Abstract
The potential of a three-way randomly amplified polymorphic DNA (RAPD) procedure (RAPD typing) for typing Salmonella enterica strains assigned to 12 serotypes was analyzed. The series of organisms used included 235 strains (326 isolates) collected mainly from clinical samples in the Principality of Asturias and 9 reference strains. RAPD typing was performed directly with broth cultures of bacteria by using three selected primers and optimized PCR conditions. The profiles obtained with the three primers were used to define RAPD types and to evaluate the procedure as a typing method at the species and serotype levels. The typeability was 100%; the reproducibility and in vitro stability could be considered good. The concordance of RAPD typing methods with serotyping methods was 100%, but some profiles obtained with two of the three primers were obtained with strains assigned to different serotypes. The discrimination index (DI) within the series of organisms was 0.94, and the DI within serotypes Typhimurium, Enteritidis, and Virchow were 0.72, 0.52, and 0.66, respectively. Within these serotypes the most common RAPD types were differentiated into phage types and vice versa; combining the types identified by the two procedures (RAPD typing and phage typing) resulted in further discrimination (DI, 0.96, 0.74, and 0.87, respectively). The efficiency, rapidity, and flexibility of the RAPD typing method support the conclusion that it can be used as a tool for identifying Salmonella organisms and as a typing method that is complementary to serotyping and phage typing methods.
Typing methods are useful tools for performing epidemiological surveys of pathogenic bacteria. They are used for the following two main purposes: to discriminate between epidemiologically unrelated isolates belonging to the same microbial species or taxon based on phenotypic or genotypic characteristics or traits called epidemiological markers and to recognize a close relationship among isolates derived from the same outbreak or chain of transmission, reflecting the fact that the isolates are recent derivatives of a simple ancestor cell (14, 17). The usefulness of a trait for typing is related to its stability in a given strain and its diversity in the strains forming one species. The organisms of the genospecies Salmonella enterica (Salmonella choleraesuis) are grouped into more than 2,300 serotypes or serovars as determined by the Kauffmann-White serological scheme (7). However, serotyping is normally inadequate as a single typing method for epidemiological purposes for the following two main reasons: (i) typological cataloging of surface exposed antigens can provide little information concerning the overall genetic relationships of strains belonging to different serovars (9, 10, 13, 15); and (ii) most human salmonellosis episodes and outbreaks, as well as livestock outbreaks, are caused by a few serotypes. Over the last few years several genetic typing methods for Salmonella spp. have been evaluated, and these methods appear to be useful tools for epidemiological and phylogenetic purposes. A rapid method that is used universally, randomly amplified polymorphic DNA (RAPD) segment analysis (21, 22) performed with different primers, has been proposed as a tool for characterizing organisms belonging to some Salmonella serovars (3, 5, 8, 10, 16).
In this paper we describe the results of a RAPD analysis performed directly with aliquots of water-diluted overnight cultures of bacteria; in this analysis we used three selected primers, optimized PCR conditions, and a large series of organisms, including both epidemiologically related and unrelated isolates assigned to 12 serotypes and collected in the Principality of Asturias (Spain).
MATERIALS AND METHODS
Bacterial strains.
This study was performed with 326 isolates that were grouped into 235 epidemiologically unrelated Salmonella strains and were isolated in the Principality of Asturias from 1984 to 1998. A total of 202 isolates (199 strains) were associated with sporadic human salmonellosis episodes that occurred at different times and/or in different hospitals, and another 108 isolates (21 strains) were associated with 21 outbreaks; 4 strains were isolated from foods, and 11 strains were isolated from water samples not associated with outbreaks. The isolates belonged to three serogroups. The serogroup B isolates included 73 serotype Typhimurium isolates (in 59 strains), 6 serotype Bredeney strains, 5 serotype Brandenburg strains, and 6 serotype Derby strains. The serogroup C isolates included 7 serotype Infantis strains, 18 serotype Hadar strains, 57 serotype Virchow isolates (in 20 strains), 20 serotype Ohio isolates (in 16 strains), 4 serotype Muenchen strains, and 3 serotype Newport strains. The serogroup D isolates included 83 serotype Enteritidis isolates (in 54 strains) and 44 serotype Panama isolates (in 37 strains). Serotyping of organisms other than members of serotypes Enteritidis and Typhimurium, as well as phage typing of serotype Enteritidis, Typhimurium, and Virchow organisms (by using the methods described by Ward et al. [20], Anderson et al. [1], and Chambers et al. [2], respectively) were carried out by workers at the Centro Nacional de Microbiología, Majadahonda, Madrid, Spain. In addition, nine strains obtained from different collections representing the three serogroups and their most common serotypes and three outgroup strains (Escherichia coli ATCC O111-B4, Yersinia enterocolitica ATCC 27729, and Lactobacillus plantarum ATCC 1497) were analyzed and used as reference strains.
RAPD fingerprinting.
The assays were performed with the following three types of DNA templates: (i) 100 ng of DNA which was isolated with a Nucleon BACC-3 for blood and cell culture kit (Amersham Pharmacia Biotech), (ii) 15 μl of a 10-fold distilled water dilution of a Luria-Bertani broth overnight culture, and (iii) 15 μl from a single colony grown on nutrient agar (the colony was picked and resuspended in 150 μl of distilled water; the suspension was boiled for 5 min; and the supernatant was collected after centrifugation for 2 min). RAPD reactions were carried out with the following three primers: primer S (5′-TCACGATGCA-3′), which was described by Williams et al. (22), and primers OPB-6 (5′-TGCTCTGCCC-3′) and OPB-17 (5′-AGGGAACGAG-3′), which were described by Lin et al. (10). In this study the latter two primers were designated primers B and C, respectively. The conditions that were selected as the optimal conditions for obtaining accurate amplified band profiles with the three primers were as follows. Assays were performed in 50-μl reaction mixtures containing an amplification buffer (10 mM Tris-HCl [pH 8.8], 1.5 mM MgCl2, 50 mM KCl, 0.1% Triton X-100), each deoxynucleoside triphosphate (Roche Diagnostics, Barcelona, Spain) at a concentration of 200 μM, 0.9 μM primer (Amersham Pharmacia Biotech), 2 U of DyNAzyme II DNA polymerase (Finnzymes OY, Espoo, Finland), and 100 ng of template DNA or 15 μl of a bacterial dilution in water. The temperature cycling program used with a Perkin-Elmer Gene Amp PCR system (models 2400 and 9600) was as follows: 2 initial cycles consisting of 94°C for 4 min, 35°C for 2 min, and 72°C for 2 min, followed by 35 cycles consisting of 94°C for 30 s, 35°C for 1 min, and 72°C for 2 min and a final extension step consisting of 72°C for 5 min. The reaction products were analyzed by electrophoresis on 1.5% agarose gels, stained with ethidium bromide, and photographed under UV light. Lambda DNA digested with PstI was used as the molecular weight marker. Each amplified band profile was defined by the presence or absence of bands at particular positions on the gel. Profiles were considered different when at least one polymorphic band was identified. The profiles were labelled with the letters assigned to the primers followed by Arabic numerals.
Reproducibility was examined by comparing the band profiles obtained in at least three RAPD analyses of representative strains that produced each band profile. The intermethod concordance (IMC) was defined as the maximum proportion (percentage) of strains that were grouped together into unique types by RAPD typing and by another method (17). The discrimination index (DI) (i.e., the probability that two unrelated strains obtained from the population would be placed in different typing groups) was calculated by using Simpson’s index of diversity (6).
PstI-SphI (PS) ribotyping was performed as described by Landeras and Mendoza (8). The method used consisted of double digestion of DNA with PstI and SphI, hybridization with an rrn operon, and use of a nonradioactive DNA labelling and detection kit (Roche Diagnostics).
RESULTS
Selection of primers and optimal PCR conditions.
In the first step, primers were selected (from a set of seven aleatory oligonucleotides) and PCR conditions were optimized in order to maximize the discriminatory power of RAPD analysis and to test the reproducibility, precision, and ease of interpretation of amplified band profiles. This analysis was performed by using purified DNA and a series of 30 Salmonella strains representing common serotypes (serotypes Enteritidis, Typhimurium, Hadar, and Virchow) and uncommon serotypes (serotypes Derby, Bredeney, and Muenchen), as well as 3 outgroup strains (E. coli ATCC O111-B4, Y. enterocolitica ATCC 27729, and L. plantarum ATCC 1497). Different concentrations of the DNA template primer and Mg2+, different numbers of cycles (29 to 35 cycles), and different annealing temperatures (35 to 40°C) were examined. The primers selected were primers S, B, and C, and they were selected for three reasons: (i) under the conditions described above they yielded the most accurate amplified band profiles; (ii) the band profiles obtained with each of the primers exhibited high levels of similarity with respect to the size and number of amplified bands but differed greatly from the band profiles obtained for the three non-Salmonella strains; and (iii) strains of different serotypes yielded different band profiles with the three primers (data not shown). The reproducibility of the technique when each primer was used was initially examined by comparing the fingerprint profiles obtained from three RAPD analyses of the 30 Salmonella strains and 3 non-Salmonella strains.
In order to simplify the procedure, DNA templates were obtained in two other ways: from the supernatant of a boiled colony and from a water-diluted overnight culture of bacteria. Under the conditions used, the band profiles generated by the two procedures were the same, were reproducible, and were the same as the results obtained with template DNA. Thus, for the subsequent steps, the last option was chosen.
RAPD analysis performed with selected primers.
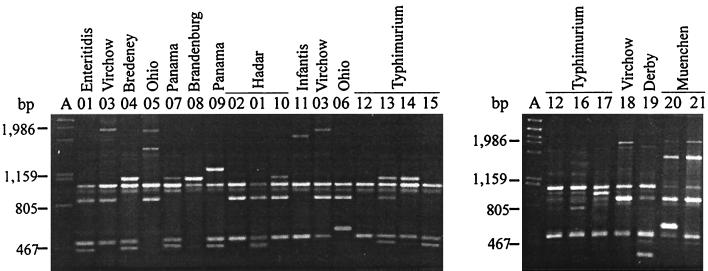
The Salmonella organisms which we studied (326 isolates and 9 reference strains) were analyzed by performing a RAPD analysis with the primers and PCR conditions described above. With primer S, 21 amplified DNA band profiles were differentiated; each of these profiles included two to five fragments which were 250 to 2,000 bp long. Two fragments, which were about 500 and 900 bp long, were found in 17 and 20 band profiles, respectively, and in 93.5 and 99.5% of the isolates, respectively (Fig. 1 and Table 1). Different amplified fragments appeared to be characteristic of different serotypes (a ca. 1,800-bp fragment for serotype Infantis, a 250-bp fragment for serotype Derby, and a 1,900-bp fragment for serotype Virchow). Five profiles (profiles S01, S07, S11, S12, and S15) were produced by strains belonging to different serotypes. The strains of serotypes Enteritidis, Infantis, and Bredeney produced single profiles (profiles S01, S11, and S04, respectively), while the serotype Typhimurium strains produced seven profiles, the serotype Panama and Hadar strains produced three profiles each, and the strains of serotypes Virchow, Ohio, Brandenburg, Derby, Muenchen, and Newport produced two profiles each.
FIG. 1.
Amplified band profiles generated with primer S for S. enterica. Lanes A, phage λ DNA digested with PstI; lanes 01 to 21, primer S profiles. Profile S12 (representing serotype Typhimurium strain ATCC 14028) (lanes 12) was included in both gels. The distribution of strains in serotypes and primer S profiles is shown in Table 1.
TABLE 1.
Differentiation of Salmonella serotypes into RAPD types
| Serogroup | Serotype | No. of strains | DI | RAPD profiles
|
Phage type(s)a | Strains
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Primer S | Primer B | Primer C | No. | Group(s)b | Reference strain(s)c | |||||
| B | Typhimurium | 62 | 0.715 | 12 | 03 | 11 | 5, 23, 28, 66, 80, 104, 110, 124, 133, 193, 195, 203, 204, NPT | 32d | 6 | ATCC 14028 |
| 12 | 104, 104b, NPT | 8 | ||||||||
| 14 | 104, 104b, NPT | 5 | ||||||||
| 16 | 96, NPT | 2 | ||||||||
| 10 | 193 | 1 | ||||||||
| 15 | 96 | 1d | 7 | |||||||
| 15 | 03 | 11 | 2, 193 | 3 | ||||||
| 19 | 193 | 1 | ||||||||
| 14 | 03 | 13 | NPT | 2 | BC 25268/75 | |||||
| 18 | 195 | 1 | ||||||||
| 17 | 03 | 12 | NPT | 1 | ||||||
| 14 | NPT | 1 | ||||||||
| 13 | 03 | 15 | 104 | 1 | LT2 | |||||
| 16 | 03 | 11 | NRP | 1 | ||||||
| 12 | 01 | 10 | 120 | 1 | ||||||
| 01 | 03 | 17 | 193 | 1 | ||||||
| B | Derby | 6 | 19 | 12 | 20 | 4 | ||||
| 11 | 13 | 21 | 2 | |||||||
| B | Bredeney | 6 | 04 | 09 | 22 | 3 | ||||
| 23 | 2 | |||||||||
| 10 | 22 | 1 | ||||||||
| B | Brandenburg | 5 | 07 | 05 | 24 | 1d | ||||
| 08 | 05 | 25 | 1 | |||||||
| 26 | 3 | |||||||||
| C | Virchow | 21 | 0.66 | 03 | 04 | 31 | 8, 34 | 11d | CECT 4454 | |
| 32 | 4a, 19, 31 | 5 | 3 | |||||||
| 33 | 16 | 1 | ||||||||
| 34 | 8 | 1 | ||||||||
| 35 | 17 | 1 | ||||||||
| 06 | 36 | 31 | 1 | |||||||
| 18 | 04 | 31 | 33 | 1 | ||||||
| C | Hadar | 18 | 0.78 | 02 | 03 | 29 | 7d | |||
| 01 | 29 | 5 | ||||||||
| 11 | 29 | 1 | ||||||||
| 10 | 03 | 29 | 3d | |||||||
| 01 | 29 | 1 | ||||||||
| 01 | 03 | 30 | 1 | |||||||
| C | Ohio | 16 | 0.13 | 05 | 01 | 37 | 15 | 2 | ||
| 06 | 01 | 37 | 1 | |||||||
| C | Infantis | 7 | 11 | 06 | 27 | 5 | ||||
| 28 | 2 | |||||||||
| C | Muenchen | 4 | 20 | 08 | 30 | 2 | ||||
| 21 | 08 | 30 | 1 | |||||||
| 38 | 1 | |||||||||
| C | Newport | 4 | 15 | 14 | 40 | 3 | ||||
| 01 | 04 | 39 | 1 | |||||||
| D | Enteritidis | 59 | 0.52 | 01 | 01 | 01 | 1, 4, 5a, 6, 6a, 7, NPT, NRP | 40d | 4, 5 | CPHL PT4, CPHL PT6a |
| 06 | 6a, NPT | 8d | 1 | |||||||
| 04 | NRP | 5d | ||||||||
| 03 | 11 | 1 | ||||||||
| 05 | NRP | 1 | ||||||||
| 02 | 02 | 1, 8, 13a | 3 | ATCC 13076, CPHL PT8, CPHL PT13a | ||||||
| 01 | NRP | 1 | ||||||||
| D | Panama | 37 | 0.60 | 07 | 08 | 08 | 19d | |||
| 12 | 08 | 08 | 14 | |||||||
| 02 | 09 | 3 | ||||||||
| 09 | 07 | 07 | 1 | |||||||
NPT, non-phage typeable; NRP, nonrecognized pattern.
Each group contains epidemiologically related isolates, which are described in the text.
ATCC, American Type Culture Collection; BC, Bayer AG Collection (PH-FZ, Wuppertal, Germany); CECT, Colección Española de Cultivos Tipo; CPHL, Central Public Health Laboratory (London, United Kingdom).
Strains obtained from food and/or water were included.
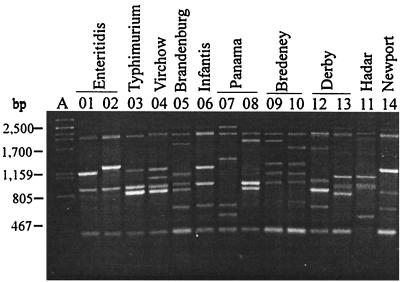
With primer B, 14 amplified DNA band profiles were differentiated; each of these profiles included four to seven fragments which were 400 to 2,500 bp long. Two fragments, which were about 400 and 2,000 bp long, were found in 13 profiles, and a third fragment, which was about 900 bp long, was present in 10 profiles and 94.72% of the isolates (Fig. 2 and Table 1). Six profiles (profiles B01, B02, B03, B04, B06, and B08) were produced by strains belonging to different serotypes. The serotype Hadar and Panama strains produced three profiles each; the serotype Enteritidis, Virchow, Typhimurium, Bredeney, Derby, and Newport strains produced two profiles each; and the strains of the resting serotypes each produced a single profile.
FIG. 2.
Amplified band profiles generated with primer B for S. enterica. Lane A, phage λ DNA digested with PstI; lanes 01 to 14, primer B profiles. The distribution of strains in serotypes and primer B profiles is shown in Table 1.
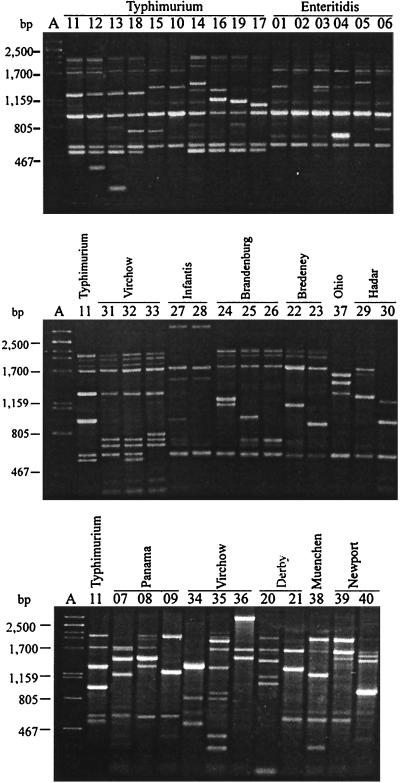
With primer C, 40 amplified DNA band profiles were obtained. Most of the profiles were defined by considering only bands that were 1,700 bp long or smaller. The only exceptions were profiles C27, C28, and C36, which contained a clear band at about 3,000 bp, which was also considered; for the rest of the profiles the bands that were located between 1,700 and 2,000 bp were not reproducible. Thus, the profiles defined included three to seven fragments which were 200 to 3,000 bp long. No fragment was found in all of the profiles, but two fragments, at about 510 and 1,700 bp, were found in 34 and 35 profiles, respectively, and in 90 and 96.6% of the isolates, respectively (Fig. 3). No profile was produced by strains belonging to different serotypes. Only serotype Infantis strains produced a fragment (at about 3,000 bp) that was characteristic of a serotype. The strains belonging to 11 serotypes (all of the serotypes except serotype Ohio) could be differentiated with this primer (Table 1).
FIG. 3.
Amplified band profiles generated with primer C for S. enterica. Lane A, phage λ DNA digested with PstI; lanes 01 to 40, primer C profiles. Profile C11 (representing serotype Typhimurium strain ATCC 14028) (lanes 11) was included in all three gels. The distribution of strains in serotypes and primer C profiles is shown in Table 1.
On the other hand, it is noteworthy that when a strain initially classified as a Salmonella strain produced a band profile without fragments found in the other profiles, it was suspected of not being a Salmonella strain and was reclassified. In all of these cases the strains were not Salmonella strains. Similarly, when a strain initially assigned to a specific serotype produced (with the three primers) band profiles characteristic of another serotype, it was reserotyped, confirming that it had been registered with an erroneous serotype.
Concordance of RAPD typing with serotyping and phage typing.
RAPD typing results were compared with serotyping results (see above) and, for serotypes Enteritidis, Typhimurium, and Virchow, with phage typing results. The IMC was 100% for RAPD typing and serotyping, but the IMC could be lower with other series of organisms because strains belonging to different serotypes sometimes produce the same profiles (Table 1). Phage typing differentiated the serotype Enteritidis strains (n = 42) into the following nine phage types: PT1 (6 strains); PT4 (14 strains); PT6a (10 strains); and PT5a, PT6, PT7, PT8, PT11, and PT13a (1 strain each). Four strains were non-phage typeable (NPT), and two other strains produced a nonrecognized lysis pattern (NRP). Serotype Typhimurium strains (n = 59) were differentiated into the following 15 phage types: DT104 (17 strains); DT193 (8 strains); DT195 (2 strains); DT80 (2 strains); DT96 (2 strains); DT23 (2 strains); and DT2, DT5, DT28, DT66, DT110, DT120, DT124, DT133, and DT204 (1 strain each). Seventeen other strains were NPT. Serotype Virchow strains (n = 17) were differentiated into the following eight phage types: PT4a (3 strains); PT8 (7 strains); PT31 (2 strains); and PT16, PT17, PT19, PT33, and PT34 (1 strain each). Within the three serotypes the IMC between RAPD typing and phage typing was low. In fact, within each serotype the most common RAPD profiles were differentiated into several phage types, and conversely, the most frequent phage types were differentiated into RAPD types, with the following two relevant exceptions: the 13 strains (26 isolates) belonging to serotype Enteritidis PT4 fell into RAPD type S01 B01 C01, and seven of the eight serotype Virchow PT8 strains fell into RAPD type S03 B04 C31 (the eighth strain differed only in the profile generated with the third primer, C34) (Table 2).
TABLE 2.
Subdivision of serotype Enteritidis, Typhimurium, and Virchow RAPD types into phage types and subdivision of phage types into RAPD types
| Serotype | No. of strains | DI | RAPD type-phage type analysis
|
Phage type-RAPD type analysis
|
||||
|---|---|---|---|---|---|---|---|---|
| RAPD type | Phage type | No. of strains | Phage type | RAPD type | No. of strains | |||
| Enteriditis | 42 | 0.74 | S01 B01 C01 | 1 | 5 | 1 | S01 B01 C01 | 5 |
| 4 | 14a | S01 B02 C02 | 1a | |||||
| 6a | 4a | 6a | S01 B01 C01 | 4a | ||||
| NPT | 2 | S01 B01 C06 | 6 | |||||
| Others | 4b | NPT | S01 B01 C01 | 2 | ||||
| S01 B01 C06 | 6a | 6 | S01 B01 C06 | 2 | ||||
| NPT | 2 | NRPc | S01 B01 C01 | 1 | ||||
| S01 B02 C02 | 1 | 1a | S01 B01 C04 | 1 | ||||
| 8 | 1a | Others | 20 | |||||
| 13a | 1a | |||||||
| Others | 2b | |||||||
| Virchow | 17 | 0.87 | S03 B04 C31 | 8 | 6 | 8 | S03 B04 C31 | 6 |
| 34 | 1 | S03 B04 C34 | 1 | |||||
| S03 B04 C32 | 4a | 3 | 31 | S03 B04 C32 | 1 | |||
| Others | 2b | S03 B06 C36 | 1 | |||||
| Others | 5 | Others | 8 | |||||
| Typhimurium | 59 | 0.96 | S12 B03 C11 | NPT | 7 | 96 | S12 B03 C15 | 1 |
| 23 | 2 | S12 B03 C16 | 1 | |||||
| 80 | 2 | 104 | S12 B03 C11 | 8 | ||||
| 104 | 8 | S12 B03 C12 | 6 | |||||
| 193 | 3 | S12 B03 C14 | 2 | |||||
| Others | 8b | S13 B03 C15 | 1 | |||||
| S12 B03 C12 | 104 | 6 | 193 | S12 B03 C11 | 3 | |||
| NPT | 2 | S15 B03 C11 | 2 | |||||
| S12 B03 C14 | 104 | 2 | Others | 3d | ||||
| NPT | 3 | 195 | S12 B03 C11 | 1 | ||||
| S12 B03 C16 | 96 | 1 | S14 B03 C18 | 1a | ||||
| NPT | 1 | NPT | S12 B03 C11 | 7 | ||||
| S16 B03 C11 | 2 | 1 | S12 B03 C12 | 2 | ||||
| 193 | 2 | S12 B03 C14 | 3 | |||||
| Others | 11 | S14 B03 C13 | 2 | |||||
| Others | 3d | |||||||
| Others | 13 | |||||||
Reference strains were included.
One strain of each phage type.
NRP, nonrecognized lysis pattern; NPT, non-phage typeable.
One strain of each RAPD type.
Epidemiological concordance, in vivo stability, and correlation of RAPD typing with PS ribotyping.
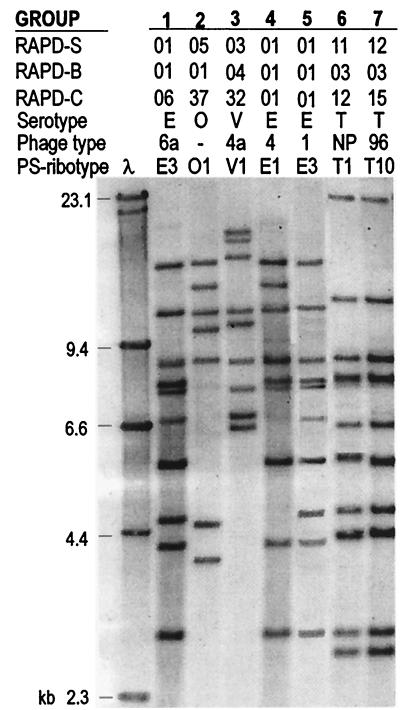
Three other criteria for RAPD typing (epidemiological concordance, in vivo stability, and correlation with another genetic typing system [PS ribotyping]) were examined by comparing results obtained with seven groups of epidemiologically related isolates. Each group included sequential isolates recovered from a single patient or isolates collected from different patients and foods associated with previously diagnosed outbreaks in our laboratories. The PS ribotypes and their relationships with the other markers are shown in Fig. 4, and the following data are noteworthy. In group 1, three serotype Enteritidis isolates collected from one urine sample and two feces samples from a single female fell into RAPD profile S01 B01 C06, ribotype E-PS3, and phage type PT6a. In group 2, four serotype Ohio isolates collected over a 3-month period from the feces of a single child fell into RAPD profile S05 B01 C37 and ribotype O-PS1. In group 3, three serotype Virchow isolates collected from the feces of an infant over a 3-month period fell into RAPD profile S03 B04 C32, ribotype V-PS1, and phage type PT4a. In group 4, serotype Enteritidis isolates collected from the feces of nine patients and one food handler, as well as from four contaminated foods (two cakes, soup, and lamb), associated with a restaurant outbreak fell into RAPD profile S01 B01 C01, ribotype E-PS1, and phage type PT4. In group 5, serotype Enteritidis isolates collected over a 3-month period from the feces of 14 elderly people living in a rest home fell into RAPD profile S01 B01 C01, ribotype E-PS3, and phage type PT1. In group 6, serotype Typhimurium isolates that were associated with a family outbreak caused by a piece of defectively cured ham and were collected from the feces of the patients and from the ham fell into RAPD profile S11 B03 C12 and ribotype T-PS1 and were NPT. In group 7, serotype Typhimurium isolates that were collected from the diarrheic feces of seven young women living in a drug rehabilitation center and were associated with an outbreak related to water as the infection vehicle fell into RAPD profile S12 B03 C15, ribotype T-PS10, and phage type DT96.
FIG. 4.
Correlation of RAPD typing with serotyping, phage typing, and PS ribotyping for seven groups of epidemiologically related Salmonella isolates. RAPD-S, primer S RAPD profile; RAPD-B, primer B RAPD profile; RAPD-C, primer C RAPD profile; NP, non-phage typeable; E, Enteritidis; O, Ohio; V, Virchow; T, Typhimurium.
DISCUSSION
The use of PCR-based techniques has had a revolutionary impact on the diagnosis of infectious diseases. Because these techniques can detect or analyze minute amounts of microbial DNA or RNA sequences, they are highly sensitive and specific methods for identifying pathogens. The RAPD fingerprinting technique, in which arbitrary oligonucleotides are used to promote DNA synthesis at low annealing temperatures in order to determine genomic diversity, is a particularly powerful typing method. Unlike traditional PCR analysis, which requires specific knowledge of DNA sequences and the use of target-specific sequences, the RAPD technique does not require any specific knowledge of the DNA sequences of the target organisms. This makes it a flexible tool that has great power and general applicability (3, 10, 12, 16, 17, 21, 22).
The results presented in this paper show that RAPD analysis performed with selected primers under well-defined conditions can be used to reproducibly amplify random fragments of DNA from Salmonella genomes in order to differentiate between and within serotypes. The performance of the RAPD technique was evaluated by using several criteria, including typeability, reproducibility, typing system concordance, epidemiological concordance, ease of interpretation of the amplified band profiles, and discriminatory power. With the three primers used, all of the Salmonella isolates examined could be assigned to amplified band profile groups. With regard to reproducibility, a weakness frequently reported for RAPD analysis (19), three facts must be emphasized. (i) The method has been shown to exhibit total reproducibility in distributing strains into amplified profile groups, and it has been shown to be a very useful tool for epidemiological purposes, which differentiates strains assigned to different serotypes. (ii) In different experiments the amplified band profile of a given strain could include one or more bands that were poorly defined or were not visualized, but the profile was still different from other profiles. The PCR conditions used in this work minimized but did not always eliminate weak and nonconstant amplified fragments in some of the profiles, mainly profiles obtained with primer C. The nonconstant fragments were not used during differentiation of amplified band profiles. (iii) There were variations in RAPD profiles obtained in different laboratories. Moreover, each laboratory is free to establish which amplified fragments are considered in order to define profiles. The concordance between serotyping and RAPD typing was 100%. However, it is important to note that some profiles generated with primers S and B were produced by strains belonging to different serotypes.
The discriminatory power of the method was tested in the following two ways: (i) by considering the number of profiles generated both with each primer separately and combining the results obtained with the three primers (RAPD types), in both cases within the series of organisms and within each serotype, and (ii) by calculating the DI. For the latter analysis, epidemiologically related isolates (isolates collected from different samples from a single patient diagnosed as having extraintestinal or persistent-recidivant intestinal salmonellosis, as well as isolates collected from the feces and/or blood of different patients and food handlers and from foods associated with specific outbreaks) having identical traits (serotype, phage type, PS ribotype, and RAPD type) were assigned to single strains. The 326 isolates were grouped into 235 strains (in addition, 9 reference strains were also included). The numbers of profiles obtained with primers S, B, and C were 21 (DI, 0.84), 14 (DI, 0.78), and 40 (DI, 0.92), respectively. By combining the profiles obtained with the three primers, we obtained 57 RAPD types and a DI of 0.94 for the series of organisms; it is noteworthy that the 12 serotypes were subdivided into two or more RAPD types and that the DI for the six most common serotypes ranged between 0.13 and 0.78. When RAPD types were combined with phage types, further differentiation occurred, and the DI for serotypes Enteritidis, Typhimurium, and Virchow were 0.74, 0.96, and 0.87, respectively. For this analysis the NPT and NRP strains were considered two more different types (Table 2).
With regard to convenience, one advantage of the three-way procedure is its rapidity; it can be performed directly with aliquots of a water-diluted bacterial culture, and the same conditions are used with the three primers. A second advantage is that it is easy to interpret the resulting amplified band profiles; the profiles obtained with each primer exhibit a certain level of similarity, and many profiles are serotype specific, thus revealing the relatedness of Salmonella organisms. In addition to rapidity, the procedure is also convenient because it is accessible, flexible and easy to perform. Other genetic procedures, including procedures based on pulsed-field gel electrophoresis or restriction-hybridization fragment length polymorphism, such as ribotyping and IS200 typing, have been evaluated and proposed as typing procedures for several serotypes (4, 8, 11–13, 18), but they are more complex, costly, and technically demanding than RAPD typing.
ACKNOWLEDGMENTS
We thank M. A. Usera and the Centro Nacional de Microbiología for providing the serotyped and phage-typed Salmonella strains and the personnel of the microbiology laboratories of the Hospital Central de Asturias (Oviedo, Spain), the Hospital San Agustin (Avilés, Spain), the Hospital de Jarrio, the Hospital Cabueñes (Gijón, Spain), and the Hospital Carmen y Severo Ochoa (Cangas del Narcea, Spain) for providing clinical isolates.
This work was supported by a grant from the Fondo de Investigación Sanitaria (Ref. 98/0296). S. M. Soto was the recipient of a grant from the Ministry of Culture and Education of Spain (F.P.I./AP98/09429078).
REFERENCES
- 1.Anderson E S, Ward L R, de Saxe M J, de Sa J D H. Bacteriophage typing designations of Salmonella typhimurium. J Hyg. 1977;78:297–300. doi: 10.1017/s0022172400056187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers R M, McAdam P, de Sa J D H, Ward L R, Rowe B. A phage-typing scheme for Salmonella virchow. FEMS Microbiol Lett. 1987;40:155–157. [Google Scholar]
- 3.Fald A A, Nguyen A V, Khan M I. Analysis of Salmonella enteritidis isolates by arbitrary primed PCR. J Clin Microbiol. 1995;33:987–989. doi: 10.1128/jcm.33.4.987-989.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerra B, Landeras E, González-Hevia M A, Mendoza M C. A three-way ribotyping scheme for Salmonella serotype Typhimurium and its usefulness for phylogenetic and epidemiological purposes. J Med Microbiol. 1997;46:307–313. doi: 10.1099/00222615-46-4-307. [DOI] [PubMed] [Google Scholar]
- 5.Hilton A C, Banks J G, Penn C W. Random amplification of polymorphic DNA (RAPD) of Salmonella: strain differentiation and characterization of amplified sequences. J Appl Bacteriol. 1996;81:575–584. doi: 10.1111/j.1365-2672.1996.tb03550.x. [DOI] [PubMed] [Google Scholar]
- 6.Hunter P R, Gaston M A. Numerical index of the discriminatory ability of typing systems: an application of Simpson’s index of diversity. J Clin Microbiol. 1988;26:2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kauffmann F. The bacteriology of Enterobacteriaceae. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. 9th ed. Baltimore, Md: Williams and Wilkins Co.; 1994. pp. 186–187. [Google Scholar]
- 8.Landeras E, Mendoza M C. Evaluation of PCR-based methods and ribotyping performed with a mixture of PstI and SphI to differentiate strains of Salmonella serotype Enteritidis. J Med Microbiol. 1998;47:427–434. doi: 10.1099/00222615-47-5-427. [DOI] [PubMed] [Google Scholar]
- 9.Landeras E, González-Hevia M A, Mendoza M C. Molecular epidemiology of Salmonella serotype Enteritidis. Relationships between food, water and pathogenic strains. Int J Food Microbiol. 1998;43:81–90. doi: 10.1016/s0168-1605(98)00092-0. [DOI] [PubMed] [Google Scholar]
- 10.Lin A W, Usera M A, Barret T J, Goldsby R A. Application of random amplified polymorphic DNA analysis to differentiate strains of Salmonella enteritidis. J Clin Microbiol. 1996;34:870–876. doi: 10.1128/jcm.34.4.870-876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maslow J N, Slutsky A M, Arbeit R D. The application of pulsed field gel electrophoresis to molecular epidemiology. In: Persing D H, Smith T F, White T J, editors. Diagnostic molecular microbiology: principles and applications. Washington, D.C.: American Society for Microbiology; 1993. pp. 563–572. [Google Scholar]
- 12.Mendoza M C, Landeras E. Molecular epidemiological methods for differentiation of Salmonella enterica serovar Enteritidis strains. In: Saeed A M, Gast R K, Potter M E, Wall P G, editors. Salmonella enterica serovar Enteritidis in humans and animals. Epidemiology, pathogenesis, and control. Ames: Iowa State University Press; 1999. pp. 125–140. [Google Scholar]
- 13.Olsen J E, Brown D J, Skov M N, Christensen J P. Bacterial typing methods suitable for epidemiological analysis. Applications in investigations of salmonellosis among livestock. Vet Q. 1993;15:125–135. doi: 10.1080/01652176.1993.9694390. [DOI] [PubMed] [Google Scholar]
- 14.Orskov F, Orskov I. Summary of a workshop on the clone concept. Epidemiology, taxonomy and evolution of the Enterobacteriaceae and other bacteria. J Infect Dis. 1993;148:346–357. doi: 10.1093/infdis/148.2.346. [DOI] [PubMed] [Google Scholar]
- 15.Selander R K, Caugant D A, Ochman H, Musser J M, Gilmour M N, Whittam T S. Methods of multilocus enzyme electrophoresis for bacterial population genetics systematics. Appl Environ Microbiol. 1989;51:873–874. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shangkuan Y-H, Lin H C. Application of random amplified polymorphic DNA analysis to differentiate strains of Salmonella typhi and other Salmonella species. J Appl Microbiol. 1998;85:693–702. doi: 10.1111/j.1365-2672.1998.00582.x. [DOI] [PubMed] [Google Scholar]
- 17.Struelens M J, et al. Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clin Microbiol Infect. 1996;2:2–11. doi: 10.1111/j.1469-0691.1996.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 18.Threlfall E J, Chart H. Interrelationships between strains of Salmonella enteritidis. Epidemiol Infect. 1993;111:1–8. doi: 10.1017/s0950268800056612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyler K D, Wang G, Tyler S D, Johnson W M. Factors affecting reliability and reproducibility of amplification-based DNA fingerprinting of representative bacterial pathogens. J Clin Microbiol. 1997;35:339–346. doi: 10.1128/jcm.35.2.339-346.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward L R, de Sa J D H, Rowe B. A phage-typing scheme for Salmonella enteritidis. Epidemiol Infect. 1987;99:291–294. doi: 10.1017/s0950268800067765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welsh J, McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990;18:7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams J G K, Kubelin A R, Livak K J, Rafalski J A, Tingey S V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990;18:6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]