Abstract
Background
SARS-CoV-2 infection results in a broad spectrum of COVID-19 disease, from mild or no symptoms to hospitalization and death. COVID-19 disease severity has been associated with some pre-existing conditions and the magnitude of the adaptive immune response to SARS-CoV-2, and a recent genome-wide association study (GWAS) of the risk of critical illness revealed a significant genetic component. To gain insight into how human genetic variation attenuates or exacerbates disease following SARS-CoV-2 infection, we implicated putatively functional COVID risk variants in the cis-regulatory landscapes of human immune cell types with established roles in disease severity and used high-resolution chromatin conformation capture to map these disease-associated elements to their effector genes.
Results
This functional genomic approach implicates 16 genes involved in viral replication, the interferon response, and inflammation. Several of these genes (PAXBP1, IFNAR2, OAS1, OAS3, TNFAIP8L1, GART) were differentially expressed in immune cells from patients with severe versus moderate COVID-19 disease, and we demonstrate a previously unappreciated role for GART in T cell-dependent antibody-producing B cell differentiation in a human tonsillar organoid model.
Conclusions
This study offers immunogenetic insight into the basis of COVID-19 disease severity and implicates new targets for therapeutics that limit SARS-CoV-2 infection and its resultant life-threatening inflammation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13059-022-02691-1.
Background
SARS-CoV-2 induces a strong immune response dominated by CD4+ and CD8+ T cells reactive to spike antigen-derived epitopes [1, 2] and accompanied by elevated lymphokines and reduced frequencies of T and B cells in the blood. Pan-lymphopenia and higher cytokine levels are associated with severe disease [3–9], and milder disease is associated with higher frequencies of circulating SARS-CoV-2-specific CD4+ and CD8+ T cells [2, 10–13]. The lungs of COVID-19 patients are also enriched for T cells, and SARS-CoV-2-infected monocyte-derived alveolar macrophages and neutrophils producing T cell chemokines are more abundant in patients with severe disease [10, 14]. During anti-viral immune responses, CD4+ T follicular helper cells (TFH) migrate into germinal centers (GC) to help GC B cells differentiate into high affinity antibody-producing plasmablasts [15]. Circulating SARS-CoV-2-specific TFH, plasmablasts, and high-affinity Ab are detected in COVID-19 patients, and the frequency of activated TFH and plasmablasts in the blood is associated with neutralizing IgG levels [11, 16–20]. SARS-CoV-2 infection in macaques induces a similar cellular dynamic in the spleen [21, 22], and the frequency of circulating plasmablasts, naive CD4+ T cells, and TFH in humans is associated with disease severity [6, 11, 17–19, 23]. The immune dynamics of SARS-CoV-2 infection suggests that genetically encoded factors regulating the differentiation and function of CD4+ T cells, TFH, and germinal center B cells (GCB) likely influence the severity of COVID-19 disease. Recent genome-wide association studies (GWAS) for critically ill COVID-19 patients have revealed a number of loci associated with the trait [24, 25]. However, GWAS does not identify causal effector genes at non-coding signals, and these loci are often presumptively named after the nearest gene. To implicate putative causal variants and their corresponding effector genes at COVID-19 GWAS loci, we leveraged a 3D genomic variant-to-gene mapping approach using disease-relevant, human immune cell types.
Results
As an initial step, we used ATAC-seq to identify accessible SNPs in linkage disequilibrium (LD) with GWAS sentinel signals and high-resolution promoter-focused Capture-C (PCC) to connect them to the genes they likely regulate. Because of their connection to COVID-19 disease severity, we chose to perform these analyses in primary naive B cells, naïve CD4+ T cells, follicular helper T cells [26], and germinal center B cells from human tonsil, and circulating monocytes (Additional file 1 Fig S1A, Table S1). We included hESC as a non-immune comparator [27]. The number (range: 55k–91k open regions) and genomic distribution (mean range: 496–655 bp) of open chromatin regions (OCR) were comparable among cell types (Additional file 1 Fig S1B-E). To put these OCR in the context of the 3-dimensional structure of the genome, we performed high-resolution PCC targeting the majority of coding and non-coding genes in the human genome [26, 28–31]. The quantity and quality of promoter interactions were similar among immune cell types (Additional file 1 Fig S2, Table S2). One-third to one-half of the open chromatin landscapes were connected to gene promoters (Fig. 1A), and genes whose promoters interact with distal open chromatin regions were expressed ~ 10-fold higher than genes not physically associated with open chromatin (Fig. 1B). These results indicate that promoter-connected OCR represent cis-regulatory elements engaged in active control of gene expression. The promoter-connected open chromatin landscape of naive CD4+ T cells was significantly enriched for COVID-19 disease risk heritability (~ 28-fold, FDR = 0.045, Fig. 1C). TFH and monocyte gene regulatory architectures showed a similar magnitude but more variable enrichment for COVID-19 disease risk variants, and B cells and ESC landscapes did not show enrichment (Fig. 1C). These results are consistent with the growing evidence that dynamics relevant to multiple immune cell types may contribute to disease severity and suggest that COVID-19-associated variants in CD4+ T cell and monocyte open chromatin may have the strongest influence on disease severity.
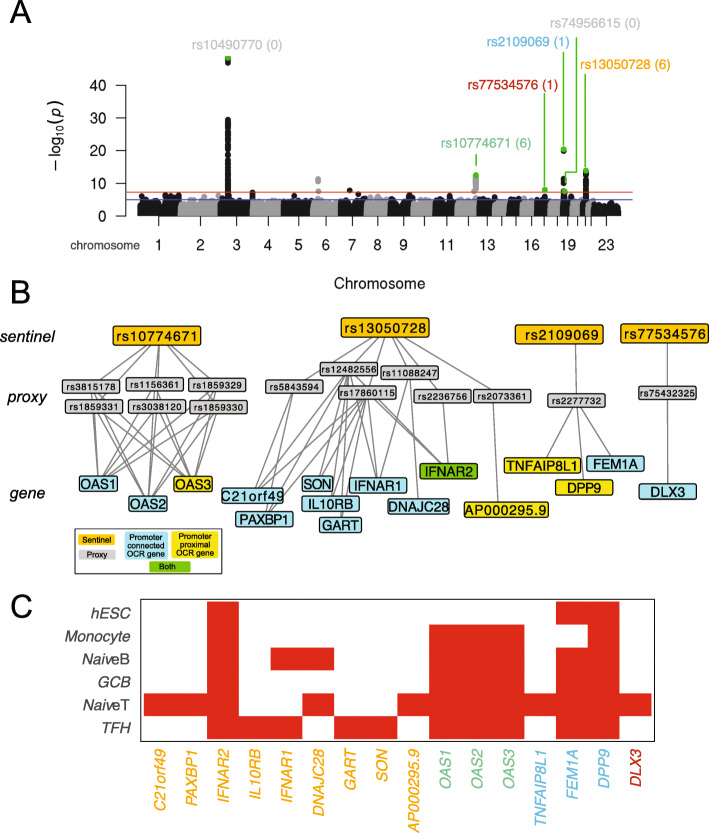
Fig. 1.
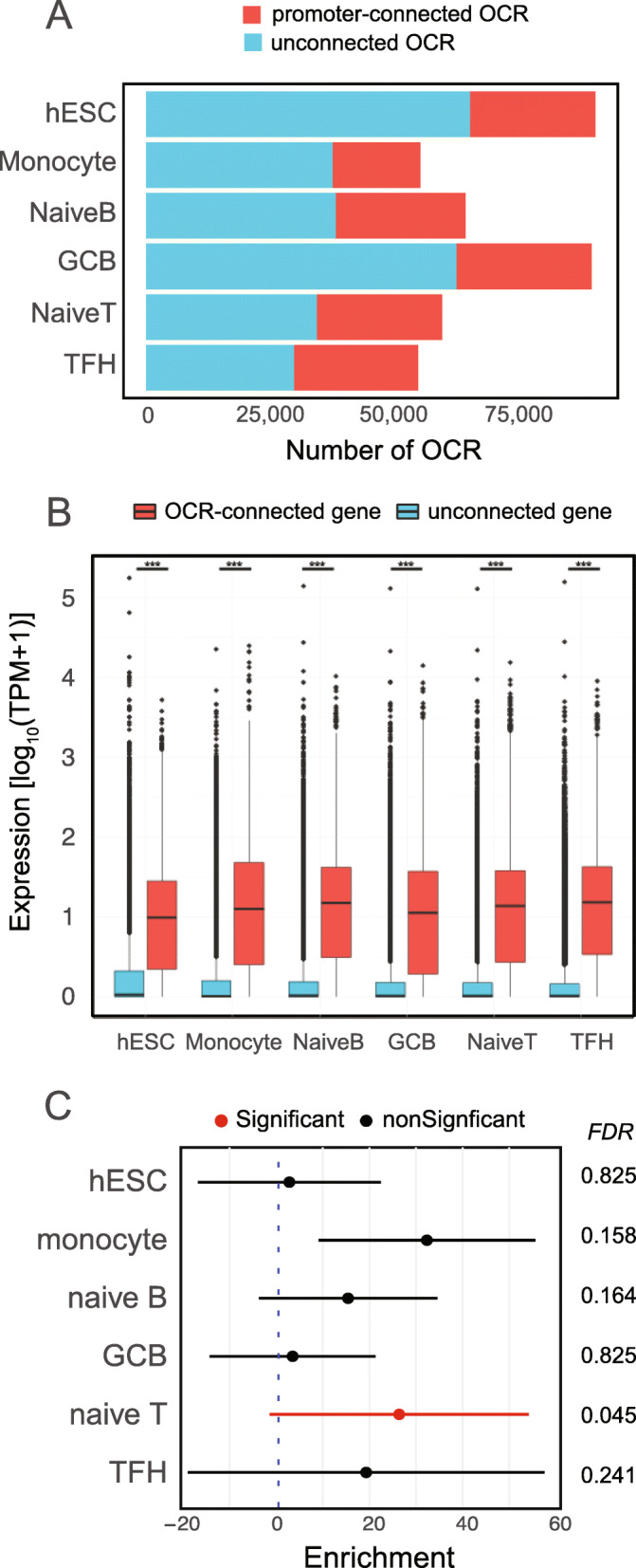
Promoter-connected open chromatin is enriched for highly expressed genes and COVID-19 disease risk heritability. A The number of OCRs contacting promoters determined by Capture C and those without promoter contacts. B Expression measured by transcripts per kilobase million (TPM) of genes with at least one OCR-promoter contact (red) vs. genes without promoter-OCR contacts (blue). Boxplots represent the median expression for each category. Statistical significance was determined using two-sided Wilcoxon rank-sum tests. TPM range contacted 0–25,499.71, non-contacted 177,277.33. Medians: hESC contacting = 8.86, non-contacting 0.0621, monocyte contacting = 11.627, non-contacting 0.0123, naïve B contacting = 14.0, non-contacting 0.0289, GCB contacting = 14.0, non-contacting 0.0289, naïve CD4 T contacting = 12.8, non-contacting 0.0244 TFH contacting = 14.3, non-contacting = 0.216. C Enrichment of estimated COVID-19 GWAS heritability determined by partitioned score regression for the open chromatin landscape for each cell type. Points indicate calculated enrichment and whiskers indicate 95% confidence interval. The associated FDR for each enrichment is depicted on the right
To map potentially functional COVID-19 variants to their target genes, we intersected our ATAC-seq and PCC data with the most recent COVID-19 disease risk GWAS [25] (Fig. 2A and B, Table S3). We examined proxy SNPs in high LD (R2 > 0.8) with the six independent genome-wide significant signals associated with COVID19 severity and identified 16 genes whose promoters physically interact with accessible COVID-19-associated variants in immune cells (Fig. 2B, C, Additional file 1 Fig S3). We identified accessible proxy SNPs in LD with the COVID-19 sentinel rs10774671 in the promoter of OAS3, and accessible proxies interacting with OAS1 and OAS2 in all immune cell types analyzed (Fig. 2C). The DPP9 sentinel rs2109069 is in LD with accessible proxies in the DPP9 promoter in each immune cell type, but also to distal accessible proxies interacting with FEM1A in the T and B cells, and to distal proxies interacting with TNFAIP8L1 specifically in naive CD4+ T cells (Fig. 2C). The sentinel rs13050728 is in LD with accessible proxies interacting with PAXBP1, C21orf49, and AP000295.9 in naive CD4+ T cells; IFNAR1 in TFH and naïve B cells; DNAJC28 in naive T and B cells; GART, IL10RB, and SON in TFH cells; and IFNAR2 in all cell types (Fig. 2C). A proxy SNP in LD with the sentinel rs77534576 is connected to the DLX3 gene in naive CD4+ T cells (Fig. 2C).
Fig. 2.
Chromosome capture-based variant-to-gene mapping identifies candidate effector genes at COVID-19 GWAS loci. A Manhattan plot generated using the summary statistics from the COVID-19 severity GWAS. Genome-wide significant signals are shown together with the number of accessible gene-annotated proxies associated. B Depiction of the statistical sentinel-proxy SNP linkages and the PCC-derived physical gene-proxy connections identified in this study. Genes in yellow were implicated by an accessible proxy in the promoter regions, genes in blue were implicated through chromatin-based contact between the promoter region and a distal accessible proxy, and green indicates implication by both promoter and distal proxies. C Heatmap depicting genes implicated by variant-to-gene mapping in each cell type in red. Color of each gene corresponds to the signal shown in A
Gene ontology analyses of the set of COVID-19 variant-connected genes showed enrichment for pathways involved in coronavirus pathogenesis (-logP = 8.97), viral hypercytokinemia (-logP = 8.95), viral/bacterial pattern recognition (-logP = 4.0), interferon signaling (-logP = 5.93), and T cell exhaustion (-logP = 2.39). This set of genes was also enriched (P < 0.0003) for factors involved in viral infection, RNA virus replication, anti-viral response, multiple sclerosis, psoriasis, and necrosis (Additional file 1 Fig S4, Table S4). We note that our approach did not implicate genes for two COVID-19-associated GWAS signals (rs10490770 & rs74956615). rs10490770 is the strongest signal located on chromosome 3, and its proxy SNP rs17713054 has been reported to contact the LZTFL1 promoter in lung epithelial cells, suggesting that these risk loci act outside the immune cells [32].
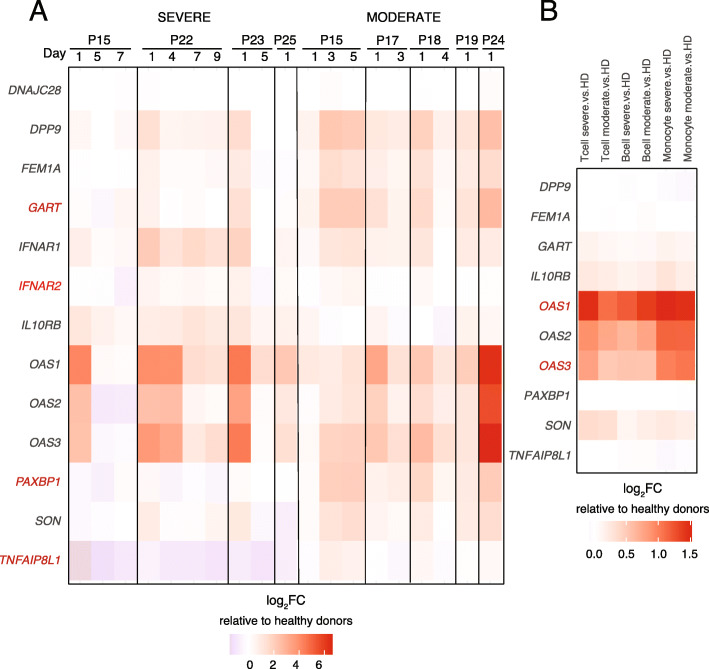
To explore the functional significance of genes implicated through their connection to COVID-19-associated immune regulatory architectures, we compared their expression in bulk blood leukocytes [33] or at the single-cell level [34] from COVID-19 patients vs. healthy donors from published datasets. We found nearly two-thirds (10/16) of these implicated genes were differentially expressed in circulating leukocytes of SARS-CoV-2-infected humans (Fig. 3A, B). OAS1, OAS2, OAS3, IL10RB, FEM1A, GART, SON, and IFNAR2 were significantly (FDR < 0.05) upregulated in lymphocytes and monocytes from COVID-19 patients compared to healthy controls, while TNFAIP8L1 was significantly downregulated in monocytes from COVID-19 patients (Fig. 3A, B). Importantly, six of these genes also exhibited severity-associated patterns of expression. IFNAR2 was significantly upregulated in severe COVID-19 patients (FDR = 0.0075, Fig. 3A), and both OAS1 and OAS3 were upregulated in T cells from severe COVID-19 patients compared to those with mild disease (Fig. 3B). PAXBP1 was significantly downregulated (FDR = 0.0067) in severe vs. mild COVID patients, and GART and TNFAIP8L1 were nominally downregulated in severe patients (p = 0.047, FDR = 0.127).
Fig. 3.
Immune genes implicated through contact with COVID-19 variants are differentially expressed in patients with SARS-CoV-2 infection and severe COVID-19 disease. Differential gene expression in mild/moderate and severe COVID-19 patients relative to healthy donors quantified by A bulk RNA-seq of whole blood leukocytes at various timepoints from Galani et al. [33] and B single-cell RNA-seq from peripheral blood from Zhang et al. [34] Values represent log2FC of TPM for each gene relative to the mean of healthy donors, and genes showing disease severity-associated expression are shown in red. Data in B represent pseudo-bulk RNA-seq of T cells, B cells, or monocytes clustered by single-cell transcript patterns. Genes indicated in red in A and B exhibited severity-associated expression patterns
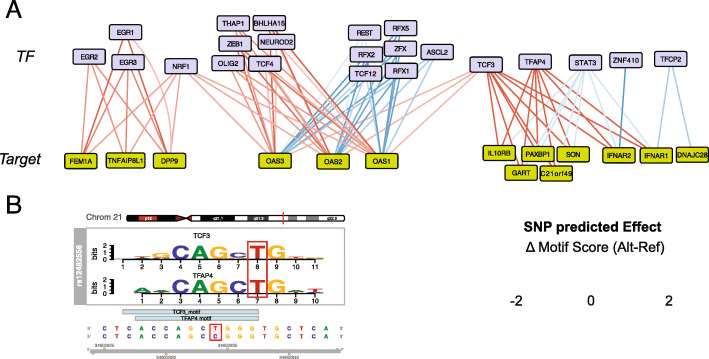
The putatively causal variants identified in this study likely influence COVID-19 risk by directly contacting and altering the expression of their target genes in immune cell types. To test whether these COVID-19 variants may influence gene transcription by affecting the binding of transcription factors, we used a modeling approach to predict their impact on the affinity of transcription factors for consensus DNA motifs present in the regulatory landscape of COVID-19-relevant immune cell types. This approach identified 9 COVID-19-associated SNPs predicted to impact binding of 22 expressed transcription factors to 14 of the 16 genes identified in this study (Fig. 4 and Table S5). COVID-19 risk-associated sequence variation at IFNAR2 may increase binding of ZNF410, a zinc finger protein involved in repression of fetal hemoglobin in erythroid cells [35], and risk variants also increase the predicted affinity of STAT3 for elements connected to IFNAR1, IFNAR2, PAXBP1, GART, C21ORF49, SON, and IL10RB. Variants connected to OAS1, OAS2, and OAS3 may affect the binding of 15 distinct transcription factors, including the RFX family of transcription factors involved in expression of MHC class II genes, and the plasmablast and TFH factors MIST1 (BHLHA15) [36] and ASCL2 [37]. This set of affected transcription factors form a network of highly co-regulated activities downstream of the TCR, particularly centered around STAT3 and EGR1 (Additional file 1 Fig S5), and enriched for roles in hematopoietic, B and T cell development, differentiation, and function (Table S6).
Fig. 4.
In silico prediction of transcription factor binding site disruption by accessible COVID-19 associated proxies. A Transcription factors (blue) with binding motifs likely to be disrupted by accessible COVID-19 SNPs and their connected target genes (green). The predicted effect of the SNP on TF binding is indicated in red for decreased affinity and in blue for increased affinity. COVID-19 risk-associated sequence variation at an element connected to IFNAR2 is predicted to increase binding of ZNF410, a zinc finger protein involved in repression of fetal hemoglobin in erythroid cells. Risk variants also increased the predicted affinity of STAT3 for elements at six implicated genes including PAXBP1. Risk variants at PAXBP1 and five other implicated genes were also predicted to reduce binding of the MYC-induced AP4 (TFAP4) oncoprotein and E2A (TCF3), a central transcription factor in lymphocyte development and malignancy. COVID-19 disease variants connected to OAS1 and OAS3 were predicted to affect the binding of 15 distinct transcription factors, including the E proteins TCF3, TCF4, TCF12, and NEUROD2, the RFX family of transcription factors involved in expression of a variety of immune factors including the MHC class II genes, and the plasmablast and TFH factors MIST1 (BHLHA15) and ASCL2. B An example of the predicted impact of the COVID-19 risk allele of rs12482556 (red) on binding of TCF3 and TFAP4
Discussion
Together, our results suggest that genes central to viral genome sensing, host control of viral replication, the interferon response, and immune inflammation are likely under genetic control by common variants associated with COVID-19 disease risk. This study implicated multiple genes at all but one locus. While methods for fine-mapping GWAS signals generally assume a single causal variant acting at one effector gene, this assumption is not always valid. We observe clear evidence for pleiotropic effects of COVID-19 disease risk-associated genetic variation at the level of multiple proxies in open chromatin at each locus in the same and/or distinct cell types, and individual accessible proxies contacting multiple genes in one or across more cell types (Supplemental Figure 3). Similar pleiotropy was observed for FTO obesity variants on the dynamics and lineage-specific expression of distal genes such as IRX3 and IRX5 [38].
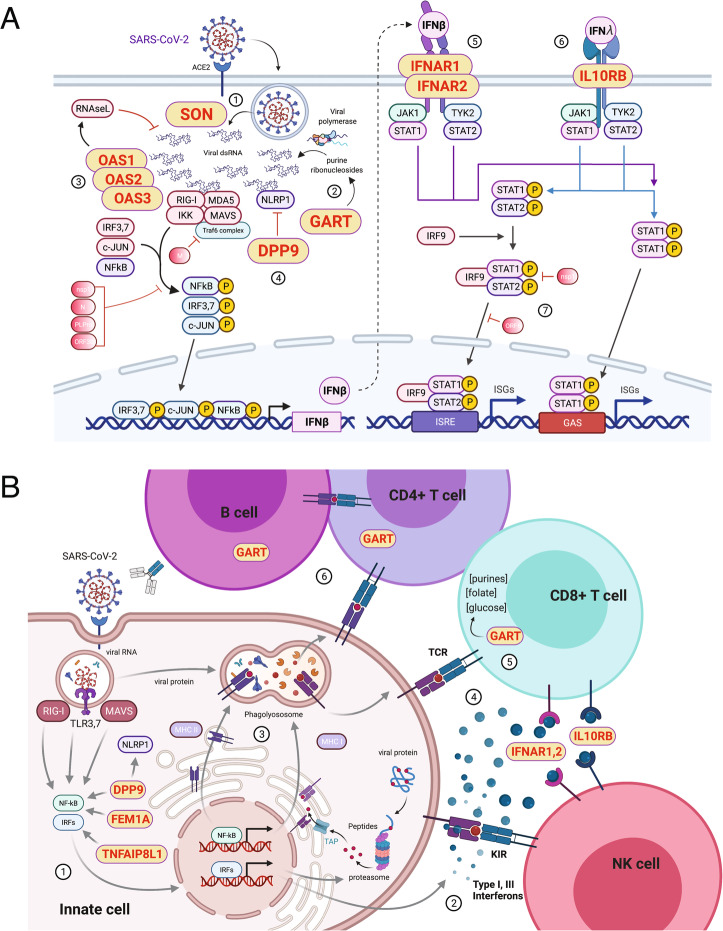
The genes identified through physical association with accessible COVID-19 variants have known roles in viral replication, the interferon response, and inflammation. The genes GART and SON encode factors that may directly impact SARS-CoV-2 replication (Fig. 5A). SON encodes a factor that regulates HBV influenza A replication [39, 40], while GART controls de novo purine pools required for coronavirus RNA replication that may also drive evolution of viral variants over the course of the pandemic [41, 42]. Interferons (IFN) are important for the control of early virus replication and in determining moderate vs. severe inflammatory disease [43]. SARS-CoV2 induces type I and type III interferons [44] that signal through IFNAR1, IFNAR2, and IL10RB (Fig. 5A), but SARS-CoV2 also encodes factors that can inhibit type I and III responses [45–47]. Thus, many SARS-CoV-2-infected individuals exhibit blunted and/or delayed interferon responses [20, 48–50] and experience more severe disease than COVID-19 patients with strong interferon responses [32, 50, 51]. SARS-CoV-2 dsRNA genomes are sensed by the RIG-I/MDA5 and RNAseL pathways [52, 53]. OAS1, OAS2, and OAS3 encode crucial regulators of dsRNA degradation by RNAseL, and DPP9 regulates the activity of NLRP1, a dsRNA-sensing component of the inflammasome [54] (Fig. 5A). Gain of function mutations in OAS1 lead to autoinflammatory disease in humans [55]; polymorphisms at the OAS1 locus are associated with type 2 diabetes [56], a pre-existing condition associated with severe COVID-19 disease [57]; and genetic variation at DPP9 is associated with the risk of developing pulmonary fibrosis [58]. Cytokine release syndrome is a major inflammatory complication in patients with severe COVID-19 disease [59–61]. Receptors for type I (IFNAR1 and 2) and III (IL10RB) interferons drive inflammation mediated by NK and CD8+ T cells, and IL-10RB binds IL-10 whose levels are a severity predictor in COVID-19 [62] (Fig. 5B). FEM1A encodes a negative regulator of NFkB activation [63], and TNFAIP8L1 regulates expression of the chemokine MCP-1 [64]. DNAJC28 is a mitochondrial Hsp40 family member and cofactor of Hsp70 heat shock proteins [65]. PAXBP1 encodes a regulator of ROS and p53 [66], and DLX3 encodes a homeobox protein known to function downstream of the TGFB, BMP, and WNT pathways in tooth and placental development [67], but immune roles for these factors have not been established.
Fig. 5.
Potential mechanisms by which V2G-implicated genes impact COVID-19 disease severity. A SON may control release and processing of SARS-CoV-2 RNA genomes (1), and GART is involved in de novo generation of the purine precursors required for SARS-CoV-2 RNA replication (2). Sensing of dsRNA regulated by OAS1, OAS2, OAS3, and DPP9 may lead to degradation of SARS-CoV-2 genomes and activation of the inflammasome (3, 4). SARS-CoV-2-induced activation of NFkB, IRFs, and Jun, dampened by SARS-CoV-2-encoded factors (4 - M, nsp1, N, PLPro, ORF3b), induces IFNB. Anti-viral signaling is mediated by type I interferon receptors encoded by IFNAR1 and IFNAR2 (5), and the type III interferon receptor encoded by IL10RB (6). These processes are known to be inhibited by the SARS-CoV-2-encoded factors nsp1 and ORF6 (7). B Sensing of SARS-CoV-2 RNA genomes released upon infection of lung epithelial cells or alveolar macrophages increases expression of components of the antigen processing and presentation machinery, interferons, cytokines, and chemokines (1–3). These processes are regulated by TNFAIP8L1, FEM1A, and DPP9. Type I interferon receptors IFNAR1 and IFNAR2 control T and NK cell function, and IL10RB affects responsiveness to type III interferons and other cytokines (4). The enzymatic activity of GART regulates metabolic and epigenetic processes important for lymphocyte activation, proliferation, and differentiation (5, 6)
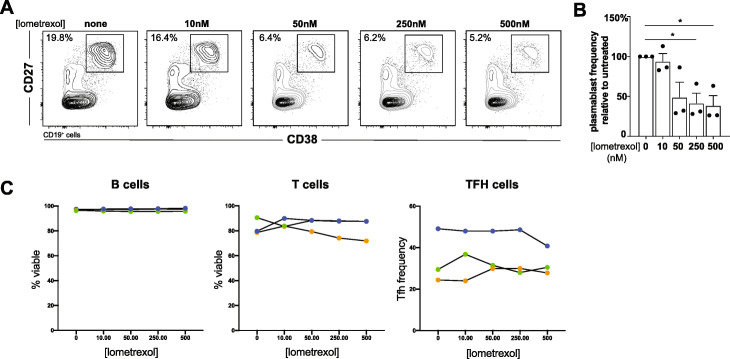
GART encodes an enzyme involved in purine biosynthesis, and its folate-derived metabolites have roles in DNA methylation and mitochondrial redox, processes that regulate immune cell function [68] (Fig. 5B). To test for a role for GART in adaptive immune responses associated with susceptibility to severe COVID-19, we used the GART inhibitory drug lometrexol in an in vitro human tonsillar organoid model of T cell-dependent germinal center B cell differentiation [32]. After 7 days in culture, T-B interactions in control organoids supported the differentiation of CD27 + CD38+ GCB cell plasmablasts (Fig. 6A and B) capable of producing high-affinity class-switched antibodies in this mod [32]. The GART inhibitor lometrexol abrogated plasmablast differentiation in a dose-dependent manner (Fig. 6A and B) without affecting B or T cell survival or TFH frequency (Fig. 6C). These results indicate that GART has a previously unappreciated role in T cell-B cell germinal center reactions, and further link GART to immune processes associated with COVID-19 disease severity.
Fig. 6.
GART inhibition abrogates germinal center plasmablast output in tonsillar organoids. A Day 7 plasmablast frequencies from untreated or lometrexol-treated tonsillar organoids from a representative tonsil donor. B Plasmablast frequency diminishment in day 7 lometrexol drugged organoids relative to untreated counterparts from three tonsil donors. *P ≤ 0.05. C B and T cell viability and TFH frequency from these same experiments. Data from each donor are depicted separately
Conclusions
This work implicates genetic variation in the cis-regulatory architecture of immune cells as a potential source of the observed variation in COVID-19 disease severity. The initial GWAS implicated candidate effector genes using metrics of linear proximity to the GWAS signal and public expression quantitative trait loci (eQTLs) datasets [25]. Several of our V2G attributions agreed with these analyses, including OAS1, OAS2, and OAS3 as the likely effector genes for rs10774671. DLX3, IL10RB, and IFNAR2 were also implicated in the prior study via intersection with eQTLs from various tissues and cell types. However, a number of GWAS candidate genes were not implicated in our study, and our immune-focused PCC maps identified several candidate effectors not implicated previously: IFNAR1, GART, SON, and AP00295.9 for rs1305728 and TNFAIP8L1 and FEM1A for rs77534576. In addition, our V2G mapping of genes involved in COVID severity identified GART as a novel target whose activity could potentially be promoted for better anti-viral humoral immune responses or inhibited as a potential treatment for systemic autoimmune disease. Further work is necessary to determine the roles of these genes in COVID-19, and whether genes implicated here may represent therapeutic targets for COVID-19 and other inflammatory disorders.
Methods
Antibodies
Anti-human CD19-APC-Cy7 (HIB19, cat#302218), CD27 PerCP-Cy5.5 (O323, cat#302820), and IgD-Pacific-Blue (IA6-2, cat#348225) were from Biolegend. Anti-human CD38-APC (HIT2, cat#555462) and CD21 Pe B-ly4 (557327) were from BD Biosciences.
Purification of B cells from human tonsil
Fresh tonsils were obtained as discarded surgical waste from de-identified immune-competent children undergoing tonsillectomy to address airway obstruction or a history of recurrent tonsillitis. These studies were approved by The Children’s Hospital of Philadelphia Institutional Review Board as non-human subject research. The mean age of donors was 5.6 years (range 3-16 years) and 75% were male. Tonsillar mononuclear cells were isolated from tissues by mechanical disruption (tonsils were minced and pressed through a 70-micron cell screen) followed by Ficoll-Paque centrifugation. Naïve B cells (CD19+CD21+IgD+CD38−) and GC B cells (CD19+CD21+CD38+IgD−CD27−) were then sorted using a MoFlo Astrios EQ (Beckman Coulter). The gating strategy is shown in Additional file 1 Fig S6.
Tonsillar organoid preparation and staining
Excised tonsils from three de-identified immunocompetent patients were diced and strained through a 100-μm filter. A single cell suspension of tonsillar mononuclear cells (MNCs) was created with Ficoll density gradient separation. Once isolated, MNC were counted and resuspended in organoid media (RPMI with L-glutamine, 10% FBS, 2 mM glutamine, 1X penicillin-streptomycin, 1 mM sodium pyruvate, 1X MEM non-essential amino acids, 10 mM HEPES buffer and 1 μg/ml of recombinant human B cell activating factor [BioLegend]) at a concentration of 6 × 107 cells per ml. As previously described by Wagar et al. [32], MNC’s were transferred to permeable transwells (0.4-μm pore, 12-mm diameter; Millipore). Transwells were inserted into standard 12-well polystyrene plates containing 1 ml of additional organoid media and placed in an incubator at 37 °C and 5% CO2. Lometrexol in phosphate-buffered saline was added, or not, to organoids after 48 h in culture. Organoid media with or without lometrexol was replaced every 3 days. On culture day 7, organoid MNCs were resuspended and stained at 4 °C with the anti-human CD38 (HIT2; BioLegend), CD27 (O323; BioLegend), CD19 (HIB19; BioLegend), and L/D aqua (Invitrogen). Cells were analyzed with LSRFortessa (BD Bioscience) and visualized with FlowJo software (TreeStar).
Cell fixation
We used standard methods for cell fixation [26, 28–31]. Briefly, 107 naïve or germinal center B cells were suspended in 10 mL RPMI + 10% FBS, followed by an additional 270μL of 37% formaldehyde and incubation for 10 min at RT on a platform rocker. The fixation reaction was quenched by the addition of 1.5 mL cold 1 M glycine (4 °C). Fixed cells were centrifuged at 210×g for 5 min at 4 °C and supernatants were removed. The cell pellets were washed in 10-ml cold PBS (4 °C) followed by centrifugation as above. Cell pellets were resuspended in 5-ml cold lysis buffer (10 mM Tris pH 8, 10 mM NaCl, 0.2% NP-40/Igepal supplemented with a protease inhibitor cocktail). Resuspended cell pellets were incubated for 20 min on ice, centrifuged at 680×g, and lysis buffer was removed. Cell pellets were resuspended in 1 mL of fresh lysis buffer, transferred to 1.5-mL Eppendorf tubes, and snap frozen in ethanol/dry ice or liquid nitrogen. Frozen cell pellets were stored at − 80 °C for 3C library generation.
3C library generation
We used standard methods for generation of 3C libraries [26, 28–31]. For each library, 107 fixed cells were thawed at 37 °C, followed by centrifugation at RT for 5 min at 1845×g. The cell pellet was resuspended in 1 mL of dH2O supplemented with 5 μL 200X protease inhibitor cocktail, incubated on ice for 10 min, then centrifuged. Cell pellet was resuspended to a total volume of 650 μL in dH2O. Fifty microliters of cell suspension was set aside for pre-digestion QC, and the remaining sample was divided into 3 tubes. Both pre-digestion controls and samples underwent a pre-digestion incubation in a Thermomixer (BenchMark) with the addition of 0.3%SDS, 1x NEB DpnII restriction buffer, and dH2O for 1 h at 37 °C shaking at 1000 rpm. A 1.7% solution of Triton X-100 was added to each tube and shaking was continued for another hour. After pre-digestion incubation, 10 μL of DpnII (NEB, 50 U/μL) was added to each sample tube only and continued shaking along with pre-digestion control until the end of the day. An additional 10 μL of DpnII was added to each digestion reaction and digested overnight. The next day, a further 10 μL DpnII was added and continue shaking for another 2–3 h. One hundred microliters of each digestion reaction was then removed, pooled into one 1.5-mL tube, and set aside for digestion efficiency QC. The remaining samples were heat inactivated incubated at 1000 rpm in a MultiTherm for 20 min at 65 °C to inactivate the DpnII and cooled on ice for 20 additional minutes. Digested samples were ligated with 8 μL of T4 DNA ligase (HC ThermoFisher, 30 U/μL) and 1X ligase buffer at 1000 rpm overnight at 16 °C in a MultiTherm. The next day, an additional 2 μL of T4 DNA ligase was spiked into each sample and incubated for another few hours. The ligated samples were then de-crosslinked overnight at 65 °C with Proteinase K (20 mg/mL, Denville Scientific) along with pre-digestion and digestion control. The following morning, both controls and ligated samples were incubated for 30 min at 37 °C with RNase A (Millipore), followed by phenol/chloroform extraction, ethanol precipitation at − 20 °C, the 3C libraries were centrifuged at 1000×g for 45 min at 4 °C to pellet the samples. The controls were centrifuged at 1845×g. The pellets were resuspended in 70% ethanol and centrifuged as described above. The pellets of 3C libraries and controls were resuspended in 300 μL and 20 μL dH2O, respectively, and stored at − 20 °C. Sample concentrations were measured by Qubit. Digestion and ligation efficiencies were assessed by gel electrophoresis on a 0.9% agarose gel and also by quantitative PCR (SYBR green, Thermo Fisher).
Promoter-Capture-C design
Our promoter-Capture-C approach was designed to leverage the four-cutter restriction enzyme DpnII in order to give high resolution restriction fragments of a median of ~250bp [26, 28–31]. This approach also allows for scalable resolution through in silico fragment concatenation. Custom capture baits were designed using Agilent SureSelect RNA probes targeting both ends of the DpnII restriction fragments containing promoters for coding mRNA, non-coding RNA, antisense RNA, snRNA, miRNA, snoRNA, and lincRNA transcripts (UCSC lincRNA transcripts and sno/miRNA under GRCh37/hg19 assembly) totaling 36,691 RNA baited fragments through the genome. The capture library was re-annotated under gencodeV19 at both 1-fragment and 4-fragment resolution.
Promoter-Capture-C assay
Isolated DNA from 3C libraries was quantified using a Qubit fluorometer (Life technologies), and 10 μg of each library was sheared in dH2O using a QSonica Q800R to an average fragment size of 350bp [26, 28–31]. QSonica settings used were 60% amplitude, 30s on, 30s off, 2-min intervals, for a total of 5 intervals at 4 °C. After shearing, DNA was purified using AMPureXP beads (Agencourt). DNA size was assessed on a Bioanalyzer 2100 using a DNA 1000 Chip (Agilent) and DNA concentration was checked via Qubit. SureSelect XT library prep kits (Agilent) were used to repair DNA ends and for adaptor ligation following the manufacturer’s protocol. Excess adaptors were removed using AMPureXP beads. Size and concentration were checked by Bioanalyzer using a DNA 1000 Chip and by Qubit fluorometer before hybridization. One microgram of adaptor-ligated library was used as input for the SureSelect XT capture kit using manufacturer protocol and our custom-designed 41 K promoter Capture-C library. The quantity and quality of the captured library was assessed by Bioanalyzer using a high sensitivity DNA Chip and by Qubit fluorometer. SureSelect XT libraries were then paired-end sequenced on 8 lanes of Illumina Hiseq 4000 platform (100 bp read length).
ATAC-seq library generation
The tonsillar T cells [26], monocytes [69], and B cell subsets were processed in the same manner. A total of 50,000 to 100,000 sorted cells were centrifuged at 550 g for 5 min at 4 °C. The cell pellet was washed with cold PBS and resuspended in 50 μL cold lysis buffer (10 mM Tris-HCl, pH 7.4, 10 mM NaCl, 3 mM MgCl2, 0.1% NP-40/IGEPAL CA-630) and immediately centrifuged at 550 g for 10 min at 4 °C. Nuclei were resuspended in the Nextera transposition reaction mix (25 μL 2x TD Buffer, 2.5 μL Nextera Tn5 transposase (Illumina Cat #FC-121-1030), and 22.5 μL nuclease free H2O) on ice, then incubated for 45 min at 37 °C. The tagmented DNA was then purified using the Qiagen MinElute kit eluted with 10.5 μL Elution Buffer (EB). Ten microliters of purified tagmented DNA was PCR amplified using Nextera primers for 12 cycles to generate each library. PCR reaction was subsequently cleaned up using 1.5x AMPureXP beads (Agencourt), and concentrations were measured by Qubit. Libraries were paired-end sequenced on the Illumina HiSeq 4000 platform (100 bp read length).
ATAC-seq analysis
ATAC-seq peaks from libraries of tonsillar T cells [26], monocytes [69], and B cell subsets were called using the ENCODE ATAC-seq pipeline (https://www.encodeproject.org/atac-seq/). Briefly, pair-end reads from three biological replicates for each cell type were aligned to hg19 genome using bowtie2, and duplicate reads were removed from the alignment. Narrow peaks were called independently for each replicate using macs2 (-p 0.01 --nomodel --shift -75 --extsize 150 -B --SPMR --keep-dup all --call-summits) and ENCODE blacklist regions (ENCSR636HFF) were removed from peaks in individual replicates. The IDR optimal peak set for each cell type was used to define open chromatin regions in this study.
Promoter-focused Capture-C analysis
Paired-end reads from three biological replicates were pre-processed using the HiCUP pipeline (v0.5.9) [70], with bowtie2 as aligner and hg19 as the reference genome. Significant promoter interactions at 1-DpnII fragment resolution were called using CHiCAGO (v1.1.8) [71] with default parameters except for binsize set to 2500. Significant interactions at 4-DpnII fragment resolution were also called using CHiCAGO with artificial .baitmap and .rmap files in which DpnII fragments were concatenated in silico into 4 consecutive fragments using default parameters except for removeAdjacent set to False. The significant interactions (CHiCAGO score > 5) from both 1-fragment and 4-fragment resolutions were exported in .ibed format and merged into a single file using custom a PERL script to remove redundant interactions and to keep the max CHiCAGO score for each interaction.
COVID-19 GWAS data integration
We curated the lead SNPs from the recent COVID-19 severity GWAS from the COVID-19 Host Genetics Initiative [25] (Freeze 5). The six genome-wide significant SNPs associated with critical illness and COVID-19 severity were selected for investigation in our study. We identified proxies in LD with significant COVID-19 severity loci using LDLinkR with R2 > 0.8 in EUR ancestry. We next intersected the COVID-19 sentinel and proxy SNPs with the set of OCR annotated to promoter regions (−1500/+ 500 bp of TSS) and OCR overlapping promoter interacting regions identified by Capture C. Genomic coordinate overlaps were identified using the R package GenomicRanges (ver 1.42) against the human genome reference hg19.
Partitioned heritability LD score regression enrichment analysis
Partitioned heritability LD score regression (v1.0.0) was used to identify enrichment of GWAS summary statistics among open accessible regions identified in each cell type. The baseline analysis was performed using LDSCORE data (https://data.broadinstitute.org/alkesgroup/LDSCORE) with LD scores, regression weights, and allele frequencies from 1000G Phase1 and summary statistics from the COVID-19 Host Genetics Initiative [25]. We generated partitioned LD score regression annotations for each cell type using the coordinates of the all promoter OCR + promoter-interacting OCR. Finally, the cell-type-specific partitioned LD scores were compared to baseline LD scores to measure enrichment in each cell type independently.
Transcription factor motif analysis
Transcription factor binding site motifs overlapping with proxies implicated in by variant to gene mapping analysis were identified using the R package motifbreakR (v2.0.0) [72] using the Jaspar2018 database as our reference set of position weight matrices [73]. Results were filtered to TFs that expressed in the implicated cell type (TPM > 1) and were visualized using Cytoscape (v3.8.2) [74].
RNA-seq library generation and analysis
RNA was isolated from ~ 1 million of each cell type using Trizol Re- agent (Invitrogen), purified using the Directzol RNA Miniprep Kit (Zymo Research), and depleted of contaminating genomic DNA using DNAse I. Purified RNA was checked for quality on a Bioanlayzer 2100 using the Nano RNA Chip and samples with RIN > 7 were used for RNA-seq library preparation. RNA samples were depleted of rRNA using QIAseq Fastselect RNA removal kit (Qiagen). Samples were then processed for the preparation of libraries using the SMARTer Stranded Total RNA Sample Prep Kit (Takara Bio USA) according to the manufacturer’s instructions. Briefly, the purified first-strand cDNA is amplified into RNA-seq libraries using SeqAmp DNA Polymerase and the Forward and the Reverse PCR Primers from the Illumina Indexing Primer Set HT for Illumina. Quality and quantity of the libraries was assessed using the Agilent 2100 Bioanalyzer system and Qubit fluorometer (Life Technologies). Sequencing of the finalized libraries was performed on the NovaSeq 6000 platform at the CHOP Center for Spatial and Functional Genomics. For analysis, TPM values of bulk RNA-seq data were calculated using the pseudo-aligner Kallisto 0.46.2 [75]. Gene level expression was calculated by combining individual TPM values. The comparison of gene expression level between those with promoters with contacts to OCRs or those lacking contacts with OCRs was performed using an unpaired two-sided Wilcoxon rank-sum test, implemented in the R function wilcox.test.
COVID-19 V2G gene integration with external RNA-seq datasets
We retrieved bulk RNA-seq fastq datasets associated with Galani et al. [33] from the Sequence Read Archive using sra-tools (SRA Toolkit Development Team; http://ncbi.github.io/sra-tools/) and plotted the log2 fold change data reported for severe or mild COVID-19 compared to the mean of 5 healthy donors. Single-end fastq files were retrieved with fastq-dump. TPM values were calculated using kallisto with the following parameters: --single -l 200 -s 20. The log2FC for the genes implicated in our immune cell types was calculated for each COVID19 patient compared. We retrieved processed data for pseudo-bulk comparisons between severe and mild COVID-19 relative to healthy donors from Zhang et al. [34].
Ingenuity pathway analysis
Ingenuity pathway analysis (IPA, QIAGEN) was used for gene ontology analysis.
Supplementary Information
Additional file 1: Figures S1-S6 and Supplemental Table Legends. Figure S1. Summary of ATAC-seq datasets. Figure S2. Summary of Capture C datasets. Figure S3. Genomic Tracks indicating the position of SNPs of interest. Figure S4. Top IPA gene ontology network for genes implicated by COVID-19 V2G. Figure S5. The top IPA gene ontology network for transcription factors whose binding is predicted to be influenced by COVID-19-associated SNPs. Figure S6. Gating strategy for sorting of naïve B cells and GCB cells for this study.
Additional file 2: Table S1 – Summary of ATAC-seq peak calls.
Additional file 3: Table S2 – Summary of interactions called in PCC.
Additional file 4: Table S3 – COVID-19 variant-to-gene mapping (V2G) using Capture C and ATAC-seq.
Additional file 5: Table S4 - COVID-19 V2G gene set IPA analysis.
Additional file 6: Table S5 – Modeling COVID-19-associated SNP effects on TF.
Additional file 7: Table S6 – COVID-19 SNP-effected TF gene set IPA analysis.
Additional file 8: Review history – Peer review history.
Acknowledgements
The authors acknowledge Margot Field and Lihua Shi for excellent technical assistance.
Code availability
Publicly available analysis software and code were used as described in the methods section.
Peer review information
Andrew Cosgrove was the primary editor of this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Review history
The review history is available as Additional file 8.
Authors’ contributions
M.P. conducted epigenomic and transcriptomic analyses and assisted with the preparation of the manuscript; C.S. conducted and contributed to the design of the epigenomic and transcriptomic analyses; A.C. designed the custom Capture-C promoter probe set; C.L.C. and N.R. provided human tonsillar T and B cells and performed the tonsillar organoid experiments; L.S. and K.S. provided human monocytes; P.S, R.M.T., M.E.J, and J.A.P. generated and sequenced the epigenomic and transcriptomic libraries; S.F.G. directed the genomic and epigenomic studies; A.D.W. directed the epigenomic, transcriptomic, and immunologic studies and wrote the manuscript. S.F.G and A.D.W. are co-senior authors. The authors read and approved the final manuscript.
Funding
This work was supported by The Center for Spatial and Functional Genomics at The Children’s Hospital of Philadelphia (ADW and SFG), the Daniel B. Burke Endowed Chair for Diabetes Research (SFG), the Jeffrey Modell Foundation (NR), and NIH grants AI137143 (ADW), DK122586 (ADW, SFG), HG010067 (SFG), and AI146026 (NR).
Availability of data and materials
Monocyte and naïve and germinal center B cell raw ATAC seq and Capture C datasets are deposited in GEO with the accession number GSE174658 [76]. The naïve CD4+ T cell and TFH datasets are available at ArrayExpress (https://www.ebi.ac.uk/arrayexpress/) with accession numbers E-MTAB-6621 (promoter-Capture-C) and E-MTAB-6617 (ATAC-seq) [77]. COVID-19 severity-associated sentinel SNPs were retrieved from Supplemental Table 2 of the COVID-19 Host Genetics Initiative. COVID19 summary stats were retrieved from Freeze 5 of COVID19 Host initiative (https://www.covid19hg.org/results/r5/) [78]. Gene expression data for severe COVID19 was retrieved from the SRA from BioProject PRJNA638753 [79].
Declarations
Ethics approval and consent to participate
The use of de-identified discarded tonsil tissue from pediatric patients undergoing tonsillectomy at The Children’s Hospital of Philadelphia for treatment of sleep apnea was approved by the CHOP Institutional Review Board. The use of de-identified peripheral blood mononuclear cells obtained by the Penn Human Immunology Core from apheresis donors was approved by the University of Pennsylvania Institutional Review Board.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Struan F. A. Grant and Andrew D. Wells jointly supervised this work.
References
- 1.Braun J, et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020:1–8. 10.1038/s41586-020-2598-9. [DOI] [PubMed]
- 2.Grifoni A, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, Wang Q, Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, Wherry EJ. T cell responses in patients with COVID-19. Nat Rev Immunol. 2020;20(9):529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcos-Jiménez A, Sánchez-Alonso S, Alcaraz-Serna A, Esparcia L, López-Sanz C, Sampedro-Núñez M, Mateu-Albero T, Sánchez-Cerrillo I, Martínez-Fleta P, Gabrie L, Campo Guerola L, Rodríguez-Frade JM, Casasnovas JM, Reyburn HT, Valés-Gómez M, López-Trascasa M, Martín-Gayo E, Calzada MJ, Castañeda S, Fuente H, González-Álvaro I, Sánchez-Madrid F, Muñoz-Calleja C, Alfranca A. Deregulated cellular circuits driving immunoglobulins and complement consumption associate with the severity of COVID-19 patients. Eur J Immunol. 2021;51(3):634–647. doi: 10.1002/eji.202048858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y, et al. Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome; n.d. 10.1101/2020.03.02.20029975.
- 8.Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C, Yue J, Zhang Z, Renz H, Liu X, Xie J, Xie M, Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chua RL, Lukassen S, Trump S, Hennig BP, Wendisch D, Pott F, Debnath O, Thürmann L, Kurth F, Völker MT, Kazmierski J, Timmermann B, Twardziok S, Schneider S, Machleidt F, Müller-Redetzky H, Maier M, Krannich A, Schmidt S, Balzer F, Liebig J, Loske J, Suttorp N, Eils J, Ishaque N, Liebert UG, von Kalle C, Hocke A, Witzenrath M, Goffinet C, Drosten C, Laudi S, Lehmann I, Conrad C, Sander LE, Eils R. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. Nat Biotechnol. 2020;38(8):970–979. doi: 10.1038/s41587-020-0602-4. [DOI] [PubMed] [Google Scholar]
- 10.Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Cheng L, Li J, Wang X, Wang F, Liu L, Amit I, Zhang S, Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26(6):842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 11.Moderbacher CR, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekine T, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158–168.e14. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther. 2020;5(1):84. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grant RA, et al. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature. 2021:1–10. 10.1038/s41586-020-03148-w. [DOI] [PMC free article] [PubMed]
- 15.Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50(5):1132–1148. doi: 10.1016/j.immuni.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaebler C, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021:1–10. 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed]
- 17.Mathew D, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369:eabc8511. doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juno JA, Tan HX, Lee WS, Reynaldi A, Kelly HG, Wragg K, Esterbauer R, Kent HE, Batten CJ, Mordant FL, Gherardin NA, Pymm P, Dietrich MH, Scott NE, Tham WH, Godfrey DI, Subbarao K, Davenport MP, Kent SJ, Wheatley AK. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat Med. 2020;26(9):1428–1434. doi: 10.1038/s41591-020-0995-0. [DOI] [PubMed] [Google Scholar]
- 19.Yang X, Dai T, Zhou X, Qian H, Guo R, Lei L, Zhang X, Zhang D, Shi L, Cheng Y, Hu J, Guo Y, Zhang B. Naturally activated adaptive immunity in COVID-19 patients. J Cell Mol Med. 2020;24(21):12457–12463. doi: 10.1111/jcmm.15771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arunachalam PS, Wimmers F, Mok CKP, Perera RAPM, Scott M, Hagan T, Sigal N, Feng Y, Bristow L, Tak-Yin Tsang O, Wagh D, Coller J, Pellegrini KL, Kazmin D, Alaaeddine G, Leung WS, Chan JMC, Chik TSH, Choi CYC, Huerta C, Paine McCullough M, Lv H, Anderson E, Edupuganti S, Upadhyay AA, Bosinger SE, Maecker HT, Khatri P, Rouphael N, Peiris M, Pulendran B. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science. 2020;369(6508):1210–1220. doi: 10.1126/science.abc6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakshmanappa YS, et al. SARS-CoV-2 induces robust germinal center CD4 T follicular helper cell responses in rhesus macaques. Nat Commun. 2021;12(1):541. doi: 10.1038/s41467-020-20642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Wu Q, Liu Z, Wang Q, Wu J, Hu Y, Bai T, Xie T, Huang M, Wu T, Peng D, Huang W, Jin K, Niu L, Guo W, Luo D, Lei D, Wu Z, Li G, Huang R, Lin Y, Xie X, He S, Deng Y, Liu J, Li W, Lu Z, Chen H, Zeng T, Luo Q, Li YP, Wang Y, Liu W, Qu X. Spike-specific circulating T follicular helper cell and cross-neutralizing antibody responses in COVID-19-convalescent individuals. Nat Microbiol. 2021;6(1):51–58. doi: 10.1038/s41564-020-00824-5. [DOI] [PubMed] [Google Scholar]
- 23.Kuri-Cervantes L, et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol. 2020;5:eabd7114. doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pairo-Castineira E, Clohisey S, Klaric L, Bretherick AD, Rawlik K, Pasko D, Walker S, Parkinson N, Fourman MH, Russell CD, Furniss J, Richmond A, Gountouna E, Wrobel N, Harrison D, Wang B, Wu Y, Meynert A, Griffiths F, Oosthuyzen W, Kousathanas A, Moutsianas L, Yang Z, Zhai R, Zheng C, Grimes G, Beale R, Millar J, Shih B, Keating S, Zechner M, Haley C, Porteous DJ, Hayward C, Yang J, Knight J, Summers C, Shankar-Hari M, Klenerman P, Turtle L, Ho A, Moore SC, Hinds C, Horby P, Nichol A, Maslove D, Ling L, McAuley D, Montgomery H, Walsh T, Pereira AC, Renieri A, GenOMICC Investigators., ISARIC4C Investigators., COVID-19 Human Genetics Initiative., 23andMe Investigators., BRACOVID Investigators., Gen-COVID Investigators. Shen X, Ponting CP, Fawkes A, Tenesa A, Caulfield M, Scott R, Rowan K, Murphy L, Openshaw PJM, Semple MG, Law A, Vitart V, Wilson JF, Baillie JK. Genetic mechanisms of critical illness in Covid-19. Nature. 2020;591(7848):92–98. doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- 25.COVID-19 Host Genetics Initiative. Mapping the human genetic architecture of COVID-19. Nature. 2021. 10.1038/s41586-021-03767-x. [DOI] [PMC free article] [PubMed]
- 26.Su C, Johnson ME, Torres A, Thomas RM, Manduchi E, Sharma P, Mehra P, le Coz C, Leonard ME, Lu S, Hodge KM, Chesi A, Pippin J, Romberg N, Grant SFA, Wells AD. Mapping effector genes at lupus GWAS loci using promoter Capture-C in follicular helper T cells. Nat Commun. 2020;11(1):3294. doi: 10.1038/s41467-020-17089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pahl MC, et al. Cis-regulatory architecture of human ESC-derived hypothalamic neuron differentiation aids in variant-to-gene mapping of relevant complex traits. Biorxiv. 2020:2020.07.06.146951. 10.1101/2020.07.06.146951. [DOI] [PMC free article] [PubMed]
- 28.Chesi A, Wagley Y, Johnson ME, Manduchi E, Su C, Lu S, Leonard ME, Hodge KM, Pippin JA, Hankenson KD, Wells AD, Grant SFA. Genome-scale Capture C promoter interactions implicate effector genes at GWAS loci for bone mineral density. Nat Commun. 2019;10(1):1260. doi: 10.1038/s41467-019-09302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su C, et al. 3D promoter architecture re-organization during iPSC-derived neuronal cell differentiation implicates target genes for neurodevelopmental disorders. Prog Neurobiol. 2021:102000. 10.1016/j.pneurobio.2021.102000. [DOI] [PMC free article] [PubMed]
- 30.Hammond RK, Pahl MC, Su C, Cousminer DL, Leonard ME, Lu S, Doege CA, Wagley Y, Hodge KM, Lasconi C, Johnson ME, Pippin JA, Hankenson KD, Leibel RL, Chesi A, Wells AD, Grant SFA. Biological constraints on GWAS SNPs at suggestive significance thresholds reveal additional BMI loci. Elife. 2021;10:e62206. doi: 10.7554/eLife.62206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lasconi C, Pahl MC, Cousminer DL, Doege CA, Chesi A, Hodge KM, Leonard ME, Lu S, Johnson ME, Su C, Hammond RK, Pippin JA, Terry NA, Ghanem LR, Leibel RL, Wells AD, Grant SFA. Variant-to-gene-mapping analyses reveal a role for the hypothalamus in genetic susceptibility to inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2020;11(3):667–682. doi: 10.1016/j.jcmgh.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagar LE, Salahudeen A, Constantz CM, Wendel BS, Lyons MM, Mallajosyula V, Jatt LP, Adamska JZ, Blum LK, Gupta N, Jackson KJL, Yang F, Röltgen K, Roskin KM, Blaine KM, Meister KD, Ahmad IN, Cortese M, Dora EG, Tucker SN, Sperling AI, Jain A, Davies DH, Felgner PL, Hammer GB, Kim PS, Robinson WH, Boyd SD, Kuo CJ, Davis MM. Modeling human adaptive immune responses with tonsil organoids. Nat Med. 2021;27(1):125–135. doi: 10.1038/s41591-020-01145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galani I-E, Rovina N, Lampropoulou V, Triantafyllia V, Manioudaki M, Pavlos E, Koukaki E, Fragkou PC, Panou V, Rapti V, Koltsida O, Mentis A, Koulouris N, Tsiodras S, Koutsoukou A, Andreakos E. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat Immunol. 2021;22(1):32–40. doi: 10.1038/s41590-020-00840-x. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J-Y, Wang XM, Xing X, Xu Z, Zhang C, Song JW, Fan X, Xia P, Fu JL, Wang SY, Xu RN, Dai XP, Shi L, Huang L, Jiang TJ, Shi M, Zhang Y, Zumla A, Maeurer M, Bai F, Wang FS. Single-cell landscape of immunological responses in patients with COVID-19. Nat Immunol. 2020;21(9):1107–1118. doi: 10.1038/s41590-020-0762-x. [DOI] [PubMed] [Google Scholar]
- 35.Lan X, et al. ZNF410 uniquely activates the NuRD component CHD4 to silence fetal hemoglobin expression. Mol Cell. 2021;81:239–254.e8. doi: 10.1016/j.molcel.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capoccia BJ, Lennerz JKM, Bredemeyer AJ, Klco JM, Frater JL, Mills JC. Transcription factor MIST1 in terminal differentiation of mouse and human plasma cells. Physiol Genomics. 2011;43(3):174–186. doi: 10.1152/physiolgenomics.00084.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, Chen X, Zhong B, Wang A, Wang X, Chu F, Nurieva RI, Yan X, Chen P, van der Flier LG, Nakatsukasa H, Neelapu SS, Chen W, Clevers H, Tian Q, Qi H, Wei L, Dong C. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature. 2014;507(7493):513–516. doi: 10.1038/nature12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobreira DR, Joslin AC, Zhang Q, Williamson I, Hansen GT, Farris KM, Sakabe NJ, Sinnott-Armstrong N, Bozek G, Jensen-Cody SO, Flippo KH, Ober C, Bickmore WA, Potthoff M, Chen M, Claussnitzer M, Aneas I, Nóbrega MA. Extensive pleiotropism and allelic heterogeneity mediate metabolic effects of IRX3 and IRX5. Science. 2021;372(6546):1085–1091. doi: 10.1126/science.abf1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karlas A, Machuy N, Meyer TF. Human host cell factors crucial for influenza virus replication identified by genome-wide RNAi screen. New Biotechnol. 2010;27:S84. doi: 10.1016/j.nbt.2010.01.234. [DOI] [PubMed] [Google Scholar]
- 40.Sun C-T, Lo WY, Wang IH, Lo YH, Shiou SR, Lai CK, Ting LP. Transcription repression of human hepatitis B virus genes by negative regulatory element-binding protein/SON*. J Biol Chem. 2001;276(26):24059–24067. doi: 10.1074/jbc.M101330200. [DOI] [PubMed] [Google Scholar]
- 41.Danchin A, Marlière P. Cytosine drives evolution of SARS-CoV-2. Environ Microbiol. 2020;22(6):1977–1985. doi: 10.1111/1462-2920.15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo R, Wong Y-S, Lam T-W. Tracking cytosine depletion in SARS-CoV-2. Biorxiv. 2020:2020.10.26.354787. 10.1101/2020.10.26.354787.
- 43.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin X, Riva L, Pu Y, Martin-Sancho L, Kanamune J, Yamamoto Y, Sakai K, Gotoh S, Miorin L, de Jesus PD, Yang CC, Herbert KM, Yoh S, Hultquist JF, García-Sastre A, Chanda SK. MDA5 governs the innate immune response to SARS-CoV-2 in lung epithelial cells. Cell Rep. 2021;34(2):108628. doi: 10.1016/j.celrep.2020.108628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuen C-K, et al. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg Microbes Infec. 2020;9:1–29. doi: 10.1080/22221751.2020.1780953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu J, Shi Y, Pan X, Wu S, Hou R, Zhang Y, Zhong T, Tang H, du W, Wang L, Wo J, Mu J, Qiu Y, Yang K, Zhang LK, Ye BC, Qi N. SARS-CoV-2 ORF9b inhibits RIG-I-MAVS antiviral signaling by interrupting K63-linked ubiquitination of NEMO. Cell Rep. 2021;34(7):108761. doi: 10.1016/j.celrep.2021.108761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lokugamage KG, Hage A, de Vries M, Valero-Jimenez AM, Schindewolf C, Dittmann M, et al. Type I interferon susceptibility distinguishes SARS-CoV-2 from SARS-CoV. J Virol. 2020;94(23). 10.1128/JVI.01410-20. [DOI] [PMC free article] [PubMed]
- 48.Blanco-Melo D, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bastard P, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Acharya D, Liu G, Gack MU. Dysregulation of type I interferon responses in COVID-19. Nat Rev Immunol. 2020;20(7):397–398. doi: 10.1038/s41577-020-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, Péré H, Charbit B, Bondet V, Chenevier-Gobeaux C, Breillat P, Carlier N, Gauzit R, Morbieu C, Pène F, Marin N, Roche N, Szwebel TA, Merkling SH, Treluyer JM, Veyer D, Mouthon L, Blanc C, Tharaux PL, Rozenberg F, Fischer A, Duffy D, Rieux-Laucat F, Kernéis S, Terrier B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Q, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y, Renner DM, Comar CE, Whelan JN, Reyes HM. SARS-CoV-2 induces double-stranded RNA-mediated innate immune responses in respiratory epithelial-derived cells and cardiomyocytes. PNAS. 2021;118:e2022643118. doi: 10.1073/pnas.2022643118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bauernfried S, Hornung V. DPP9 restrains NLRP1 activation. Nat Struct Mol Biol. 2021;28(4):333–336. doi: 10.1038/s41594-021-00580-y. [DOI] [PubMed] [Google Scholar]
- 55.Magg T, et al. Heterozygous OAS1 gain-of-function variants cause an autoinflammatory immunodeficiency. Sci Immunol. 2021;6:eabf9564. doi: 10.1126/sciimmunol.abf9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vujkovic M, Keaton JM, Lynch JA, Miller DR, Zhou J, Tcheandjieu C, Huffman JE, Assimes TL, Lorenz K, Zhu X, Hilliard AT, Judy RL, Huang J, Lee KM, Klarin D, Pyarajan S, Danesh J, Melander O, Rasheed A, Mallick NH, Hameed S, Qureshi IH, Afzal MN, Malik U, Jalal A, Abbas S, Sheng X, Gao L, Kaestner KH, Susztak K, Sun YV, DuVall SL, Cho K, Lee JS, Gaziano JM, Phillips LS, Meigs JB, Reaven PD, Wilson PW, Edwards TL, Rader DJ, Damrauer SM, O’Donnell CJ, Tsao PS, The HPAP Consortium. Atkinson MA, Powers AC, Naji A, Kaestner KH, Regeneron Genetics Center. Abecasis GR, Baras A, Cantor MN, Coppola G, Economides AN, Lotta LA, Overton JD, Reid JG, Shuldiner AR, Beechert C, Forsythe C, Fuller ED, Gu Z, Lattari M, Lopez AE, Schleicher TD, Padilla MS, Toledo K, Widom L, Wolf SE, Pradhan M, Manoochehri K, Ulloa RH, Bai X, Balasubramanian S, Barnard L, Blumenfeld AL, Eom G, Habegger L, Hawes A, Khalid S, Maxwell EK, Salerno WJ, Staples JC, Yadav A, Jones MB, Mitnaul LJ, VA Million Veteran Program. Aguayo SM, Ahuja SK, Ballas ZK, Bhushan S, Boyko EJ, Cohen DM, Concato J, Constans JI, Dellitalia LJ, Fayad JM, Fernando RS, Florez HJ, Gaddy MA, Gappy SS, Gibson G, Godschalk M, Greco JA, Gupta S, Gutierrez S, Hammer KD, Hamner MB, Harley JB, Hung AM, Huq M, Hurley RA, Iruvanti PR, Ivins DJ, Jacono FJ, Jhala DN, Kaminsky LS, Kinlay S, Klein JB, Liangpunsakul S, Lichy JH, Mastorides SM, Mathew RO, Mattocks KM, McArdle R, Meyer PN, Meyer LJ, Moorman JP, Morgan TR, Murdoch M, Nguyen XMT, Okusaga OO, Oursler KAK, Ratcliffe NR, Rauchman MI, Robey RB, Ross GW, Servatius RJ, Sharma SC, Sherman SE, Sonel E, Sriram P, Stapley T, Striker RT, Tandon N, Villareal G, Wallbom AS, Wells JM, Whittle JC, Whooley MA, Xu J, Yeh SS, Aslan M, Brewer JV, Brophy MT, Connor T, Argyres DP, Do NV, Hauser ER, Humphries DE, Selva LE, Shayan S, Stephens B, Whitbourne SB, Zhao H, Moser J, Beckham JC, Breeling JL, Romero JPC, Huang GD, Ramoni RB, Pyarajan S, Sun YV, Cho K, Wilson PW, O’Donnell CJ, Tsao PS, Chang KM, Gaziano JM, Muralidhar S, Chang KM, Voight BF, Saleheen D. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet. 2020;52(7):680–691. doi: 10.1038/s41588-020-0637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, Loyd JE, Cosgrove GP, Lynch D, Groshong S, Collard HR, Wolters PJ, Bradford WZ, Kossen K, Seiwert SD, du Bois RM, Garcia CK, Devine MS, Gudmundsson G, Isaksson HJ, Kaminski N, Zhang Y, Gibson KF, Lancaster LH, Cogan JD, Mason WR, Maher TM, Molyneaux PL, Wells AU, Moffatt MF, Selman M, Pardo A, Kim DS, Crapo JD, Make BJ, Regan EA, Walek DS, Daniel JJ, Kamatani Y, Zelenika D, Smith K, McKean D, Pedersen BS, Talbert J, Kidd RN, Markin CR, Beckman KB, Lathrop M, Schwarz MI, Schwartz DA. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45(6):613–620. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: A meta-analysis. J Med Virol. 2020;92(11):2283–2285. doi: 10.1002/jmv.25948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ye Q, Wang B, Mao J. Cytokine storm in COVID-19 and treatment. J Inf Secur. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mehta P, McAuley D, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, Zhang P, Liu X, Gao G, Liu F, Jiang Y, Cheng X, Zhu C, Xia Y. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infec. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Minami M, Shimizu K, Okamoto Y, Folco E, Ilasaca ML, Feinberg MW, Aikawa M, Libby P. Prostaglandin E receptor type 4-associated protein interacts directly with NFkB1 and attenuates macrophage activation. J Biol Chem. 2008;283(15):9692–9703. doi: 10.1074/jbc.M709663200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen P, Zhou J, Li J, Zhang Q, Zuo Q. TIPE1 suppresses osteosarcoma tumor growth by regulating macrophage infiltration. Clin Transl Oncol. 2019;21(3):334–341. doi: 10.1007/s12094-018-1927-z. [DOI] [PubMed] [Google Scholar]
- 65.Musskopf MK, de Mattos EP, Bergink S, Kampinga HH. Hsp40/DNAJ chaperones. eLS. 2018. 10.1002/9780470015902.a0027633.
- 66.Zhou S, Han L, Weng M, Zhu H, Heng Y, Wang G, Shen Z, Chen X, Fu X, Zhang M, Wu Z. Paxbp1 controls a key checkpoint for cell growth and survival during early activation of quiescent muscle satellite cells. PNAS. 2021;118(13):e2021093118. doi: 10.1073/pnas.2021093118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berghorn KA, Clark-Campbell PA, Han L, McGrattan M, Weiss RS, Roberson MS. Smad6 represses Dlx3 transcriptional activity through inhibition of DNA binding. J Biol Chem. 2006;281(29):20357–20367. doi: 10.1074/jbc.M603049200. [DOI] [PubMed] [Google Scholar]
- 68.Puleston DJ, Villa M, Pearce EL. Ancillary activity: beyond core metabolism in immune cells. Cell Metab. 2017;26(1):131–141. doi: 10.1016/j.cmet.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi L, et al. IL-1 transcriptional responses to lipopolysaccharides are regulated by a complex of RNA binding proteins. J Immunol Baltim Md. 1950;2020(204):1334–1344. doi: 10.4049/jimmunol.1900650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wingett S, et al. HiCUP: pipeline for mapping and processing Hi-C data. F1000Res. 2015;4:1310. doi: 10.12688/f1000research.7334.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cairns J, Freire-Pritchett P, Wingett SW, Várnai C, Dimond A, Plagnol V, Zerbino D, Schoenfelder S, Javierre BM, Osborne C, Fraser P, Spivakov M. CHiCAGO: robust detection of DNA looping interactions in Capture Hi-C data. Genome Biol. 2016;17(1):127. doi: 10.1186/s13059-016-0992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coetzee SG. Coetzee, G. A. & Hazelett, D. J. motifbreakR: an R/Bioconductor package for predicting variant effects at transcription factor binding sites. Bioinformatics. 2015;31(23):3847–3849. doi: 10.1093/bioinformatics/btv470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khan A, et al. JASPAR 2018: update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 2017;46:gkx1126. doi: 10.1093/nar/gkx1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bray NL, Pimentel H, Melsted P, Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol. 2016;34(5):525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- 76.Pahl, M.C., Le Coz, C., Su, C., Sharma, P., Thomas, R.M., Pippin, J.A., Cruz Cabrera, E., Johnson, M.E., Leonard, M.E., Lu, S., Chesi, A., Sullivan, K.E., Romberg, N., Grant. S.F.A., and Wells, A.D. Implicating effector genes at COVID-19 GWAS loci using promoter-focused Capture-C in disease-relevant immune cell types. Monocyte and naïve and germinal center B cell raw ATAC seq and Capture C datasets. Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE174658 [DOI] [PMC free article] [PubMed]
- 77.Wells, A.D., Chesi, A., Manduchi, E., Johnson, M.E., Leonard, M.E., Romberg, N., Lu, S., Grant. S.F.A. Promoter capture-C of primary human T Follicular Helper (TFH) cells and naive CD4-positive helper T cells from tonsils of healthy volunteers. Naïve CD4+ T cell and TFH raw ATAC seq and Capture C datasets. https://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-6621/
- 78.COVID19 Host initiative. COVID19 severity associated sentinel SNPs from Supplemental Table 2 of the COVID-19 Host Genetics Initiative. COVID19 summary stats Freeze 5. https://www.covid19hg.org/results/r5/
- 79.Galani I, Rovina N, Lampropoulou V, Triantafyllia V, Manioudaki M, Pavlos E, et al. COVID-19 transcriptional response in blood (PRJNA638753). Bioproject. https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA638753. 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figures S1-S6 and Supplemental Table Legends. Figure S1. Summary of ATAC-seq datasets. Figure S2. Summary of Capture C datasets. Figure S3. Genomic Tracks indicating the position of SNPs of interest. Figure S4. Top IPA gene ontology network for genes implicated by COVID-19 V2G. Figure S5. The top IPA gene ontology network for transcription factors whose binding is predicted to be influenced by COVID-19-associated SNPs. Figure S6. Gating strategy for sorting of naïve B cells and GCB cells for this study.
Additional file 2: Table S1 – Summary of ATAC-seq peak calls.
Additional file 3: Table S2 – Summary of interactions called in PCC.
Additional file 4: Table S3 – COVID-19 variant-to-gene mapping (V2G) using Capture C and ATAC-seq.
Additional file 5: Table S4 - COVID-19 V2G gene set IPA analysis.
Additional file 6: Table S5 – Modeling COVID-19-associated SNP effects on TF.
Additional file 7: Table S6 – COVID-19 SNP-effected TF gene set IPA analysis.
Additional file 8: Review history – Peer review history.
Data Availability Statement
Monocyte and naïve and germinal center B cell raw ATAC seq and Capture C datasets are deposited in GEO with the accession number GSE174658 [76]. The naïve CD4+ T cell and TFH datasets are available at ArrayExpress (https://www.ebi.ac.uk/arrayexpress/) with accession numbers E-MTAB-6621 (promoter-Capture-C) and E-MTAB-6617 (ATAC-seq) [77]. COVID-19 severity-associated sentinel SNPs were retrieved from Supplemental Table 2 of the COVID-19 Host Genetics Initiative. COVID19 summary stats were retrieved from Freeze 5 of COVID19 Host initiative (https://www.covid19hg.org/results/r5/) [78]. Gene expression data for severe COVID19 was retrieved from the SRA from BioProject PRJNA638753 [79].