Abstract
Background:
Concurrent radiation therapy (RT) with cetuximab, an anti-EGFR monoclonal antibody (mAb), is a standard treatment for locally advanced head and neck squamous carcinoma (HNSCC). CTLA-4+ T regulatory cells (Treg) dampen cellular immunity and correlate negatively with clinical outcomes. This phase I study added ipilimumab, an anti-CTLA-4 mAb, to cetuximab-RT.
Methods:
A [3 + 3] design was used to establish the recommended phase II dose (RP2D) of ipilimumab, added at week 5 for four, q3-week doses to fixed, standard cetuximab-RT. Eligible subjects had stage III-IVb, high-risk (HPV-) or intermediate-risk (HPV+) HNSCC. Dose limiting toxicity (DLT) was defined as any grade 4 adverse event (AE) except in-field radiation dermatitis or immune-related (ir) AE requiring ≥ 2 weeks of systemic steroids. Baseline tumor and serial blood specimens were collected for immune correlatives.
Results:
From July 2013-May 2016, 18 patients enrolled. Two of 6 in cohort 1 (ipilimumab 3 mg/kg) experienced grade 3 dermatologic DLTs, triggering de-escalation of ipilimumab to 1 mg/kg. Dose Level -1 was expanded to N = 12 without DLT. irAE included: grade 1, 2, and 3 dermatitis (2, 1, and 3 cases), grade 4 colitis (1), and grade 1 hyperthyroidism (1). Three-year disease-free survival (DFS) and overall survival (OS) were 72% (90% CI: 57–92%) and 72% (90% CI: 56–92%). High expression of co-inhibitory receptors PD1/LAG3/CD39 on baseline tumor-infiltrating Treg was associated with worse DFS (HR=5.6, 95% CI: 0.83–37.8, p=0.08).
Conclusions:
The RP2D for ipilimumab plus standard cetuximab-RT is 1mg/kg in weeks 5, 8, 11, and 14. The regimen is tolerable and yields acceptable survival without cytotoxic chemotherapy.
Keywords: CTLA-4, Head and Neck Cancer, immunotherapy
Introduction
Head and neck squamous cell carcinoma (HNSCC) is among the most immunosuppressive malignancies. HNSCC is characterized by impaired functions of immune effector cells, dysregulated cytokine profiles and an increased frequency of intra-tumoral and circulating regulatory T cells (Treg)1–4. Furthermore, Treg in HNSCC patients express high levels of immune checkpoint ligands such as CTLA4 and effectively downregulate anti-tumor functions of cytotoxic T cells5,6. The anti-tumor immune profile of HNSCC patients with advanced disease is especially compromised, and restoration of immune competence is expected to have beneficial effects on disease control6. Immune therapies aiming to rejuvenate patients’ immune response are, therefore, a critical addition to standard of care therapy for patients with HNSCC. Recently, immune checkpoint inhibitors targeting programmed death (PD)-1 were U.S. FDA approved for recurrent/metastatic HNSCC7–10. While therapeutic targeting of cytotoxic T lymphocyte antigen (CTLA)-4 alone or in combination with anti-PD1/L1 monoclonal antibodies (mAb) has been successful in several cancers, including melanoma and lung cancer, data for the combination have been negative in recurrent/metastatic HNSCC to date 11,12. The use of anti-CTLA-4 mAb to overcome immune suppression during curative-intent multimodality treatment has not been reported in locally advanced HNSCC.
The EGFR-specific mAb cetuximab represents an established immunotherapy for patients with HNSCC13,14, and improves survival when added to radiation therapy (RT) in locally advanced HNSCC or cytotoxic chemotherapy in recurrent/metastatic HNSCC15,16. However, the response rate for cetuximab monotherapy in advanced HNSCC has been relatively low, i.e. less than 15%.16 In a recent study investigating the effects of cetuximab on various immune cell populations in patients with HNSCC, we showed that although cetuximab treatment increased NK cell and CD8+ T cell activation and cytotoxicity, these effects were associated with a simultaneous increase of CTLA4+ T regulatory cells (Treg) in clinical nonresponders17. Therefore, we hypothesized that the addition of the anti-CTLA-4 mAb, ipilimumab, to cetuximab-RT might promote the ablation of Treg and contribute to better clinical outcomes. With this rationale in mind, we conducted a phase I clinical trial evaluating the safety and preliminary efficacy of this combination in patients with intermediate or high-risk, locally advanced HNSCC. Immune modulation was a secondary objective of the trial, and participants were immunologically monitored upon trial entry and at specified time points during and after therapy.
Materials and Methods
Study design
This single-center phase I clinical trial was designed to identify the recommended phase II dose (RP2D) of ipilimumab when administered in combination with standard, fixed cetuximab-RT for high- or intermediate-risk patients with locally advanced HNSCC (NCT01935921). A 3+3 phase I design was used, with escalation and de-escalation cohorts specified in Table 1. Ipilimumab was added at the specified dose during weeks 5, 8, 11, and 14 of standard cetuximab-RT, and only ipilimumab was escalated or de-escalated per design. Cetuximab-RT was fixed in all cohorts. Cetuximab was dosed at 400 mg/m2 loading dose one week prior to the start of RT, then at 250 mg/m2/week concurrent with RT. Intensity-modulated RT was used to prescribe the planning target volume (PTV) to 70 to 74 Gy delivered in 35 to 37 fractions at 2 Gy per fraction. The studies detailed in this manuscript were conducted in accordance with the Declaration of Helsinki ethical guidelines.
Table 1.
Treatment Regimen
| Treatment type | Week of Treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 11 | 14 | |
| IMRT | X | X | X | X | X | X | X | |||
| 70–74 Gy, standard fractionation (35 fractions of 2 Gray over 7 weeks) | ||||||||||
|
| ||||||||||
| Cetuximab | X | X | X | X | X | X | X | X | ||
| 400 mg/m2 load then 250 mg/m2/week | ||||||||||
|
| ||||||||||
| Ipilimumab | X | X | X | X | ||||||
| Cohort −1: 1 mg/kg | ||||||||||
| Cohort 1 (starting dose): 3 mg/kg | ||||||||||
| Cohort 2: 10 mg/kg | ||||||||||
Study population
All patients provided written, informed consent. Key eligibility criteria included: AJCC v.7 stage III-IVb previously untreated, locally advanced HNSCC (pharynx, larynx); high risk (human papillomavirus-negative (HPV-)) or intermediate risk disease (HPV+ and either: ≥ 10 pack-year tobacco and ≥ N2 disease; or T4 or N3 disease); ECOG performance status 0–2. HPV status was determined by p16 IHC staining for oropharyngeal primary cancers as well as in situ hybridization as described18.
Objectives
The primary objective was to determine the RP2D for the combination of ipilimumab, cetuximab and RT as defined by the rate of dose-limiting toxicity (DLT). DLT was defined as an adverse event (AE) at least possibly attributable to ipilimumab and meeting one of the following criteria: any grade 4 adverse event (AE) except in-field radiation dermatitis or asymptomatic, correctable lab abnormality; any grade ≥ 3 immune-related (ir)AE requiring ≥ 2 weeks of systemic immunosuppression; any AE resulting in the delay of RT by more than 10 fractions. The DLT observation window was from first administration of ipilimumab (week 5) through 4 weeks post completion of RT. This DLT observation window was selected to include the period of overlap of all three modalities, RT, cetuximab, and ipilimumab, as the toxicities of cetuximab-RT and ipilimumab monotherapy are known. An additional four weeks post RT were included, the period of time when acute toxicities from standard cetuximab-RT have begun to clear. Secondary objectives were to describe the toxicities of the combination with particular focus on irAE; to describe preliminary oncologic efficacy; and to evaluate tumor microenvironment and peripheral blood biomarkers of T cell and Treg activation.
Statistical analysis
Progression-free survival (PFS) was defined as the elapsed time from first treatment until a progression event, defined as disease recurrence or death with or without disease recurrence. Disease-free survival (DFS) was defined as time from first treatment to documented disease progression. Overall survival (OS) was defined as elapsed time from first treatment until death or loss to follow-up. Patients lost to follow up were censored on their last date of contact. PFS, DFS, and OS were estimated by the Kaplan-Meier method with Greenwood 90% confidence intervals. Survival summary statistics combined both dose cohorts of ipilimumab. Serial samples of PBL and TIL collected at baseline, week 5 (start of ipilimumab) and week 14 (end of ipilimumab) were tested for time-dependent within-patient change using Friedman’s test. Individual baseline values of PBL and TIL were tested for association with PFS (maximum follow-up of 28 months) with proportional hazards regression.
Collection and processing of PBMC and TIL
Tumor and blood samples were processed, and lymphocytes isolated as previously described13. Both tumor infiltrating lymphocytes (TIL) and peripheral blood mononuclear cells (PBMC) were frozen and stored in liquid nitrogen until completion of clinical trial accrual. For analysis, samples were thawed, stained with fluorochrome tagged antibodies against surface markers, washed, and fixed. Fixed cells were permeabilized for intracellular staining with FoxP3 staining fixation/permeabilization buffer kit (eBioscience). Appropriate dilutions for staining were determined from healthy donor ex vivo and activated lymphocytes prior to staining of clinical trial samples.
The human investigations were performed after approval by the University of Pittsburgh IRB (PRO13020549) and in accordance with an assurance filed with and approved by the U.S Department of Health and Human Services. Readers may request available clinical trial data or original correlative biomarker data from the corresponding author.
Flow cytometric analyses
Three staining panels were designed to analyze proportions of cells expressing inhibitory receptors (IRs) and other functional markers to correlate with activation and proliferation status pre- and post-treatment. All antibodies were from Biolegend, unless otherwise specified. Cell population surface markers were determined using the following antibodies: CD4 (clone RPTA4), CD8 (clone RPTA4), CD3 (clone HIT3a), and FoxP3 (clone PCH101, Thermofisher Scientific). IR antibodies included CD39 (clone A1), PD1 (clone J105, Thermo-Fisher Scientific), CTLA4 (clone 14D3, Thermofisher Scientific), LAG3 (relatlimab, BMS), TIM3 (clone F38232), and TIGIT (clone A15153G). IFN-γ (clone 4S.B3) was assessed by intracellular staining using the ebioscience fixation/permeabilization kit. Flow cytometry was performed on a Fortessa analyzer (Becton Dickinson), and FACS data files were analyzed using FlowJo software. Gating scheme for immune cells began with a lymphocyte gate from forward and side scatter and then further by viable cells and then by appropriate population markers.
Results
Study design and patient enrollment
From July 2013 to May 2016, 18 patients were enrolled and treated. Five patients had a primary tumor localized in the larynx, 3 in the hypopharynx and 10 in the oropharynx (3 HPV- and 7 HPV+). Two patients had stage III, 13 had stage IVa, and 3 had stage IVb disease. Fourteen of the 18 patients were smokers, with >10 pack-year tobacco history15. Patient characteristics and demographics are summarized in Table 2.
Table 2.
Patient Characteristics and Demographics
| Characteristic | N (%) |
|---|---|
|
| |
| Age (Median, Range) | 57 (43–74 years) |
| Race/Ethnicity | |
| Non-Hispanic White | 18 (95%) |
| Black | 1 (5%) |
| Sex | |
| Male | 18 (95%) |
| Female | 1 (5%) |
| ECOG Performance Status | |
| 0 | 15 (79%) |
| 1 | 4(21%) |
| Primary Tumor Site | |
| Oral Cavity/Oropharynx (Overlapping) | 2 (11%) |
| Oropharynx | 9 (47%) |
| Hypopharynx | 3 (16%) |
| Larynx | 5 (26%) |
| Stage | |
| III | 2 (11%) |
| IVa | 14 (74%) |
| IVb | 3 (16%) |
| HPV Status (Oropharynx or overlapping, N=11) | |
| p16+ | 8 (73%) |
| p16− | 3 (27%) |
| Tobacco Use | |
| ≥ 10 pack-years | 16 (84%) |
| < 10 pack-years | 3 (16%) |
Determination of RP2D
A summary of all AEs occurring across both cohorts is tabulated in Table 3. In Cohort 1 (ipilimumab 3 mg/kg), 2 of 6 (33%) patients experienced a grade 3 dermatologic irAE meeting the definition for DLT (Figure 1). Two dose-limiting, skin-related grade III adverse events were observed in the first 6 patients: perforating folliculitis and autoimmune dermatitis. Due to this unacceptable rate of DLT, per protocol dose-de-escalation to Dose Level −1 (1 mg/kg in weeks 5, 8, 11, and 14) was implemented. No DLTs were observed in the first 6 patients, thus this cohort was expanded to a total of 12. Among the 12 evaluable patients enrolled to Dose Level −1, no grade III DLT was observed during the pre-specified observation window. One patient developed an irAE of interest, grade 4 colitis treated with steroids then infliximab and ultimately successfully with colectomy after the DLT observation window. He died subsequently from myocardial infarction without disease progression. Based on the trial design, the RP2D for the addition of ipilimumab to standard cetuximab-RT is 1 mg/kg given in weeks 5, 8, 11, and 14. During expansion, one patient developed anaphylaxis to the first cetuximab dose, was withdrawn from study, and replaced, resulting in 12 evaluable patients in Dose Level −1, and a total of 18 evaluable patients across the study including the starting and de-escalation cohorts.
Table 3:
Summary of Treatment-Related Adverse Events
| NCI CTCAE GRADE | ||
|---|---|---|
|
| ||
| IMMUNE-RELATED | Grade 1–2 | Grade 3–4 |
|
| ||
| Dermatologic | ||
| Rash | 13 (72%) | 5 (28%) |
| Gastrointestinal | ||
| Diarrhea | 0 | 1 (6%) |
| Colitisa | 0 | 1 (6%) |
| Transaminitis | 1 (6%) | 0 |
| Endocrine | ||
| Acute thyroiditisb | 1 (6%) | 0 |
|
| ||
| REGIMEN-RELATED | Grade 1–2 | Grade 3–4 |
|
| ||
| Mucositis | 6 (33%) | 12 (67%) |
| Radiation Dermatitis | 14 (78%) | 4 (22%) |
| Hypothyroidism | 6(33%) | 0 |
One patient in the expansion cohort developed grade 4 colitis following the third dose of ipilimumab. Colitis was refractory to corticosteroids, infliximab, mycophenolate mofetil, and tacrolimus. The patient ultimately required colectomy which was successful. He subsequently died from myocardial infarction without disease progression.
One patient in the expansion cohort developed anti-TPO antibody-mediated thyroiditis that presented as subclinical hyperthyroidism then progressed to hypothyroidism. No acute intervention was required; physiologic thyroid hormone replacement was initiated without complication.
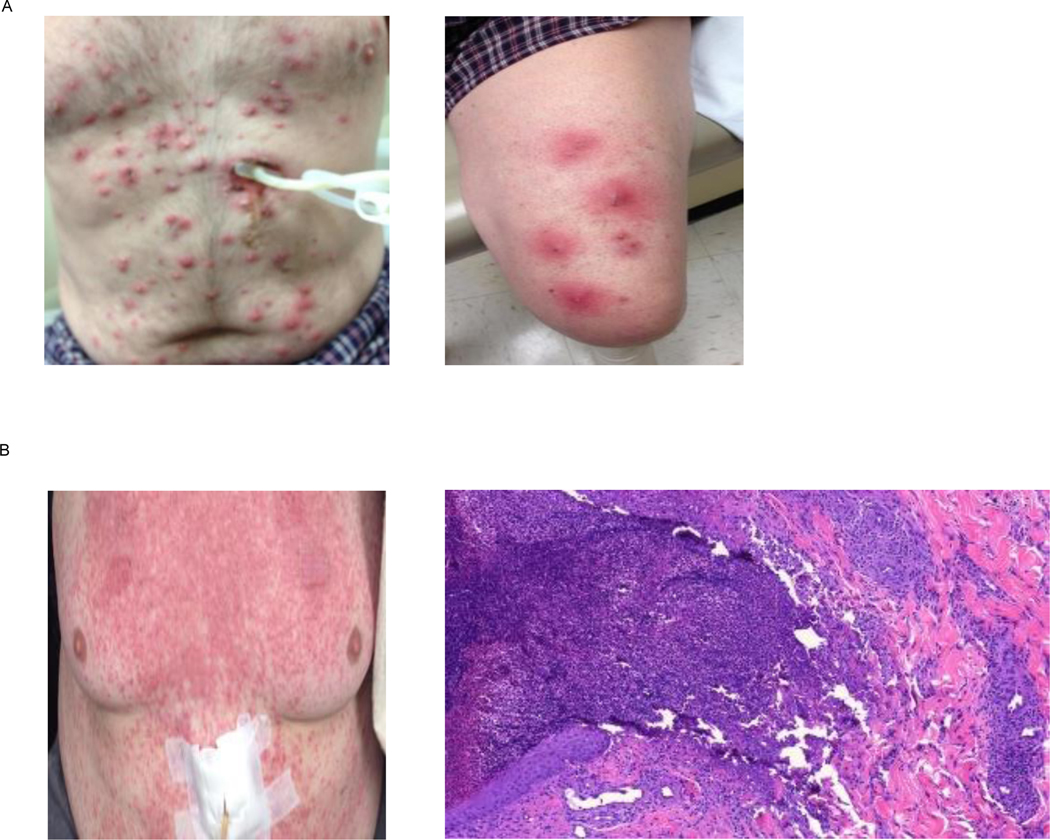
Figure 1.
a demonstrates gross findings of an erythematous papular rash with pustules involving the trunk, chest and extremities. Histologic findings (not shown) from this patient showed predominantly suppurative inflammation with a lesser granulomatous infiltrate within the dermis and superficial subcutis with accompanying necrobiosis. No changes to the basement membrane were noted, and there was alack of microorganisms.
b is of a patient presenting with a fine maculopapular rash over the chest and trunk. A representative histologic section showed focal excoriation with neutrophilic scale and parakeratosis overlying a dense superficial and mid dermal perivascular infiltrate composed of lymphocytes, histiocytes, neutrophils, and scattered eosinophils.
Regimen compliance
Intensity-modulated RT was prescribed 70 to 74 Gy at 2.0 Gy per fraction delivered over 35 to 37 fractions with sequential cone-downs after 50 Gy and 60 Gy. All 18 patients were prescribed RT per protocol with a mean dose of 70.6 (range 66–74) Gy over 52 (range 46–84) elapsed days. One patient refused the final 2 fractions of treatment completing at 66.0 Gy while two separate patients had a prolonged course resulting in 62 and 84 elapsed days secondary to non-compliance and toxicity deemed unrelated to ipilimumab. The mean number of administered cetuximab doses was 7.5 (range 2–10) with 16 of 18 (89%) subjects receiving ≥ 7 doses. The mean number of administered ipilimumab doses was 3.6 (range 2–4), with 14 of 18 (78%) subjects receiving all 4 doses.
Oncologic outcomes
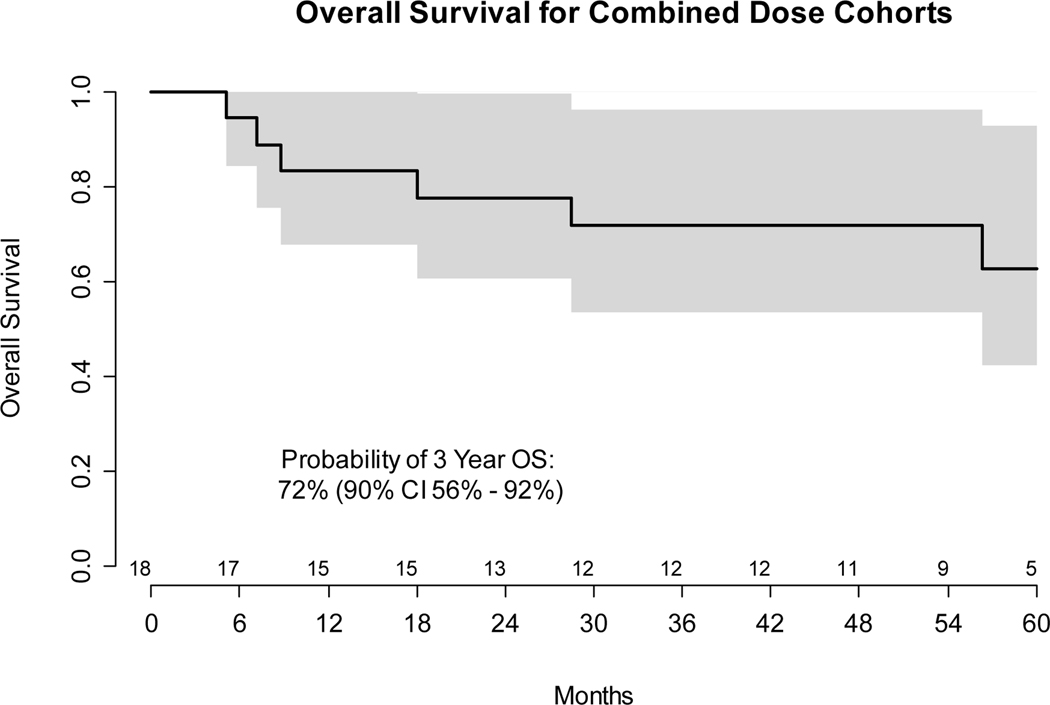
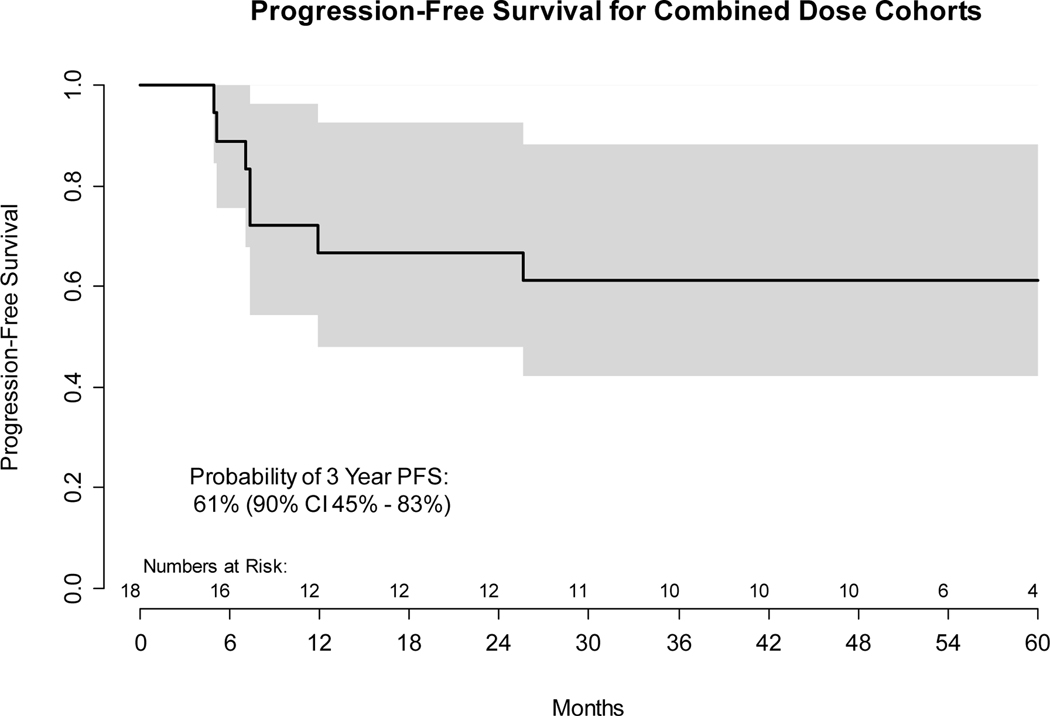
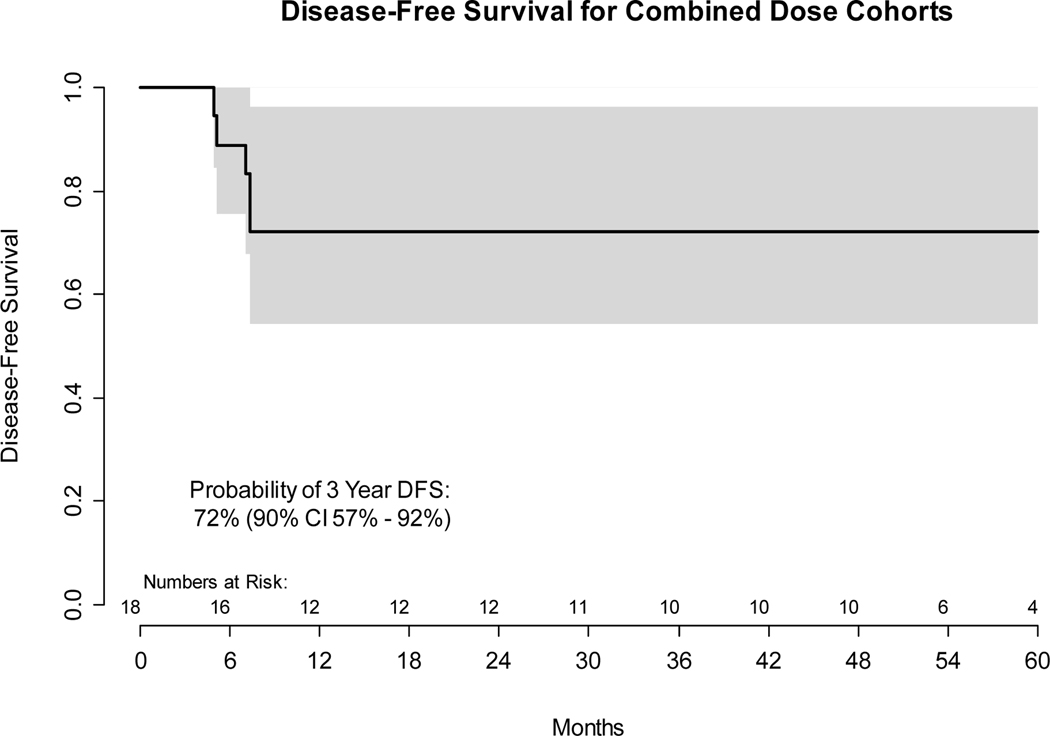
All 18 enrolled and evaluable patients were followed for disease progression, as determined by clinical evaluation and cross-sectional imaging in accordance with RECIST 1.1 criteria. The final survival sweep was conducted on January 20, 2021 with median follow up for the 11 (61%) patients without progression of 55 months (range 11 – 87). Among the 7 (39%) progression events, 5 patients had documented disease progression between 5 and 7 months while 2 HPV- patients died without disease progression 12 and 26 months after initial treatment, from myocardial infarction without disease progression and from a second primary lung cancer, respectively. Per protocol and standard definitions, all deaths were considered progression events for determination of PFS, irrespective of disease status or cause. However, deaths from another cause without evidence of disease progression were not considered progression events for determination of DFS. As depicted in Figure 2, the probability of 3-year PFS was 61% (90% confidence interval 45% - 83%), of 3-year DFS was 72% (90% confidence interval 57% - 92%), and of 3-year OS was 72% (90% confidence interval 56% - 92%). Among the 5 patients with documented disease progression, 2 had HPV+ and 3 had HPV- HNSCC. The site of recurrence was: 2 locoregional only; 1 distant only; and 2 both locoregional and distant. Two PFS events were death without disease progression.
Figure 2.
A: Kaplan-Meier plot of progression-free survival from first treatment. Both dose cohorts are combined. Gray bands show the 90% confidence interval. Numbers of patients at risk at six month intervals are shown above the x axis.
B: Kaplan-Meier plot of overall survival from first treatment. Both dose cohorts are combined. Gray bands show the 90% confidence interval. Numbers of patients at risk at six month intervals are shown above the x axis.
C: Kaplan-Meier plot of progression-free survival from first treatment. Both dose cohorts are combined. Gray bands show the 90% confidence interval. Numbers of patients at risk at six month intervals are shown above the x axis.
T cell and Treg modulation and function
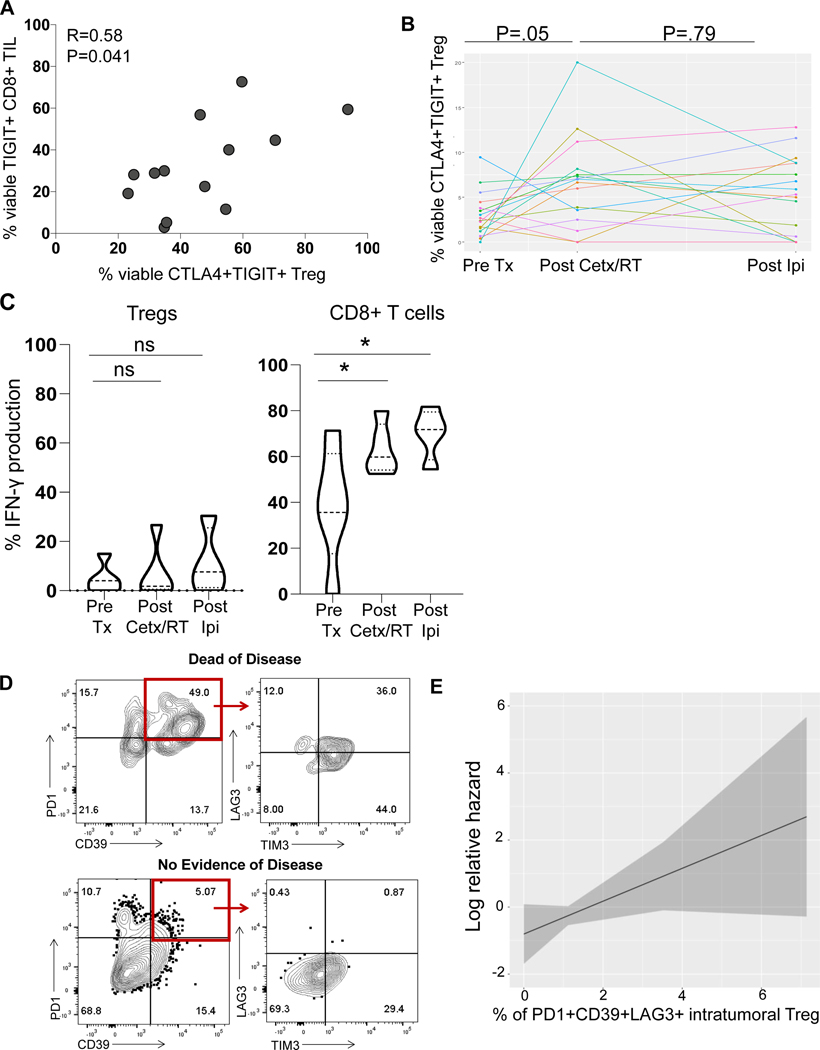
Of the 18 clinical trial patients, baseline TIL and serial PBL were available for analysis from 13 and 16 patients, respectively. We initially analyzed baseline TIL for key T cell and Treg markers that could be predictive of immune modulation. At baseline, CD8+ T cells from the HNSCC TME had increased expression of TIGIT, which correlated with expression of CTLA4+TIGIT+ Tregs in the TME (Figure 3A). These results are not surprising given previous studies and the role that TIGIT has on both CD8+ T cell and Treg function19–21. We then analyzed serial PBL from all timepoints to understand if CTLA4+TIGIT+ Tregs provided a systemic signature for Treg modulation. The CTLA4+TIGIT+ Treg signature increased in some patients post cetuximab/RT (p=0.05), and was decreased in a fraction of patients post treatment with ipilimumab (Figure 3B). We also assessed differences in other inhibitory receptors on intratumoral Tregs from baseline TIL and correlated with disease outcome. Patients with no evidence of disease (NED) had decreased co-expression of key inhibitory receptors on intratumoral Tregs i.e. PD1, CD39, LAG3compared to those patients that died of disease (DOD) (Figure 3C–D). Indeed, only 1 of 59 phenotypes tested in tumor tissue, high expression of PD1/LAG3/CD39 on baseline TIL Treg [Hazard ratio (HR) = 5.6, 95% CI = .83 – 37.8, p = .0762], was possibly associated with an increased risk of disease recurrence (Figure 3E), and this was not correlated with baseline systemic signatures on Tregs (Figure 3F). This likely indicates the inability to detect the importance of other immunological targets on Tregs in the HNSCC TME given the small sample size.
Figure 3:
(A) Evaluation of pre-treatment tumor infiltrating lymphocytes (TIL) demonstrates correlation of TIGIT+CD8+ T cells with CTLA4+TIGIT+ Tregs (n=13) (B) Linear analysis of CTLA4+TIGIT+ Tregs in the peripheral blood of patients pre-treatment, post cetux/RT and post Ipi (n=16). Red dotted line indicates a patient with SAE, severe grade III skin reaction. (C) Representative flow cytometry plots of intratumoral Treg (gated on CD4+CD3+FoxP3+ TIL) showing higher percentage of double positive PD1+CD39+ Tregs expressing LAG3 from a patient that died of disease after treatment. Representative flow cytometry plots of intratumoral Treg demonstrating a lower percentage of double positive PD1+CD39+ Tregs expressing LAG3 from a patient that continues to have no evidence of disease on most recent follow up. Patient flow plots in figures D had similar baseline characteristics and risk factors (HPV negative, stage II HNSCC). (D) Summary data of intratumoral Treg expressing PD1+CD39+ Tregs expressing LAG3. (E) Unbiased biostatistical analysis of all combinatorial parameters of patient baseline TIL and serial PBL. Patients with higher percentage of intratumoral Treg expressing PD1+LAG3+CD39+ had a higher hazard ratio of disease recurrence (F) Correlation of PD1+CD39+LAG3+ Treg signature in patient TIL vs. PBL.
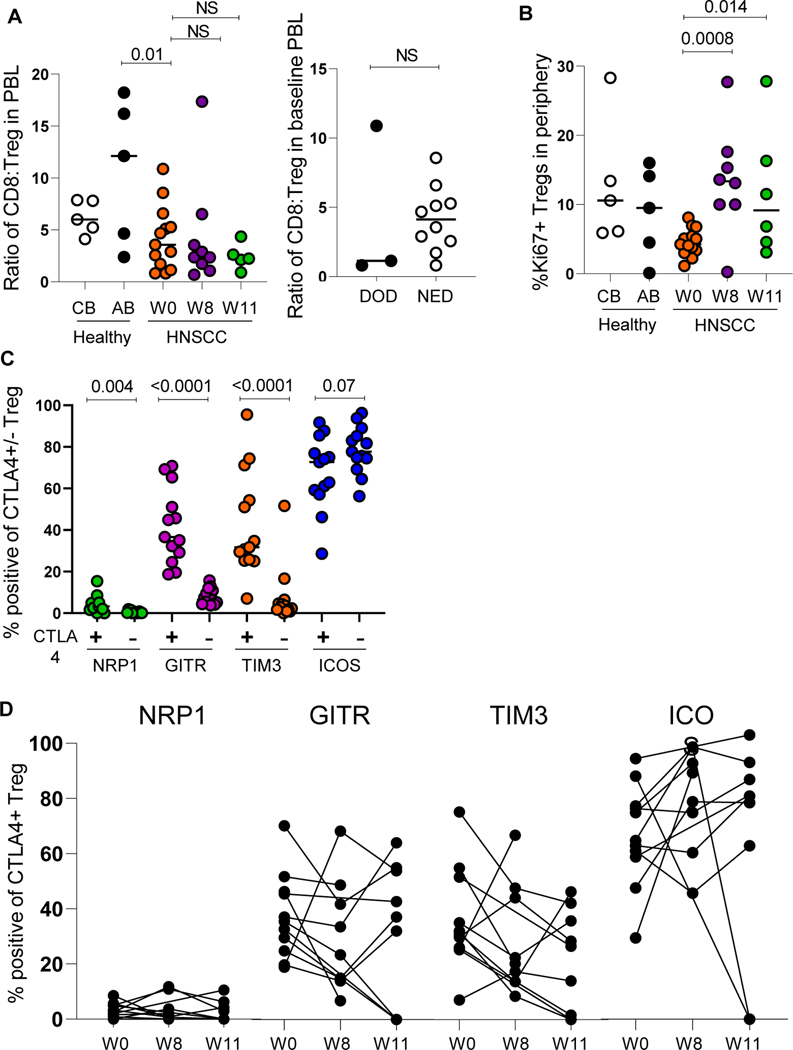
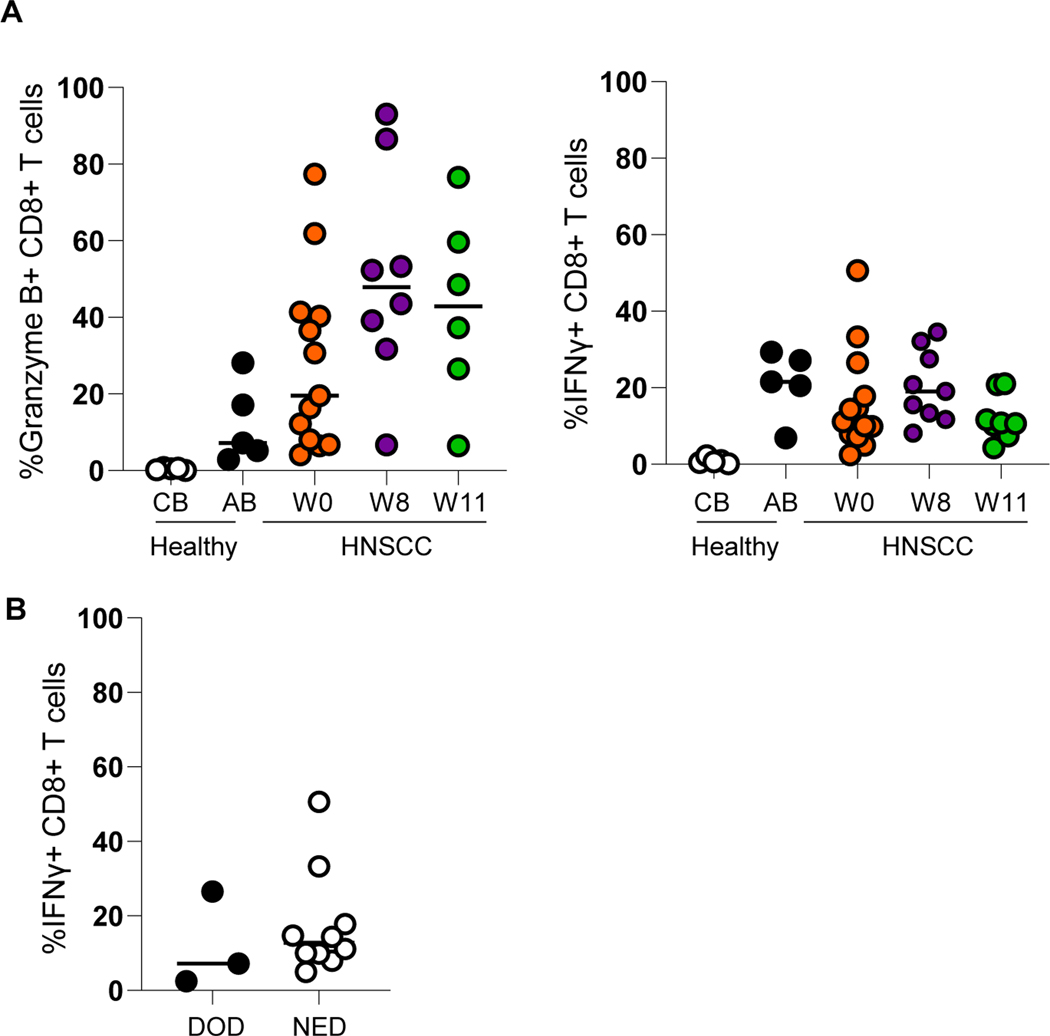
Given the ability to assay immune populations systemically within this unique trial, we interrogated Treg stability and function in the serial PBL. We first assessed fluctuations of CD8:Treg ratios in the periphery of patients compared to healthy cord and adult blood. Overall, CD8:Treg ratios were reduced in the periphery of HNSCC patients and decreased over time (week 8 and 11). Further, the CD8:Treg ratios in those patients with NED were higher than those who were DOD (Figure 4A). We also assessed Treg proliferation and stability as it relates to CTLA4 expression. Tregs from the periphery of HNSCC patients had increased proliferation post treatment (week 8 and 11, Figure 4B). Further, key molecules (NRP1, GITR, TIM3, and ICOS) that confer Treg stability were mostly increased on the CTLA4+ Treg population compared to the CTLA4- Treg population (Figure 4C). Finally, in further evaluating the fluctuations of these key molecules on CTLA4+ Tregs with treatment, we observed mixed trends of increased and decreased expression over time, which could reflect differential modulation of Tregs in HNSCC patients (Figure 4D). Finally, to supplement our further Treg analysis, we evaluated differences in Granzyme B and IFNγ production by CD8+ T cells with treatment (Figure 5A). While not statistically significant, numerically increased Granzyme B production by CD8+ T cells was observed with treatment and increased IFNγ production by CD8+ T cells from patients with NED (Figure 5B).
Figure 4:
(A) Evaluation of CD8:Treg ratio in the periphery of HNSCC patients with treatment (n=13), CB= healthy cord blood, AB= healthy adult blood and ratio of CD8:Treg in the periphery of HNSCC patients with survival (n=13). DOD=dead of disease, NED= no evidence of (B) Proliferation of Tregs in the periphery of HNSCC patients with treatment (n=13) (C) Co-expression of CTLA4 and other molecules related to Treg stability in the peripheral blood of HNSCC patients (n=13) (D) Modulation of molecules associated with Treg stability on peripheral CTLA4+ Tregs in HNSCC patients (n=13).
Figure 5:
(A) Evaluation of granzyme B and IFNγ production in the periphery by CD8+ T cells of HNSCC patients with treatment (n=13), CB= healthy cord blood, AB= healthy adult blood (B) Correlation of IFNγ production by CD8+ T cells in periphery of HNSCC patients with survival (n=13). DOD=dead of disease, NED= no evidence of disease.
Readers may request available clinical trial data or original correlative biomarker data from the corresponding author
Discussion
Recent clinical trials using immunotherapy for treatment of recurrent/metastatic and chemotherapy refractory HNSCC led to FDA approval of two mAbs targeting the PD-1 pathway, nivolumab and pembrolizumab8,22. Given the success of immunotherapy in a heavily pretreated patient population, current efforts are focusing on introducing this new type of therapy earlier in treatment, as an intensification strategy in high-risk patients for whom current standard of care therapy is not sufficiently efficacious. Concurrent cetuximab-RT is an evidence-based standard for previously untreated, locally advanced HNSCC, though was found recently to be inferior to concurrent cisplatin-RT in patients with HPV+ disease in two randomized, controlled trials23,24. Cetuximab’s tolerability was recently challenged in the same head-to-head studies where the rates of severe acute and late toxicity were similar among HPV+ patients23,24. Nonetheless, cetuximab-RT is the predominant therapeutic strategy in the United States for patients who are elderly, frail, or otherwise not candidates for cisplatin chemotherapy25,26. Although a level 1 standard of care, cetuximab-RT results in suboptimal disease control in intermediate or high risk, locally advanced HNSCC. This is the first trial combining CTLA-4 targeting with cetuximab-RT in HNSCC and is novel in that it assesses cancer immunotherapy in the high-risk locally advanced setting, the next frontier for integrating immune checkpoint receptor-targeted immunotherapy into this disease. As shown in Table 1, we designed the schedule of the combined regimen to introduce ipilimumab at week 5 of cetuximab-RT, which as the standard of care, curative backbone, was fixed. This schedule permitted us to discern the toxicities attributable to ipilimumab that may overlap with cetuximab-RT, particularly dermatologic AE, since cetuximab classically causes acneiform rash and potentiates radiation dermatitis. Indeed, dermatologic irAEs were unique to this combination and dose-limiting, resulting in de-escalation of ipilimumab to Dose Level −1, which was tolerable without DLT in the expanded cohort of 12. Thus, the RP2D for the addition of ipilimumab to cetuximab-RT is 1mg/kg in weeks 5, 8, 11, and 14.
Overall, HNSCC patients tolerated this treatment at RP2D relatively well, however specific irAEs of interest warrant additional discussion. As noted above, the potential for additive or synergistic dermatologic AEs was anticipated by the design of the study by the sequential addition of ipilimumab at Week 5, given skin toxicity is caused by all three modalities under study. The two dose-limiting dermatologic irAEs in the starting cohort, grade 3 perforating folliculitis and grade 3 diffuse autoimmune dermatitis, were unusual for either cetuximab or ipilimumab monotherapy. Of note, both dermatologic DLTs occurred shortly after completion of cetuximab, were clinically and histologically distinct from cetuximab-induced acneiform rash, and responded to systemic corticosteroids. Although the skin biopsy results were somewhat nonspecific, these findings have been seen rarely in prior reports of patients treated with ipilimumab27,28. Similar to other reports, these rashes resolved with cessation of ipilimumab and systemic steroid therapy. The possibility that cetuximab potentiated both immune-related dermatologic DLTs is possible mechanistically; as we have shown, cetuximab induces anti-EGFR CD8+ T cells following antibody-dependent cell-mediated cytotoxicity29,30. Another irAE of interest, auto-immune colitis, occurred in one patient treated at RP2D, after the DLT observation period. This rate of colitis, 1 of 18, does not appear different from that observed in ipilimumab monotherapy27. Colitis is a known and expected irAE for ipilimumab that typically resolves with cessation of drug and administration of steroids, with or without infliximab, however this patient was refractory to both interventions. Ultimately, he required a colectomy, and died subsequently from myocardial infarction without disease progression.
As a treatment intensification design, this phase 1 study enrolled patients with intermediate-risk HPV+ or high-risk HPV-, locally advanced disease, where 3-year OS are 71% and 46%, respectively, when treated with concurrent cisplatin-RT15. Here, the 3-year PFS of 72% and OS of 71% appear to overlap with these results, of interest given this phase I regimen omitted the cytotoxic, cisplatin. While preliminary, our survival data appear favorable when compared to the cetuximab-RT arm of the randomized phase III trial leading to its FDA indication (3-year PFS and OS of 42% and 55% respectively), even though this landmark study also included patients with good-risk HPV+ disease15.
Our recent data show that cetuximab induces cell-mediated immunity, but also increases the number of immunosuppressive Treg as well as the expression of CTLA-4 on Treg, making this lymphocyte population even more suppressive. Radiation has also been shown to increase the percentage of intratumoral Tregs expressing CTLA-4. It is well established that a high percentage of intratumoral Tregs correlates negatively with clinical outcomes31. We hypothesize that ipilimumab may overcome the suppressive CTLA4+ Treg population and improve outcomes from standard cetuximab-RT in patients with intermediate or high-risk, locally advanced HNSCC. Given these findings, we evaluated the safety and preliminary efficacy of the sequential addition of ipilimumab, an anti-CTLA-4 mAb targeting Treg, to cetuximab-RT. We did not see a dramatic or convincing decline in CTLA4+ Treg (Fig 3B), except in 3 patients, and indeed saw more variability than expected, likely due the small trial cohort preventing adequate power to make conclusions and two dose levels. Therefore, we began exploring additional IIR expression, as shown in Fig 3.
Patients with HNSCC express altered peripheral immune cells compared to healthy individuals 32, however whether this is due to tumor antigen exposure initiating a peripheral memory cell population or cytokines leading to modulation of immune cell populations outside of the tumor is unknown. In this study, neither IR expression on tumor-infiltrating Treg nor CD8+ T cells demonstrated similarities to IR expression on the corresponding cells in the peripheral blood. The lack of significant differences in baseline CTLA4+ Treg in patients that responded to treatment compared to those who did not suggests that other IRs may confer different properties to CTLA4+ Treg that make them more or less suppressive. Elimination of a less suppressive Treg population with the use of ipilimumab therefore may flip the balance of immune surveillance and immune escape in favor of the tumor. For example, baseline overexpression of PD1/LAG3/CD39 in TIL Treg, possibly associated with worse PFS in this study may suggest tumoral reliance upon non-CTLA-4 immune escape mechanisms. Patients with a high baseline PD1+LAG3+CD39+ Treg may also have had different immune modulatory responses during treatment compared to patients with a lower number of this cell population at baseline. We conclude that the addition of an inhibitor to PD1 or other emerging IR targets of T cell dysfunction (e.g. CD39, LAG3, TIM3) may be important in combination immune-based therapies to properly modulate Tregs and subsequently increase CD8+ T cell function in HNSCC. Immunophenotyping of circulating lymphocyte populations indicated variations in CD8/Treg ratio as well as proliferation of Tregs measured by Ki67 positivity, suggesting modulation of Treg subsets (Figure 4). We also noted increased Granzyme B and, to a lesser extent, IFN-γ positivity in the CD8 T cell compartment, suggesting enhanced activity of these cytotoxic T cells (Figure 5).
This phase I trial has some important limitations. First, the trial was designed to evaluate safety and RP2D. The total sample size of 18, including 12 treated at RP2D, translates to broad confidence intervals surrounding estimates of oncologic efficacy. This limitation was partially offset by enforcing a minimum extended follow up period of 36 months after the last patient’s last dose, in order to report actual rather than estimated DFS, PFS, and OS. Despite having a small sample size, this trial did result in some significant findings regarding our secondary endpoint of oncologic outcome as well as a potential intratumoral biomarker that could predict oncologic outcome. Further study is needed to fully understand the mechanisms as to why baseline CTLA4+ Treg does not correlate with treatment response. On-trial biopsies may have given some additional phenotypic analyses to understand the changes of IR expression on Treg with the combination of therapies. It is also possible that a significant correlation between TIL and PBL cell populations was not uncovered due to a small sample size and a study that was not designed for such purposes. Despite the study limitations, this unique study is the first to evaluate the addition of ipilimumab to cetuximab-RT in the definitive treatment setting for HNSCC patients. The results are highly relevant, given that JAVELIN-100 H&N using avelumab plus CRT in locally advanced, previously untreated HNSCC was recently reported as a negative phase III trial21.
In summary, the addition of ipilimumab to standard cetuximab-RT at the defined RP2D is safe and appears to have sufficient clinical activity to warrant a phase II study. In light of two recent studies demonstrating the superiority of cisplatin to cetuximab in the setting of definitive RT for HPV+ HNSCC23,24, the developmental path may be most relevant in non-cisplatin candidates. Given the more favorable AE profile of anti-PD1 over anti-CTLA4 mAb, along with current FDA indications in recurrent/metastatic HNSCC, the current focus within the HNSCC field is to integrate PD1/L1 checkpoint inhibitors into curative-intent, multimodality settings. The first reported phase III randomized controlled trial in the definitive, intermediate- to high-risk HNSCC population evaluated bolus cisplatin-RT with avelumab, an anti-PDL1 mAb, vs. placebo 33. Notably, this study was negative for the primary endpoint of PFS. Multiple other studies are ongoing. While further development of this ipilimumab regimen is not currently planned, due to prioritization of anti-PD1/L1 approaches across the field, anti-CTLA4 approaches to overcome immunosuppression resulting from cetuximab and/or RT should be reconsidered, particularly given their different mechanism of action as immune checkpoints and potential for differential therapeutic resistance. In future studies evaluating ipilimumab or other checkpoint inhibitors added to RT, where an immune mechanism is hypothesized, pre and on-treatment tumor biopsies would be helpful to evaluate if intratumoral Treg or other immune cell populations are modulated. This is the approach we are taking in an ongoing trial of pembrolizumab, cisplatin, and RT (NCT02777385).
Statement of Translational Relevance.
Concurrent radiation therapy (RT) with cetuximab, an anti-EGFR monoclonal antibody (mAb), is a standard treatment for locally advanced head and neck squamous cell carcinoma (HNSCC), however disease control in high-risk disease is suboptimal. CTLA-4+ T regulatory cells (Treg) dampen cellular immunity and correlate negatively with clinical outcomes. We conducted a phase I trial to evaluate the safety and preliminary efficacy of the CTLA-4 targeting mAb, ipilimumab, with standard, fixed cetuximab-RT. We identified the recommended phase II dose, ipilimumab 1mg/kg in weeks 5, 8, 11, and 14 of cetuximab-RT, and observed acceptable PFS and OS without cytotoxic chemotherapy. High expression of co-inhibitory receptors PD1/LAG3/CD39 on baseline tumor-infiltrating Treg was marginally associated with worse PFS. This is the first trial to combine CTLA-4 targeting with definitive cetuximab-RT in HNSCC, as the field moves towards rational integration of immune checkpoint inhibitors into curative-intent RT platforms.
Acknowledgements:
Supported by NCI CTEP, the University of Pittsburgh/UPMC Hillman Cancer Center SPORE in Head and Neck Cancer (Ferris, P50 CA097190), and the Shared Resources of the UPMC Hillman Cancer Center Support Grant (P30 CA047904).
Conflict of Interest
Dr. Ferris has received compensation for consulting/advisory boards from Aduro Biotech Inc, EMD Serano, MacroGenics Inc, Merck, Novasenta (stock), Numab Therapeutics AG and Pfizer. Through contracts with the UPMC Hillman Cancer Center, his laboratory has also been funded to perform sponsored research by Astra-Zeneca/Medimmune, Bristol Myers Squibb, Novasenta and Tesaro.
Footnotes
Clinical trial information: NCT01935921
REFERENCES
- 1.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137–48. [DOI] [PubMed] [Google Scholar]
- 2.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991–8. [DOI] [PubMed] [Google Scholar]
- 3.Jie HB, Gildener-Leapman N, Li J, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. British journal of cancer 2013;109:2629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramer JD, Burtness B, Ferris RL. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol 2019;99:104460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annual review of immunology 2011;29:235–71. [DOI] [PubMed] [Google Scholar]
- 6.Reichert TE, Strauss L, Wagner EM, Gooding W, Whiteside TL. Signaling abnormalities, apoptosis, and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research 2002;8:3137–45. [PubMed] [Google Scholar]
- 7.Ferris RL, Blumenschein G, Fayette J Jr, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. The New England journal of medicine 2016;375:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferris RL, Blumenschein G, Fayette J Jr, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 2018;81:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albers A, Abe K, Hunt J, et al. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer research 2005;65:11146–55. [DOI] [PubMed] [Google Scholar]
- 10.Gillison ML, Blumenschein G, Fayette J Jr, et al. CheckMate 141: 1-Year Update and Subgroup Analysis of Nivolumab as First-Line Therapy in Patients with Recurrent/Metastatic Head and Neck Cancer. Oncologist 2018;23:1079–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siu LL, Even C, Mesia R, et al. Safety and Efficacy of Durvalumab With or Without Tremelimumab in Patients With PD-L1-Low/Negative Recurrent or Metastatic HNSCC: The Phase 2 CONDOR Randomized Clinical Trial. JAMA Oncol 2019;5:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferris RL, Haddad R, Even C, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol 2020;31:942–50. [DOI] [PubMed] [Google Scholar]
- 13.Argiris A, Heron DE, Smith RP, et al. Induction docetaxel, cisplatin, and cetuximab followed by concurrent radiotherapy, cisplatin, and cetuximab and maintenance cetuximab in patients with locally advanced head and neck cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010;28:5294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2010;28:4390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. The New England journal of medicine 2006;354:567–78. [DOI] [PubMed] [Google Scholar]
- 16.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. The New England journal of medicine 2008;359:1116–27. [DOI] [PubMed] [Google Scholar]
- 17.Jie HB, Schuler PJ, Lee SC, et al. CTLA-4+ Regulatory T Cells are Increased in Cetuximab Treated Head and Neck Cancer Patients, Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer research 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L, Mao L, Liu JF, et al. Blockade of TIGIT/CD155 Signaling Reverses T-cell Exhaustion and Enhances Antitumor Capability in Head and Neck Squamous Cell Carcinoma. Cancer Immunol Res 2019;7:1700–13. [DOI] [PubMed] [Google Scholar]
- 20.Mao L, Wu X, Gong Z, Yu M, Huang Z. PDIA6 contributes to aerobic glycolysis and cancer progression in oral squamous cell carcinoma. World J Surg Oncol 2021;19:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gameiro SF, Ghasemi F, Barrett JW, et al. Treatment-naive HPV+ head and neck cancers display a T-cell-inflamed phenotype distinct from their HPV- counterparts that has implications for immunotherapy. Oncoimmunology 2018;7:e1498439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915–28. [DOI] [PubMed] [Google Scholar]
- 23.Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 2019;393:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehanna H, Robinson M, Hartley A, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet 2019;393:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ang KK, Chen A, Curran WJ, Jr., et al. Head and neck carcinoma in the United States: first comprehensive report of the Longitudinal Oncology Registry of Head and Neck Carcinoma (LORHAN). Cancer 2012;118:5783–92. [DOI] [PubMed] [Google Scholar]
- 26.Ward MC, Reddy CA, Adelstein DJ, Koyfman SA. Use of systemic therapy with definitive radiotherapy for elderly patients with head and neck cancer: A National Cancer Data Base analysis. Cancer 2016. [DOI] [PubMed] [Google Scholar]
- 27.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dika E, Ravaioli GM, Fanti PA, et al. Cutaneous adverse effects during ipilimumab treatment for metastatic melanoma: a prospective study. Eur J Dermatol 2017;27:266–70. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava RM, Lee SC, Andrade Filho PA, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research 2013;19:1858–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srivastava RM, Trivedi S, Concha-Benavente F, et al. CD137 Stimulation Enhances Cetuximab-Induced Natural Killer: Dendritic Cell Priming of Antitumor T-Cell Immunity in Patients with Head and Neck Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 2017;23:707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jie HB, Schuler PJ, Lee SC, et al. CTLA-4(+) Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer research 2015;75:2200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferris RL. Immunology and Immunotherapy of Head and Neck Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33:3293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee NY, Ferris RL, Psyrri A, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol 2021;22:450–62. [DOI] [PubMed] [Google Scholar]