Abstract
Baicalein is a flavonoid extracted from the root of Scutellaria baicalensis (Chinese Skullcap) and is consumed as part of this botanical dietary supplement to reduce oxidative stress, pain and inflammation. We previously reported that baicalein can also modify receptor signaling through the progesterone receptor (PR) and glucocorticoid receptor (GR) in vitro, which is interesting due to the well-established roles of both PR and GR in reducing inflammation. To understand the effects of baicalein on PR and GR signaling in vivo in the uterus, ovariectomized CD-1 mice were treated with DMSO, progesterone (P4), baicalein, P4 with baicalein, and P4 with RU486, a PR antagonist, for a week. The uteri were collected for histology and RNA sequencing. Our results showed that baicalein attenuated the anti-proliferative effect of P4 on luminal epithelium as well as on the PR target genes HAND2 and ZBTB16. Baicalein did not change levels of PR or GR RNA or protein in the uterus. RNA sequencing data indicated that many transcripts significantly altered by baicalein were regulated in the opposite direction by P4. Similarly, a large portion of GO/KEGG terms and GSEA gene sets were altered in the opposite direction by baicalein as compared to P4 treatment. Treatment of baicalein did not change body weight, organ weight, or blood glucose level. In summary, baicalein functioned as a PR antagonist in vivo and therefore may oppose P4 action under certain conditions such as uterine hyperplasia, fibroids, and uterine cancers.
Graphical Abstract
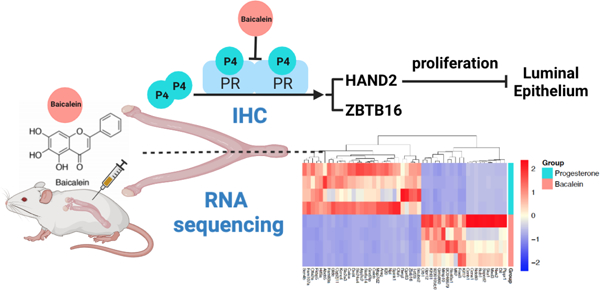
Progesterone is a steroid hormone that plays a vital role in female reproductive health, particularly in uterine biology.1 Progesterone exerts its transcriptional regulation through the nuclear progesterone receptor (PRA and PRB) in tissues such as the uterus, breast, ovary and pituitary.2 Upon binding to its ligand progesterone, PR dimerizes and translocates into the nucleus to regulate gene transcription.3 Progesterone signaling is critical for early pregnancy events such as implantation and decidualization and it is essential for maintaining pregnancy.4 In the uterus, progesterone is known to counteract the proliferative effects of estrogen on the epithelial cells and thus exerts a protective function.5 In addition, expression of PR is associated with longer survival times in endometrial and ovarian cancer patients.6,7 Because progesterone is not bioavailable orally unless formulated as micronized progesterone, synthetic analogs of progesterone are often prescribed to patients with fibroids and endometrial cancer.8,9 Selective progesterone receptor modulators (SPRMs) are a class of synthetic molecules capable of interacting with PR with agonist and antagonist properties. For instance, onapristone and ulipristal acetate both act as PR antagonist and have been used for treatment of endometriosis, fibroids and breast cancer10,11 However, synthetic PR ligands usually have some affinity to the androgen receptor (AR) or glucocorticoid receptor (GR) and interactions with these are associated with side effects such as cardiovascular diseases, weight gain and stroke.12,13 Thus, the identification of progestins with minimal side effects would be beneficial. Botanical supplements possessing progestin-like compounds and anti-inflammatory characteristics have not been studied extensively for this biological activity.
Herbal dietary supplements are becoming increasingly popular as people turn to them for health benefits. The herbal dietary supplements market increased by 8.6% to $9.6 billion in total US sales in 2019 from the previous year.14 In 2020, amid the COVID-19 pandemic, the market for herbal supplements was estimated at $10.8 billion and projected to reach $16.9 billion by 2027.15 It is common for women to consume botanical supplements to treat gynecological conditions such as menopausal or post-menopausal symptoms, cyclic mastalgia, infertility or endometriosis.16 Plants with estrogenic activities used in dietary supplements include soy, red clover, hops, chasteberry, flaxseed and licorice.17 Plant based compounds with estrogenic activities are known as phytoestrogens, and they can bind to the estrogen receptor (ER) to trigger transcriptional activation due to their similar structures or functions to estrogens.18 Herbal supplements containing phytoestrogens have been used for alleviating menopausal symptoms, however, botanical supplements are usually composed of mixtures of compounds and recent data suggest that some botanicals contain ligands that act on PR and GR.17,19 For instance, studies have shown that apigenin and kaempferol from botanical supplements counteract the proliferative effects of genistein in the uterus.20,21 These compounds can bind to PR and trigger transcriptional action similar to progesterone, thus are termed as phytoprogestins.
Baicalein is isolated from Scutellaria baicalensis Georgi, or Chinese skullcap, a medicinal plant used in traditional Chinese medicine.22 Baicalein is one of the most abundant compounds and bioactive flavonoids in Chinese skullcap, which has been shown to possess anti-cancer and anti-inflammatory properties.23 Baicalein is mainly extracted from the root and represents approximately 10% of the total Chinese skullcap extract.24 Baicalein is known to possess anti-inflammatory functions by inhibiting hyperpermeability, expression of cell adhesion molecule (CAM), and migration of leukocytes for vascular inflammatory diseases.25 It reduced lipopolysaccharide induced inflammation via suppressing Janus protein tyrosine kinase/Signal transducers and activators of transcription (JAK/STATs) activation and ROS production in macrophages. Baicalein induced apoptosis in epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKI)-resistant lung cancer.26 In undifferentiated thyroid cancer cells, baicalein induced apoptosis through blocking the ERK/PI3K/Akt pathway27. In breast cancers, baicalein inhibited cell growth and induced apoptosis of cancer cells, and suppressed breast cancer cell adhesion, migration and invasion.24,28,29 However, the effects of baicalein as a phytoprogestin on the uterus, a PR rich tissue, remain poorly understood. In a previous study, we screened compounds with structural similarity to apigenin and kaempferol, and identified baicalein using a fluorescence polarization competitive binding assay for PR and GR with IC50 values of 15 and 19 μM, respectively.30 Baicalein activated a hormone responsive luciferase construct and regulated gene expression in both uterine and breast cancer cell lines. In the present study, we investigated the effects of baicalein on the uterine tissue in vivo for the first time since PR and GR signaling can play an important role in the uterus, particularly as it relates to fibroids, endometriosis and inflammation.
RESULTS AND DISCUSSION
Baicalein Attenuates The Inhibitory Effect Of Progesterone on Uterine Epithelial Cell Proliferation.
We previously reported that baicalein acts as a PR antagonist in vitro and that it increased expression of the GR target gene, glucocorticoid-induced leucine zipper (Gilz) in the uterus in vivo, which could be blocked by the PR and GR antagonist, RU486.31 To investigate the effects of baicalein on uterine cell proliferation, CD-1 mice (n=5/group) were injected IP with 10% DMSO, 1 mg/kg progesterone (P4), 25 mg/kg baicalein, 1 mg/kg P4 combined with 25 mg/kg baicalein, or 1 mg/kg P4 combined with 10 mg/kg RU486 for 7 days. In the control group, immunohistochemistry for PCNA staining found that 49% of the luminal and glandular epithelial cells were proliferating (Figure. 1A), but in the P4 treated group, a significant number of the epithelial cells (93%) were quiescent (Figure 1B). When treated with P4 and baicalein, there were more proliferating epithelial cells (32%) compared to the P4 group (7%), which were not different than control (Figure. 1C). Baicalein alone resulted in 47% of the luminal epithelial cells stained for proliferation marker PCNA. This result indicated that baicalein alone does not reduce the percentage of PCNA positively stained luminal epithelium, but baicalein attenuated the anti-proliferative effects of P4 on epithelial proliferation. Mice were also treated with P4 in combination with the PR antagonist, RU486, and the uteri had 23% proliferating epithelial cells, which was not statistically different compared to the control group (Figure 1D). In the uterus, it is well understood that steroid hormones, such as estradiol (E2) and P4, have opposing actions in regulating cell proliferation and differentiation in a paracrine manner.32–35 Estradiol, acting through stromal ER alpha, drives epithelial proliferation through the secretion of factors such as Fgf10, Hox10a and Bmp8a.36 On the other hand, P4 inhibits expression of Fibroblast growth factors (FGFs) and blocks estrogen-induced epithelial proliferation through PR.37 Treating with baicalein combined with P4 reduced the anti-proliferative effect of P4. In addition, studies showed that baicalein blocked proliferation and induced apoptosis in the cervical carcinoma Hela cell line.38,39 Women with certain gynecological diseases such as endometriosis and uterine fibroids are treated with progestin antagonists.40,41 These diseases often involve inflammation that contributes to abdominal pain.42,43
Figure 1. Baicalein attenuates the inhibitory effect of progesterone on luminal and glandular epithelial cell proliferation.
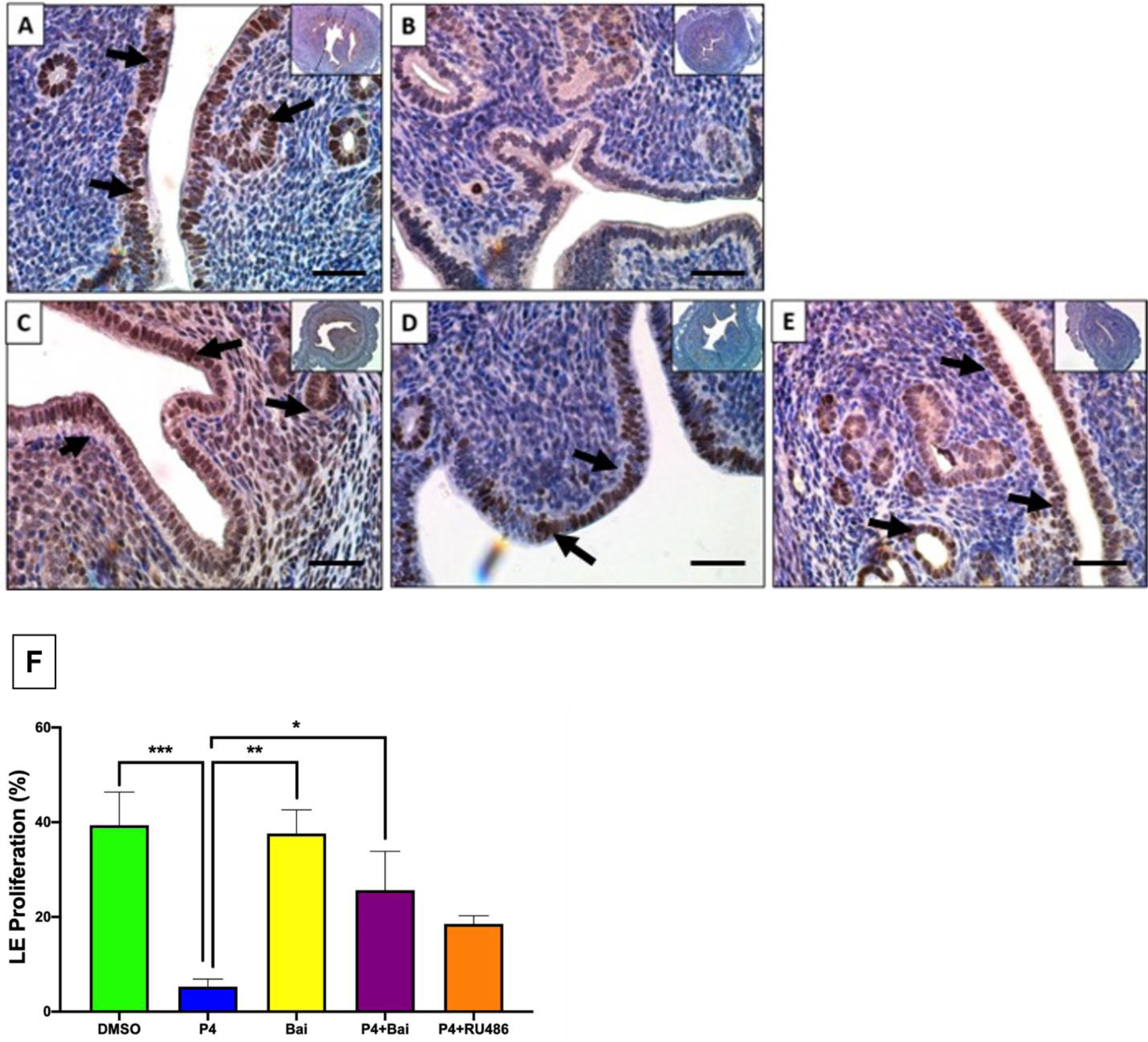
Immunohistochemical staining against proliferation marker PCNA on uterine cross sections of 10% DMSO (A), 1 mg/kg progesterone (B), 25 mg/kg baicalein and 1 mg/kg progesterone (C), 1 mg/kg progesterone and 10 mg/kg RU486 (D) and 25 mg/kg baicalein (E) treated mice. (F) Percentage of proliferating luminal epithelial cells in each treatment group. Arrows indicate positive stains. Scale bar = 300 μm. N=5/group. Inset images were taken at 4x magnification. Asterisks indicate * p≤0.05, ** p≤0.01,*** p≤0.001.
Baicalein Decreased P4 Induced HAND2 Expression.
Heart and neural crest derivatives-expressed protein 2 (HAND2) is a transcription factor that is exclusively expressed in the sub-epithelial stromal regions in the uterus and has a critical role in regulating uterine epithelial functions.44 HAND2 is induced by P4 in ovariectomized mice and blocked by RU486.37 We evaluated HAND2 protein expression by immunohistochemistry (IHC) after mice were treated with baicalein and our results showed that HAND2 protein was limited to a thin layer of sub-epithelial stromal cells in the control group (Figure. 2A), but its expression was upregulated and extended to deep regions of the stromal in the mouse uteri treated with P4 (Figure. 2B). In mice treated with the combination of P4 and baicalein, HAND2 staining was more diffuse than in the P4 group (Figure. 2C). HAND2 protein levels were comparable between the control group and the P4 combined with RU486 group (Figure. 2D). HAND2 expression was limited to the small region close to epithelium when treated with baicalein (Figure. 2E), indicating that baicalein alone did not increase HAND2 abundance compared the control group. Studies have reported that P4 upregulates HAND2, and HAND2 inhibits the expression of stromal FGFs that induce luminal epithelial proliferation through the ERK1/2 pathway in a paracrine manner.37,45 Together with the proliferation data, the data indicate that P4 induces HAND2 and inhibits luminal epithelial proliferation, while baicalein blocks the P4-induced HAND2 expression and the inhibition of proliferation similar to RU486, the established PR antagonist.
Figure 2. Baicalein decreased progesterone induced HAND2 expression in mouse uteri.
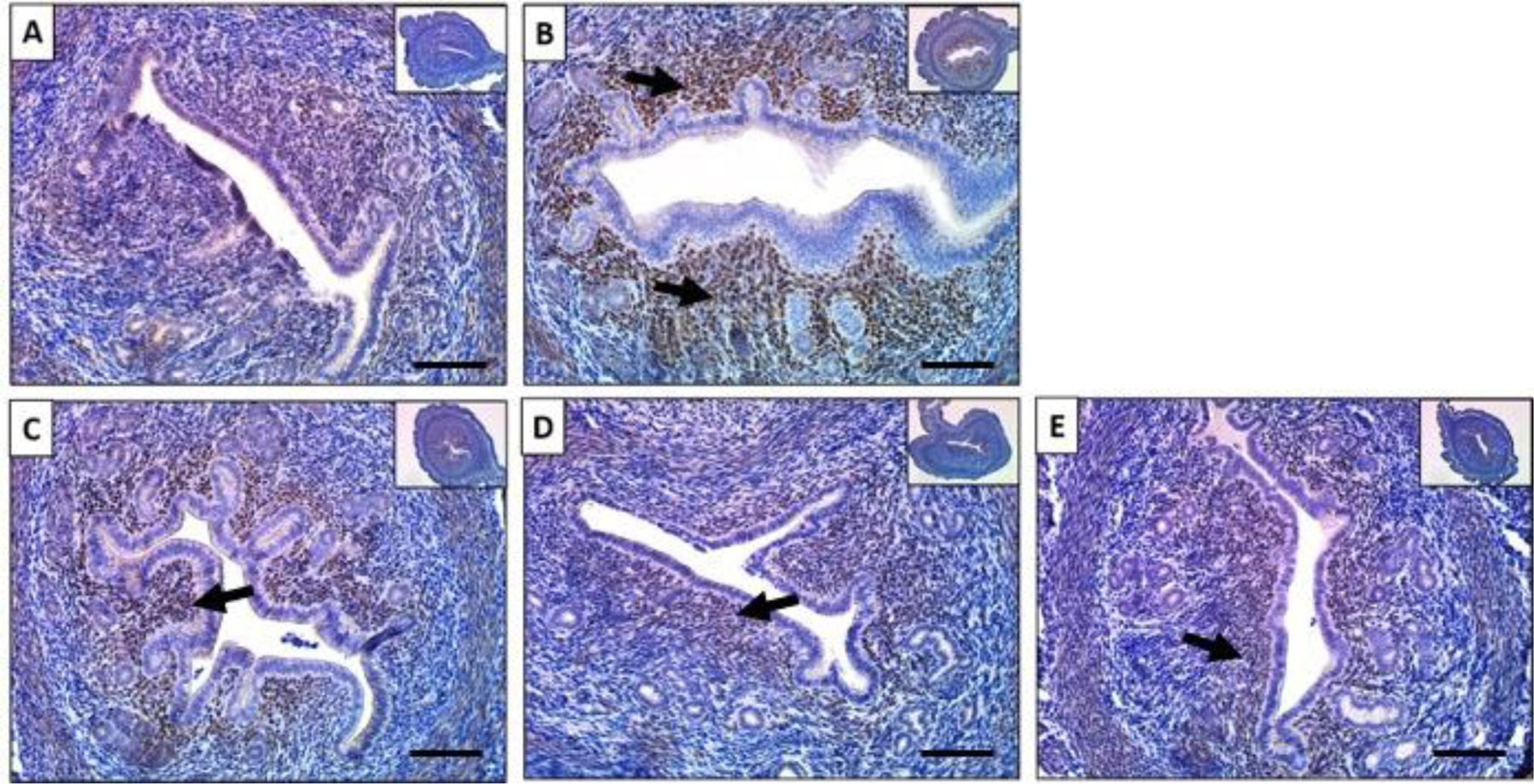
Immunohistochemical staining against progesterone receptor target HAND2 on uterine cross sections of 10% DMSO (A), 1 mg/kg progesterone (B), 25 mg/kg baicalein and 1 mg/kg progesterone (C), 1 mg/kg progesterone and 10 mg/kg RU486 (D) and 25 mg/kg baicalein (E) treated mice. Arrows indicate positive stains. Scale bar = 150 μm. Inset images were taken at 4x magnification.
Baicalein Decreased Progesterone Induced ZBTB16 Expression.
Zinc finger and BTB domain-containing 16 (ZBTB16) is a transcriptional factor that belongs to the family of Krüppel‐like zinc finger proteins and is involved in cell cycle control differentiation of myeloid cells, and spermatogenesis.46 ZBTB16 is induced by P4 in the female reproductive tract and is essential for stromal cell decidualization.47 Kommagani and colleagues reported in a study of human endometrial stromal cells that ChIP-Seq identified more than 10 progesterone response elements within the Zbtb16 gene, indicating that it may be a direct target of PR signaling.48 We evaluated the effects of baicalein on ZBTB16 abundance, and our results showed that there was very little ZBTB16 in the control group (Figure. 3A), but its abundance was elevated in the P4 treated group (Figure. 3B). When treated with P4 and baicalein, the abundance level of ZBTB16 was reduced compared to P4 treatment alone, similar to the P4 and RU486 group (Figure. 3D). When treated with baicalein alone, ZBTB16 was at basal level, similar to the control (Figure. 3E). These results suggest that baicalein acts as a PR antagonist that decreases P4-induced ZBTB16 expression in the uterus. Furthermore, Qiu and colleagues reported that ZBTB16 was one of the downstream targets promoted by FOXA1 and higher FOXA1 expression was correlated with a higher incidence of endometrial cancer.49 ZBTB16 was also found to be regulated by both GR and PR and it functioned as a tumor suppressor that inhibits proliferation and metastasis in breast cancer cells. 50,51
Figure 3. Baicalein decreased progesterone induced ZBTB16 expression in mouse uteri.
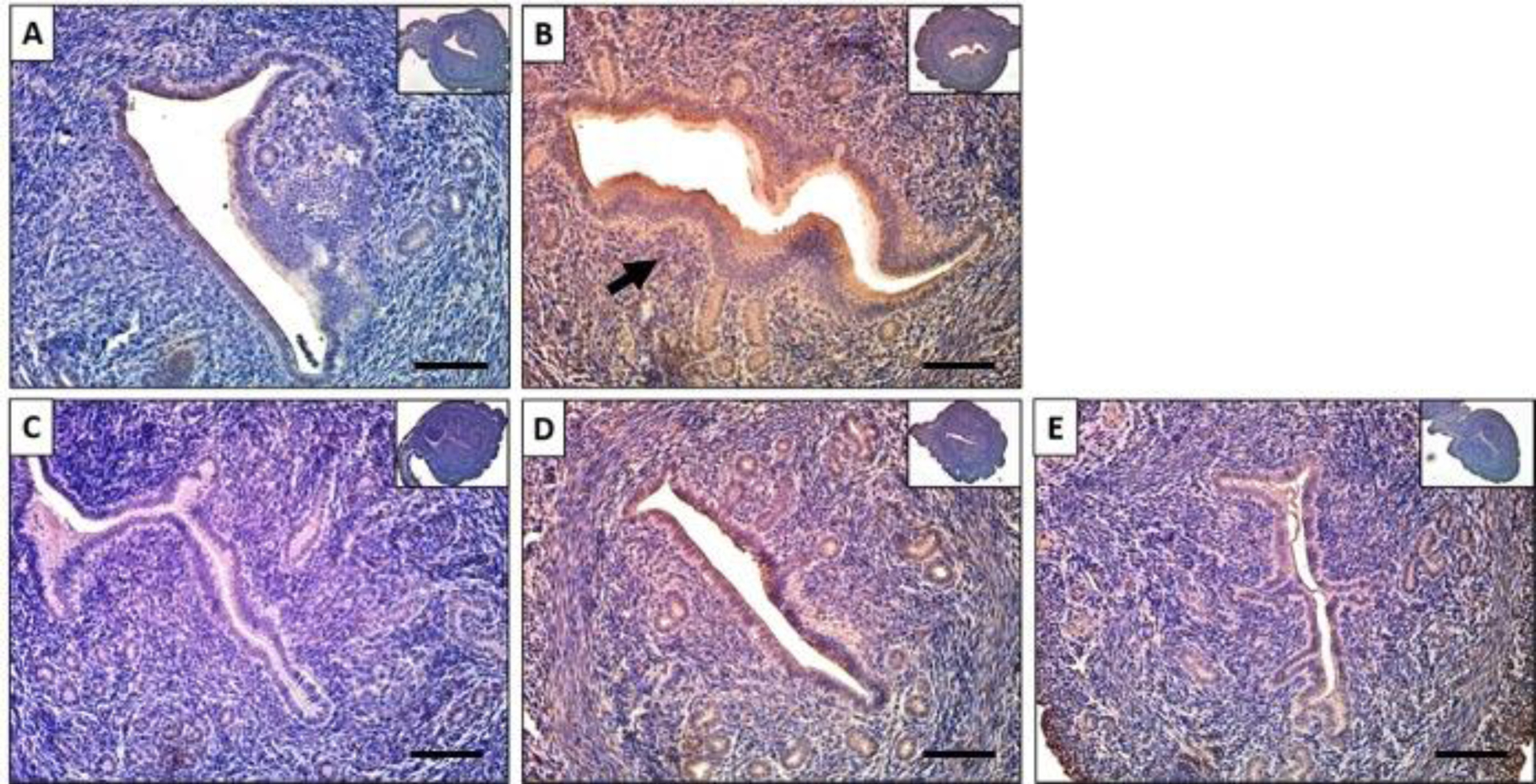
Immunohistochemical staining against progesterone receptor target ZBTB16 on uterine cross sections of 10% DMSO (A), 1 mg/kg progesterone (B), 25 mg/kg baicalein and 1 mg/kg progesterone (C), 1 mg/kg progesterone and 10 mg/kg RU486 (D) and 25 mg/kg baicalein (E) treated mice. Arrows indicate positive stains. Scale bar = 150 μm. Inset images were taken at 4x magnification.
Baicalein Did Not Alter PR Protein Levels.
To evaluate the effects of baicalein on PR protein levels, uterine sections were immunostained for PR. The results showed that PR was expressed uniformly in the luminal and glandular epithelial cells in the control group (Figure. 4A). Treatment of P4, P4 with baicalein, or P4 with RU486 did not alter PR expression (Figure. 4B-D). Similarly, treatment of baicalein alone did not cause a difference in PR expression in the uterus compared to the control or P4 group (Figure. 4E). In our previous study, we also showed that baicalein did not change PR expression in breast cancer cells.31 The results are consistent with the in vitro data.
Figure 4. Baicalein did not alter PR protein levels in mouse uteri.
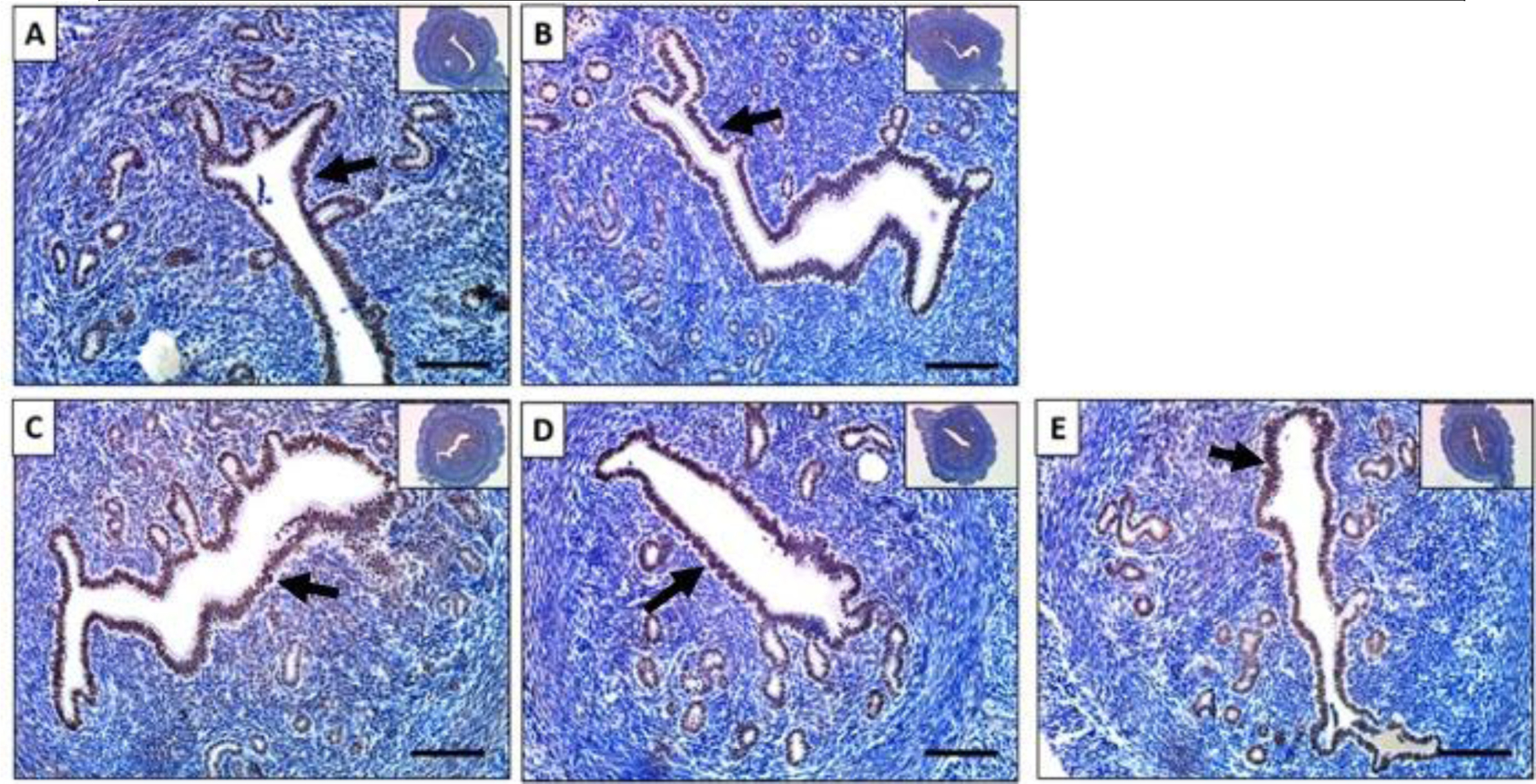
Immunohistochemical staining against progesterone receptor on uterine cross sections of 10% DMSO (A), 1 mg/kg progesterone (B), 25 mg/kg baicalein and 1 mg/kg progesterone (C), 1 mg/kg progesterone and 10 mg/kg RU486 (D) and 25 mg/kg baicalein (E) treated mice. Arrows indicate positive stains. Scale bar = 150 μm. Inset images were taken at 4x magnification.
Baicalein’s Effect on GR Expression.
GR is a constitutively expressed transcription factor.52 GR is expressed in the uterus of both mouse and human.53 To evaluate the effect of baicalein on GR protein, uterine sections were stained for GR. The results showed that GR was abundantly expressed in both the stromal and epithelial cells in the control (Figure. 5A). In the P4 treated group, GR was expressed in the uterine stromal cells, but very little was in the luminal epithelial cells (Figure. 5B). When mice were treated with P4 and baicalein, GR was expressed intensively in the stroma as well as luminal epithelium, similar to the P4 and RU486 (Figure. 5 C and D). When mice were treated with baicalein alone, GR was expressed in both stroma and epithelium, resembles the control group (Figure. 5E). These data suggest that baicalein alone does not alter the expression of GR, but it could reverse the P4 induced stromal-only expression of GR to both stromal and epithelial expression. The exact effects of P4 on GR localization is not clear and the biological significance of epithelial and stromal expression remains unknown, however, GR expression in the uterus is associated with poor prognosis in ER-expressing endometrial tumors.54 Future studies are required to understand the role of GR in the uterus.
Figure 5. Baicalein’s effect on GR expression in mouse uteri.
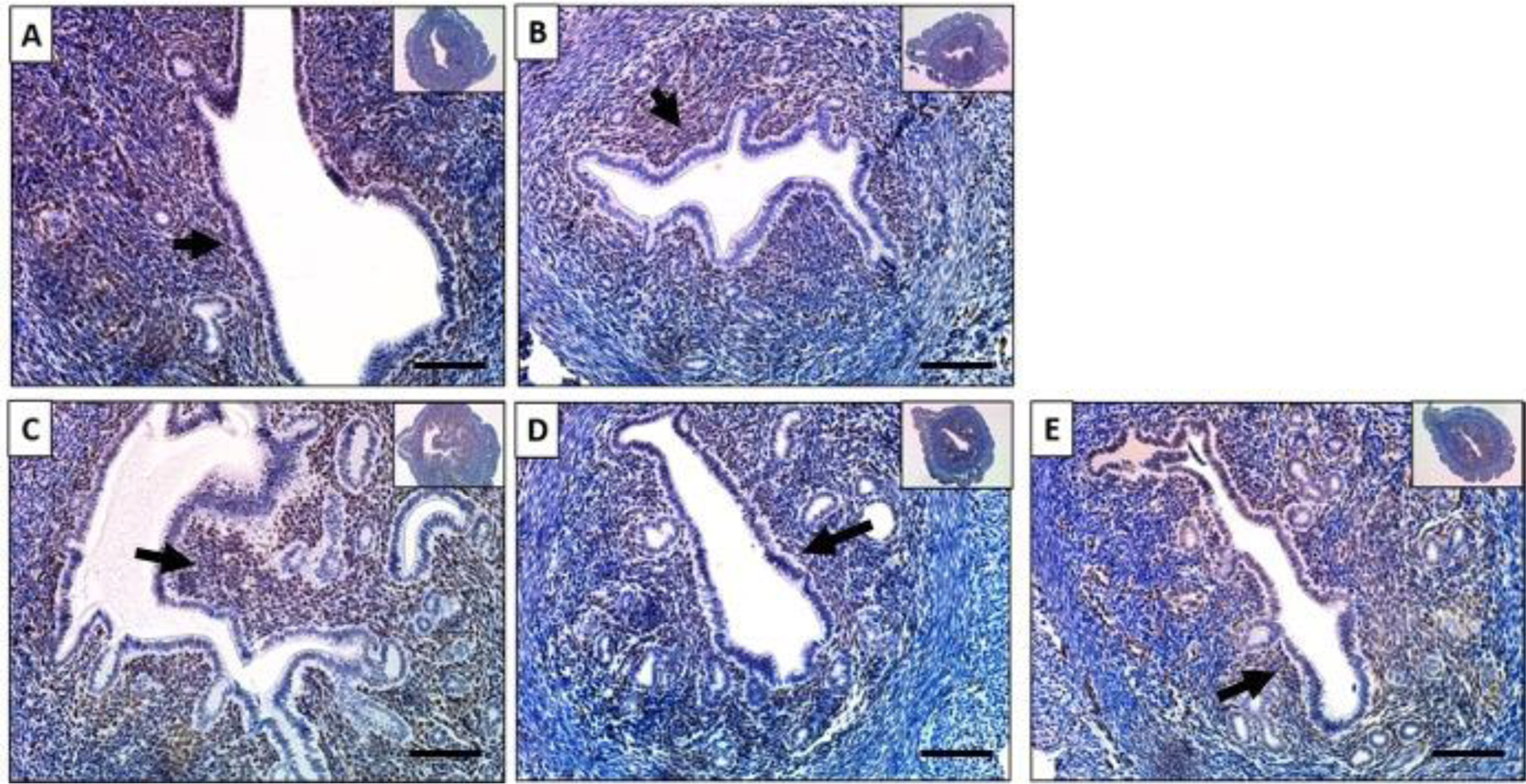
Immunohistochemical staining against glucocorticoid receptor on uterine cross sections of 10% DMSO (A), 1 mg/kg progesterone (B), 25 mg/kg baicalein and 1 mg/kg progesterone (C), 1 mg/kg progesterone and 10 mg/kg RU486 (D) and 25 mg/kg baicalein (E) treated mice. Arrows indicate positive stains. Scale bar = 150 μm. Inset images were taken at 4x magnification.
Common Genes And Pathways Altered Oppositely By Progesterone And Baicalein.
To evaluate and compare the transcriptomic profiles of the mouse uteri, we extracted mRNA from the control, P4, and baicalein groups and subjected it to RNA sequencing (n=4). Results for significantly altered transcripts are summarized in Figure 6. Baicalein treatment significantly upregulated 46 genes and downregulated 131 genes. We found a large portion of genes that were upregulated by baicalein were downregulated by P4. Of the 131 mRNA significantly downregulated by baicalein, 44 were upregulated by P4; while 18 of 46 transcripts significantly upregulated by baicalein were downregulated by P4. The full list of commonly altered transcripts is presented in Table 1. Full lists of significantly altered mRNA by each group are available as Supporting Information tables. DAVID functional annotation analysis showed that 46% (24 of 52) GO terms downregulated by baicalein were upregulated by P4, and 48% (13 of 27) KEGG pathways downregulated by baicalein were upregulated by P4. The top three oppositely regulated common KEGG pathways are focal adhesion, proteoglycan in cancer and cGMP-PKG signaling pathway, with nine, five and five genes in the pathway respectively. Full lists of altered GO/KEGG terms by each group are available as Supporting Information tables. Among these common genes changed in the opposite direction by baicalein and P4, studies have reported that many of them are regulated by P4 in uterine biology.55–57 For instance, Cdkl2 transcript was upregulated by baicalein treatment, but downregulated in the P4 treatment group. A previous study also found that Cdk12 was downregulated by P4 in mouse uterus.58 In human endometrial stromal cells (HESC), knockdown of PR leads to downregulation of Ccdc69.59 Our data showed baicalein treatment downregulated Ccdc69 similar to knockdown of PR. In another study with microarray data, Cdr2 expression was enhanced by P4, but it was downregulated by baicalein in our study.60 Progesterone is critical for successful pregnancy, and PR signals regulate many genes that important for implantation and decidualization.4,61 We found that baicalein downregulated integrin Itga7 in the mouse uterus, but it was upregulated in human myometrium and decidualized stromal cells during pregnancy, which again is consistent with working to oppose P4 action.62,63 Similarly, Tcf23, another baicalein-downregulated gene, was shown to be critical and upregulated in decidualization in HESC by P4.64 Baicalein also regulated genes involved in uterine pathologies such as fibroids and endometrial cancer. Fibroids are benign smooth muscle tumors originated from the myometrium. Their development is highly dependent on ovarian hormones, and PR actions play a key role in fibroid growth.9 A genomic and transcriptomic study revealed Hspb7 was associated with cell proliferation in fibroids, but it was downregulated by baicalein in our study.65 Baicalein upregulated Mal2 in the mouse uterus, and Mal2 was previously found to be linked with proliferation, migration, and invasion in endometrial cancer, which is known to be related to reduced P4 action.66 Interestingly, a few lncRNAs involved in PR signaling or tumorigenesis were also oppositely regulated by baicalein and P4. For example, Fam107a was downregulated by baicalein, but it was reported to be upregulated by MPA in myometrial explants in pregnant women.67 Fam212b has been shown to be one of the core regulators of endometrial carcinogenesis, and was downregulated by baicalein treatment in our study.68
Figure 6. Common genes and pathways altered oppositely in the uteri of mice treated with progesterone and baicalein.
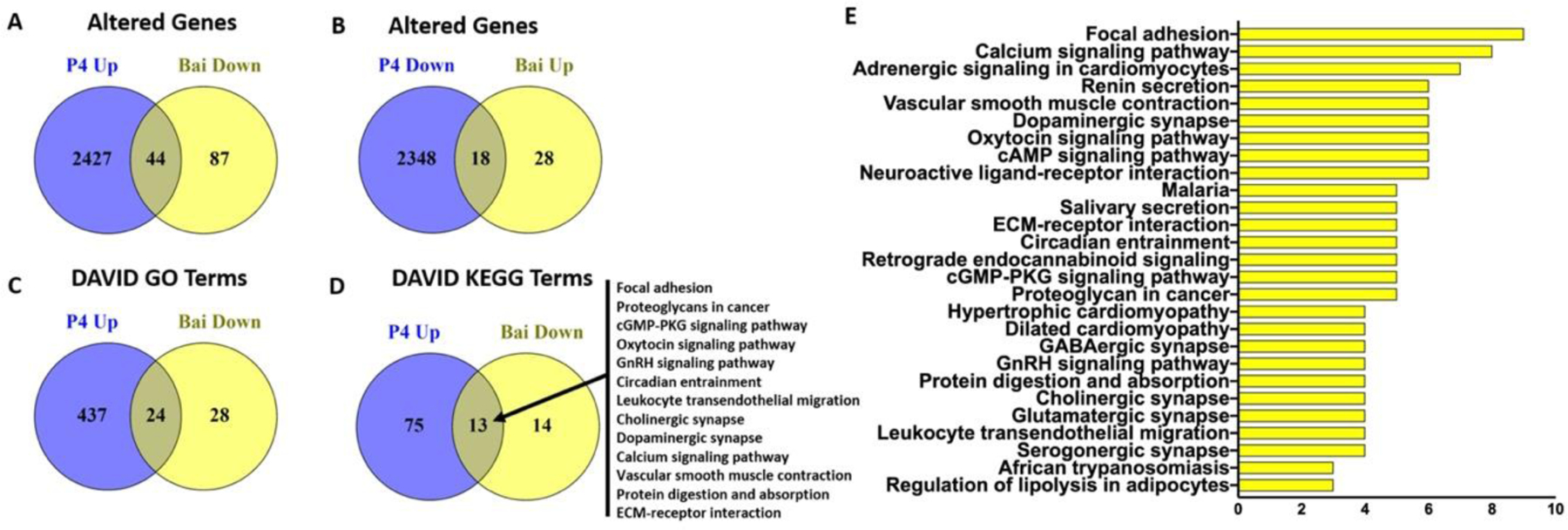
(A) 44 common genes were upregulated by progesterone but downregulated by baicalein treatment. (B) 18 common genes were downregulated by progesterone but upregulated by baicalein treatment. DAVID analysis revealed 24 GO terms (C) and 13 KEGG terms (D) that were altered oppositely by progesterone and baicalein treatment. (E) DAVID KEGG pathways downregulated by baicalein treatment. Full list of genes and GO terms can be found in Supplementary Info.
Table 1.
Genes Upregulated in P4 but Downregulated in Baicalein Treatment
| Gene Symbol | Entrez ID | Description | Fold Change | p Value | FDR Adj p Value |
|---|---|---|---|---|---|
| Plin4 | 57435 | perilipin 4 | −1.43 | 3.03E-08 | 5.77E-05 |
| Zbtb16 | 235320 | zinc finger and BTB domain containing 16 | −1.35 | 3.75E-06 | 2.28E-03 |
| Tcf23 | 69852 | transcription factor 23 | −1.14 | 1.94E-08 | 4.20E-05 |
| Nexn | 68810 | nexilin | −1.13 | 7.41E-07 | 6.11E-04 |
| Fam107a | 268709 | family with sequence similarity 107, member A | −1.11 | 1.32E-04 | 2.67E-02 |
| Gnao1 | 14681 | guanine nucleotide binding protein, alpha O | −1.07 | 3.74E-05 | 1.14E-02 |
| Mir145a | 387163 | microRNA 145a | −1.07 | 3.88E-04 | 4.12E-02 |
| Cytl1 | 231162 | cytokine-like 1 | −1.07 | 1.79E-04 | 2.84E-02 |
| Ccdc187 | 329366 | coiled-coil domain containing 187 | −1.05 | 1.15E-04 | 2.49E-02 |
| Npas4 | 225872 | neuronal PAS domain protein 4 | −1.05 | 2.14E-07 | 2.32E-04 |
| Bvht | 545261 | braveheart long non-coding RNA | −1.03 | 4.63E-06 | 2.43E-03 |
| Jph2 | 59091 | junctophilin 2 | −1.01 | 4.19E-05 | 1.22E-02 |
| Asb2 | 65256 | ankyrin repeat and SOCS box-containing 2 | −0.99 | 1.39E-04 | 2.70E-02 |
| Slit3 | 20564 | slit homolog 3 (Drosophila) | −0.98 | 2.23E-05 | 7.57E-03 |
| Ptger3 | 19218 | prostaglandin E receptor 3 (subtype EP3) | −0.96 | 1.82E-04 | 2.84E-02 |
| Ptprb | 19263 | protein tyrosine phosphatase, receptor type, B | −0.94 | 7.24E-05 | 1.68E-02 |
| Fstl3 | 83554 | follistatin-like 3 | −0.93 | 4.19E-04 | 4.32E-02 |
| Cdr2 | 12585 | cerebellar degeneration-related 2 | −0.91 | 2.68E-04 | 3.44E-02 |
| Itga7 | 16404 | integrin alpha 7 | −0.90 | 1.50E-04 | 2.70E-02 |
| Hspb7 | 29818 | heat shock protein family, member 7 (cardiovascular) | −0.89 | 1.23E-04 | 2.59E-02 |
| Mn1 | 433938 | meningioma 1 | −0.89 | 1.47E-04 | 2.70E-02 |
| Ccdc69 | 52570 | coiled-coil domain containing 69 | −0.89 | 1.55E-04 | 2.74E-02 |
| Tubb6 | 67951 | tubulin, beta 6 class V | −0.87 | 2.09E-04 | 2.96E-02 |
| Pdlim3 | 53318 | PDZ and LIM domain 3 | −0.86 | 3.98E-04 | 4.20E-02 |
| Speg | 11790 | SPEG complex locus | −0.86 | 1.82E-06 | 1.31E-03 |
| Smoc2 | 64074 | SPARC related modular calcium binding 2 | −0.85 | 5.45E-04 | 4.98E-02 |
| Fam212b | 109050 | family with sequence similarity 212, member B | −0.82 | 2.63E-04 | 3.41E-02 |
| Clmp | 71566 | CXADR-like membrane protein | −0.81 | 3.51E-04 | 3.95E-02 |
| Prkca | 18750 | protein kinase C, alpha | −0.80 | 8.95E-05 | 2.01E-02 |
| S1pr1 | 13609 | sphingosine-1-phosphate receptor 1 | −0.79 | 2.64E-04 | 3.41E-02 |
| Igsf9b | 235086 | immunoglobulin superfamily, member 9B | −0.76 | 2.00E-04 | 2.90E-02 |
| Wipf3 | 330319 | WAS/WASL interacting protein family, member 3 | −0.73 | 2.65E-05 | 8.82E-03 |
| Gja4 | 14612 | gap junction protein, alpha 4 | −0.70 | 1.47E-04 | 2.70E-02 |
| Neurl1a | 18011 | neuralized E3 ubiquitin protein ligase 1A | −0.69 | 3.69E-04 | 4.02E-02 |
| Dmpk | 13400 | dystrophia myotonica-protein kinase | −0.68 | 2.61E-04 | 3.41E-02 |
| Col6a3 | 12835 | collagen, type VI, alpha 3 | −0.64 | 2.76E-04 | 3.50E-02 |
| Myrip | 245049 | myosin VIIA and Rab interacting protein | −0.64 | 1.34E-04 | 2.67E-02 |
| Thbs3 | 21827 | thrombospondin 3 | −0.61 | 6.15E-05 | 1.50E-02 |
| Msn | 17698 | moesin | −0.56 | 1.63E-04 | 2.83E-02 |
| Ehbp1l1 | 114601 | EH domain binding protein 1-like 1 | −0.55 | 1.70E-05 | 6.39E-03 |
| Esyt2 | 52635 | extended synaptotagmin-like protein 2 | −0.54 | 2.77E-04 | 3.50E-02 |
| Ppp1r12c | 232807 | protein phosphatase 1, regulatory subunit 12C | −0.53 | 2.73E-05 | 8.93E-03 |
| Cap2 | 67252 | CAP, adenylate cyclase-associated protein, 2 (yeast) | −0.50 | 4.66E-04 | 4.57E-02 |
| Tgm2 | 21817 | transglutaminase 2, C polypeptide | −0.48 | 3.10E-04 | 3.65E-02 |
Significantly downregulated transcripts by baicalein were subjected to DAVID KEGG pathway analysis and our result identified 27 pathways. In addition to the common pathways oppositely regulated by baicalein and P4, baicalein downregulated 14 other pathways including cAMP signaling pathway, oxytocin signaling pathway GnRH signaling pathway and ECM-receptor interaction (Figure. 6E). The role of baicalein in these pathways needs further investigation. Overall, these data suggest that baicalein regulates a subset of genes that are important in uterine physiology and pathology in the opposite direction of P4 and has antagonistic effect on PR in the uterus in vivo.
Gene Sets Regulated Oppositely By Progesterone And Baicalein.
RNAseq data was further analyzed by GSEA to evaluate significantly altered gene sets (Table 3 and Figure. 7). Our data showed that 2 of 8 hallmark gene sets were negatively enriched in baicalein group but positively enriched in P4 group. These gene sets were UV response and myogenesis, and the normalized enrichment scores were 1.76 and 1.47 for P4, −1.94 and −2.29 for baicalein, respectively. Three of 12 hallmark genes were positively enriched in the baicalein group but negatively enriched in the P4 group. These gene sets were E2F targets, G2M checkpoint and DNA repair, and the normalized enrichment scores were −2.59, −2.26 and −1.61 for P4 and 1.95, 1.88 and 1.41 for the baicalein group respectively. The full list of significantly enriched gene sets is shown in Table 3. These overlapping gene sets indicate a role of baicalein in regulating cell cycle and proliferation. Furthermore, some of the significantly enriched gene sets by baicalein alone are also involved in gynecological diseases. For instance, it is well understood that Epithelial To Mesenchymal Transition (EMT) plays an important role in the progression of many cancers including endometrial cancer.69,70 Baicalein suppresses metastasis of breast cancer cells by inhibiting EMT 71. Wnt/beta-catenin signaling is involved in several aspects of the genesis of fibroids, and inactivation of Wnt/beta-catenin signaling suppresses endometrial cancer cell growth in vitro.72,73 Interestingly, our GSEA data showed both EMT and Wnt/beta-catenin signaling gene sets were negatively enriched by baicalein, suggesting a potential benefit on gene regulated in fibroids and endometrial cancer. These data suggest that baicalein has additional functions other than mediating PR signaling. Future studies are needed to elucidate the effects of baicalein on other gene sets and pathways.
Table 3.
Significantly Enriched Hallmark Gene Sets by Baicalein Treatment
| Gene Sets | Normalized Enrichment Score | FDR q-value |
|---|---|---|
| Xenobiotic metabolism | 2.18 | <0.001 |
| Oxidative phosphorylation | 1.96 | 0.001 |
| E2F targets | 1.96 | <0.001 |
| G2M checkpoint | 1.88 | 0.001 |
| Coagulation | 1.72 | 0.004 |
| Bile acid metabolism | 1.65 | 0.007 |
| Complement | 1.59 | 0.012 |
| Reactive oxygen species pathway | 1.57 | 0.010 |
| Estrogen response late | 1.56 | 0.011 |
| Estrogen response early | 1.48 | 0.022 |
| Peroxisome | 1.41 | 0.042 |
| DNA repair | 1.41 | 0.040 |
|
| ||
| Myogenesis | −2.30 | <0.001 |
| Epithelial mesenchymal transition | −2.25 | <0.001 |
| UV response | −1.94 | <0.001 |
| Notch signaling | −1.74 | 0.004 |
| Wnt beta-catenin signaling | −1.63 | 0.007 |
| Kras signaling | −1.59 | 0.009 |
| Myc target | −1.52 | 0.018 |
| Apical junction | −1.48 | 0.023 |
Figure 7. Common GSEA genes sets altered oppositely in the uteri of mice treated with progesterone and baicalein.
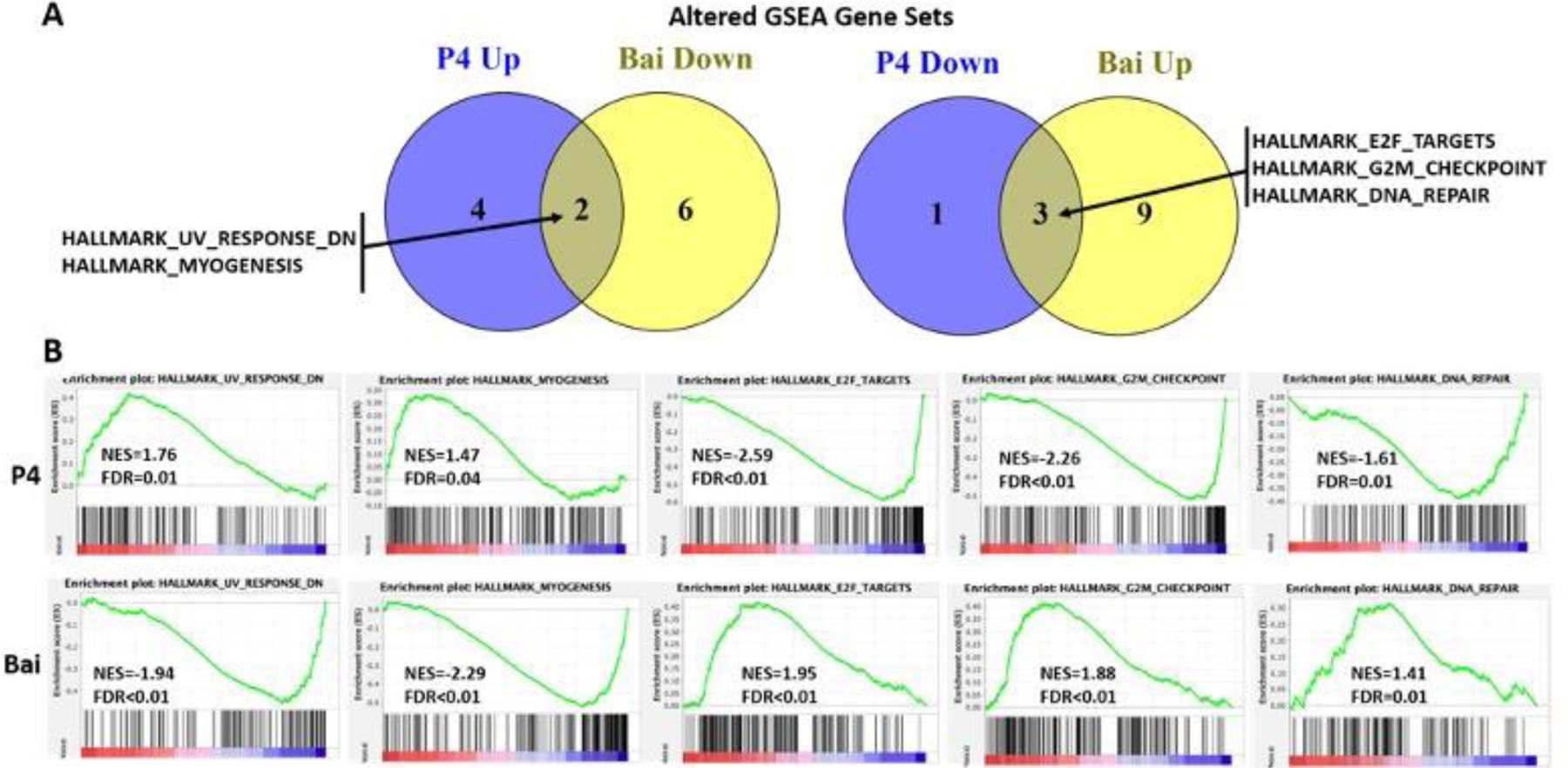
(A) Two gene sets were upregulated by progesterone but downregulated by baicalein treatment (left) and three gene sets were downregulated by progesterone but upregulated by baicalein treatment (right). (B) Enrichment plots of the common gene sets altered oppositely by progesterone (top) and baicalein (bottom) treatment. NES: normalized enrichment score. FDR<0.05 is noted as significant.
Baicalein Did Not Alter Body Weight, Organ Weights Or Blood Glucose Level.
In order to evaluate whether baicalein could exert glucocorticoid side effects such as weight gain and diabetes, the body weight, and organ weights were measured at the end of the animal study. The weights of the body, uteri and liver for each group are summarized in Table 4. There was no significant difference in body weight or organ weights in any treated group. The blood level of glucose was also measured and there was no difference in glucose level in any treated group. Although common glucocorticoid side effects include obesity, antagonism of insulin action and osteoporosis, our results showed no difference in body weight or serum glucose level of the mice. More research is warranted to investigate the role of baicalein on GR.
Table 4.
Body Weights, Organ Weights and Blood Glucose Levels
| Group | DMSO | P4 | Bai | P4+Bai | P4+RU486 |
|---|---|---|---|---|---|
| Body Weight (g) | 30±2 | 30±2 | 30±2 | 30±1 | 29±2 |
| Ut Weight (g) | 0.033±0.010 | 0.026±0.001 | 0.021±0.001 | 0.024±0.001 | 0.021±0.002 |
| MG Weight (g) | 0.22±0.02 | 0.26±0.02 | 0.24±0.02 | 0.23±0.04 | 0.17±0.01 |
| Liver Weight (g) | 1.3±0.04 | 1.4±0.06 | 1.4±0.07 | 1.2±0.04 | 1.2±0.04 |
| Glucose level (mg/dL) | 231.6±18.66 | 196.2±13.52 | 209.4±13.57 | 179.8±22.11 | 198.2±16.84 |
Chinese Skullcap has been widely used as a medicinal plant in Asian countries for centuries, and the main bioactive compound is baicalein.22 Baicalein is orally consumed and well absorbed from the stomach and small intestine, and the plasma concentration of baicalein reaches maximum 0.75–3 h after administration. It is predominantly metabolized in the liver and small intestine by glucuronidation via uridine 5’-diphospho-glucuronosyl-transferase systems.74 In two studies where healthy adults were given baicalein chewable tablets at either a single dose of 100–2800 mg or multiple doses of 200–800 mg daily, researchers found no signs of liver or kidney toxicity and minimal mild side effects, indicating oral intake of baicalein was safe and well tolerated.75,76 Our data show that baicalein opposes P4 action on the luminal epithelium and it blocks the expression of PR target genes HAND2 and ZBTB16. RNA sequencing analysis indicates that baicalein regulates a subset of PR target genes in the opposite direction of P4. This study is the first to show baicalein can repress some actions of PR in the murine uterus.
EXPERIMENTAL SECTION
Animal Study and Chemicals
Ovariectomized CD1 mice (age 6–8 weeks from Envigo) were used for the animal studies. Baicalein (purity ≥ 95%) was purchased from Cayman (70610, Cayman Chemical, Ann Arbor, MI). In the first animal study, five mice were randomly assigned into each treatment group and received 10% DMSO, 1 mg/kg progesterone, or 25 mg/kg baicalein for 7 days through IP injection. RNA was extracted from the uteri of these mice for RNA sequencing analysis. In the second animal study, five mice were randomly assigned into each treatment group and received 10% DMSO, 1 mg/kg progesterone, 25 mg/kg baicalein, 1 mg/kg progesterone with 25 mg/kg baicalein, or 1 mg/kg progesterone with 10 mg/kg RU486 for 7 days through IP injection. After treatment, the animals were weighted and euthanized. The uterine tissue was collected and weighted. One uterine horn was snap-frozen in liquid nitrogen and stored at −80 °C for RNA extraction and the other uterine horn was fixed in 10 mL of 10% buffered formalin for 24 h, transferred into 70% EtOH and processed for histology using a Shandon 1000 Processor (Thermo) as described before.77 The processed tissue was embedded with paraffin into 5 mm thick blocks and then sectioned into 5 μm thick sections using a microtome. The slides were dried for at least 24 h before being processed for further analysis. All animal studies were approved by the UIC Institutional Animal Care and Use Committee (Protocol number 18–205).
Blood Glucose Level Analysis
Serum samples were collected for blood glucose measurement at the end of the animal study. Briefly, blood was drawn immediately after euthanasia from the posterior vena cava and cooled on ice for 30 min, then 15 min at room temperature (rt) to clot. The samples were centrifuged at 1000 x g for 10 min to remove the clot, and the supernatant liquid component (serum) was submitted to the Diagnostic Laboratory of the Biologic Resources Laboratory at UIC for measurement. Five samples per group were measured.
Immunohistochemical Staining
Immunohistochemistry (IHC) was performed for PCNA, HAND2, FKBP5 on uterine samples as previous described.78 Briefly, slides of uterine horns were deparaffinized using three xylenes washes and rehydrated though a series of decreasing concentrations of EtOH, then subjected to heat-induced antigen retrieval with sodium citrate buffer at 100 °C for 30 min and allowed to cool to rt. This was followed by inactivation of endogenous peroxidase activity with 0.3% H2O2/MtOH for 15 min in dark. The samples were then rinsed with phosphate buffered saline with Tween-20 (PBST) and incubated in blocking solution consisting of 5% horse serum (Vectastain ABC kit, Vector Laboratories, Inc.) diluted in 1%BSA/PBST at RT for 60 min. The tissue sections were incubated with following primary antibodies overnight at 4 °C: PCNA (1:200, 13110 Cell Signaling), FKBP5 (1:200, 14155–1 Protein Tech), PR (1:200, AB101688 Abcam), GR (1:100, 12041 Cell Signaling), HAND2 (1:200, ab200040 Abcam) and ZBTB16 (1:200, PA5–112862 Invitrogen). Next day, slides were rinsed with PBST prior to incubation with anti-goat biotinylated secondary antibody (Vectastain ABC kit, Vector Laboratories, Inc.) at 1:200 dilution in PBST for 60 min at rt. Slides were then rinsed and incubated in ABC solution (PBS: A: B=50:1:1) (Vectastain ABC kit, Vector Laboratories, Inc.) for 30 min at rt. For visualization of the immunoreactivity, all slides were subjected to chromogen 3’3-diaminobenzidine (DAB) (Vector Laboratories, Inc. Burlingame, CA) for 30 seconds. Slides were rinsed in tap water for 10 min to stop the DAB reaction. Thereafter, the slides were counterstained with hematoxylin for 1 min followed by dehydration and cover-slipping. After drying for 24 h, the slides were cleaned and imaged using Nikon E600 Eclipse microscope with CMOS C-Mount microscope camera.
RNA Isolation and RNA Sequencing Profiling
Uteri of mice treated with 10% DMSO, 1 mg/kg progesterone, or 25 mg/kg baicalein for 7 days in the first animal study were subjected to RNA isolation and RNA sequencing. RNA sequencing of uterine tissue was profiled using n=4 per treatment group. Total RNA was extracted from uterine tissues of mice using the Qiagen RNeasy Mini kit (Qiagen #74104) according to the manufacturer’s instructions. The concentration of mRNA was determined by a nanodrop. RNA libraries (three technical replicates/treatment) were created. RNA quality determination, mRNA enrichment, library construction, sequencing, and transcriptome statistical analysis were performed at the Genomics Core Facility at Northwestern University. Samples with RINs of 7 or greater were prepared with TruSeq mRNA-Seq Library Prep (Illumina) with 1 ug of RNA and 12 cycles of PCR amplification. The libraries were barcoded, pooled and sequenced on the HiSeq Sequencing 50 followed by statistical analysis.
Statistical and Bioinformatics Analysis
For RNA-Seq data, gene set enrichment of differentially expressed genes was performed using DAVID Webservice and GSEA. Gene sets with an FDR adjusted p-value <0.01 were considered significant. All data were analyzed utilizing GraphPad Prism software 8 (GraphPad Prism). Data are presented as means ± standard error of the means (SEM). Unpaired student’s t test and one-way ANOVA were performed. Tuckey’s test was used for multiple comparison. A statistical significance was assigned at p≤0.05.
Supplementary Material
Table 2.
Genes Downregulated in P4 but Upregulated in Baicalein Treatment
| Gene Symbol | Entrez ID | Description | Fold Change | p Value | FDR Adj p Value |
|---|---|---|---|---|---|
| Bcl2l15 | 229672 | BCLl2-like 15 | 1.10 | 2.36E-04 | 3.22E-02 |
| Nsg2 | 18197 | neuron specific gene family member 2 | 0.88 | 1.76E-04 | 2.84E-02 |
| Fgf16 | 80903 | fibroblast growth factor 16 | 0.88 | 5.18E-04 | 4.85E-02 |
| Tjp3 | 27375 | tight junction protein 3 | 0.87 | 5.06E-05 | 1.30E-02 |
| Mal2 | 105853 | mal, T cell differentiation protein 2 | 0.83 | 1.46E-05 | 5.74E-03 |
| Lamb3 | 16780 | laminin, beta 3 | 0.79 | 3.80E-04 | 4.08E-02 |
| Zfp7 | 223669 | zinc finger protein 7 | 0.74 | 3.84E-04 | 4.11E-02 |
| Bnipl | 171388 | BCL2/adenovirus E1B 19kD interacting protein like | 0.74 | 2.45E-04 | 3.28E-02 |
| Fxyd3 | 17178 | FXYD domain-containing ion transport regulator 3 | 0.73 | 4.52E-04 | 4.57E-02 |
| Rab11fip4 | 268451 | RAB11 family interacting protein 4 (class II) | 0.72 | 4.86E-05 | 1.30E-02 |
| Krt80 | 74127 | keratin 80 | 0.71 | 1.20E-04 | 2.56E-02 |
| Ctsh | 13036 | cathepsin H | 0.64 | 4.97E-05 | 1.30E-02 |
| Tacstd2 | 56753 | tumor-associated calcium signal transducer 2 | 0.63 | 2.97E-04 | 3.65E-02 |
| Spint1 | 20732 | serine protease inhibitor, Kunitz type 1 | 0.61 | 3.52E-04 | 3.95E-02 |
| Ngef | 53972 | neuronal guanine nucleotide exchange factor | 0.60 | 1.54E-04 | 2.74E-02 |
| 2010300C02Rik | 72097 | RIKEN cDNA 2010300C02 gene | 0.53 | 4.65E-04 | 4.57E-02 |
| Tpd52 | 21985 | tumor protein D52 | 0.50 | 1.32E-04 | 2.67E-02 |
| Cdkl2 | 53886 | cyclin-dependent kinase-like 2 (CDC2-related kinase) | 0.46 | 5.32E-04 | 4.93E-02 |
ACKNOWLEDGMENTS
We would like to thank Northwestern University Sequencing Core for the assistance with RNAseq experiments and analysis. We also thank UIC Biologic Resources Laboratory for testing glucose levels.
Funding Sources
This study was supported by RO1 AT008824 to JEB and BTM and fellowship T32 AT007533 to JRA from the National Center for Complementary and Integrative Health (NCCIH).
Footnotes
ASSOCIATED CONTENT
Full RNAseq data and GSEA results are found in the Supporting Information. This material is available free of charge on the internet at http://pubs.acs.org.
The authors declare no conflicts of interest.
REFERENCES
- 1.Scarpin K. Progesterone action in human tissues: regulation by progesterone receptor (PR) isoform expression, nuclear positioning and coregulator expression. Nucl Recept Signal 2009;7:1–13. doi: 10.1621/nrs.07009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng Z, Wen H, Ju X, et al. Hormone receptor expression profiles differ between primary and recurrent high-grade serous ovarian cancers. Oncotarget 2017;8(20):32848–32855. doi: 10.18632/oncotarget.15858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laudet V, Gronemeyer H, Laudet V, Gronemeyer H. Pr. Nucl Recept Factsb Published online 2002:375–390. doi: 10.1016/B978-012437735-6/50034-5 [DOI] [Google Scholar]
- 4.H Wang SD. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 2006;7:185–199. [DOI] [PubMed] [Google Scholar]
- 5.Diep CH, Daniel AR, Mauro LJ, Knutson TP, Lange CA. Progesterone action in breast, uterine, and ovarian cancers. J Mol Endocrinol 2015;54(2):R31–R53. doi: 10.1530/JME-14-0252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo H, Li S, Zhao M, Sheng B, Zhu H, Zhu X. Prognostic value of progesterone receptor expression in ovarian cancer: a meta-analysis. Oncotarget 2017;8(22):36845–36856. doi: 10.18632/oncotarget.15982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Zhao D, Gong C, et al. Prognostic role of hormone receptors in endometrial cancer: A systematic review and meta-analysis. World J Surg Oncol 2015;13(1):1–12. doi: 10.1186/s12957-015-0619-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll JS, Hickey TE, Tarulli GA, Williams M, Tilley WD. Deciphering the divergent roles of progestogens in breast cancer. Nat Rev Cancer 2017;17(1):54–64. doi: 10.1038/nrc.2016.116 [DOI] [PubMed] [Google Scholar]
- 9.Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev 2013;34(1):130–162. doi: 10.1210/er.2012-1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reis FM, Coutinho LM, Vannuccini S, Batteux F, Chapron C, Petraglia F. Progesterone receptor ligands for the treatment of endometriosis: the mechanisms behind therapeutic success and failure. Hum Reprod Update 2020;26(4):565–585. doi: 10.1093/HUMUPD/DMAA009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Islam MS, Afrin S, Jones SI, Segars J. Selective Progesterone Receptor Modulators—Mechanisms and Therapeutic Utility. Endocr Rev 2020;41(5):643–694. doi: 10.1210/ENDREV/BNAA012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Africander DJ, Storbeck KH, Hapgood JP. A comparative study of the androgenic properties of progesterone and the progestins, medroxyprogesterone acetate (MPA) and norethisterone acetate (NET-A). J Steroid Biochem Mol Biol 2014;143:404–415. doi: 10.1016/j.jsbmb.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 13.Louw-du Toit R, Perkins MS, Hapgood JP, Africander D. Comparing the androgenic and estrogenic properties of progestins used in contraception and hormone therapy. Biochem Biophys Res Commun 2017;491(1):140–146. doi: 10.1016/j.bbrc.2017.07.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Herbal Supplement Sales Increase by 8.6% in 2019,. Accessed July 1, 2021. https://www.globenewswire.com/en/news-release/2020/08/31/2086400/0/en/US-Herbal-Supplement-Sales-Increase-by-8-6-in-2019-Record-Breaking-Sales-Predicted-for-2020.html
- 15.Global Herbal Supplements Industry (2020 to 2027) - Market Accessed July 1, 2021. https://www.globenewswire.com/en/news-release/2021/05/06/2224598/28124/en/Global-Herbal-Supplements-Industry-2020-to-2027-Market-Trajectory-Analytics.html
- 16.Dennehy CE. The Use of Herbs and Dietary Supplements in Gynecology:An Evidence-Based Review. J Midwifery Women’s Heal 2006;51(6):402–409. doi: 10.1016/j.jmwh.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 17.Dietz BM, Hajirahimkhan A, Dunlap TL, Bolton JL. Botanicals and their bioactive phytochemicals for women’s health. Pharmacol Rev 2016;68(4):1026–1073. doi: 10.1124/pr.115.010843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Front Neuroendocrinol 2010;31(4):400–419. doi: 10.1016/j.yfrne.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dean M, Murphy BT, Burdette JE. Phytosteroids beyond estrogens: Regulators of reproductive and endocrine function in natural products. Mol Cell Endocrinol 2017;442:98–105. doi: 10.1016/j.mce.2016.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dean M, Austin J, Jinhong R, Johnson ME, Lantvit DD, Burdette JE. The Flavonoid Apigenin Is a Progesterone Receptor Modulator with In Vivo Activity in the Uterus. Horm Cancer 2018;9(4):265–277. doi: 10.1007/s12672-018-0333-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fern Toh M, Mendonca E, Eddie SL, et al. Kaempferol Exhibits Progestogenic Effects in Ovariectomized Rats Published online 2014. doi: 10.4172/2157-7536.1000136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Q, Chen XY, Martin C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci Bull 2016;61(18):1391–1398. doi: 10.1007/s11434-016-1136-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dinda B, Dinda S, DasSharma S, Banik R, Chakraborty A, Dinda M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur J Med Chem 2017;131:68–80. doi: 10.1016/j.ejmech.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 24.Wang CZ, Li XL, Wang QF, Mehendale SR, Yuan CS. Selective fraction of Scutellaria baicalensis and its chemopreventive effects on MCF-7 human breast cancer cells. Phytomedicine 2010;17(1):63–68. doi: 10.1016/j.phymed.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee W, Ku SK, Bae JS. Anti-inflammatory Effects of Baicalin, Baicalein, and Wogonin In Vitro and In Vivo. Inflammation 2015;38(1):110–125. doi: 10.1007/s10753-014-0013-0 [DOI] [PubMed] [Google Scholar]
- 26.Park HJ, Park SH, Choi YH, Chi GY. The root extract of scutellaria baicalensis induces apoptosis in egfr tki-resistant human lung cancer cells by inactivation of stat3. Int J Mol Sci 2021;22(10). doi: 10.3390/ijms22105181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Qiu S, Qin J. Baicalein Induced Apoptosis and Autophagy of Undifferentiated Thyroid Cancer Cells by the ERK/PI3K/Akt Pathway Vol 11. e-Century Publishing Corporation; 2019. Accessed July 1, 2021. www.ajtr.org [PMC free article] [PubMed] [Google Scholar]
- 28.Yan W, Ma X, Zhao X, Zhang S. Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des Devel Ther 2018;12:3961–3972. doi: 10.2147/DDDT.S181939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Ling Y, Chen Y, et al. Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett 2010;297(1):42–48. doi: 10.1016/j.canlet.2010.04.022 [DOI] [PubMed] [Google Scholar]
- 30.Austin JR, Kirkpatrick BJ, Rodríguez RR, Johnson ME, Lantvit DD, Burdette JE. Baicalein Is a Phytohormone that Signals Through the Progesterone and Glucocorticoid Receptors. Horm Cancer 2020;11(2):97–110. doi: 10.1007/s12672-020-00382-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin JR, Kirkpatrick BJ, Rodríguez RR, Johnson ME, Lantvit DD, Burdette JE. Baicalein Is a Phytohormone that Signals Through the Progesterone and Glucocorticoid Receptors. Horm Cancer 2020;11(2):97–110. doi: 10.1007/s12672-020-00382-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooke PS, Buchanan DL, Young P, et al. Stromal Estrogen Receptors Mediate Mitogenic Effects of Estradiol on Uterine Epithelium Vol 94.; 1997. Accessed August 1, 2019. https://www.pnas.org/content/pnas/94/12/6535.full.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooke PS, Buchanan DL, Lubahn DB, Cunha GR. Mechanism of Estrogen Action: Lessons from the Estrogen Receptor-α Knockout Mouse1. Biol Reprod 1998;59(3):470–475. doi: 10.1095/biolreprod59.3.470 [DOI] [PubMed] [Google Scholar]
- 34.Chung D, Das SK. Mouse Primary Uterine Cell Coculture System Revisited: Ovarian Hormones Mimic the Aspects of in Vivo Uterine Cell Proliferation Published online 2011. doi: 10.1210/en.2011-0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wada-Hiraike O, Hiraike H, Okinaga H, et al. Role of Estrogen Receptor in Uterine Stroma and Epithelium: Insights from Estrogen Receptor / Mice; 2006. Accessed August 1, 2019. www.pnas.orgcgidoi10.1073pnas.0608861103 [DOI] [PMC free article] [PubMed]
- 36.Chung D, Gao F, Jegga AG, Das SK. Estrogen mediated epithelial proliferation in the uterus is directed by stromal Fgf10 and Bmp8a. Mol Cell Endocrinol 2015;400:48–60. doi: 10.1016/J.MCE.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC. The Antiproliferative Action of Progesterone in Uterine Epithelium Is Mediated by Hand2. Science (80- ) 2011;331(February):912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia X, Xia J, Yang H, et al. Baicalein blocked cervical carcinoma cell proliferation by targeting CCND1 via Wnt/β-catenin signaling pathway. Artif Cells, Nanomedicine Biotechnol 2019;47(1):2729–2736. doi: 10.1080/21691401.2019.1636055 [DOI] [PubMed] [Google Scholar]
- 39.Y P, C G, Y Y, et al. Baicalein induces apoptosis of human cervical cancer HeLa cells in vitro. Mol Med Rep 2015;11(3):2129–2134. doi: 10.3892/MMR.2014.2885 [DOI] [PubMed] [Google Scholar]
- 40.Murdoch M, Roberts M. Selective progesterone receptor modulators and their use within gynaecology. Obstet Gynaecol 2014;16(1):46–50. doi: 10.1111/tog.12072 [DOI] [Google Scholar]
- 41.Liang B, Wu L, Xu H, et al. Efficacy, safety and recurrence of new progestins and selective progesterone receptor modulator for the treatment of endometriosis: a comparison study in mice doi: 10.1186/s12958-018-0347-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chegini N. Proinflammatory and profibrotic mediators: Principal effectors of leiomyoma development as a fibrotic disorder. Semin Reprod Med 2010;28(3):180–203. doi: 10.1055/s-0030-1251476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Augoulea A, Alexandrou A, Creatsa M, Vrachnis N, Lambrinoudaki I. Pathogenesis of endometriosis: The role of genetics, inflammation and oxidative stress. Arch Gynecol Obstet 2012;286(1):99–103. doi: 10.1007/s00404-012-2357-8 [DOI] [PubMed] [Google Scholar]
- 44.Wu X, Howard MJ. Transcripts encoding hand genes are differentially expressed and regulated by BMP4 and GDNF in developing avian gut. Gene Expr 2002;10(5–6):279–293. doi: 10.3727/000000002783992361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Q, Kannan A, DeMayo FJ, et al. The antiproliferative action of progesterone in uterine epithelium is mediated by hand2. Science (80- ) 2011;331(6019):912–916. doi: 10.1126/science.1197454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Šeda O, Šedová L, Vcelák J, Vanková M, Liška F, Bendlová B. ZBTB16 and metabolic syndrome: A network perspective. Physiol Res 2017;66(3):S357–S365. doi: 10.33549/physiolres.933730 [DOI] [PubMed] [Google Scholar]
- 47.Fahnenstich J, Nandy A, Milde-Langosch K, Schneider-Merck T, Walther N, Gellersen B. Promyelocytic leukaemia zinc finger protein (PLZF) is a glucocorticoid- and progesterone-induced transcription factor in human endometrial stromal cells and myometrial smooth muscle cells. Mol Hum Reprod 2003;9(10):611–623. doi: 10.1093/molehr/gag080 [DOI] [PubMed] [Google Scholar]
- 48.Kommagani R, Szwarc MM, Vasquez YM, et al. The Promyelocytic Leukemia Zinc Finger Transcription Factor Is Critical for Human Endometrial Stromal Cell Decidualization. PLoS Genet 2016;12(4). doi: 10.1371/journal.pgen.1005937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiu M, Bao W, Wang J, et al. FOXA1 promotes tumor cell proliferation through AR involving the Notch pathway in endometrial cancer. BMC Cancer 2014;14(1). doi: 10.1186/1471-2407-14-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He J, Wu M, Xiong L, et al. BTB/POZ zinc finger protein ZBTB16 inhibits breast cancer proliferation and metastasis through upregulating ZBTB28 and antagonizing BCL6/ZBTB27. Clin Epigenetics 2020;12(1):82. doi: 10.1186/s13148-020-00867-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wasim M, Carlet M, Mansha M, et al. PLZF/ZBTB16, a glucocorticoid response gene in acute lymphoblastic leukemia, interferes with glucocorticoid-induced apoptosis. J Steroid Biochem Mol Biol 2010;120(4–5):218–227. doi: 10.1016/j.jsbmb.2010.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weikum ER, Knuesel MT, Ortlund EA, Yamamoto KR. Glucocorticoid receptor control of transcription: Precision and plasticity via allostery. Nat Rev Mol Cell Biol 2017;18(3):159–174. doi: 10.1038/nrm.2016.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whirledge SD, Kisanga EP, Oakley RH, Cidlowski JA. Neonatal genistein exposure and glucocorticoid signaling in the adult mouse uterus. Environ Health Perspect 2018;126(4):047002–1-047002–047017. doi: 10.1289/EHP1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vahrenkamp JM, Yang CH, Rodriguez AC, et al. Clinical and Genomic Crosstalk between Glucocorticoid Receptor and Estrogen Receptor α In Endometrial Cancer. Cell Rep 2018;22(11):2995–3005. doi: 10.1016/j.celrep.2018.02.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JH, Ulrich B, Cho J, Park J, Kim CH. Progesterone Promotes Differentiation of Human Cord Blood Fetal T Cells into T Regulatory Cells but Suppresses Their Differentiation into Th17 Cells. J Immunol 2011;187(4):1778–1787. doi: 10.4049/jimmunol.1003919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dinh DT, Breen J, Akison LK, et al. Tissue-specific progesterone receptor-chromatin binding and the regulation of progesterone-dependent gene expression. Sci Rep 2019;9(1):1–14. doi: 10.1038/s41598-019-48333-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li F, Miao X, Chen Y, Curry TE. CXADR-like membrane protein (CLMP) in the rat ovary: Stimulation by human chorionic gonadotrophin during the periovulatory period. Reprod Fertil Dev 2016;28(6):742–749. doi: 10.1071/RD14201 [DOI] [PubMed] [Google Scholar]
- 58.Pan H, Deng Y, Pollard JW. Progesterone blocks estrogen-induced DNA synthesis through the inhibition of replication licensing. Proc Natl Acad Sci U S A 2006;103(38):14021–14026. doi: 10.1073/pnas.0601271103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pabona JMP, Simmen FA, Nikiforov MA, et al. Krüppel-like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: Implications for the pathogenesis of endometriosis. J Clin Endocrinol Metab 2012;97(3):E376. doi: 10.1210/jc.2011-2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banerjee D, Martin N, Nandi S, et al. A genome-wide steroid response study of the major human fungal pathogen Candida albicans. Mycopathologia 2007;164(1):1–17. doi: 10.1007/s11046-007-9025-8 [DOI] [PubMed] [Google Scholar]
- 61.Bhurke AS, Bagchi IC, Bagchi MK. Progesterone-Regulated Endometrial Factors Controlling Implantation. Am J Reprod Immunol 2016;75(3):237–245. doi: 10.1111/aji.12473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mazur EC, Vasquez YM, Li X, et al. Progesterone receptor transcriptome and cistrome in decidualized human endometrial stromal cells. Endocrinology 2015;156(6):2239–2253. doi: 10.1210/en.2014-1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burkin HR, Rice M, Sarathy A, Thompson S, Singer CA, Buxton ILO. Integrin upregulation and localization to focal adhesion sites in pregnant human myometrium. Reprod Sci 2013;20(7):804–812. doi: 10.1177/1933719112466303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kommagani R, Szwarc MM, Kovanci E, et al. A Murine uterine transcriptome, responsive to steroid receptor coactivator-2, reveals transcription factor 23 as essential for decidualization of human endometrial stromal cells. Biol Reprod 2014;90(4). doi: 10.1095/biolreprod.114.117531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cirilo PDR, Marchi FA, Barros Filho M de C, et al. An Integrative Genomic and Transcriptomic Analysis Reveals Potential Targets Associated with Cell Proliferation in Uterine Leiomyomas. Hoheisel JD, ed. PLoS One 2013;8(3):e57901. doi: 10.1371/journal.pone.0057901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhandari A, Shen Y, Sindan N, et al. MAL2 promotes proliferation, migration, and invasion through regulating epithelial-mesenchymal transition in breast cancer cell lines. Biochem Biophys Res Commun 2018;504(2):434–439. doi: 10.1016/j.bbrc.2018.08.187 [DOI] [PubMed] [Google Scholar]
- 67.Cordeaux Y, Tattersall M, Stephen Charnock-Jones D, Smith GCS. Effects of medroxyprogesterone acetate on gene expression in myometrial explants from pregnant women. J Clin Endocrinol Metab 2010;95(12). doi: 10.1210/jc.2010-1541 [DOI] [PubMed] [Google Scholar]
- 68.Xu J, Qian Y, Ye M, et al. Distinct expression profile of lncRNA in endometrial carcinoma. Oncol Rep 2016;36(6):3405–3412. doi: 10.3892/or.2016.5173 [DOI] [PubMed] [Google Scholar]
- 69.K E, S Y, S P, et al. Hyaluronic Acid-Functionalized Nanomicelles Enhance SAHA Efficacy in 3D Endometrial Cancer Models. Cancers (Basel) 2021;13(16). doi: 10.3390/CANCERS13164032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao H, Zhang Z, Peng D, Wei C, Ma B. Type II transmembrane serine proteases 4 (TMPRSS4) promotes proliferation, invasion and epithelial–mesenchymal transition in endometrial carcinoma cells (HEC1A and Ishikawa) via activation of MAPK and AKT. Animal Cells Syst (Seoul) 2021;25(4):211. doi: 10.1080/19768354.2021.1944311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma XC, Yan W, Dai Z, et al. Baicalein suppresses metastasis of breast cancer cells by inhibiting EMT via downregulation of SATB1 and Wnt/β-catenin pathway. Drug Des Devel Ther 2016;10:1419–1441. doi: 10.2147/DDDT.S102541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.C L, Y Y, F D, W L, X Y, L D. Inhibition of NCAPG expression inactivates the Wnt/β-catenin signal to suppresses endometrial cancer cell growth in vitro. Environ Toxicol Published online September 3, 2021:tox.23364. doi: 10.1002/TOX.23364 [DOI] [PubMed] [Google Scholar]
- 73.M ES, SK S, S A, MS I, MA B. Wnt/β-catenin signaling pathway in uterine leiomyoma: role in tumor biology and targeting opportunities. Mol Cell Biochem 2021;476(9):3513–3536. doi: 10.1007/S11010-021-04174-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang L, Li C, Lin G, Krajcsi P, Zuo Z. Hepatic Metabolism and Disposition of Baicalein via the Coupling of Conjugation Enzymes and Transporters—In Vitro and In Vivo Evidences. AAPS J 2011 133 2011;13(3):378–389. doi: 10.1208/S12248-011-9277-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pang H, Xue W, Shi A, et al. Multiple-Ascending-Dose Pharmacokinetics and Safety Evaluation of Baicalein Chewable Tablets in Healthy Chinese Volunteers. Clin Drug Investig 2016 369 2016;36(9):713–724. doi: 10.1007/S40261-016-0418-7 [DOI] [PubMed] [Google Scholar]
- 76.Li M, Shi A, Pang H, et al. Safety, tolerability, and pharmacokinetics of a single ascending dose of baicalein chewable tablets in healthy subjects. J Ethnopharmacol 2014;156:210–215. doi: 10.1016/j.jep.2014.08.031 [DOI] [PubMed] [Google Scholar]
- 77.Li K, Liszka M, Zhou C, Brehm E, Flaws JA, Nowak RA. Prenatal exposure to a phthalate mixture leads to multigenerational and transgenerational effects on uterine morphology and function in mice. Reprod Toxicol 2020;93:178–190. doi: 10.1016/j.reprotox.2020.02.012 [DOI] [PubMed] [Google Scholar]
- 78.Salem W, Li K, Krapp C, et al. Imatinib treatments have long-term impact on placentation and embryo survival. Sci Rep 2019;9(2535). doi: 10.1038/s41598-019-39134-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


