Abstract
Background
We previously reported that two differentially methylated region (DMR) networks identified by DMR and co-methylation analyses are strongly correlated with the fibrosis stages of nonalcoholic fatty liver disease (NAFLD). In the current study, we examined these DMR networks in viral hepatitis and hepatocellular carcinoma (HCC).
Methods
We performed co-methylation analysis of DMRs using a normal dataset (GSE48325), two NAFLD datasets (JGAS000059 and GSE31803), and two HCC datasets (GSE89852 and GSE56588). The dataset GSE60753 was used for validation.
Results
One DMR network was clearly observed in viral hepatitis and two HCC populations. Methylation levels of genes in this network were higher in viral hepatitis and cirrhosis, and lower in HCC. Fatty acid binding protein 1 (FABP1), serum/glucocorticoid regulated kinase 2 (SGK2), and hepatocyte nuclear factor 4 α (HNF4A) were potential hub genes in this network. Increased methylation levels of the FABP1 gene may be correlated with reduced protection of hepatocytes from oxidative metabolites in NAFLD and viral hepatitis. The decreased methylation levels of SGK2 may facilitate the growth and proliferation of HCC cells. Decreased methylation levels of HNF4A in HCC may be associated with tumorigenesis. The other DMR network was observed in NAFLD, but not in viral hepatitis or HCC. This second network included genes involved in transcriptional regulation, cytoskeleton organization, and cellular proliferation, which are specifically related to fibrosis and/or tumorigenesis in NAFLD.
Conclusions
Our results suggest that one DMR network was associated with fibrosis and tumorigenesis in both NAFLD and viral hepatitis, while the other network was specifically associated with NAFLD progression. Furthermore, FABP1, SGK2, and HNF4A are potential candidate targets for the prevention and treatment of HCC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-022-02360-4.
Keywords: Epigenetics, NAFLD, Hepatocellular carcinoma, Network modeling, DNA methylation
Background
Nonalcoholic fatty liver disease (NAFLD) includes a wide spectrum of liver diseases, ranging from the benign non-progression condition non-alcoholic fatty liver to nonalcoholic steatohepatitis (NASH), which can progress to liver cirrhosis and hepatocellular carcinoma (HCC) [1, 2]. Similar to that in other developed countries, NAFLD has become a common disorder associated with metabolic syndrome in Japan [3, 4]. HCC is a significant cause of morbidity and mortality in Japan [5]. Furthermore, HCC is the most common malignancy in patients with NAFLD/NASH, and a higher incidence of HCC has been reported in patients with advanced/severe fibrosis. While the treatment of viral hepatitis improves, NAFLD and NASH are rapidly becoming the leading causes of HCC in Japan. Accordingly, the investigation of NAFLD pathogenesis, especially in relation to HCC, and the discovery of novel treatment targets are of utmost concern.
Recent progress in genetic and epigenetic analyses has provided new insights into NAFLD [6, 7]. Indeed, previous genome-wide association studies by us and others have established a definitive genetic background associated with the development of the disease [8–10]. In addition to genetic effects, changes in epigenetic status are also important for the development of NAFLD [7, 11]. To evaluate the epigenetic status of the NAFLD liver, we previously performed whole hepatic mRNA sequencing followed by weighted gene co-expression network analysis (WGCNA) [12]. We identified two core gene networks involved in NAFLD progression: one contains a scale-free network with four hub genes associated with increases in fibrosis and tumorigenesis and the other consists of a random network associated with mitochondrial dysfunction. One of the most important methods for evaluating the epigenetic status of NAFLD in the liver is DNA methylation analysis. We performed genome-wide hepatic DNA methylation analysis and identified 610 differentially methylated regions (DMRs) that are associated with the progression of NAFLD [13]. We subsequently performed co-methylation analyses of the 610 DMRs and identified two DMR networks associated with NAFLD progression [14]. The annotated genes of one of the networks includes genes involved in transcriptional regulation, cytoskeleton organization, and cellular proliferation and are thus potentially associated with tumorigenesis. The annotated genes of the second network are potentially associated with metabolic dysfunction.
Our previous studies demonstrated that analysis of DMR networks is a powerful tool for understanding the pathogenesis of liver disease and for identifying treatment targets. In the present study, we investigated the methylation levels of fibrosis-associated DMR networks in viral hepatitis and HCC using DNA methylation datasets.
Methods
DNA methylation analysis datasets
For DNA methylation analysis, we used the following datasets: our recently published Japanese hepatic DNA methylation data of NAFLD (JGAS000059, http://trace.ddbj.nig.ac.jp/jga/index.html) [13], American hepatic DNA methylation data of NAFLD (NCBI Gene Expression Omnibus accession number GSE31803) [15], Japanese hepatic DNA methylation data of viral hepatitis and HCC (GSE89852) [16], Italian hepatic DNA methylation data of HCC (GSE56588) [17], and German normal hepatic DNA methylation data (GSE48325) [18]. For a validation study of the identified methylated region, we used GSE60753 [19]. Genome-wide DNA methylation levels were determined using the Infinium HumanMethylation450 BeadChip (Illumina, San Diego, CA, USA).
Co-methylation analysis of DMRs
A total of 610 DMRs, including 3683 CpGs sites, that are differentially methylated between mild fibrosis (F0–F2, fibrosis stages 0 - 2) and advanced fibrosis (F3–F4, fibrosis stages 3 or 4) across the Japanese and American NAFLD cohorts were identified using Probe Lasso [20] as previously reported [13]. Co-methylation analysis was performed as previously described [14] for 610 DMRs of German control (n = 34), Japanese NAFLD (n = 60), American NAFLD (n = 56), Japanese viral hepatitis (n = 37), Japanese HCC (n = 37), and Italian HCC liver (n = 224) samples. Briefly, a co-methylation analysis was performed using WGCNA R package to assess inter-correlation among the 3683 CpG sites [21, 22]. In this analysis, a network was represented by the adjacency matrix A = [αij], where αi,j was |PCCi,j|β, PCCi,j was the Pearson’s correlation coefficient between the methylation levels of CpG probes i and j across samples, and β was a soft threshold. The β threshold was set at 16 for the Japanese NAFLD, 14 for the American NAFLD, 7 for the Japanese viral hepatitis, 6 for the Japanese HCC, 6 for the Italian HCC, and 14 for the German control samples, which were the smallest values at which a co-methylation network exhibited scale-free properties with a model-fitting index of R2 > 0.70. Topological overlap measures were obtained, which were a measure of co-methylation interconnectedness between probes [23]. To evaluate co-methylation interconnectedness between the DMRs, average values of topological overlap measures of the CpG matrix combining two different DMRs were calculated. To extract the DMR networks, the cutoff values of the average values of the topological overlap measures were set to 0.15 for the Japanese NAFLD, American NAFLD, and German control groups, 0.35 for the Japanese viral hepatitis group, 0.30 for the Japanese HCC group, and 0.28 for the Italian HCC group. The networks were visualized using the Cytoscape software platform [24].
Other statistical analysis
Methylation levels of DMRs between two groups were evaluated using Hotelling’s T-squared test of the Hotelling R-package (version 1.0-5).
Results
NAFLD-related networks in viral hepatitis and HCC
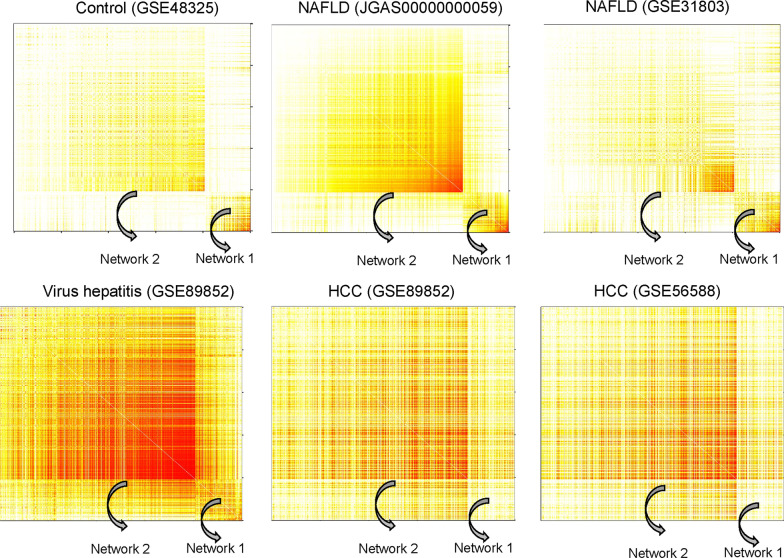
We have previously reported two DMR networks associated with the progression of NAFLD [14], as shown in Fig. 1. DMR network 1 was observed in the control and the two NAFLD populations. However, this network was not observed in the non-cancerous viral hepatitis or the two HCC populations, suggesting this DMR network was specific to normal and NAFLD conditions. Meanwhile, DMR network 2 was not obvious in the normal group, but was observed in the two NAFLD populations and in the non-cancerous viral hepatitis and two HCC populations.
Fig. 1.
Heatmaps of differentially methylated regions (DMRs) in the control, nonalcoholic fatty liver disease (NAFLD), viral hepatitis, and hepatocellular carcinoma (HCC) datasets. Average values of topological overlap measures for the CpG matrix combining two different DMRs were calculated as described in the “Methods” section. Heatmaps of 610 DMRs were constructed using the German control, Japanese NAFLD, American NAFLD, Japanese viral hepatitis, Japanese HCC, and Italian HCC datasets. The order of the DMRs was the same in each of the six heatmaps. Light colors represent low topological overlap with the progressively darker red color indicating increasing overlap
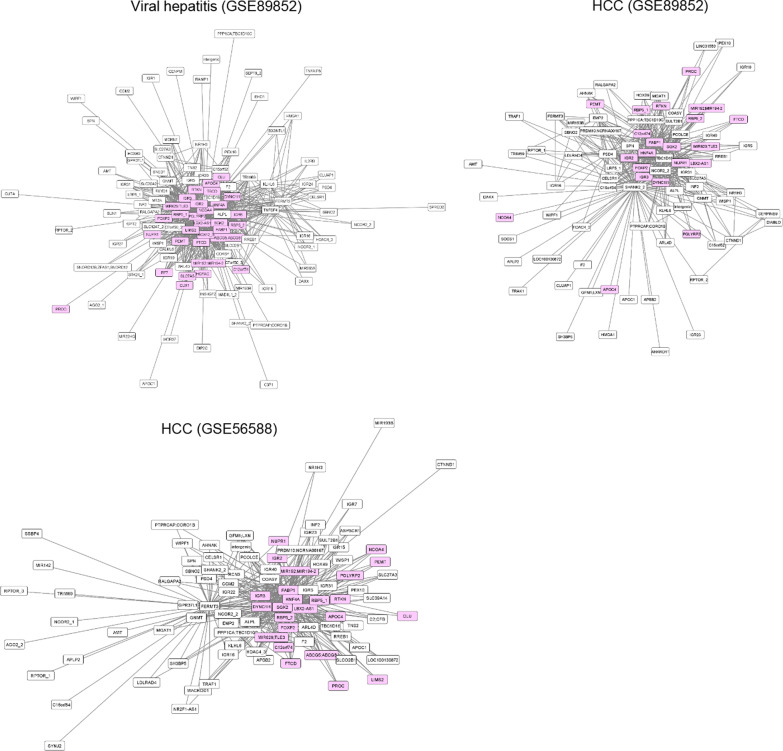
After visualization using the Cytoscape platform, it was determined that all the genes of the DMR network 2 in NAFLD were present in the DMR network observed in viral hepatitis (Fig. 2). Moreover, many of the genes of DMR network 2 were also observed in HCC (Fig. 2). The top 10 genes (nodes) connected with many other genes in the two HCC populations are shown in Table 1. Fatty acid binding protein 1 (FABP1), serum/glucocorticoid regulated kinase 2 (SGK2), and hepatocyte nuclear factor 4 α (HNF4A) were identified as potential hub genes of DMR network 2 in HCC. Detailed information regarding the CpG sites of DMR network 2, which were commonly observed in NAFLD and HCC populations, is provided in Additional file 1: Table S1.
Fig. 2.
Differentially methylated region (DMR) network 2 in the Japanese viral hepatitis, Japanese hepatocellular carcinoma (HCC), and Italian HCC datasets. The DMR network 2 of Japanese viral hepatitis, Japanese HCC, and Italian HCC were extracted as described in the “Methods” section. Nodes (DMRs) identified in the Japanese and American nonalcoholic fatty liver disease (NAFLD) groups in our previous study [14], are indicated in pink
Table 1.
Top 10 nodes (genes) connected with many other nodes observed in networks of hepatocellular carcinoma (HCC)
| GSE89852 | GSE56588 | ||
|---|---|---|---|
| Node (gene) | No. of edges (%) | Node (gene) | No. of edges (%) |
| SHANK2 | 81 (9.8) | FABP1 | 82 (9.9) |
| SGK2 | 54 (6.6) | SGK2 | 79 (9.5) |
| FABP1 | 51 (6.2) | FERMT3 | 61 (7.3) |
| TBC1D16 | 50 (6.1) | DYNC1I1 | 54 (6.5) |
| PSD4 | 33 (4.0) | LBX2-AS1 | 53 (6.4) |
| NCOR2 | 31 (3.8) | HNF4A | 44 (5.3) |
| HNF4A | 28 (3.4) | RBP5_2 | 39 (4.7) |
| FOXP2 | 27 (3.3) | ALPL | 25 (3.0) |
| IGR2 | 27 (3.3) | C12orf74 | 12 (1.4) |
| IGR3 | 27 (3.3) | MIR192;MIR194-2 | 10 (1.2) |
| Total | 824 (100) | 832 (100) | |
IGR intergenic region
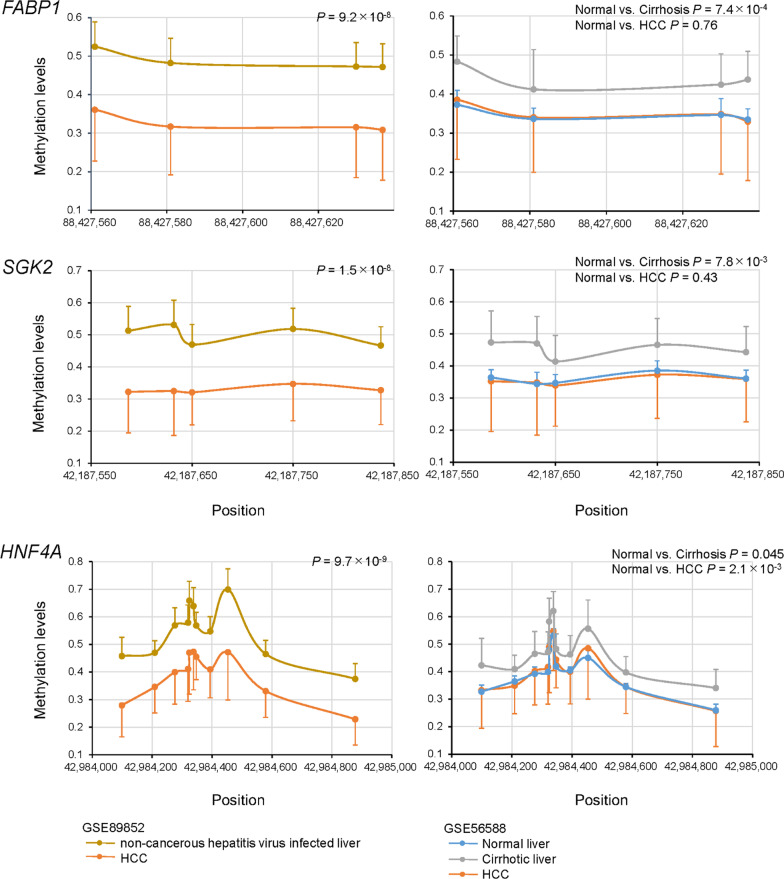
Methylation levels of DMR network 2 genes in HCC
Next, we examined the methylation levels of FABP1, SGK2, and HNF4A (Fig. 3). The levels of methylation of these genes in HCC (GSE89852) were significantly decreased compared to those in non-cancerous paired samples of hepatitis virus-infected liver (Fig. 3). The GSE56588 dataset includes methylation levels for 11 normal liver samples and 10 cirrhotic liver samples. Although the sample sizes of normal and cirrhotic livers were small, the levels of FABP1, SGK2, and HNF4A methylation in cirrhotic livers were higher than those in normal livers. Methylation levels for HCC samples were comparable to normal levels. The methylation levels of genes in DMR network 2 correlated with increased fibrosis stages in NAFLD [13, 14]. Therefore, the increased methylation levels of these genes in viral hepatitis and cirrhotic liver could also be related to increased fibrosis. Methylation levels of the other 18 genes of the DMR network 2 were higher in non-cancerous hepatitis virus-infected liver samples compared to those in HCC samples (Additional file 2: Fig. S1, GSE89852). Evaluation of the GSE56588 dataset revealed the methylation levels of these 18 genes in cirrhotic livers were higher than those in normal livers. Methylation levels of these genes were decreased in HCC tissues.
Fig. 3.
Differentially methylated regions (DMRs) of genes, FABP1, SGK2, and HNF4A in livers of the patients with hepatocellular carcinoma (HCC). Data are expressed as mean ± standard deviation. The left panels show a comparison between paired liver samples from viral hepatitis and HCC. The right panels show a comparison of liver samples from normal, cirrhosis and HCC groups. P values were calculated using Hotelling’s T-squared test
Methylation levels of FABP1, SGK2, and HNF4A in other cirrhosis and HCC liver samples as well as HCC cell lines
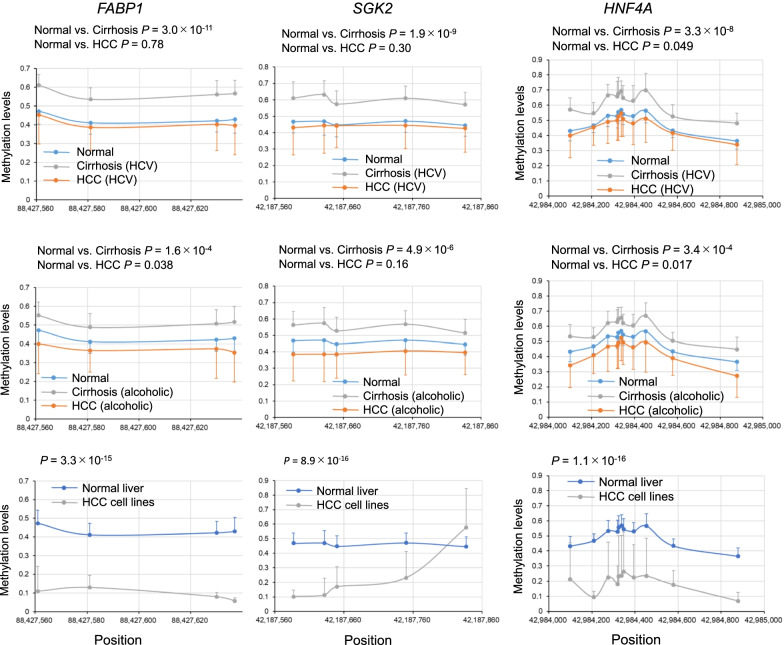
To evaluate whether FABP1, SGK2, and HNF4A related to cirrhosis and HCC, we examined the methylation levels of these gene using the GSE60753 dataset. The GSE60753 dataset includes methylation levels for 34 normal liver samples, 39 hepatitis C virus (HCV)-infected and 21 alcoholic cirrhotic liver samples, and 12 HCV-infected and 15 alcohol-related HCC samples [19]. As observed in the GSE56588 dataset, the methylation levels of FABP1, SGK2, and HNF4A in cirrhotic livers were higher than those in normal livers. The higher methylation levels were observed in cirrhosis of different causes, such as HCV virus and alcohol (Fig. 4). Methylation levels for HCC samples were comparable to normal levels, even if the causes of HCC were different. These data indicated the dynamic change of the methylation levels of FABP1, SGK2, and HNF4A, which were increased during fibrosis progression and decreased after HCC development.
Fig. 4.
Differentially methylated regions (DMRs) of genes, FABP1, SGK2, and HNF4A in cirrhotic livers, and livers from patients with hepatocellular carcinoma (HCC), and HCC cell lines. Data are expressed as mean ± standard deviation. Data were analyzed using the GSE60753 dataset. The upper panels show a comparison of liver samples from control patients, patients with HCV-infected cirrhosis, and patients with HCC. The middle panels show a comparison of liver samples from control patients, patients with alcoholic cirrhosis, and patients with HCC arising from chronic alcoholism. The lower panels show a comparison between normal liver tissues and HCC cell lines. P values were calculated using Hotelling’s T-squared test
Using data of eight established HCC cell lines obtained from GSE60753, we found that the methylation levels of FABP1, SGK2, and HNF4A were significantly lower compared to those of normal liver tissues (Fig. 4). The changes in methylation levels of these genes were confirmed at the cell level.
Discussion
Epigenetic studies are considered to be important for better understanding the pathogenesis of liver fibrosis and HCC. Many studies have been conducted [7, 11, 13–19]; however, the changes in methylation levels during liver disease progression are still controversial. Different from an expression analysis, many CpG sites affecting expression are located in one gene. To evaluate the methylation levels of multiple consecutive CpG sites, a DMR analysis was proposed and its methods have been developed [20, 25, 26]. Gene network analysis is effective to extract key genes related to the development and progression of diseases [21–23]. For applying the network analysis to evaluate methylation levels in NAFLD, we focused on using the average values of topological overlap measures for the CpG matrix in DMR units to extract DMR networks by combining two different DMRs [14]. Our method could evaluate multiple methylation sites on a gene-by-gene basis and found two DMR networks that strongly correlate with the fibrosis stages of NAFLD.
In the current study, we examined whether these DMR networks were present in viral hepatitis and HCC. DMR network 2 was clearly observed in the viral hepatitis and two HCC populations that were evaluated. The levels of CpGs methylation in DMR network 2 were increased in viral hepatitis and cirrhosis, consistent with that in advanced NAFLD [14]. This suggests that DMR network 2 may play important roles in viral hepatitis fibrosis, as well as NAFLD fibrosis. In contrast to methylation levels in advanced NAFLD, viral hepatitis, and cirrhosis, the methylation levels in HCC were decreased, even though DMR network 2 was conserved in HCC. These findings suggest that DMR network 2 plays an important role in carcinogenesis in advanced NAFLD and viral hepatitis.
The main genes involved in DMR network 2 were FABP1, SGK2, and HNF4A. The methylation levels of these gene were increased in liver fibrosis due to HCV infection or chronic alcoholism as well as NAFLD, and were decreased in HCC with HCV infection or alcoholism. FABP1 is primarily expressed in the liver, where it is involved in the binding, transport, and metabolism of long-chain fatty acids. Furthermore, FABP1 plays important roles in changes of cellular lipid metabolic homeostasis that are associated with liver diseases, such as NAFLD, viral hepatitis, cirrhosis, and HCC. FABP1 may also play a protective role in hepatocytes by controlling the availability of long-chain fatty acids and their oxidative metabolites, which are potentially cytotoxic [27]. Therefore, increased methylation levels of FABP1 could deteriorate the protection of hepatocytes from oxidative metabolites in NAFLD as well as in viral and alcoholic hepatitis, while decreased FABP1 methylation levels in HCC may be related to the promotion of cell growth.
SGK2 is an isoform of the serum and glucocorticoid kinase (SGK) family of serine/threonine kinases [28]. Expression of SGK2 is induced in response to signals that activate phosphatidylinositol 3-kinase and is implicated in the regulation of cell growth, proliferation, survival, and migration. Thus, increased methylation of SGK2 may suppress abnormal cell growth in NAFLD as well as in viral and alcoholic hepatitis, while decreased methylation may facilitate cell growth and proliferation in HCC. Baldwin et al. reported that the loss of p53 or SGK2 alone has little effect on cell viability, whereas loss of both p53 and SGK2 leads to cell death, indicating synthetic lethality [29]. Accordingly, SGK2 could theoretically be a target for tumor suppressor-specific drug discovery.
HNF4A is a transcription factor with important roles in liver development, hepatocyte differentiation, and lipid and glucose metabolism [30]. The expression of HNF4A is decreased in NAFLD [13, 14], as well as in viral hepatitis and cirrhosis [30]. The increased methylation of HNF4A in viral and alcoholic cirrhosis could be related to the metabolic dysfunction in these diseases. HNF4A also has oncogenic roles. For instance, HNF4A induces multidrug‑resistance via cell apoptosis, promotes oncogenic cellular metabolism, and reduces the levels of reactive oxygen species (ROS) to enhance genomic alterations and promote cell proliferation, invasion, metastasis, and angiogenesis [31]. Decreased methylation levels of HNF4A in HCC were similar to those of normal levels, which may be related to carcinogenesis in cases of advanced hepatitis, viral hepatitis, and chronic alcoholism. The overall findings indicate that the formation of DMR networks involving FABP1, SGK2, and HNF4A could cooperatively and/or synergistically affect fibrosis and carcinogenesis in advanced NAFLD and viral and alcoholic hepatitis.
In contrast to DMR network 2, DMR network 1 was observed in normal and NAFLD livers, but not observed in viral hepatitis and HCC. The methylation levels of CpGs in DMR network 1 are strongly correlated with patient age and blood glucose levels, in addition to the stages of fibrosis [14]. Patients with NAFLD are often affected by obesity and type 2 diabetes. Thus, long-term hyperglycemia induced by excess calorie intake was considered to promote DMR network 1 in NAFLD. HCV is often accompanied by metabolic alterations, such as dyslipidemia, hepatic steatosis, insulin resistance, obesity, and type 2 diabetes [32]; however, DMR network 1 was not observed in viral hepatitis or in HCC developed from viral hepatitis. HCC develops in the presence of cirrhosis for most cases with viral etiologies, while patients with NAFLD can develop HCC in the absence of cirrhosis [5]. Therefore, DMR network 1 could be related to the carcinogenesis in NAFLD without cirrhosis. This is consistent with the DMR network 1 including genes involved in transcriptional regulation, such as zinc finger and BTB domain containing 38 (ZBTB38), transducing-like enhancer of split 3 (TLE3), and castor zinc finger 1 (CASZ1), and the oncogene agrin (AGRN), which enhances cellular proliferation, migration, and oncogenic signaling [14, 33–36]. Accordingly, these genes in DMR network 1 may be potential targets for the protection from NAFLD progression to HCC.
Conclusions
By performing DMR network analyses, we identified the NAFLD-specific DMR network 1 and DMR network 2, which was present in NAFLD, viral hepatitis, and HCC. We also determined that the methylation levels of DMR network 2 changed cooperatively and/or synergistically during the development of HCC. Common changes in the methylation levels of FABP1, SGK2, and HNF4A from fibrosis to cancer were found in NAFLD, hepatitis virus, and alcoholic hepatitis. Our findings provide new insights into the pathogenesis of HCC, with FABP1, SGK2, and HNF4A being candidate targets for the prevention and treatment of HCC.
Supplementary Information
Additional file 1. Table S1. Commonly observed genes in HCC (GSE89852 and GSE56588) and NAFLD (JGAS00000000059 and GSE31803).
Additional file 2. Fig. S1. DMRs of the genes in network 2 in livers of the Japanese and Italian HCC patients.
Acknowledgements
This work was supported by a grant from the Japan Society for the Promotion of Science (JSPS) KAKENHI to KH (Grant Number 19K08979).
Abbreviations
- AGRN
agrin
- CASZ1
Castor zinc finger 1
- DMR
Differentially methylated region
- F0–F2
fibrosis stages 0 - 2
- F3–F4
fibrosis stages 3 or 4
- FABP1
Fatty acid binding protein 1
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- HNF4A
Hepatocyte nuclear factor 4 α
- NAFLD
Nonalcoholic fatty liver disease
- ROS
Reactive oxygen species
- SGK2
Serum/glucocorticoid regulated kinase 2
- TLE3
Transducing-like enhancer of split 3
- WGCNA
Weighted gene co-expression network analysis
- ZBTB38
BTB domain containing 38
Author contributions
HK designed and coordinated the study; SK, MY, YO, YH, TK, KI, SS, AN, and HK acquired and analyzed data; SK, MY, YO, YH, TK, KI, SS, AN, and HK interpreted the data; SK and HK wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by a grant from the Japan Society for the Promotion of Science (JSPS) KAKENHI to KH (Grant Number 19K08979).
Availability of data and materials
The data that support the findings of this study are available from DDBJ (http://trace.ddbj.nig.ac.jp/jga/index.html, accession number JGAS000059), and NCBI Gene Expression Omnibus (accession number GSE31803, GSE89852, GSE56588, GSE48325, and GSE60753).
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and the Japanese Ethical Guidelines for Human Genome and Gene Analysis Research. The protocol was approved by the ethics committee of Osaka Ohtani University, Osaka, Japan (No. BE-0045-19, July 2, 2019, and No. BE-0062-21, August 2, 2021) and the ethics committee of Yokohama City University, Yokohama, Japan (A171200002, December 26, 2017).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. The authors report no financial or non-financial competing interests associated with this research. We used database.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 3.Farrell GC. Non-alcoholic steatohepatitis: what is it, and why is it important in the Asia-Pacific region? J Gastroenterol Hepatol. 2003;18:124–138. doi: 10.1046/j.1440-1746.2003.02989.x. [DOI] [PubMed] [Google Scholar]
- 4.Okanoue T, Umemura A, Yasui K, Itoh Y. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in Japan. J Gastroenterol Hepatol. 2011;1:153–162. doi: 10.1111/j.1440-1746.2010.06547.x. [DOI] [PubMed] [Google Scholar]
- 5.Eguchi Y, Wong G, Lee I-H, Akhtar O, Lopes R, Sumida Y. Hepatocellular carcinoma and other complications of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in Japan: a structured review of published works. Hepatol Res. 2021;51:19–30. doi: 10.1111/hepr.13583. [DOI] [PubMed] [Google Scholar]
- 6.Anstee QM, Day CP. The genetics of NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:645–655. doi: 10.1038/nrgastro.2013.182. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Friso S, Choi SW. Epigenetic mechanisms underlying the link between non-alcoholic fatty liver diseases and nutrition. Nutrients. 2014;6:3303–3325. doi: 10.3390/nu6083303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitamoto T, Kitamoto A, Yoneda M, Hyogo H, Ochi H, Nakamura T, et al. Genome-wide scan revealed that polymorphisms in the PNPLA3, SAMM50, and PARVB genes are associated with development and progression of nonalcoholic fatty liver disease in Japan. Hum Genet. 2013;132:783–792. doi: 10.1007/s00439-013-1294-3. [DOI] [PubMed] [Google Scholar]
- 9.Hotta K, Yoneda M, Hyogo H, Ochi H, Mizusawa S, Ueno T, et al. Association of the rs738409 polymorphism in PNPLA3 with liver damage and the development of nonalcoholic fatty liver disease. BMC Med Genet. 2010;11:1–10. doi: 10.1186/1471-2350-11-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitamoto T, Kitamoto A, Yoneda M, Hyogo H, Ochi H, Mizusawa S, et al. Targeted next-generation sequencing and fine linkage disequilibrium mapping reveals association of PNPLA3 and PARVB with the severity of nonalcoholic fatty liver disease. J Hum Genet. 2014;59:241–246. doi: 10.1038/jhg.2014.17. [DOI] [PubMed] [Google Scholar]
- 11.Kitamoto T, Kitamoto A, Ogawa Y, Honda Y, Imajo K, Saito S, et al. Targeted-bisulfite sequence analysis of the methylation of CpG islands in genes encoding PNPLA3, SAMM50, and PARVB of patients with non-alcoholic fatty liver disease. J Hepatol. 2015;63:494–502. doi: 10.1016/j.jhep.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 12.Hotta K, Kikuchi M, Kitamoto T, Kitamoto A, Ogawa Y, Honda Y, et al. Identification of core gene networks and hub genes associated with progression of nonalcoholic fatty liver disease by RNA sequencing. Hepatol Res. 2017;47:1445–1458. doi: 10.1111/hepr.12877. [DOI] [PubMed] [Google Scholar]
- 13.Hotta K, Kitamoto T, Kitamoto A, Ogawa Y, Honda Y, Kessoku T, et al. Identification of the genomic region under epigenetic regulation during non-alcoholic fatty liver disease progression. Hepatol Res. 2018 doi: 10.1111/hepr.12992. [DOI] [PubMed] [Google Scholar]
- 14.Hotta K, Kitamoto A, Kitamoto T, Ogawa Y, Honda Y, Kessoku T, et al. Identification of differentially methylated region (DMR) networks associated with progression of nonalcoholic fatty liver disease. Sci Rep. 2018 doi: 10.1038/s41598-018-31886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy SK, Yang H, Moylan CA, Pang H, Dellinger A, Abdelmalek MF, et al. Relationship between methylome and transcriptome in patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:1076–1087. doi: 10.1053/j.gastro.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuramoto J, Arai E, Tian Y, Funahashi N, Hiramoto M, Nammo T, et al. Genome-wide DNA methylation analysis during non-alcoholic steatohepatitis-related multistage hepatocarcinogenesis: comparison with hepatitis virus-related carcinogenesis. Carcinogenesis. 2017;38:261–270. doi: 10.1093/carcin/bgx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villanueva A, Portela A, Sayols S, Battiston C, Hoshida Y, Méndez-Gonzáez J, et al. DNA methylation-based prognosis and epidrivers in hepatocellular carcinoma. Hepatology. 2015;61:1945–1956. doi: 10.1002/hep.27732. [DOI] [PubMed] [Google Scholar]
- 18.Ahrens M, Ammerpohl O, von Schönfels W, Kolarova J, Bens S, Itzel T, et al. DNA methylation analysis in nonalcoholic fatty liver disease suggests distinct disease-specific and remodeling signatures after bariatric surgery. Cell Metab. 2013;18:296–302. doi: 10.1016/j.cmet.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Hlady RA, Tiedemann RL, Puszyk W, Zendejas I, Roberts LR, Choi JH, et al. Epigenetic signatures of alcohol abuse and hepatitis infection during human hepatocarcinogenesis. Oncotarget. 2014;5:9425–9443. doi: 10.18632/oncotarget.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris TJ, Butcher LM, Feber A, Teschendorff AE, Chakravarthy AR, Wojdacz TK, et al. ChAMP: 450k chip analysis methylation pipeline. Bioinformatics. 2014;30:428–430. doi: 10.1093/bioinformatics/btt684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinform. 2008 doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravasz E, Somera AL, Mongru DA, Oltvai ZN, Barabási AL. Hierarchical organization of modularity in metabolic networks. Science. 2002;297:1551–1555. doi: 10.1126/science.1073374. [DOI] [PubMed] [Google Scholar]
- 23.Langfelder P, Horvath S. Eigengene networks for studying the relationships between co-expression modules. BMC Syst Biol. 2007 doi: 10.1186/1752-0509-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang T, Pan Q, Lin L, Szulwach KE, Song CX, He C, et al. DNA hydroxymethylation changes are associated with neurodevelopmental genes in the developing human cerebellum. Hum Mol Genet. 2012;21:5500–5510. doi: 10.1093/hmg/dds394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziller MJ, Gu H, Muller F, Donaghey J, Tsai LT, Kohlbacher O, et al. Charting a dynamic DNA methylation landscape of the human genome. Nature. 2013;500:477–481. doi: 10.1038/nature12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang G, Bonkovsky HL, de Lemos A, Burczynski FJ. Recent insights into the biological functions of liver fatty acid binding protein 1. J Lipid Res. 2015;56:2238–2247. doi: 10.1194/jlr.R056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruhn MA, Pearson RB, Hannan RD, Sheppard KE. Second AKT: the rise of SGK in cancer signalling. Growth Factors. 2010;28:394–408. doi: 10.3109/08977194.2010.518616. [DOI] [PubMed] [Google Scholar]
- 29.Baldwin A, Grueneberg DA, Hellner K, Sawyer J, Grace M, Li W, et al. Kinase requirements in human cells: V. Synthetic lethal interactions between p53 and the protein kinases SGK2 and PAK3. Proc Natl Acad Sci U S A. 2010;107:12463–12468. doi: 10.1073/pnas.1007462107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeh MM, Bosch DE, Daoud SS. Role of hepatocyte nuclear factor 4-alpha in gastrointestinal and liver diseases. World J Gastroenterol. 2019;25:4074–4091. doi: 10.3748/wjg.v25.i30.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Zhang Y, Zhang J, Deng Q, Liang H. Controversial roles of hepatocyte nuclear receptor 4 α on tumorigenesis. Oncol Lett. 2021 doi: 10.3892/ol.2021.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shengir M, Elgara M, Sebastiani G. Metabolic and cardiovascular complications after virological cure in hepatitis C: what awaits beyond. World J Gastroenterol. 2021;27:1959–1972. doi: 10.3748/wjg.v27.i17.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miotto B, Chibi M, Xie P, Koundrioukoff S, Moolman-Smook H, Pugh D, et al. The RBBP6/ZBTB38/MCM10 axis regulates DNA replication and common fragile site stability. Cell Rep. 2014;7:575–587. doi: 10.1016/j.celrep.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 34.Flack JE, Mieszczanek J, Novcic N, Bienz M. Wnt-dependent inactivation of the Groucho/TLE co-repressor by the HECT E3 ubiquitin ligase Hyd/UBR5. Mol Cell. 2017;67:181–193. doi: 10.1016/j.molcel.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charpentier MS, Christine KS, Amin NM, Dorr KM, Kushner EJ, Bautch VL, et al. CASZ1 promotes vascular assembly and morphogenesis through the direct regulation of an EGFL7/RhoA-mediated pathway. Dev Cell. 2013;25:132–143. doi: 10.1016/j.devcel.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakraborty S, Lakshmanan M, Swa HLF, Chen J, Zhang X, Ong YS, et al. An oncogenic role of Agrin in regulating focal adhesion integrity in hepatocellular carcinoma. Nat Commun. 2015 doi: 10.1038/ncomms7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. Commonly observed genes in HCC (GSE89852 and GSE56588) and NAFLD (JGAS00000000059 and GSE31803).
Additional file 2. Fig. S1. DMRs of the genes in network 2 in livers of the Japanese and Italian HCC patients.
Data Availability Statement
The data that support the findings of this study are available from DDBJ (http://trace.ddbj.nig.ac.jp/jga/index.html, accession number JGAS000059), and NCBI Gene Expression Omnibus (accession number GSE31803, GSE89852, GSE56588, GSE48325, and GSE60753).