Abstract
Background
The relationship between anaesthetic technique and graft patency after open lower limb revascularization is unclear. The aim of this study was to evaluate the association between 30-day graft patency after elective infrainguinal bypass and anaesthetic technique (regional anaesthesia (RA, i.e. neuraxial and/or peripheral nerve blockade) compared with general anaesthesia (GA)).
Methods
Patients who underwent elective infrainguinal bypass in the 2014–2019 National Surgical Quality Improvement Program Vascular Procedure Targeted Lower Extremity Open data set were included. Excluded patients were those under 18 years old, those who did not receive RA or GA, and/or had an international normalized ratio of 1.5 of greater, a partial thromboplastin time more than 35 s, or a platelet count less than 80 × 109/L. The primary outcome was primary graft patency without reintervention. The relationship between anaesthetic technique and patency was analysed with multivariable logistic regression.
Results
Included were 8893 patients with a mean(s.d.) age of 68(11) years and 31.5 per cent female. Within the cohort, 7.7 per cent (n = 688) patients received RA only, 90.4 per cent (n = 8039) GA only, and 1.9 per cent (n = 166) both GA and RA. In the RA-only group, 91.7 per cent (631 of 688) received neuraxial anaesthesia. The primary patency rate was 93.2 per cent (573 of 615) for RA only, and 91.5 per cent (6390 of 6983) for GA only (standardized mean difference, 0.063). RA was not associated with a higher rate of patency compared with GA (adjusted OR, 1.16; 95 per cent c.i., 0.83 to 1.63; P = 0.378).
Conclusion
There was no association between anaesthetic technique and 30-day graft patency after elective infrainguinal bypass surgery. Further prospective studies would be useful to study the impact of anaesthesia technique on important patient-centred outcomes such as long-term patency and non-home discharge.
Graft patency after revascularization is associated with higher quality of life scores and is an important patient-centred outcome. We evaluated the association between 30-day graft patency after elective infrainguinal bypass surgery and anaesthetic technique (regional anaesthesia (neuraxial and/or peripheral nerve blockade) compared with general anaesthesia). Multivariable logistic regression did not show an association between anaesthetic technique and 30-day graft patency.
Introduction
Lower limb (infrainguinal) revascularization surgeries are performed for patients with arterial occlusion, with the goals of improving pain and function1,2. Graft patency is associated with higher quality of life scores3; however, open lower limb revascularization is associated with a significant risk of graft failure, with 30-day patency rates ranging from 78.6 per cent for patients with acute limb ischaemia4 to 92.7 per cent in non-emergent revascularizations5. Patients with loss of graft patency may require further surgeries and/or amputation2. A regional multicentre retrospective cohort of 2036 infrainguinal bypass surgeries from 2003 to 2007 reported a 1-year permanent graft occlusion rate of 12 per cent, and 42 per cent of these patients required major amputation6.
Multiple anaesthesia options exist for elective infrainguinal bypass, including general anaesthesia (GA) and regional anaesthesia (RA). RA includes spinal, epidural, and combined spinal epidural anaesthesia as well as peripheral nerve blockade7. Rarely, a patient may receive both GA and RA, such as conversion to GA after failed RA. Clinically, major determinations for the choice between RA or GA include contraindications to regional anaesthesia (such as coagulopathy), patient preference, patient factors leading to a higher risk of complications with GA, and surgical factors including duration, complexity, and need for vein harvest from an upper extremity.
As increasingly complex patients undergo infrainguinal revascularization8, further additional evidence is required to define the relationship between anaesthetic technique and graft patency, which represents a potentially modifiable factor. Early studies suggested a possible association between neuraxial anaesthesia and decreased rates of reoperation9,10 and graft failure10,11, but subsequent studies (using data from before 2011) did not find an association between anaesthesia technique and 7-day12 or 30-day graft failure5. Plausible mechanisms by which RA may impact graft patency include sympathetic blockade, decreased catecholamine release, and improved lower limb blood flow5. In the context of arteriovenous fistula creation, regional anaesthesia is associated with improved graft patency compared with general13 or local anaesthesia14.
The primary objective of this study was to determine the association between anaesthetic technique (RA versus GA) and 30-day primary graft patency in patients undergoing elective infrainguinal bypass surgery. Secondary, aims were to analyse the relationship between anaesthetic technique and the rates of major reintervention, amputation, bleeding requiring transfusion, or secondary procedure, pneumonia, composite thromboembolism (venous thromboembolism (VTE), myocardial infarction (MI) or stroke), mortality, composite morbidity and mortality, and non-home discharge.
Methods
With approval from the University of British Columbia Clinical Research Ethics Board (25 January 2021, H20-03437) and a waiver of informed consent, a retrospective cohort study was performed using the National Surgical Quality Improvement Program (NSQIP®) data set. The NSQIP general data set was linked with the ‘Vascular Procedure Targeted Datasets Lower Extremity Open (LEO)’. The NSQIP data set is a large, multicentre data set with prospectively collected variables up to 30 days after surgery15. The study was registered before undertaking the analysis (registration number: NCT04730310 (http://www.clinicaltrials.gov); 29 January 2021).
Study population
All patients aged 18 years and older undergoing elective lower extremity open revascularization cases within the NSQIP® LEO data set between 2014 and 2019 were included. Exclusion criteria were patients with ASA physical status V (defined as ‘5-Moribund’), anaesthesia other than GA or RA (‘unknown’, ‘other’, or missing data for both principal and additional anaesthesia techniques), non-elective surgery status, missing procedure type, or those who did not receive a graft (only femoral endarterectomy or profundaplasty). Patients with a platelet count less than 80 × 109/L within 90 days before surgery16, international normalized ratio (INR) of 1.5 or higher, or partial thromboplastin time (PTT) greater than 35 s were excluded as they would likely be considered ineligible for RA based on guidelines17,18.
Anaesthetic technique
The exposure to either RA or GA was modelled as a binary variable in the primary analysis (Appendix S1 includes detailed definitions). RA was defined as any of spinal, epidural, and/or peripheral nerve block. In NSQIP, RA with monitored anaesthesia care (MAC) was coded as MAC for the principal technique and was included as RA for the analysis. Those receiving both GA and RA (GA + RA) were excluded from the primary analysis but included in planned sensitivity analysis.
Primary outcome
All outcomes were measured within 30 days after surgery, except for hospital length of stay (LOS) which was the total LOS15. The primary outcome, primary graft patency, was defined by NSQIP as one of the following: most severe procedural outcome; clinically patent graft; patent graft, no stenosis; or patent graft with stenosis. Non-patency was defined by having the ‘most severe procedural outcome’ being death, image-proven graft thrombosis, or clinically evident thrombosis with no planned intervention, major amputation, new bypass in the treated arterial segment, not documented, other, revised graft with stenosis, or revised graft, no current stenosis; or untreated loss of patency being ‘yes’ (not patent and no procedure was performed). In the LEO data set, patency documentation included imaging (CT, angiogram, or duplex ultrasound), physical exam, and surgeon’s diagnosis. In NSQIP, the ‘revised graft’ refers to the outcome of requiring graft revision within 30 days after the primary abstracted procedure, not whether the primary procedure itself consisted of a graft revision. NSQIP does not abstract a surgery as the primary procedure if it is performed due to a complication within 30 days of a previous procedure or the same hospitalization.
Secondary outcomes
Secondary outcomes included major reintervention, amputation, bleeding requiring transfusion, or secondary procedure, VTE, MI or stroke, pneumonia, postoperative LOS, readmission rate, and death15, as well as a derived variable of non-home discharge (Appendix S1). Two composite secondary outcomes were created: arterial or venous thromboembolism, and composite morbidity and mortality (bleeding, arterial or venous thromboembolism, pneumonia, or death).
Potential covariates
In building the multivariable logistic regression model, several potential confounding variables were considered based on both previous literature and baseline differences between the two cohorts: age5,19,20, bleeding diathesis5, severe chronic obstructive pulmonary disease (COPD)5,19,20, smoking status, renal failure19, functional status5,6, diabetes20, total operating time5,19, year of surgery, high-risk physiological and anatomical risk factors as defined by NSQIP15, and procedure type (Appendix S1).
Statistical analysis
Patient characteristics for preoperative, intraoperative, and postoperative variables in the RA, GA, and GA + RA groups were compared with the use of descriptive statistics. Continuous variables are presented as mean(s.d.) and median (interquartile range (i.q.r.)) for parametric and non-parametric data respectively. Categorical variables are presented as frequency (per cent). Standardized mean difference or HodgesLehmann statistic was presented. Utilization of RA between 2014 and 2019 is displayed graphically. Complete case analyses without imputation for missing values were performed.
To assess the association among anaesthesia type and patency, a multivariable logistic regression was performed, adjusting for potential confounders as above. The significance level was set at P < 0.05 for all analyses. The same exposure and confounder variables were used in the multivariable logistic regressions for secondary outcomes, except that LOS was modelled by way of multivariable linear regression with logarithmic transformation of LOS due to its non-normal distribution. The degree of unmeasured confounding was assessed by way of the E value21.
In a planned sensitivity analysis, several subgroups were analysed: neuraxial anaesthesia compared with GA, surgical site (femoral versus femoropopliteal versus popliteal), and previous intervention using the NSQIP variable ‘high-risk factors—anatomical features’. It was not possible to perform subgroup analyses for patients receiving peripheral nerve blocks due to low frequency and lack of detailed block information within NSQIP. In another a priori sensitivity analysis, the GA + RA group was analysed as a distinct category in addition to GA-only or RA-only, as well as by grouping GA + RA into either the GA or RA group. Statistical analyses were performed with SAS 9.4 (SAS Institute, Cary, North Carolina, USA). Standardized differences were calculated with the SAS macro ‘Stddiff’22.
Sample size calculation
Grip and colleagues previously reported a 30-day patency rate of 78.6 per cent (mean (s.d.) 1.8 per cent)3, although this estimate was not stratified by anaesthesia type. Based on previous NSQIP infrainguinal revascularization studies4,6, a 10 per cent rate of RA utilization was assumed. To detect an increase in patency rate by 4 per cent in the RA group (versus GA), with an α of 0.05 and power of 0.9, it was calculated that 11 600 patients were required. Data from 2014 to 2019 were included in an attempt to achieve this sample size.
Results
Cohort characteristics
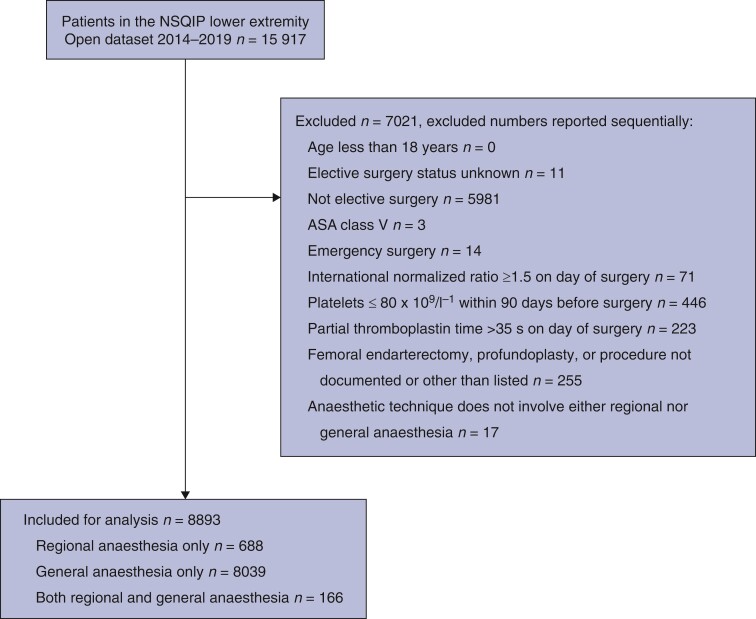
The cohort included 8893 patients (Fig. 1), with a mean(s.d.) age of 68(11) years, and 31.5 per cent (2799 of 8893) were female. Variables with more than 2 per cent missing data were patency (13.0 per cent; n = 1155), race (19.3 per cent; n = 1713), additional anaesthesia technique (a NSQIP variable for any additional anaesthesia technique that was not considered the primary anaesthesia technique) (90.3 per cent; n = 8031), preoperative PTT (46.2 per cent; n = 4114), and INR (30.1 per cent; n = 2673). The frequencies of missing data were 10.6 per cent (73 of 688) and 13.1 per cent (1056 of 6390) for the RA-only and GA-only groups respectively.
Fig. 1.
Study inclusion and exclusion flow chart
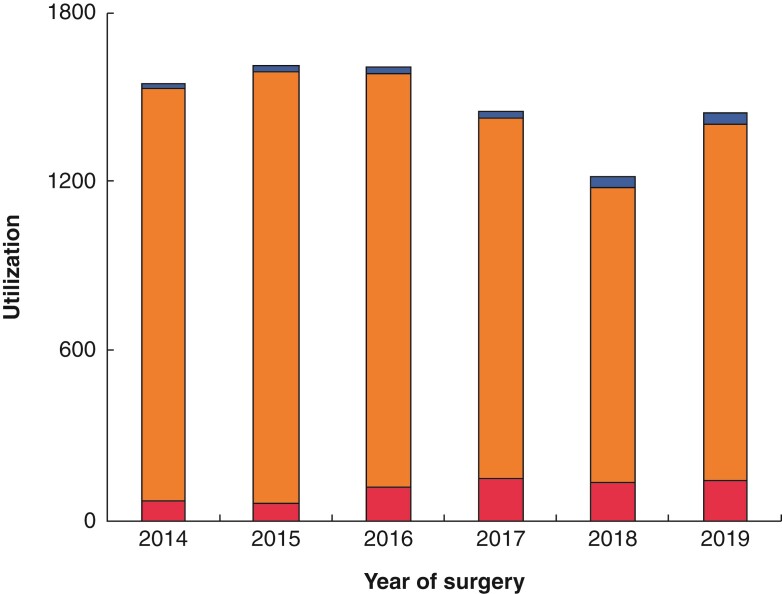
The breakdown of the cohort by anaesthesia technique is 7.7 per cent (n = 688) of patients received RA-only, 90.4 per cent (n = 8039) received GA-only, and 1.9 per cent (n = 166) received both GA and RA. Of those who received any RA, 85.8 per cent (733 of 854) received neuraxial anaesthesia. Of those with RA-only, neuraxial use was 91.7 per cent (631 of 688; n = 561 spinal and n = 159 epidural anaesthesia), and 4.4 per cent (30 of 688) received ‘regional anaesthesia’. In the GA + RA group, 61.5 per cent (102 of 166) involved neuraxial anaesthesia, with 36.7 per cent (61 of 166) receiving ‘regional anaesthesia’ (definitions in Appendix S1). Fig. 2 shows utilization of RA between 2014 and 2019.
Fig. 2.
Regional anaesthesia utilization versus year of surgery The utilization of regional anaesthesia (red, lower section of bar), general anaesthesia (orange, middle section of bar), and both general and regional anaesthesia (blue, top section of bar) are displayed. The percentage of revascularization surgeries performed with regional anaesthesia only between 2014 and 2019 (per year) were 4.7 (72 of 1549), 4.1 (66 of 1613), 7.5 (121 of 1609), 10.2 (148 of 1453), 11.0 (135 of 1224), and 10.1 (146 of 1445) respectively (P < 0.0001, Cochran–Armitage trend test, two-sided)
The study population characteristics are summarized in Table 1. Compared with patients in the GA-only group, patients in the RA-only group were more likely to have high-risk physiological factors (definition in Appendix S1). In contrast, patients receiving GA-only were more likely to have bleeding disorders, previous intervention, longer duration of surgery, and complex grafts. The characteristics of patients in the GA + RA group are presented in Table S1.
Table 1.
Cohort characteristics
| Characteristic | Regional only N = 688 | General only N = 8039 | P | Standardized difference |
|---|---|---|---|---|
| Age (years), mean(s.d.) | 71.2(10.2) | 67.2(10.5) | <0.001 | 0.389 |
| Female sex | 205 (29.8) | 2548 (31.7) | 0.304 | −0.041 |
| ASA physical status | <0.001 | 0.166 | ||
| I | 0 | 14 (0.2) | ||
| II | 29 (4.2) | 363 (4.5) | ||
| III | 469 (68.2) | 5981 (74.4) | ||
| IV | 187 (27.2) | 1671 (20.8) | ||
| Preoperative functional health status | 0.083 | 0.153 | ||
| Independent | 668 (97.1) | 7644 (95.1) | ||
| Partially dependent | 18 (2.6) | 348 (4.3) | ||
| Totally dependent | 0 | 23 (0.3) | ||
| Current smoker, within 1 year before surgery | 246 (35.8) | 3540 (44.0) | <0.001 | −0.170 |
| History of severe COPD | 102 (14.8) | 988 (12.3) | 0.054 | 0.074 |
| Bleeding disorders and anticoagulants | 29 (4.2) | 1579 (19.6) | <0.001 | −0.490 |
| Preoperative antiplatelet medication | 533 (77.5) | 6822 (84.9) | <0.001 | −0.189 |
| Platelets (10 9 /l), median (i.q.r.) | 231 (187–284) | 235 (191–288) | 0.469 | 2 (−8 to 4)* |
| Congestive heart failure, within 30 days before surgery | 7 (1.0) | 146 (1.8) | 0.126 | −0.068 |
| Hypertension requiring medication | 551 (80.1) | 6530 (81.2) | 0.463 | −0.029 |
| Preoperative renal failure | 248 (36.1) | 2521 (31.4) | 0.011 | 0.099 |
| Acute renal failure within 24 h before surgery | 2 (0.3) | 49 (0.6) | 0.292 | −0.048 |
| Currently on dialysis | 28 (4.1) | 340 (4.2) | 0.842 | −0.008 |
| Diabetes | 0.614 | 0.041 | ||
| On insulin | 151 (22.0) | 1879 (23.4) | ||
| On non-insulin medication | 131 (19.0) | 1563 (19.4) | ||
| Symptomatology | 0.012 | 0.131 | ||
| Asymptomatic | 33 (4.8) | 299 (3.7) | ||
| Claudication | 251 (36.5) | 2855 (35.5) | ||
| Critical limb ischaemia: rest pain | 148 (21.5) | 2190 (27.2) | ||
| Critical limb ischaemia: tissue loss | 249 (36.2) | 2602 (32.4) | ||
| Procedure type | <0.001 | 0.438 | ||
| Femoral distal bypass with prosthetic/spliced vein/composite | 29 (4.2) | 791 (9.8) | ||
| Femoral distal bypass with single segment saphenous vein | 155 (22.5) | 1489 (18.5) | ||
| Femoropopliteal bypass with prosthetic/spliced vein/composite | 122 (17.7) | 2281 (28.4) | ||
| Femoropopliteal bypass with single segment saphenous vein | 326 (47.4) | 2812 (35.0) | ||
| Popliteal distal bypass with prosthetic/spliced vein/composite or non-saphenous conduit | 2 (0.3) | 155 (1.9) | ||
| Popliteal distal with single segment saphenous vein | 54 (7.9) | 511 (6.4) | ||
| High-risk anatomical factors | <0.001 | 0.238 | ||
| Prior ipsilateral bypass involving currently treated segment | 95 (13.8) | 1568 (19.5) | ||
| Prior ipsilateral percutaneous intervention involving currently treated segment | 84 (12.2) | 1434 (17.8) | ||
| High-risk physiological factors | 181 (26.3) | 1328 (16.5) | <0.001 | 0.240 |
| Total operating time (min), median (i.q.r.) | 168 (132.5–223.5) | 210 (154–284) | <0.001 | −38 (−44 to −31)* |
| Race | <0.001 | 0.875 | ||
| White | 270 (39.2) | 5401 (67.2) | ||
| Black or African American | 39 (5.7) | 1281 (15.9) | ||
| Unknown/not reported | 366 (53.2) | 1280 (15.9) | ||
| Asian, Native Hawaiian, or Pacific Islander, American Indian or Alaska Native | 13 (1.9) | 77 (1.0) |
Hodges–Lehmann estimator midpoint (95 per cent c.i.). Numbers are n (%) unless otherwise specified. COPD, chronic obstructive pulmonary disease; i.q.r., interquartile range.
Graft patency
After excluding patients with missing patency data, the rate of patent graft for the overall cohort was 91.6 per cent (7086 of 7738). By anaesthetic technique, the patency rate was 93.2 per cent (573 of 615) for RA and 91.5 per cent (6390 of 6983) for GA (standardized mean difference, 0.063). The characteristics of patients with missing data for patency are listed in Table S2 and differed from the main cohort in terms of more asymptomatic patients and fewer previous bypasses. The patency rates for different procedure types are presented in Table 2.
Table 2.
Patency rates by surgery type
| Procedure | n (% total cohort) n = 8893 | Patency (n, % row total) | ||
|---|---|---|---|---|
| Missing n = 1155 (13.0) | No n = 652 (7.3) | Yes n = 7086 (79.7) | ||
| Femoral distal bypass | ||||
| with single segment saphenous vein | 1687 (19.0) | 182 (10.8) | 176 (10.4) | 1329 (78.8) |
| with prosthetic/spliced vein/composite | 832 (9.4) | 108 (13.0) | 105 (12.6) | 619 (74.4) |
| Femoropopliteal bypass | ||||
| with single segment saphenous vein | 3198 (36.0) | 455 (14.2) | 186 (5.8) | 2557 (80.0) |
| with prosthetic/spliced vein/composite | 2449 (27.5) | 303 (12.4) | 127 (5.2) | 2019 (82.4) |
| Popliteal distal bypass | ||||
| with single segment saphenous vein | 568 (6.4) | 83 (14.6) | 41 (7.2) | 444 (78.2) |
| with prosthetic/spliced vein/composite or non-saphenous conduit | 159 (1.8) | 24 (15.1) | 17 (10.7) | 118 (74.2) |
Outcomes and multivariable regression
The use of RA only compared with GA only, was not associated with a higher rate of patency (adjusted OR, 1.16; 95 per cent c.i., 0.83 to 1.63; P = 0.378). The model had a receiver operating characteristic (ROC) area under the curve of 0.645, with a Nagelkerke R2 of 0.0446.
The ORs of RA compared with GA for the primary and secondary outcomes are listed in Table 3, after adjusting for covariates listed above. The ORs for patency comparing RA versus GA in subgroups of surgery by location and by history of previous intervention are listed in Table 4. Sensitivity analysis using different groupings of RA, GA, and GA + RA did not change the associations.
Table 3.
Outcomes and logistic regression ORs for primary and secondary outcomes
| Outcome | Regional only N = 688 Number (%) |
General only N = 8039 Number (%) | Standardized difference | Adjusted* OR for regional only (95% c.i.) | P† |
|---|---|---|---|---|---|
| Patency | 573 (93.2) | 6390 (91.5) | 0.063 | 1.16 (0.83 to 1.63) | 0.378 |
| Major reintervention on the bypass | 18 (2.6) | 332 (4.1) | −0.084 | 0.71 (0.43 to 1.16) | 0.173 |
| Major amputation (transtibial/proximal) | 6 (0.9) | 132 (1.6) | −0.069 | 0.60 (0.26 to 1.39) | 0.237 |
| Unplanned readmission | 105 (15.3) | 1104 (13.7) | 0.043 | 1.19 (0.95 to 1.50) | 0.126 |
| Non-home discharge | 120 (17.5) | 1673 (20.9) | −0.086 | 0.74 (0.59 to 0.93) | 0.009 |
| Composite (morbidity and mortality) | 100 (14.5) | 1250 (15.6) | −0.028 | 1.10 (0.87-1.39) | 0.429 |
| Death | 7 (1.0) | 88 (1.1) | −0.008 | 0.76 (0.34-1.70) | 0.505 |
| Bleeding requiring transfusion or procedure | 76 (11.1) | 1010 (12.6) | −0.047 | 1.12 (0.0.86-1.46) | 0.409 |
| Pneumonia | 8 (1.2) | 76 (1.0) | 0.021 | 1.34 (0.62 to 2.90) | 0.452 |
| Arterial and venous thromboembolism‡ | 26 (3.8) | 267 (3.3) | 0.025 | 1.12 (0.73 to 1.72) | 0.605 |
*Covariates adjusted for were age, bleeding disorder, severe chronic obstructive pulmonary disease, smoking status, renal failure, functional status, diabetes, total operating time, year of surgery, high-risk physiological and anatomical risk factors as defined by National Surgical Quality Improvement Program, and procedure type. †Multivariable logistic regression. ‡Myocardial infarction, stroke, venous thromboembolism.
Table 4.
Subgroup analysis for patency with multivariable regressions
| Subgroup | N modelled | Adjusted* OR (95% c.i.) for regional versus general | P |
|---|---|---|---|
| Neuraxial anaesthesia with/without general† versus general anaesthesia only | 7638 | 1.08 (0.79–1.48) | 0.638 |
| Neuraxial only versus general only | 7546 | 1.25 (0.88–1.80) | 0.218 |
| Spinal only versus general only | 7481 | 1.25 (0.85–1.83) | 0.251 |
| Femoral bypass | 2179 | 1.50 (0.84–2.67) | 0.172 |
| Femoropopliteal bypass | 4803 | 0.92 (0.60–1.41) | 0.692 |
| Popliteal bypass | 616 | 2.35 (0.54–10.31) | 0.258 |
| Previous bypass‡ | 2792 | 0.96 (0.54–1.72) | 0.889 |
Covariates adjusted for were age, bleeding disorder, severe chronic obstructive pulmonary disease, smoking status, renal failure, functional status, diabetes, total operating time, year of surgery, high-risk physiological and anatomical risk factors as defined by National Surgical Quality Improvement Program, and procedure type. †Includes patients receiving only neuraxial or both general and neuraxial anaesthesia. ‡Defined as previous ipsilateral bypass or percutaneous intervention involving currently treated segment in National Surgical Quality Improvement Program. Values are n (%) unless stated otherwise.
The median (i.q.r.) (range) for LOS was 4 (3–6) (0 to 35) and 4 (3–6) (0 to 88) days for RA only and GA only respectively, with a Hodges–Lehmann statistic of 0.5 days (95 per cent c.i. 0 to 1). Due to the right skewed non-normal distribution, LOS was analysed using log transformed LOS in a multivariable linear regression. The RA-only technique was associated with a 12.5 per cent increase in LOS (95 per cent c.i. 7 to 16 per cent) in the regional group (P < 0.0001). Analyses of LOS using a Poisson regression or multivariable linear regression without log transformation produced similar results. Exploratory analysis with inclusion of ASA physical status and sex did not meaningfully alter the association between anaesthesia technique and patency, non-home discharge, or LOS.
Discussion
There have been conflicting data on the relationship between anaesthesia technique and graft patency. In this analysis of 8893 patients from a multicentre cohort undergoing elective lower limb revascularization, there was no association between anaesthesia technique and 30-day primary graft patency. Although the study cannot exclude a small effect from anaesthetic technique, the results suggest that any association is not large. Additional sensitivity analyses of surgical subtypes, and specific patient groups revealed similar results, further confirming the robustness of the results.
Primary patency rate was used as the primary outcome as it is a patient-centred outcome that is correlated with quality of life3. Compared with the literature, the 30-day patency rate in the current cohort was higher than the 78.6 per cent reported in patients with acute limb ischaemia4, but similar to the 92.7 per cent reported in non-emergent patients in the 2005–2008 NSQIP dataset5. The literature on the association between anaesthesia technique and graft patency is mixed. In a 1993 randomized trial of 100 patients undergoing elective lower extremity vascular surgery, patients who received GA had higher rates of the secondary outcome of reoperation within the 6-month follow-up interval than patients who received epidural anaesthesia8. Subsequent 199323 and 199511 retrospective re-analyses of different randomized trials showed conflicting results. A retrospective single-centre study of 822 patients did not find a difference in 7-day graft occlusion rates, which was approximately 10 per cent in both epidural and GA groups9. A retrospective analysis of 1995–2003 Veterans Affairs NSQIP data showed that GA, compared with spinal anaesthesia, was associated with an OR of 1.43 (95 per cent c.i. 1.16 to 1.77; P = 0.001) for graft failure, although this study did not adjust for important confounders such as surgical acuity and coagulopathy10. In contrast, another 2005–2008 NSQIP analysis found no difference in the secondary outcome of patency5.
The present study adds to the literature on anaesthesia type and patency by analysing an updated, contemporary cohort of patients using a specialty-focused data set. The multicentre data set has broad generalizability. The LEO data set contains more detailed clinical information that was not present in previous studies, including clear clinical, and imaging definitions of patency in the data abstraction guides. Moreover, it was possible to control for important confounders not included in some previous studies such as duration of surgery, coagulopathy, and high-risk anatomical factors.
The literature has shown mixed results regarding the superiority of RA over GA for morbidity and mortality1,5,19,20,24. In terms of secondary outcomes, no association between regional technique and outcomes was found except for decreased non-home discharge and increased LOS; however, the differences were small, and these exploratory results should be interpreted with caution in the context of small sample size and multiple testing25. The difference in LOS may be related to the sicker population in the RA group, and the small magnitude of difference aligns with previous literature of small to no differences in LOS19,20,24. While RA was associated with decreased odds of non-home discharge, this may be related to the more complex grafts and surgeries in the GA group, though the mechanism remains to be elucidated given the lack of difference amongst rates of patency, reintervention, morbidity, and mortality outcomes in the current cohort. The E value of 1.6 for OR and 1.23 for lower 95 per cent c.i. limit suggest that any residual, unadjusted confounder (such as frailty) would have to have a more than small effect to change the association to null. A recent multivariable modelling of 10 145 patients in the Vascular Study Group of New England database found that preoperative predictors of non-home discharge were age, sex, non-White race, tissue loss, cardiac co-morbidity, partial ambulatory deficit, and insulin-dependent diabetes26. Whether anaesthesia technique is a potentially modifiable factor in decreasing non-home discharge should be confirmed in future studies.
The present study should be interpreted in the context of several limitations. In many cases it can be difficult to determine the exact RA technique used due to the coding scheme and missing data. Misclassification of anaesthesia technique was possible depending on the chart documentation and the data abstracter. The principal anaesthesia technique may have been coded as GA even if there was additional RA, and MAC/intravenous sedation when this coexists with RA15. Furthermore, additional anaesthesia technique, where RA may be included, is not a mandatory variable and had a high rate of missing data (n = 8031). Reassuringly, out of 166 patients who had MAC as the principal anaesthesia technique, 139 patients had an additional anaesthesia technique of either neuraxial anaesthesia, or RA. Sensitivity analysis with the additional grouping of the GA + RA group revealed similar results. There was also a relatively high rate of missing patency values (13 per cent), which may have biased the results by excluding more patients with patent vessels who did not seek follow-up imaging. Other limitations include residual confounding by indication, and variables that were not available in the NSQIP data set, such as hospital-specific factors and anatomical details, which may affect RA utilization and outcomes27. Similarly, it was not possible to include cardiac valvular disease as a confounder20 as it was not available, though this likely did not affect the results given the less than 2 per cent incidence of heart failure in the cohort. It was also not possible to analyse outcomes beyond 30 days after surgery. Last, the study may be underpowered to detect a small difference due to a smaller than anticipated sample size.
For patients undergoing elective open lower limb revascularization, there was no association between the anaesthetic technique (RA versus GA) and 30-day primary patency. Nevertheless, this should be interpreted with caution due to potential misclassification of anaesthesia technique within the NSQIP data set, smaller than projected sample size, and possible residual confounding. Further prospective studies would be useful to study the impact of anaesthesia technique on important patient-centred outcomes such as long-term patency and non-home discharge.
Supplementary Material
Acknowledgements
The American College of Surgeons (ACS) NSQIP and the participating hospitals are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors. J.K. was responsible for study design, data analysis, interpretation, and first draft of the manuscript. A.F. was responsible for study design, data analysis, interpretation, and manuscript revision. S.M. was responsible for study design, interpretation, and manuscript revision. S.S. was responsible for study design, interpretation, and manuscript revision. C.P. was the Principal Investigator, and was responsible for study design, interpretation, and manuscript revision. The authors thank H. Qian at the Centre for Health Evaluation & Outcome Sciences, the University of British Columbia, Vancouver, Canada for statistical consultation.
Disclosure: J.K. received salary support as the Clinical Data Lead, St. Paul’s Hospital, Canada, for a project on ‘reducing opioid use for pain management’ from the Canadian Digital Technology Supercluster and Consortium (Careteam Technologies, Thrive Health, Excelar Technologies, Providence Health Care Ventures, and Xerus). The authors declare no other conflict of interest.
Contributor Information
Janny Xue Chen Ke, Department of Anesthesia, St. Paul’s Hospital, Providence Health Care, Vancouver, British Columbia, Canada; Department of Anesthesiology, Pharmacology & Therapeutics, University of British Columbia, Vancouver, British Columbia, Canada; Department of Anesthesiology, Pain Management, and Perioperative Medicine, Dalhousie University, Halifax, Nova Scotia, Canada.
Alana M. Flexman, Department of Anesthesia, St. Paul’s Hospital, Providence Health Care, Vancouver, British Columbia, Canada Department of Anesthesiology, Pharmacology & Therapeutics, University of British Columbia, Vancouver, British Columbia, Canada.
Stephan K. W. Schwarz, Department of Anesthesia, St. Paul’s Hospital, Providence Health Care, Vancouver, British Columbia, Canada Department of Anesthesiology, Pharmacology & Therapeutics, University of British Columbia, Vancouver, British Columbia, Canada.
Shaun MacDonald, Division of Vascular Surgery, St. Paul’s Hospital, The University of British Columbia, Vancouver, British Columbia, Canada.
Christopher Prabhakar, Department of Anesthesia, St. Paul’s Hospital, Providence Health Care, Vancouver, British Columbia, Canada; Department of Anesthesiology, Pharmacology & Therapeutics, University of British Columbia, Vancouver, British Columbia, Canada.
Funding
This study was supported in part by the Departments of Surgery and Anesthesia, Providence Health Care, Vancouver, British Columbia, Canada, and the Dr. Jean Hugill Templeton Endowment for Anesthesia Memorial Fund, the University of British Columbia, Vancouver, British Columbia, Canada.
Supplementary material
Supplementary material is available at BJS Open online
Data availability
The NSQIP data set used in this study is available to participating sites upon appropriate institutional approval.
References
- 1. Barbosa FT, Jucá MJ, Castro AA, Cavalcante JC. Neuraxial anaesthesia for lower-limb revascularization. Cochrane Database Syst Rev 2013;CD007083. 10.1002/14651858.CD007083.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007;45:S5–S67 [DOI] [PubMed] [Google Scholar]
- 3. Nguyen LL, Moneta GL, Conte MS, Bandyk DF, Clowes AW, Seely BL. Prospective multicenter study of quality of life before and after lower extremity vein bypass in 1404 patients with critical limb ischemia. J Vasc Surg 2006;44:977–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grip O, Wanhainen A, Michaëlsson K, Lindhagen L, Björck M. Open or endovascular revascularization in the treatment of acute lower limb ischaemia. Br J Surg 2018;105:1598–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghanami RJ, Hurie J, Andrews JS, Harrington RN, Corriere MA, Goodney PP et al. Anesthesia-based evaluation of outcomes of lower-extremity vascular bypass procedures. Ann Vasc Surg 2013;27:199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goodney PP, Nolan BW, Schanzer A, Eldrup-Jorgensen J, Bertges DJ, Stanley AC et al. Factors associated with amputation or graft occlusion one year after lower extremity bypass in northern New England. Ann Vasc Surg 2010;24:57–68 [DOI] [PubMed] [Google Scholar]
- 7. Macfarlane AJR, Vlassakov K, Elkassabany N. Regional anesthesia for vascular surgery: does the anesthetic choice influence outcome? Curr Opin Anaesthesiol 2019;32:690–696 [DOI] [PubMed] [Google Scholar]
- 8. Conte MS, Belkin M, Upchurch GR, Mannick JA, Whittemore AD, Donaldson MC. Impact of increasing comorbidity on infrainguinal reconstruction: a 20-year perspective. Ann Surg 2001;233:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Christopherson R, Beattie C, Frank SM, Norris EJ, Meinert CL, Gottlieb SO et al. Perioperative morbidity in patients randomized to epidural or general anesthesia for lower extremity vascular surgery. Perioperative Ischemia Randomized Anesthesia Trial Study Group. Anesthesiology 1993;79:422–434 [DOI] [PubMed] [Google Scholar]
- 10. Singh N, Sidawy AN, Dezee K, Neville RF, Weiswasser J, Arora S et al. The effects of the type of anesthesia on outcomes of lower extremity infrainguinal bypass. J Vasc Surg 2006;44:964–968 [DOI] [PubMed] [Google Scholar]
- 11. Perler BA, Christopherson R, Rosenfeld BA, Norris EJ, Frank S, Beattie C et al. The influence of anesthetic method on infrainguinal bypass graft patency: a closer look. Am Surg 1995;61:784–789 [PubMed] [Google Scholar]
- 12. Wiis JT, Jensen-Gadegaard P, Altintas Ü, Seidelin C, Martusevicius R, Mantoni T. One-week postoperative patency of lower extremity in situ bypass graft comparing epidural and general anesthesia: retrospective study of 822 patients. Ann Vasc Surg 2014;28:295–300 [DOI] [PubMed] [Google Scholar]
- 13. Jorgensen MS, Farres H, James BLW, Li Z, Almerey T, Sheikh-Ali R et al. The role of regional versus general anesthesia on arteriovenous fistula and graft outcomes: a single-institution experience and literature review. Ann Vasc Surg 2020;62:287–294 [DOI] [PubMed] [Google Scholar]
- 14. Gao C, Weng C, He C, Xu J, Yu L. Comparison of regional and local anesthesia for arteriovenous fistula creation in end-stage renal disease: a systematic review and meta-analysis. BMC Anesthesiol 2020;20:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American College of Surgeons. ACS NSQIP Participant Use Data File. http://www.facs.org/quality-programs/acs-nsqip/participant-use (accessed September 2020) [Google Scholar]
- 16. Veen JJV, Nokes TJ, Makris M. The risk of spinal haematoma following neuraxial anaesthesia or lumbar puncture in thrombocytopenic individuals. Br J Haematol 2010;148:15–25 [DOI] [PubMed] [Google Scholar]
- 17. Gulur P, Tsui B, Pathak R, Koury KM, Lee H. Retrospective analysis of the incidence of epidural haematoma in patients with epidural catheters and abnormal coagulation parameters. Br J Anaesth 2015;114:808–811 [DOI] [PubMed] [Google Scholar]
- 18. Horlocker TT, Wedel DJ, Rowlingson JC, Enneking FK, Kopp SL, Benzon HT et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and pain medicine evidence-based guidelines (third edition). Reg Anesth Pain Med 2010;35:64–101 [DOI] [PubMed] [Google Scholar]
- 19. Sgroi MD, McFarland G, Mell MW. Utilization of regional versus general anesthesia and its impact on lower extremity bypass outcomes. J Vasc Surg 2019;69:1874–1879 [DOI] [PubMed] [Google Scholar]
- 20. Roberts DJ, Nagpal SK, Kubelik D, Brandys T, Stelfox HT, Lalu MM et al. Association between neuraxial anaesthesia or general anaesthesia for lower limb revascularisation surgery in adults and clinical outcomes: population based comparative effectiveness study. BMJ 2020;371:m4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R package for computing E-values. Epidemiology 2018;29:e45-–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. SAS Macro to Calculate the Standardized Difference. http://www.lerner.ccf.org/qhs/software/lib/stddiff.sas (accessed January 2021)
- 23. Pierce ET, Pomposelli FB Jr, Stanley GD, Lewis KP, Cass JL, LoGerfo FW et al. Anesthesia type does not influence early graft patency or limb salvage rates of lower extremity arterial bypass. J Vasc Surg 1997;25:226–232 [DOI] [PubMed] [Google Scholar]
- 24. Fereydooni A, O’Meara T, Popescu WM, Dardik A, Ochoa Chaar CI. Utilization and outcomes of local anesthesia and peripheral nerve block for hybrid lower extremity revascularization. J Endovasc Ther 2020;27:94–101 [DOI] [PubMed] [Google Scholar]
- 25. Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol 2002;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iannuzzi JC, Boitano LT, Cooper MA, Watkins MT, Eagleton MJ, Clouse WD et al. Risk score for nonhome discharge after lower extremity bypass. J Vasc Surg 2020;71:889–895 [DOI] [PubMed] [Google Scholar]
- 27. McIsaac DI, Wijeysundera DN, Huang A, Bryson GL, van Walraven C. Association of hospital-level neuraxial anesthesia use for hip fracture surgery with outcomes: a population-based cohort study. Anesthesiology 2018;128:480–491 [DOI] [PubMed] [Google Scholar]
- 28. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The NSQIP data set used in this study is available to participating sites upon appropriate institutional approval.