manufacturing with growing pressures on manufacturers to produce low-priced versions that would compete against innovator products such that by 1990 roughly 40% of drugs filled were now generic costing roughly 65% less than comparable innovator products [9].
The group purchasing organization (GPO) market had slow beginnings in the early 1900s, operating by leveraging competitive pricing to vendors for its member hospitals through aggregate negotiations. However, in the 1987 Medicare and Medicaid Patient Protection Action, GPOs were granted safe harbor for collecting rebates from pharmaceutical manufacturers, thus positioning GPOs for significant economic growth [10]. From 1997 to 2021, through several mergers and acquisitions, some of the largest GPOs, University HealthSystem Consortium and Voluntary Hospitals of America, combined with several more GPOs to form Vizient, Inc. [11]. With this new lopsided market, Vizient represents over half of the hospitals in the USA and 97% of academic medical centers within the USA [12]. Premier, Inc. trails in second with about half of the sales of Vizient, Inc. [13]. Concern has grown that with an oligopolistic market, the common practice by GPOs of extending exclusivity agreements to first-to-market generics will stymie additional generics coming to market, therefore leading to less redundancy in the supply chain and decreasing the resilience to market disruptions and shortages. The oligopoly economics of GPOs in the pharmaceutical industry have been covered in the literature and documented by the FDA. The consolidation of GPOs and their extraordinary abilities to negotiate lower prices are believed to put further pressure on manufacturers with pricing, creating a “race to the bottom” scenario [14–16]. It should also be stated that while the literature points to the pricing pressures exerted by GPOs as leading to potential manufacturing issues and thus supply disruptions, the largest of these GPOs, Vizient, has applied considerable pressure to the current system to address shortages and more recently created the End Drug Shortages Alliance [17].
Since 1980, the FDA has listed innovator products and their generic equivalents within a publication, which is now an online resource, called the Orange Book [18]. This system of listing therapeutically equivalent medications as “AB rated” has streamlined drug purchasing practices such that all major drug wholesalers incorporate the Orange Book into their online ordering systems. Pharmacy personnel looking to purchase a drug product can search by brand or generic name, and immediately, all applicable therapeutic equivalents will be listed. The dynamic this has created is a two-dimensional platform for medication ordering, the first being therapeutic equivalence, and the second being price.
In 19 states, if all other prescribing requirements are met, a pharmacist is required to replace a brand name drug with a generic [19]. Additionally, the majority of states requires that generic substitution be less expensive or equal in price to the brand name. With state laws in place and pharmacies pursuing lower priced generic medications when available, the de facto choice will almost always be the cheapest generic.
US consumers, buying groups, or other purchasers that inspect the packaging, labeling, and package inserts of FDA-approved medications they receive will notice that in few cases will the manufacturer indicate where the product was made. This may be a difficult task for manufacturers given that the product components could have been made in different countries and assembled in yet another country. Often the terms “Distributed by” or “Marketed by” will be used in which the address given will be a US-based location. Currently, the location that the medications are made is considered a proprietary information, and US consumers and entities, other than the FDA, are not granted this information [20]. With numerous state laws requiring the cheapest generic, and wholesalers directing lower priced generics with incorporation of the Orange Book, the transparency of where medication is being manufactured may be a missing economic factor in the consumer-driven decision-making process.
Pricing Pressures Leading to Globalization and Impact Quality
Although originally reliant on US-based manufacturing, the pharmaceutical industry gradually shifted starting by the year 2000 and by 2005, FDA-regulated sites that were foreign surpassed the number of US-based sites [21]. By 2015, the majority of API used for pharmaceuticals, with the final manufacturing processes taking place in the USA, Europe, and India, originated from China. With a faster pathway forward for manufacturers to bring generic products to market, and increased pressures to lower costs, the USA has seen a major shift in manufacturing such that roughly 80% of pharmaceuticals have either their API or final product manufactured overseas, mostly in the Asia–Pacific region [22].
By offering cheaper labor, the offshoring of pharmaceutical manufacturing allowed for lower pricing. Importantly this also created logistical difficulties with FDA regulators who needed to coordinate cGMP inspections in advance due to visa requirements. Not subject to the unannounced visits by inspectors as in the states, offshore manufacturing plants could have up to a 2-month notice before inspections occur, allowing manufacturers to control the inspection process and obfuscate evidence of data fabrication [23]. As a result, FDA has begun opening offices in these regions. A report from the House Appropriations Committee conveyed with the FY 2021 omnibus bill instructed FDA to restart a pilot program of unannounced short-term inspections in India and to set up a similar pilot program in China. An official with the FDA declared in December 2021 that the agency will soon restart unannounced onsite inspections in India and China [24].
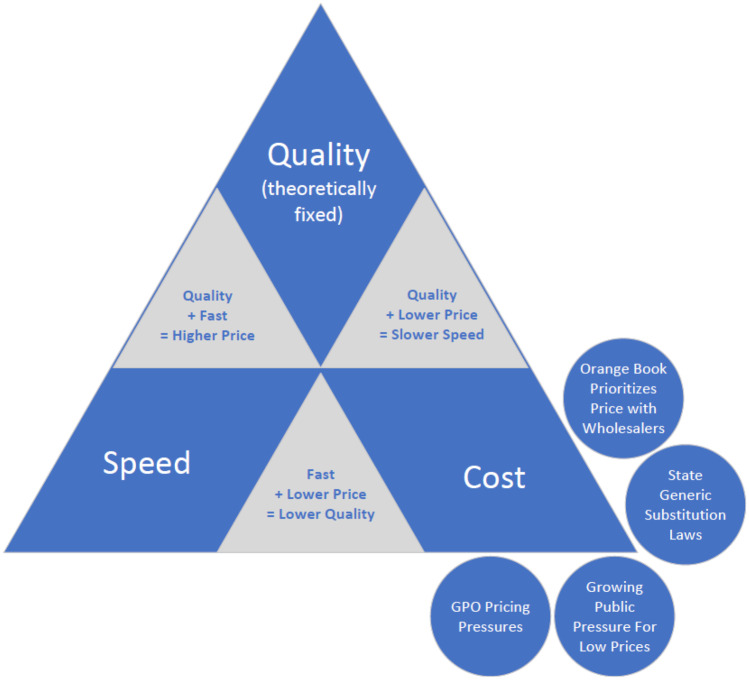
Examining the project management concept of the “iron triangle” with regard to the quality, cost, and speed of pharmaceutical manufacturing, with the understanding that the point of quality (cGMP requirements) is unmovable and generally adds 25% to the costs of production, generic manufacturers face significant pressures that could compromise the quality of production [21]. Figure 1 depicts this triangle with the concepts discussed in this review and visualizes the pressures placed on manufacturers to focus on cost that could cause the fixed point of quality to be compromised.
Fig. 1.
Triangle depicting competing priorities of cost, speed, and quality in the manufacturing process with economic forces outlined in this review
In 2018 and 2019, investigative journalist works emerged that brought the globalization of the pharmaceutical industry into focus. Rosemary Gibson’s China Rx: Exposing the Risks of America's Dependence on China for Medicine and Katherine Eban’s Bottle of Lies: The Inside Story of the Generic Drug Boom both highlighted the extreme dependence on China and India for the supply of pharmaceuticals for the USA. Eban’s account, referred to as a modern day equivalent to the 1906 Sinclair novel The Jungle, highlights the ongoing challenges the FDA faces as they tackle quality using the cGMP requirements in Title 21 CFR while operating in a globalized manufacturing environment. Eban juxtaposes the conflict within the FDA in which front-line inspectors feel that they are the last line of defense in identifying quality and safety issues. At the same time, FDA management is experiencing increasing pressures to approve generic formulations so that prices are lowered for consumers and providers, and hospitals continue to complain about patient care being disrupted by drug shortages. To highlight this conflict, Eban found that from 2013 to 2018, 78 out of 864 foreign drug plant inspections were originally found to have cGMP violations requiring an official action indicated (OAI) on their Form 483; however, every one of these was downgraded by FDA administrators later as voluntary action indicated (VAI). Downgrading from OAI to VAI would ensure no major disruptions in the manufacturers’ ability to continue to supply their pharmaceutical products to the USA. In this same time frame, out of 11,642 US-based manufacturer inspections that were found to have an OAI, only 1 was downgraded to VAI [21, 25].
One of the downgraded findings during this time period came from a finding by an FDA inspector at the Zhejiang Huahai Pharmaceuticals plant in China, the world’s largest manufacturer of the API valsartan, a drug used to lower blood pressure. The inspector found that the company failed to investigate potential impurities from test results and cited the manufacturer with an OAI on the Form 483. Four months after this filing, in a memo, the agency downgraded this finding to VAI. Then, in July 2018, a national recall was issued by FDA after an independent Connecticut-based pharmacy, Valisure, found 4 carcinogens that were the result of the impurities originally identified by the FDA inspector [21].
A key to cGMP requirements, manufacturers producing pharmaceuticals must demonstrate data to support their pharmaceutical production (i.e., ALCOA). In the 2013 case of U.S. v. Ranbaxy USA, Inc., Ranbaxy, an Indian-based generic manufacturer, admitted to “making false, fictitious, and fraudulent statements to the FDA in Annual Reports filed in 2006 and 2007 regarding the dates of stability tests conducted on certain batches of Cefaclor, Cefadroxil, Amoxicillin, and Amoxicillin and Clavulanate Potassium” manufactured at their plant in India. The settlement led to a $500 M payment by the manufacturer. The case resulted from a complaint filed by a former employee whistleblower, Dinesh Taulker, and highlighted the ability of the manufacturers to obfuscate quality concerns within their data from FDA when operating in a globalized manner [26].
Congressional Concern Regarding Present-Day FDA Inspections of Foreign Pharmaceutical Manufacturers
In a US congressional hearing with the House Committee on Energy and Commerce that followed the coverage by Eban and Gibson, Janet Woodcock, the FDA director of the Center for Drug Evaluation and Research, was questioned about the oversight and security of the US supply of medications coming from overseas, and particularly China. To the surprise of the committee, Woodcock stated, “The FDA doesn’t know whether Chinese facilities are actually producing APIs, how much they are producing, or where the APIs they are producing are being distributed worldwide, including in the United States…Similarly, we do not have information that would enable us to assess the resilience of the U.S. manufacturing base, should it be tested by China’s withdrawal from supplying the U.S. market” [26].
Gibson, who testified alongside Woodcock, listed eight recommendations moving forward to assist in improving integrity, quality, and safety of the US supply chain that is tied to China. Among these she stated “A Consumer Reports-type independent testing of every batch of every generic manufacturer’s medicine and public reporting of the results in real time will help restore the public’s trust in their medicines” [23].
Christopher Priest, the Chief of Staff for the US Defense Health Agency Operations Directorate, the agency that supports the US Department of Defense (DoD) and is responsible for the supply of its pharmaceuticals, testified at this same hearing with Woodcock and Gibson. Priest stated, “DoD is wholly dependent on the consumer market to produce and distribute pharmaceutical products it requires, spending approximately $7.5 billion annually…We are concerned about any situation where foreign actors, including China, control substantial access to critical war-fighting material. The issues raised by the increased Chinese dominance in the global API market cannot be overstated. There is risk that existing regulations, programs, and funding are insufficient to guarantee US independence from unreliable foreign suppliers. Our concern is the ability of the domestic manufacturing capability to adjust to that risk, alternate sources, if any, and how long the solutions would take to produce results” [23].
Since 1998, the Government Accountability Office (GAO) has reported concerns about the need to improve the FDA’s foreign inspection program [28]. A 2016 GAO report found that almost a third of the roughly 3000 foreign manufacturing plants had yet to be inspected by the FDA [22]. The GAO recently documented their concerns in a report released March 4, 2021, and these detailed findings were shared with the US House of Representatives Appropriations Subcommittee for Agriculture, Rural Development, Food and Drug Administration, and Related Agencies. Findings included that prior to the pandemic, with foreign inspections in countries such as India and China, the FDA is currently giving 3-month advance notice in many cases, and FDA inspectors are still relying on staff from the manufacturer facility to assist as translators. Concerns are that this continues to create a double standard so that foreign manufacturers have the ability to correct manufacturing issues just before or even during FDA inspections. Additionally, there are concerns that the current foreign inspection program does not allow for extended inspections to follow leads as is allowed in the states and in many cases inspectors are a team of one, limiting their chances of identifying issues in vastly large manufacturing facilities. From an overall volume of inspections, the GAO found that the number of inspections has generally declined since 2016, which FDA attributed to a number of vacancies [29].
Although GAO found that the FDA started, for the first time, to conduct more inspections overseas than domestic in 2015, reflecting more accurately where manufacturing is occurring, in March of 2020, the FDA paused all foreign and domestic inspections due to the COVID-19 pandemic. At this time, the FDA chose to only conduct a handful of inspections that were deemed mission critical. The GAO reported that during this pause, the FDA has relied on the following tools to continue inspection work: relying on inspections conducted by foreign regulators, reviewing manufacturer records, and sampling and testing drugs at the US border [29].
Following the FDA announcement that the agency would resume foreign inspections in February of 2022, the GAO published a 67-page report the same week stating that the foreign inspection program is in need of an overhaul [30].
Recent Efforts to Examine Quality of FDA-Approved Drugs Within the US Supply Chain
To assess the recent efforts to evaluate the drug supply chain for quality concerns, a PubMed Central (PMC) literature review was performed with the search criteria of “drug quality” and “drug contamination” between January 1, 2019, and January 1, 2022. This search only revealed a handful of organizations actively publishing results associated with the analysis of the drug supply chain. Of note were the University of Kentucky (UK) Drug Quality Study (DQS) and Valisure, LLC. These studies present data related to integrity and safety of medications found in the drug supply chain [31].
Additionally, a review of all FDA citizen petitions and FDA Freedom of Information Act (FOIA) requests submitted between January 1, 2019, and January 1, 2022, was performed to determine which organizations submitted citizen petitions or requested information related to the integrity of the drug supply chain [32, 33]. This review identified the University of Kentucky Drug Quality Study and Valisure, LLC, as the only organizations of note who either submitted a citizens petition or a FOIA request to the FDA or have demonstrated sustained efforts to assess the quality of pharmaceuticals on the US market.
The paucity of published literature and public data related to analyzing the integrity of the drug supply chain is concerning. With the recent findings published in the media by investigative journalists and highlighted by GAO reports, the FDA should be prepared to perpetuate its history of continuous quality improvement through evolving its approaches to meet the new challenges to quality that the changing manufacturing landscape has created.
Valisure
Valisure, LLC, established in 2015, is an independent analytical lab located in New Haven, CT, USA, that has partnered with the pharmacy, Medly, to dispense pharmaceuticals that have passed quality testing. Valisure operates a laboratory with ISO 17025 accreditation and is DEA- and FDA-registered. Medly is a pharmacy licensed by multiple state boards of pharmacy and per their Connecticut license dispenses typical retail prescriptions and does not compound sterile injectable drugs. The mission of the Valisure-Medly analytical pharmacy is “to bring transparency and increased quality to the pharmaceutical industry, and to deliver these benefits throughout the healthcare system” [34, 35]. Published reports on findings describe several analytical methodologies and techniques utilized to identify substandard, adulterated, and/or contaminated pharmaceuticals on the market and are outlined in the larger summary Table 1. The reported findings from both the literature and the citizen petition findings are outlined in Table 2 [34, 36–44].
Table 1.
Analytical methods utilized by Valisure as identified in various publications and sources
| Analytical methods | Details |
|---|---|
| Destructive methods | Dissolution testing |
| Simulated gastric fluid test | |
| Simulated intestinal fluid test | |
| Gas chromatography/mass spectrometry | |
| Gas chromatography flame ionization detection | |
| Liquid chromatography-high resolution mass spectrometry | |
| Non-destructive methods | Fourier transform-infrared spectrometry |
| Nuclear magnetic resonance spectrometry | |
| Raman spectrometry |
Table 2.
Summary of Valisure findings from quality surveillance*
| Medications/cosmetics | Source | Findings | |
|---|---|---|---|
| Literature | FDA citizen petition | ||
| Acetaminophen | Kenkyu 12–11-18 | Several non-prescription gelcap formulations of acetaminophen marketed as fast acting have a dissolution rate that is slower than comparable, lower priced tablets | |
| Valsartan | 6–13-19 | Contamination found in oral prescription formulations of valsartan. Identified high levels of the carcinogen N,N-dimethylformamide. Adulteration and misbranding. Multiple manufacturers and lots | |
| Ranitidine | JAMA Open Network 1–4-21 | 9–9-19 | Contamination found in oral prescription and non-prescription formulations of ranitidine. Identified high levels of the carcinogen N-nitrosodimethylamine. Misbranding. Multiple manufacturers and lots |
| Metformin | medRxiv 5–25-20 | 3–2-20 | Contamination found in oral prescription formulations of metformin. Identified high levels of the carcinogen N-nitrosodimethylamine. Adulteration and misbranding. Multiple manufacturers and lots |
| Ethanol hand sanitizer | 3–24-21 | Contamination found in non-prescription formulations of ethanol hand sanitizer. Identified high levels of the carcinogen benzene, methanol, and acetaldehyde. Adulteration and misbranding. Multiple manufacturers and lots | |
| Sunscreen and after-sun care products | 5–24-21 | Contamination of non-prescription/cosmetic formulations of sunscreen and after-sun care products. Identified high levels of the carcinogen benzene. Adulteration and misbranding. Multiple manufacturers and lots | |
| Deodorant body sprays | 11–3-21 | Contamination of non-prescription/cosmetic deodorant body sprays. Identified high levels of the carcinogen benzene. Adulteration and Misbranding. Multiple manufactures and lots | |
*Valisure also states that their pharmacy rejects more than 10% of the batches because of detected contaminants, medication dissolution issues, and incorrect doses among other issues
Valsartan
The findings of the valsartan contamination with the carcinogen N,N-dimethylformamide, as mentioned earlier in this review paper, highlighted the disconnect between FDA inspectors and officials in Silver Spring, Maryland, given they downgraded original findings from inspectors regarding impurities discovered in the manufacturing process. In a press release Dr. Woodcock stated, “We have carefully assessed the valsartan-containing medications sold in the United States, and we’ve found that the valsartan sold by these specific companies does not meet our safety standards. This is why we’ve asked these companies to take immediate action to protect patients” [21, 45].
Ranitidine
On the heels of the valsartan findings, the ranitidine contamination findings with the carcinogen N-nitrosodimethylamine (NDMA) led to much larger press coverage given the wide use of this product both in prescription form and over-the-counter. Table 3, taken from the Valisure citizen petition, lists ranitidine products found to have NDMA. An official recall was issued by FDA 6 months after Valisure submitted the petition [34, 37].
Table 3.
Ranitidine samples tested by Valisure that formed very high levels of NMDA
| 150 mg tablets or equivalent | Lot # | NDMA per tablet (ng) |
|---|---|---|
| Reference powder* | 125,619 | 2,472,531 |
| Zantac, brand OTC | 18M498M | 2,511,469 |
| Zantac (mint), brand OTC | 18H546 | 2,834,798 |
| Wal-Zan, Walgreens | 79L800819A | 2,444,046 |
| Wal-Zan (mint), Walgreens | 8ME2640 | 2,635,006 |
| Ranitidine, CVS | 9BE2773 | 2,520,311 |
| Zantac (mint), CVS | 9AE2864 | 3,267,968 |
| Ranitidine, equate | 9BE2772 | 2,479,872 |
| Ranitidine (mint), equate | 8ME2642 | 2,805,259 |
| Ranitidine, strides | 77024060A | 2,951,649 |
*Estimated NDMA scaled to equivalent of 150 mg
As shown in Table 2, Valisure continues to identify upstream manufacturing issues with products that are FDA-approved and currently in circulation to consumers through interstate commerce. Valisure, through its partnership with Medly, offers an alternative approach to accessing and screening medications commonly used by patients in the outpatient setting and are generally recognized as pioneers in taking alternative approaches to monitoring the US drug supply [34, 37]. The FDA is now very focused on the content of nitrosamines in active pharmaceutical ingredients and drug products. Although Valisure is a non-governmental organization (NGO), the number of definitive analyses performed on suspect products, and the subsequent positive responses by FDA to petitions, places Valisure closer to the role of a regulatory body.
The University of Kentucky Drug Quality Study
The University of Kentucky (UK) Drug Quality Study (DQS) was established in August of 2019 to engage in consumer-level quality assurance screening for drugs used within UK healthcare pharmacies. DQS currently screens about 300 injectable medications, using Fourier transform near-infrared (FT-NIR) and Raman spectrometry, for quality defects indicated by variability in absorbance peak intensities and locations. The mission statement of DQS is “To promote quality over price, truth over rationale, and reform over inertia in the pharmaceutical supply chain.”
The UK DQS team contacted the FDA Office of Pharmaceutical Quality, within the Center for Drug Evaluation and Research (CDER), multiple times regarding the operational setup of UK DQS. Given the stated goals, FDA advised UK DQS to pursue good laboratory practices (GLP), stipulating that as long as work did not include activities “directly related and relevant to drug manufacturing operations (including testing and drug product batch release decision by a drug manufacturer) as defined under current good manufacturing practice (CGMP) regulations,” the UK DQS is “not required to register and subject to inspections under Sect. 510(h) of the FD&C Act.” The FDA also recommended adherence to “internationally harmonized laboratory standards, e.g., ISO/IEC 17,025:2017” and to “consider seeking ISO accreditation as conforming to that standard” (Personal Communication from CDER, September 28, 2020). Based on this guidance, UK DQS is committed to pursuing compliance with the standards set forth by GxP (i.e., good laboratory practices, good clinical practices, good manufacturing practices) requirements and strives to conform to the ISO/IEC 17,025:2017 standards. It was also noted in CDER communications that findings from ongoing analyses were encouraged to be reported through the FDA MedWatch system.
Acetazolamide
Active continual scanning of injectable medications began in August of 2020. Shortly thereafter, DQS identified intralot variability within a single lot of acetazolamide for injection. Upon further investigation with destructive testing through the UK healthcare clinical laboratory and through an independent third-party lab, an FDA Medwatch report was issued, and a citizen petition was filed with the FDA on the same day, September 30, 2020. Among other things, the petition requested that the FDA recall certain lots of acetazolamide and addresses concerns that lots tested from two FDA-approved manufacturers did not meet the USP standard. Findings in the petition described the lots to be adulterated, contaminated, and misbranded. Destructive tests included melting point, liquid chromatography with tandem mass spectrometry, gas chromatography/mass spectrometry, and high-performance liquid chromatography. Completing the analyses and replicating the results at a third-party commercial lab using the regulatory assay took 2 months to complete. Vials in question were found to have, in some cases, more than six times the allowed impurities, leading to 80% API, far lower than the USP standard minimum of 97% API. Within a month of the petition being filed, there existed a national shortage of acetazolamide for injection [46, 47].
Remdesivir
In December of 2020, DQS identified irregularities among 144 samples from 7 lots of single-dose vials of Remdesivir following a process of examination with FT-NIR. The spectra of two lots indicate that the manufacturing process may have been operating outside of a state of process control. Although not confirmed with destructive testing because of the time required, the location of peaks indicates that these variations could be due to moisture content variability. Given that a USP monograph had not been published and a certified reference standard was not available at the time of these findings, no compendial testing could be performed [48, 49].
Directional Change and Additional Findings
Over a 15-month period of continuous monitoring, DQS has assembled a spectral library containing injectable medications commonly used in an inpatient care setting. Statistical analyses using DQS’ spectral library are performed to identify potential intralot and interlot variability in medications under review.
A major purpose of the CMC (chemistry, manufacturing, and controls) section of an IND (investigational new drug) application is to “draw a box” around what a manufacturer considers its drug product once it is on the market. Different analytical tests and measurements of the product can be considered different orthogonal axes of this multidimensional box. The product is what is inside this box, and everything else is outside. The definition of the box is exclusively held between the FDA and the manufacturer. No one else is allowed to know the precise definition that is in the NDA because that information is proprietary. In some cases, the US Pharmacopeia (USP) will have some information and reference standards available, but this is not always true. The challenge becomes to determine where the spectral library coincides with this box, and this is where destructive testing and compendial methods come in.
Since December 2021 DQS has solely utilized non-destructive screening using FT-NIR and Raman spectrometry as a means to identify potential concerns within the pharmaceutical supply chain, however, DQS does not complete the final destructive testing required for determining adherence to US compendial standards. This lengthy process (which can take months, by which time all that drug held by others will have been used) and the high cost of sending samples out for destructive compendial analysis were factors in this decision. Additionally, performing compendial analyses would position UK DQS more closely as a regulator. The DQS finds 1.2 unusual products/day on average. DQS reports its findings using the FDA MedWatch system and publishes its results in the scientific literature in an effort to hold manufacturers accountable to cGMP requirements and to improve patient outcomes by exerting positive pressure on the pharmaceutical supply chain. In one sense, this approach equates to UK DQS serving as a data collection service within the US supply chain that packages preliminary analyses and sends these findings to FDA. What investigative actions take place by FDA after reporting are unknown. By not completing the full suite of destructive tests, DQS leaves the FDA as the regulator, instead of the health system.
Table 4 indexes FDA MedWatch submissions that have been completed as well as any associated publications related to these submissions. In a review of MedWatch reports released from the fourth quarter of 2021, the FDA has not included medications with associated lot numbers for the 2 reports filed by DQS in December of 2021 [50, 51, 56, 57].
Table 4.
Comprehensive list of injectable medications with DQS findings reported to FDA
| Injectable medication | FDA MedWatch filing date | Publication-Pubmed Central ID |
|---|---|---|
| Acetazolamide | 09–30-2020 | Not applicable |
| Remdesivir | 11–02-2020 | PMC8679181 [48, 49] |
| Ceftaroline fosamil | 12–10-2021 | PMC8966977 [50, 56] |
| Cosyntropin | 12–27-2021 | PMC8758055 [51, 57] |
| Dacarbazine | 01–10-2022 | PMC8758066 [52, 58] |
| Levothyroxine sodium | 01–12-2022 | PMC9015687 [53, 59] |
| Micafungin sodium | 01–26-2022 | PMC8966979 [54, 60] |
| Measles, mumps, and rubella | 02–04-2022 | PMC8966980 [55, 61] |
| Piperacillin and tazobactam | 04–07-2022 | PMC9060210 [62] |
MMR Vaccine
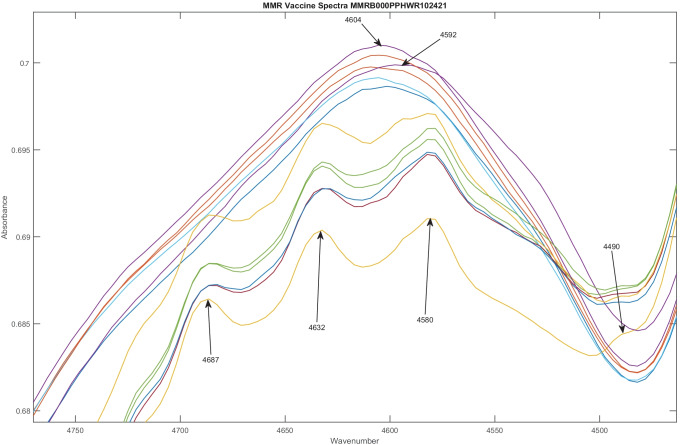
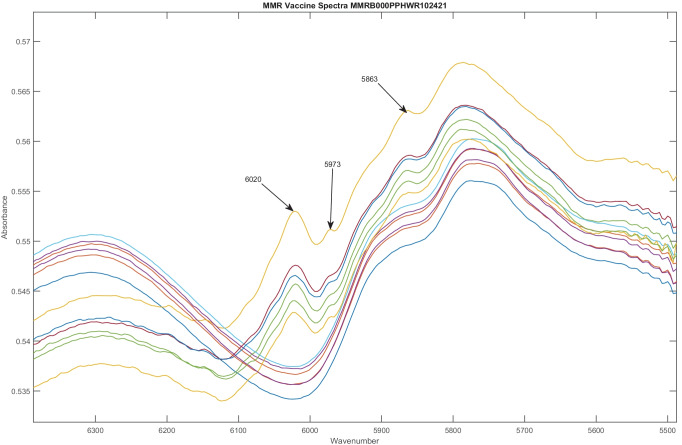
One of the more recent publications and FDA MedWatch reports, regarding the measles, mumps, and rubella (MMR) vaccine, highlights some key insights that can be gathered from continual scanning and analysis using purely non-destructive techniques. In analyzing 12 vials from a single lot, U006488, spectra from FT-NIR revealed 2 distinct groups, revealing intralot variation. Six vials had distinct peaks at 4490, 4580, 4832, and 4687 cm−1 as shown in Fig. 2 and 5863, 5973, and 6020 cm−1 as shown in Fig. 3. These were not present in the other 6 vials from the same lot. Six of the 12 vials from the same lot appeared 14.5 SDs from the other vials on a subcluster detection test, suggesting that they represent different material [55, 61]. As a result, the DQS analyzed spectra from all 198 vials of MMR vaccine scanned from 12 lots from September 2020 to January 2022.
Fig. 2.
Spectra of 12 MMR vaccine doses from the same lot, U006488, vary considerably. In general, drugs in the same lot have similar spectra. However, in this figure, two distinct groups of spectra are found, with 6 in each group. One group of spectra has peaks at 4490, 4580, 4832, and 4687 cm−1 that the other group does not have. In the other group, the location of the major peak varies, appearing sometimes at 4592 cm−1 and other times at 4604 cm−1
Fig. 3.
Spectra of 12 MMR vaccine doses from the same lot, U006488, in a different spectral region from Fig. 2. Again, 2 distinct groups of spectra are found, with 6 spectra in each group. Peaks at 5863, 5973, and 6020 cm−1 are present in one group of spectra, but absent in the other
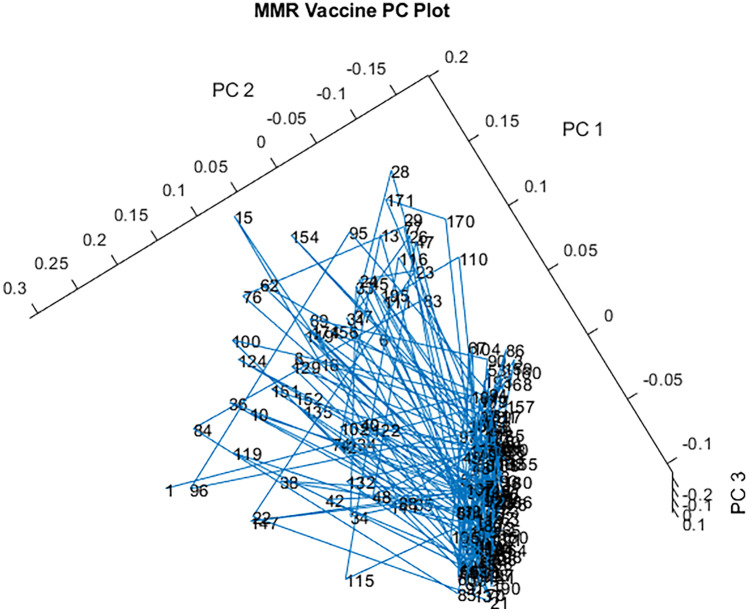
A 3-D principal component (PC) plot of all 198 vials from 12 lots of the spectral library of MMR vaccine is published in the same communication [61] and is shown in Fig. 4. The blue lines connecting the vials in the order in which they were scanned keep crossing back and forth between the main ellipsoidal cluster and the outliers, indicating that the outliers are scattered throughout the lots. As depicted in Fig. 4, the spectral library contains 140 vials in one tight ellipsoidal group, and 58 vials (30%) are outside that group (39.7 SDs using a subcluster detection test), also suggesting that the library vials contain differing materials [55, 61]. Despite the potential process control issues identified, it should be noted that the UK DQS team made no claims that the products scanned failed to meet the current compendial standards and stated they “support vaccination recommendations given by the Advisory Committee on Immunization Practices of the Center for Disease Control” and that “Given immunizations are the safest and most cost effective way of preventing disease, disability, and death, the authors support immunizations as one the greatest public health achievements” [61].
Fig. 4.
3-D PC plot of the spectral library of MMR vaccine. A total of 198 vials were scanned from 12 different lots. The tight ellipsoidal group on the right contains 140 of the 198 vials
Unlike the initial finding from acetazolamide and all of the reported findings from Valisure, DQS does not and cannot claim that their screening results with items such as the MMR vaccine indicate adulterated, misbranded, contaminated, or subtherapeutic pharmaceuticals due to the fact that destructive testing using compendial methods is not performed. At least 3 or 4 uncorrelated analytical chemistry tests are needed to confirm findings. Not listed in this publication, prior to the December 2021 timeline, the UK DQS team identified polymerization-associated irregularities with injectable ampicillin sodium dosage forms utilizing FT-NIR. The question was “Were these spectra inside the CMC box or not?” Subsequent destructive and compendial tests conducted by a third-party lab months later indicated that these dosage forms complied with compendial standards (i.e., were inside the box), and appropriate metadata were linked to the spectra in the library to mark such spectral changes as acceptable going forward. This highlights the difference between non-destructive screening and compendial analyses using USP methods. However, as demonstrated in the previous published work, and in the acetazolamide citizen petition, utilizing non-destructive techniques, such as FT-NIR, can be a tool for identifying process control concerns (e.g., inhomogeneity within batches) with pharmaceutical products that point to larger issues with quality in the production process. This knowledge of quality of individual lots can be shared via publications quickly enough to make a difference in patient care and FDA decisions. To make it easy to locate the reports, the data are published as Rapid Communications in the same journal, forming a public database of each drug and lot number tested.
Conclusion and Future Prospects
The testing conducted by Valisure, LLC, and the screening conducted by UK DQS, and their reported findings to FDA, are demonstrations of direct efforts by non-regulatory bodies to identify quality defects and potential safety concerns with FDA-approved medications already in use in the USA. The efforts of these two organizations create a level of tension between the medical community, the US public, and the FDA. There is no doubt that the FDA is still the preeminent regulator of drug quality in the world and that patients should continue to take their FDA-approved medications as prescribed by their provider. However, findings from these two groups support the idea that some level of introspection on modifying the approach for assessing drug quality is needed within the agency.
In July 2015, the FDA released a draft guidance on a proposed quality metrics (QM) reporting program for manufacturers. As recently as March 9, 2022, the FDA has announced that they are seeking feedback on changes to the previously proposed QM Reporting Program. Such solicitation by the FDA to industry will be helpful in adding an additional dimension of quality surrounding pharmaceutical products for purchase that are not solely based on price [63].
The current quality direction, cGMP, relies on both access to facilities and trustworthy data reported by manufacturers to support products being manufactured. Pricing pressures and lack or regulatory oversight will only continue a propensity for globalization among manufacturers. History has shown that pricing pressures have driven some manufacturers to produce fraudulent data when needing to demonstrate quality, as seen in the Ranbaxy incident. The alternative approaches of both Valisure, LLC, and UK DQS place no reliance on trustworthiness of data produced by globalized manufacturers. Instead, these organizations are taking the information contained in the bottles and vials of medication already on the shelf, analyzing the information, and are either using this information for their own final determination and making a citizen petition, or are sending it to the FDA for the agency to make a final determination..
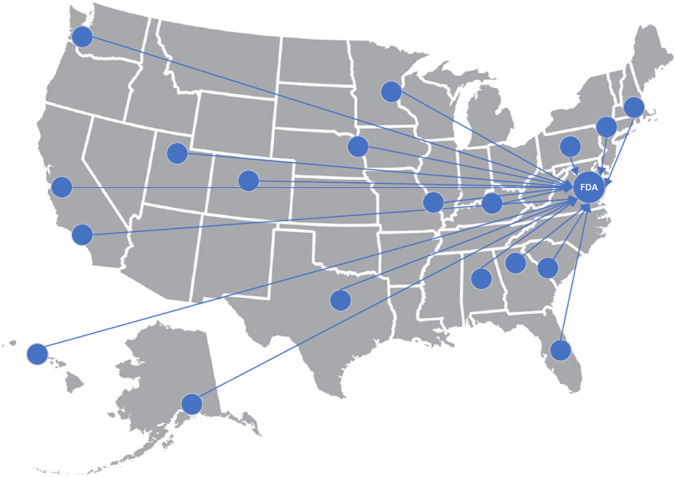
Of the two approaches outlined in this review, the first model, developed by Valisure, has the potential of creating significant market disruptions in that buying groups, distributors, or other pharmacies could feel pressured to match the rigorous approach that Valisure brings in ensuring every prescription passes their quality checks before being dispensed, and manufacturers will receive direct feedback on rejected batches and findings issued in citizen petitions. However, there may be difficulties in scaling such a level of testing across the industry given the higher costs, the length of time involved, and the concerns over being an NGO that behaves like the FDA. Alternatively, the model eventually developed by UK DQS allows for robust non-destructive analytical screening, data collection, and data sharing with the FDA without placing the data collector into the position of being another regulator due to the absence of destructive compendial testing. This sentinel screening could be scaled up with other academic health systems that contain the analytical chemistry expertise within their various colleges of pharmacy, medicine, and/or chemistry. Such a sentinel screening network of academic health systems could replicate the non-destructive data collection, analysis, and data sharing of DQS with FDA through MedWatch so that agency officials could make informed regulatory decisions or take next steps, if any, from a larger sample of data (Fig. 5). The development of such a network would also support efforts by pharmaceutical manufacturers to identify and remove counterfeit products from the supply chain. A similar operation was recently carried out by the manufacturer Gilead and the US Department of Justice, which identified counterfeit tablets of bictegravir/emtricitabine/tenofovir alafenamide, a medication used to treat HIV [64].
Fig. 5.
A sentinel screening network of academic health systems could provide independent data on drug quality to FDA
The authors do not attempt to claim that this alternative approach could amount to a replacement strategy for regulatory agencies. Quality cannot be tested into products post hoc because without cGxP anything could be in the products and an infinite number of tests would be required. A sentinel screening network (SSN), however, can be utilized to augment the current processes and mitigate the information asymmetry already present with FDA inspectors and manufacturing facilities both domestic and abroad. A similar collaboration between large academic health systems and the FDA occurred previously in the drug efficacy study that took place in the late 1960s. Although the final determination was made by agency officials for that monumental study, as it would be in this SSN, there was a symbiotic relationship that developed between agency officials and those in “universities” and “research institutes” [4]. This new SSN would also bring some of the transparency that we have with product price to product quality.
Teaser:
Two organizations offer real world evidence on how FDA, buying groups, and the US Department of Defense might augment the approach to monitoring quality in FDA-approved pharmaceuticals.
Declarations
Ethics Approval
The authors declare that no animal or human was used during the course of the review article.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.1962 Kefauver–Harris Amendment to the Federal Food, Drug, and Cosmetic Act. Pub L. No. 87–781.
- 2.1906 Pure Food and Drug Act. Pub L. No. 59–384.
- 3.Rattan AK. Data integrity: history, issues, and remediation of issues. PDA J Pharm Sci Technol. 2018;72(2):105–116. 10.5731/pdajpst.2017.007765. Epub 2017 Nov 20. PMID: 29158286. [DOI] [PubMed]
- 4.National Research Council. Drug efficacy study: final report to the commissioner of food and drugs - food and drug administration. Washington, DC: The National Academies Press 1969. 10.17226/24615
- 5.FiercePharma, Pharma manufacturing consent decrees: 16 black holes, Available from: https://www.fiercepharma.com/special-report/pharma-manufacturing-consent-decrees-16-black-holes (25 July 2016).
- 6.NPR. Senate inquiry on drug prices echoes landmark hearings held 60 years ago. Available from https://www.npr.org/sections/health-shots/2019/02/22/696967037/senate-inquiry-on-drug-prices-echoes-landmark-hearings-held-60-years-ago. Accessed March 23, 2022.
- 7.The Economist. Costly cures. Available from http://www.economist.com/news/business/21603453-american-fight-over-expensive-new-treatments-has-global-implications-costly-cures. Accessed March 23, 2022.
- 8.Drug Price Competition and Patent Term Restoration Act. Pub L. No. 98–417.
- 9.Stat News. Unaffordable prescription drugs: the real legacy of the Hatch-Waxman Act. Available from https://www.statnews.com/2020/12/16/unaffordable-prescription-drugs-real-legacy-hatch-waxman-act/. Accessed March 23, 2022.
- 10.Thomas H. Safe harbor regulations for GPOs. J Healthc Mater Manage. 1992;10(4):38–40. [PubMed] [Google Scholar]
- 11.Vizient, Inc. A health care company with deep roots. Available from: https://www.vizientinc.com/about-us. Accessed March 23, 2022.
- 12.Businesswire. Vizient recognizes suppliers and distributors in 14 categories for service excellence. Available from: https://www.businesswire.com/news/home/20211117005290/en/Vizient-Recognizes-Suppliers-and-Distributors-in-14-Categories-for-Service-Excellence. Accessed March 23, 2022.
- 13.Becker’s Hospital review. 4 of the largest GPOs | 2017. Available from: https://www.beckershospitalreview.com/finance/4-of-the-largest-gpos-2017.html. Accessed March 23, 2022.
- 14.Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320(18):1859–1860. doi: 10.1001/jama.2018.13604. [DOI] [PubMed] [Google Scholar]
- 15.Donohue JM, Hernandez I, Hershey TB. Drug shortages and group purchasing organizations—reply. JAMA. 2020;324(8):809. doi: 10.1001/jama.2020.9058. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez I, Hershey TB, Donohue JM. Drug shortages in the United States: are some prices too low? JAMA. 2020;323(9):819–820. doi: 10.1001/jama.2019.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Businesswire. Vizient calls on industry stakeholders to join alliance to end drug shortages. Available from: https://www.businesswire.com/news/home/20211108005263/en/Vizient-Calls-on-Industry-Stakeholders-to-Join-Alliance-to-End-Drug-Shortages. Accessed May 5, 2022.
- 18.US Food and Drug Administration. Orange Book preface. Available from: https://www.fda.gov/drugs/development-approval-process-drugs/orange-book-preface#:~:text=The%20Orange%20Book%20was%20distributed,FDA%20regulations%20at%20that%20time. Accessed March 23, 2022.
- 19.Sacks CA, Van de Wiele VL, Fulchino LA, Patel L, Kesselheim AS, Sarpatwari A. Assessment of variation in state regulation of generic drug and interchangeable biologic substitutions. JAMA Intern Med. 2021;181(1):16–22. doi: 10.1001/jamainternmed.2020.3588.Erratum.In:JAMAInternMed.2021Jan1;181(1):144.PMID:32865564;PMCID:PMC7489381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stockman, Farah. Our drug supply is sick. How can we fix it? New York Times, September 18, 2021, https://www.nytimes.com/2021/09/18/opinion/drug-market-prescription-generic.html.
- 21.Eban, K. (2019). Bottle of lies: the inside story of the generic drug boom.
- 22.US Gov’t Accountability Office. GAO-17–143, Drug safety: FDA has improved its foreign drug inspection program, but needs to assess the effectiveness and staffing of its foreign offices. (Dec. 2016)
- 23.The U.S.-China Economic and Security Review Commission. Hearing on “exploring the growing U.S. reliance on China’s biotech and pharmaceutical products.” July 31, 2019.
- 24.Eglovitch JS. Unannounced FDA inspections in India, China to begin soon. Regulatory Affairs Professional Society. December 15, 2021. Accessed from: https://www.raps.org/news-and-articles/news-articles/2021/12/us-fda-to-soon-start-unannounced-inspections-in-in
- 25.Gibson, R., & Singh, J. P. (2018). China Rx: exposing the risks of America’s dependence on China for medicine.
- 26.US Department of Justice. Press Release. Generic drug manufacturer ranbaxy pleads guilty and agrees to pay $500 million to resolve false claims allegations, cGMP violations and false statements to the FDA. Available from: https://www.justice.gov/opa/pr/generic-drug-manufacturer-ranbaxy-pleads-guilty-and-agrees-pay-500-million-resolve-false#:~:text=Ranbaxy%20USA%20pleaded%20guilty%20to,Paonta%20Sahib%20and%20Dewas%2C%20India. Accessed March 23, 2022.
- 27.US Food and Drug Administration. Testimony: safeguarding pharmaceutical supply chains in a global economy. Available from: https://www.fda.gov/news-events/congressional-testimony/safeguarding-pharmaceutical-supply-chains-global-economy-10302019. Accessed March 23, 2022.
- 28.US Gov’t Accountability Office. GAO/HEHS-98–21, Food and Drug Administration: improvements needed in the Foreign Drug Inspection Program. (Mar. 1998)
- 29.US Gov’t Accountability Office. GAO-21–409T, Drug safety: FDA’s future inspection plans need to address issues presented by COVID-19 backlog. (Mar. 2021)
- 30.US Gov’t Accountability Office. GAO-22–103611, Drug safety: FDA should take additional steps to improve its foreign inspection program. (Jan. 2022)
- 31.National Library of Medicine. PubMed Central. Available from: https://www.ncbi.nlm.nih.gov/pmc/. Accessed March 23, 2022.
- 32.Regulations.gov. Available from: https://www.regulations.gov/. Accessed March 23, 2022.
- 33.US Food and Drug Administration. CDER FOIA Electronic Reading Room. Available from: https://www.fda.gov/drugs/guidance-compliance-regulatory-information/cder-foia-electronic-reading-room. Accessed March 23, 2022.
- 34.Valisure. Available from: https://www.valisure.com/. Accessed March 23, 2022.
- 35.State of Connecticut. License Lookup. Available from: https://www.elicense.ct.gov/Lookup/LicenseLookup.aspx. Accessed March 23, 2022.
- 36.Kucera K, Jessop A, Alvarez N, Gortler D, Light D. Rapid and fast-release acetaminophen gelcaps dissolve slower than acetaminophen tablets. Adv Inv Pha The Medic. 2018;1:63–71. doi: 10.31872/2018/APTM-100108. [DOI] [Google Scholar]
- 37.Valisure citizen petition on valsartan. Regulations.gov. Published 2019. Accessed March 23, 2022. https://www.regulations.gov/document/FDA-2019-P-2869-0001
- 38.Braunstein LZ, Kantor ED, O'Connell K, Hudspeth AJ, Wu Q, Zenzola N, Light DY. Analysis of ranitidine-associated N-nitrosodimethylamine production under simulated physiologic conditions. JAMA Netw Open. 2021;4(1):e2034766. doi: 10.1001/jamanetworkopen.2020.34766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valisure citizen petition on ranitidine. Regulations.gov. Published 2019. Accessed March 23, 2022. http://www.regulations.gov/document/FDA-2019-P-4281-0001
- 40.Wu Q, Kvitko E, Hudspeth A, Williams S, Costantino RC, Kucera K, Light D. Analysis of crowdsourced metformin tablets from individuals reveals widespread contamination with N-nitrosodimethylamine (NDMA) and N,N-dimethylformamide (DMF) in the United States. MedRxiv. Feb 14, 2022. 10.1101/2020.05.22.20110635
- 41.Valisure citizen petition on metformin. Regulations.gov. Published 2020. Accessed March 23, 2022. https://www.regulations.gov/document/FDA-2020-P-0978-0001
- 42.Valisure citizen petition on hand sanitizer products. Regulations.gov. Published 2021. Accessed March 23, 2022. https://www.regulations.gov/document/FDA-2021-P-0338-0001
- 43.Valisure citizen petition on sunscreen and after-sun care products. Regulations.gov. Published 2021. Accessed March 23, 2022. https://www.regulations.gov/document/FDA-2021-P-0497-0001
- 44.Valisure citizen petition on deodorant body sprays. Regulations.gov. Published 2021. Accessed March 23, 2022. https://www.regulations.gov/document/FDA-2021-P-1193-0001
- 45.US Food and Drug Administration. FDA announces voluntary recall of several medicines containing valsartan following detection of an impurity. Available from: https://www.fda.gov/news-events/press-announcements/fda-announces-voluntary-recall-several-medicines-containing-valsartan-following-detection-impurity. Accessed March 23, 2022.
- 46.Acetazolamide MedWatch. FDA Form 3500 filed Sep. 30, 2020.
- 47.University of Kentucky Drug Quality Study citizen petition on acetazolamide for injection. Regulations.gov. Published 2020. Accessed March 23, 2022. https://www.regulations.gov/document/FDA-2020-P-2033-0001
- 48.Remdesivir MedWatch. FDA Form 3500 filed Nov. 2, 2020.
- 49.Almeter PJ, Isaacs JT, Schuler EE, Lodder RA. Potential process control issues with remdesivir. Contact in Context. 2021; 1-7. 10.6084/m9.figshare.16417218. PMID: 34924886; PMCID: PMC8679181. [DOI] [PMC free article] [PubMed]
- 50.Ceftaroline fosamil MedWatch. FDA Form 3500 filed Dec. 10, 2021.
- 51.Cosyntropin MedWatch. FDA Form 3500 filed Dec. 17, 2022.
- 52.Dacarbazine MedWatch. FDA Form 3500 filed Jan, 10, 2022.
- 53.Levothyroxine sodium MedWatch. FDA Form 3500 filed Jan. 13, 2022.
- 54.Micafungin sodium MedWatch. FDA Form 3500 filed Jan. 26, 2022.
- 55.Measles, mumps, and rubella MedWatch. FDA Form 3500 filed Feb. 4, 2022.
- 56.Almeter PJ, Isaacs JT, Hunter AN, Henderson BS, Lodder RA. Intra-lot and inter-lot variability in ceftaroline fosamil. Contact in Context. 2021, 1-8. http://www.contactincontext.org/files/8816/4132/5492/Ceftaroline_Rapid_Communication_-_CFRL600PLWQF103021.pdf. 10.6084/m9.figshare.17292596. PMID: 35360663 PMCID: PMC8966977 [DOI] [PMC free article] [PubMed]
- 57.Almeter PJ, Isaacs JT, Hunter AN, Henderson BS, Lodder RA. Intra-lot and inter-lot variability in cosyntropin. Contact in Context. 2021, 1-8. http://www.contactincontext.org/files/3416/4194/3542/Intra-Lot_and_Inter-Lot_Variability_in_Cosyntropin.pdf. 10.6084/m9.figshare.17363048. PMID: 35035309; PMCID: PMC8758055. [DOI] [PMC free article] [PubMed]
- 58.Isaacs JT, Almeter PJ, Henderson BS, Hunter AN, Platt TL, Lodder RA. Spectrometric results of process variations in dacarbazine. Contact in Context. 2022, 1-9. http://www.contactincontext.org/files/3016/4195/0921/Results_of_Process_Variations_in_Dacarbazine.pdf. 10.6084/m9.figshare.17868614. PMID: 35035310; PMCID: PMC8758066. [DOI] [PMC free article] [PubMed]
- 59.Isaacs JT, Almeter PJ, Henderson BS, Hunter AN, Platt TL, Lodder RA. Levothyroxine variations by process analytical technology. Contact in Context. 2022, 1-14. http://www.contactincontext.org/files/8516/4211/1241/Levothyroxine_Variations_by_Process_Analytical_Technology.pdf. 10.6084/m9.figshare.18316523. PMCID: PMC9015687 [DOI] [PMC free article] [PubMed]
- 60.Isaacs JT, Almeter PJ, Henderson BS, Hunter AN, Platt TL, Lodder RA. FTNIR spectrometry of micafungin sodium quality. Contact in Context. 2022, 1-11. http://www.contactincontext.org/files/5316/4502/9831/Micafungin_Rapid_Communication_MCFN050PGPXK11022021.pdf. 10.6084/m9.figshare.19071704. PMID: 35360460; PMCID: PMC8966979 [DOI] [PMC free article] [PubMed]
- 61.Isaacs JT, Almeter PJ, Henderson BS, Hunter AN, Platt TL, Lodder RA. Lack of content uniformity in MMR vaccine. Contact in Context. 2022, 1-27. http://www.contactincontext.org/files/2916/4558/7195/Lack_of_Content_Uniformity_in_MMR_Vaccine.pdf. 10.6084/m9.figshare.19217478. PMID: 35360461; PMCID: PMC8966980 [DOI] [PMC free article] [PubMed]
- 62.Isaacs JT, Almeter PJ, Henderson BS, Hunter AN, Platt TL, Lodder RA. Variability in content of piperacillin and tazobactam injection. Contact in Context. 2022, 1-10. http://www.contactincontext.org/files/5816/4981/7838/Variability_in_Content_of_Piperacillin_and_Tazobactam.pdf. 10.6084/m9.figshare.19561333. [DOI] [PMC free article] [PubMed]
- 63.Durivage MA. FDA seeks public comment on quality metrics reporting program. Pharmaceutical Online. Available from: https://www.pharmaceuticalonline.com/doc/fda-seeks-public-comment-on-quality-metrics-reporting-program-0001. Accessed March 23, 2022.
- 64.Gilead.com. Gilead announces actions to remove counterfeit HIV medications from U.S. supply chain. Available from: https://www.gilead.com/news-and-press/company-statements/gilead-announces-actions-to-remove-counterfeit-hiv-medications-from-us-supply-chain. Accessed May 5, 2022.