Abstract
Several lines of evidence have established that proliferation and differentiation of neural stem cells into neurons within the sub-granular zone of the dentate gyrus, a process named adult hippocampal neurogenesis, contribute to maintaining healthy cognitive functions throughout life. The rate of adult hippocampal neurogenesis decreases with aging and a premature impairment of adult hippocampal neurogenesis has been observed both in animal models of Alzheimer’s disease and human post-mortem tissues. The causal relationship between adult hippocampal neurogenesis and the development of Alzheimer’s disease pathology has, however, not been established. This is partly due to the limitation of recapitulating the development of Alzheimer’s disease pathology in rodent models and the lack of translatable biomarkers to identify tractable targets in humans. While it is tempting to postulate that adult hippocampal neurogenesis could be leveraged to improve cognitive deficits in Alzheimer’s disease, consensual results have yet to be reached to fully explore this hypothesis. In this review, we discuss how the recent progress in identifying molecular pathways in adult hippocampal neurogenesis provides a good framework to initiate strategies for drug-based intervention in neurodegenerative diseases, especially in Alzheimer’s disease. We outline how discrepancies in pre-clinical disease models and experimental methodology have resulted in contradictory findings and propose a shift towards using more translatable approaches to model neurogenesis in Alzheimer’s disease. In particular, we review how exploring novel experimental paradigms including the use of human induced pluripotent stem cells and more complex cell culture systems, as well as standardizing protocols used to investigate evidence of neurogenesis in human tissues, could deliver deeper mechanistic insights that would kick-start innovative drug discovery efforts to promote healthy aging and cellular rejuvenation.
Key Words: adult hippocampal neurogenesis, Alzheimer’s disease, cognition, human tissue, induced pluripotent stem cell, mouse models, neurodegeneration, therapeutics, tractable target
Introduction
The ongoing generation and integration of new neurons into existing hippocampal circuitry, termed adult hippocampal neurogenesis (AHN), in humans continues to be the subject of speculation. The source of these neurons is a reservoir of self-renewing neural stem cells (NSCs) and balancing the number of proliferative NSCs with those in a quiescent state (qNSCs) appears key to preserving the pool of NSCs over a lifetime, although the rate of neurogenesis declines with age. An outstanding question, however, is how dysregulation of AHN relates to the progression of neurodegenerative diseases with aging. Observations from both human studies and Alzheimer’s disease (AD) transgenic mouse models suggest that AD exacerbates the decline in AHN that occurs in physiological aging, and that enhancing neurogenesis in this context could have a beneficial effect (Disouky and Lazarov, 2021). Currently, the field lacks insight into the precise molecular mechanisms driving these distinct but related processes because 1) mouse models fail to fully recapitulate the underlying biology of the disease; 2) variability in patient post-mortem tissue protocols has led to contradictory findings. New approaches, such as standardized protocols for human post-mortem tissue and more translatable disease models including patient-derived cell lines, can be used to better understand the disease-relevant molecular mechanisms involved to yield therapeutic strategies for modulating neurogenesis in AD.
Search Strategy and Selection Criteria
References cited in this narrative review were searched on PubMed and Google Scholar using following keywords: ‘adult hippocampal neurogenesis’, ‘Alzheimer’s disease and adult hippocampal neurogenesis’, ‘adult neural stem cells, ‘animal models Alzheimer’s disease’, ‘human Adult hippocampal neurogenesis and Alzheimer’s disease’, ‘human iPSCs and adult hippocampal neurogenesis’. No restriction on publication dates was applied for search strategy.
The Relationship between Adult Hippocampal Neurogenesis and Cognitive Deficits in Alzheimer’s Disease
AHN is a complex multi-step process involving the proliferation of NSCs in the dentate gyrus, followed by the generation of neural progenitor cells which differentiate into neurons, mature to integrate within the dentate gyrus, and finally connect with the entorhinal cortex and the hippocampus (Gillotin et al., 2021). Malleability in this neuronal circuit may have a role in regulating aspects of learning and memory, particularly pattern separation in humans. This highlights the importance of maintaining the NSC pool for sustaining a lifelong AHN, which is partly achieved through the unique feature of adult NSCs shuttling between quiescence and proliferation under the control of both intrinsic and extrinsic stimuli. Among the intrinsic signaling pathways modulating AHN, some are characteristic of cellular dysfunction in physiological aging. In particular, proteostasis, regulation of redox signaling and homeostasis, lipid metabolism, and epigenetic signatures are under intense scrutiny and offer the most promising avenues for identifying targets to address the age-related and disease-associated decline in AHN (Gillotin et al., 2021). The ability of adult NSCs to shift between different cellular states recently led to the identification of distinct metabolic and functional profiles, with sub-types of NSCs harboring an increased barrier to exiting quiescence, while others are depleted from the pool (Gillotin et al., 2021). It is yet to be understood whether this represents the heterogeneity of NSC sub-types or reflects temporal molecular changes affected by signaling cues in aging and diseases. Addressing this will allow better linkage between AHN and diseases and define how to leverage AHN in therapeutic strategies to either delay symptoms or modify disease outcomes. As such, AD offers a unique paradigm with increasing evidence suggesting that impaired AHN is one of the first events in AD. This disease is characterized by progressive learning and memory deficits as well as histopathological hallmarks of amyloid-beta protein deposits and tau-associated neurofibrillary tangles with the hippocampus being one of the first affected area before spreading to other brain areas. Familial AD is almost solely caused by mutations in the genes encoding amyloid precursor protein (APP) and presenilin 1 (PSEN1) and PSEN2. Sporadic AD (SAD) has no known definitive cause but is associated with many genetic risk factors, with APOE4 status being associated with the highest single risk (Disouky and Lazarov, 2021). Assessing the causal relationship between dysregulated neurogenesis and AD is challenging due to the slow nature of disease progression, resulting in a paucity of validated molecular targets to pursue pharmacological intervention (Disouky and Lazarov, 2021). Additional limitations come from the poor translatability between species used to model AHN and from our insufficient understanding of the potential contributions of cell types other than NSCs within the neurogenic niche.
Investigating Adult Hippocampal Neurogenesis in Alzheimer’s Disease: Shifting the Paradigm from Mouse Modeling to Studies with Human Tissue
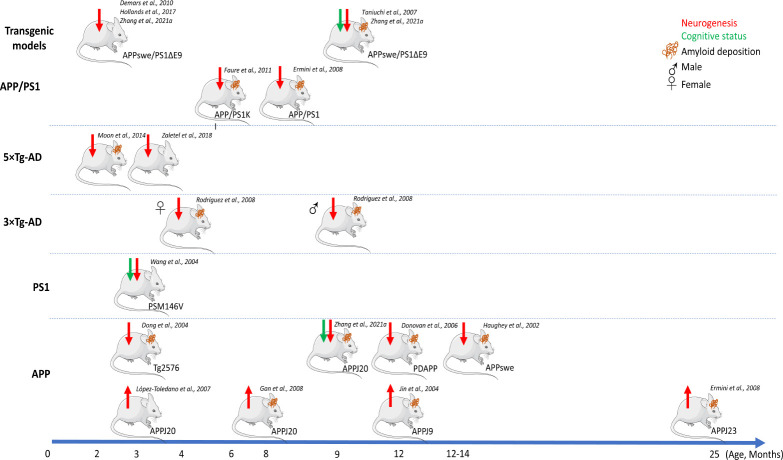
Most of our understanding of the relationship between AHN and AD is derived from observations using laboratory-bred rodents, especially mice, with the availability of genetic tools being a major advantage in using this system. AHN has been examined in many different genetic mouse models of AD and evidence of impaired neurogenesis has been found in several models overexpressing APP and PSEN1 genes harboring familial AD mutations (Figure 1). However, the timing at which deficits of adult neurogenesis occur varies between studies across different transgenic lines (Disouky and Lazarov, 2021). This could be imputed to the specificity of each genetic background and to the broad variety of cellular biomarkers used in each study to quantify changes in AHN rates. Echoing that, increased neurogenesis was also observed in an APP variant model (see APP models with double or triple mutations in Figure 1), possibly explained by a compensatory brain-repair mechanism in response to AD pathophysiology in which enhanced neurogenesis could be confounded with reactive gliogenesis. Besides methodological roadblocks with limited panels of biomarkers to thoroughly differentiate different states of NSC activity from glial cells, these conflicting results regarding the role of neurogenesis in AD highlight the difficulty in recapitulating the timeline at which AD pathology develops in mouse brains. Nonetheless, mouse AD models remain a powerful stepping stone for translation to human biology when used to address specific questions. For instance, in some models impaired neurogenesis was shown to precede AD pathology, representing valuable models to first investigate early mechanistic defects in AD etiology and second to validate findings in human post-mortem AD samples with which similar observations were made (e.g., APPswe/PS1ΔE9 or PS1 models; Figure 1). In others, plaque deposition increased with age concomitantly with an incremental impairment of neurogenesis, making these models relevant to study the association of plaque formation with impaired neurogenesis and to identify targets associated with AD progression (e.g., 3×Tg-AD and APPswe/PS1ΔE9; Figure 1). Moreover, understanding better how AD progresses differently between males and females could be modeled using some of these pre-clinical models to further explore similar observations made in humans (e.g., 3×Tg-AD; Figure 1). Finally, these models have been used to assess whether or not manipulating AHN would improve cognitive functions associated with AD. To date, contradictory results linking neurogenesis to cognition in AD at the molecular and cellular levels have been challenging for the field to resolve (Phan et al., 2021; Zhang et al., 2021b). This could be linked to low cognitive function exhibited in housed rodents but ultimately loops back to the scarcity of biomarkers. Indeed, to ablate or enhance neurogenesis in vivo, currently used driver mouse lines are not specific enough to exclusively target one cell type. Alongside improvement in the repertoire of cellular markers, more studies using aged animals would help find consensual answers to associate cognitive improvements with AHN.
Figure 1.
Alzheimer’s disease (AD) mouse models and impaired adult neurogenesis.
Schematic representation of AD mouse models showing the earliest time point of impaired neurogenesis and plaque deposition. Only selected studies aimed at associating AD pathology with adult neurogenesis impairment were depicted here. Equally, cognitive status was only represented if cognitive tests were included as part of the study. Tauopathy was not schematised due to scarcity of data to associate it with adult neurogenesis but was observed in studies by Demars et al. (2010) and Rodriguez et al. (2008) for example. Red arrows denote change in neurogenesis with up arrow corresponding to increased neurogenesis and down arrow corresponding to impaired neurogenesis. Down green arrows denote decrease in cognitive functions including learning and memory. Mice with brown lines denote coinciding amyloid-beta AD-associated pathology relative to non-transgenic controls. Genetic mutations for each model: APPswe/PS1ΔE9: APP KM670/671NL (Swedish), PSEN1: deltaE9; APP/PS1KI: APP KM670/671NL (Swedish), APP V717I, PSEN1 M233T, PSEN1 L235P; APP/PS1: APP KM670/671NL (Swedish), PSEN1 L166P; 5xTg-AD: APP KM670/671NL (Swedish), APP I716V, APP V717I, PSEN1 M146L (A>C), PSEN1 L286V; 3xTg-AD: APP KM670/671NL (Swedish), MAPT P301L, PSEN1 M146V; PS1: PSEN1 M146V; Tg2576: APP KM670/671NL (Swedish); PDAPP: APP V717F; APPswe: APP KM670/671NL (Swedish); APPJ20: APP KM670/671NL (Swedish), APP V717F; hAPPJ9: APP KM670/671NL (Swedish), APP V717F. Schematic representations of mice have been adapted from Servier Medical Art.
To translate valuable cellular and molecular mechanisms obtained from these pre-clinical models to human biology, studies using post-mortem human tissue have become the next necessary approach. This raises its own challenges with difficulties in accessing pristine tissues, a lack of shared methodologies to process these tissues, and too few biomarkers to characterize individual cell types within the neurogenic niche (Gillotin et al., 2021). Despite this, by following rigorous methods, several groups recently demonstrated that AHN occurs in human brain until old age, albeit at a very low rate (Boldrini et al., 2018; Moreno-Jiménez et al., 2019; Tobin et al., 2019). Two separate studies investigating AHN in AD patients reported that the rate is reduced in the disease compared to healthy controls and that it occurs at early onset (Boldrini et al., 2018; Moreno-Jiménez et al., 2019). Furthermore, logistic regression analysis showed a correlation between DCX+PCNA+ cells, indicative of the presence of neuroblasts, and preserved cognitive status, underlying the idea that modulating AHN could be a viable intervention for ameliorating aspects of AD (Tobin et al., 2019). In line with the difficulties mentioned above in using this approach, it was also reported that AHN drops sharply after the first year of life to low or undetectable levels in post-mortem brain samples analyzed from epilepsy patients and adult non-epileptic controls (Sorrells et al., 2018). Beyond reigniting the debate over the relevance of neurogenesis in adult humans, the lack of mechanistic understanding of how neurogenesis relates to AD calls for novel approaches aimed at investigating this relationship at the cellular and molecular levels. This will pave the way to establishing a causal link between decreased AHN and cognitive deficits in AD and advanced target identification for drug discovery in this challenging area of neurobiology (Gillotin et al., 2021).
Increasing Mechanistic Insights of Human Adult Neurogenesis in Alzheimer’s Disease
Orthogonal model systems that can replicate the complex biological landscape of AD are required to understand the interplay between AD and AHN. One approach could be to increase the use of human AD patient-derived and isogenic control of induced pluripotent stem cells lines as a tool to model neurogenesis in 2D and 3D culture systems. This strategy would further support the identification of defective signaling pathways in the first instance and ultimately offer the possibility to identify human-specific targets and biomarkers. For instance, human induced pluripotent stem cells lines harboring PSEN1 mutations exhibit a premature terminal differentiation phenotype compared to control lines, while the APP line shows overall increased neurogenesis in 2D and 3D modeling (Arber et al., 2021). This is thought to be due to reduced Notch signaling, which is a shared pathway between neurogenesis and the pathological molecular cascade in AD. Another report using similar approaches with APOE4 and SAD patient-derived human induced neural progenitor cells also identified accelerated neural differentiation and reduced proliferation compared to control lines (Meyer et al., 2019). Focusing on gene expression, the authors built upon their previous study to show that dysregulated localization of the repressor element 1-silencing transcription factor, which is an important regulator of qNSCs and transcription repressor of cell death and AD-associated genes in neurons, may contribute to disease onset (Gillotin et al., 2021). These findings support the idea that stem cell exhaustion could occur in the hippocampus as part of AD, depleting the NSC pool and decreasing cognitive reserve. Both studies highlight that mutation-specific effects should be taken into consideration when investigating neurogenesis in AD as well as patient stratification and molecular validation in post-mortem human tissue should be added to correlate neurogenic phenotype with the underlying genetic background.
The ultimate question is whether modulating neurogenesis can improve the functional consequences or delay the onset of AD. For this, addressing the contribution of cell types other than NSCs within the niche and taking into consideration the changing environment of the niche, especially during inflammation, are essential for shifting the paradigm and bringing novel mechanistic insights. As such, the use of 3D models organoids or 2D co-cultures would be ideal to begin dissecting the cross-communication between cell types. In this vein, the recent discovery that the microRNA miR-132 regulates aspects of AHN (Walgrave et al., 2021) could be a stepping stone to lead the field to new grounds. Indeed, miR-132 is known to be expressed in all central nervous system cell types and to be regulated by repressor element 1-silencing transcription factor. It is therefore tempting to wonder if this non-coding RNA would act as a ‘molecular regulator’ between cell types. Currently, it has been shown that miR-132 is required for induction of NSC proliferation and differentiation in the hippocampal niche in wild-type mice and the premature drop in levels within two AD mouse models, that coincide with a decreased neurogenesis, suggests that miR-132 levels are associated with amyloid pathology. This has been replicated in human NSC cell models using either addition of amyloid-beta oligomers and SAD patient-derived sera. Most notably, overexpression of miR-132 rescues not only neurogenesis in AD mice but also cognition. First, this study puts the relationship between AHN and AD into a molecular framework where amyloid-beta drives down-regulation of miR-132, which in turn dysregulates AHN and induces memory and learning defects. Secondly, this acts as proof of concept that enhancing neurogenesis in the context of AD can have therapeutic benefits. Whether or not targeting miR-132 alone will translate to a clinical strategy, and where it fits into the wider regulatory network acting on neurogenesis in AD, remains to be clarified but nonetheless, it represents a considerable leap forward in our understanding at the molecular level.
Discussion
Overall, the evidence favors a contribution of AHN to AD. Pushing the field towards using standardized techniques and more translatable models relating to human biology gives us the best chance of elucidating the mechanistic interactions between these two processes. This in turn will help to identify targets to modulate neurogenesis as a potential therapy for cognitive deficits associated with this disease. For this, it is vital to continue to investigate human tissues, despite its challenges. Post-mortem human tissue studies are invaluable but tissue source variability, including disease background, sample size, as well as handling and immunodetection protocols contribute to the contradictory findings that have surrounded the existence of AHN in humans. Using methodologies most likely to preserve evidence of neurogenesis and enable its detection will allow comparisons between different studies to be made more easily (Moreno-Jiménez et al., 2019). Non-human primates show greater neuroanatomical and functional similarities to humans than rodents, and extraction of fresh tissue from controlled colonies with associated histopathology and behavioral studies could greatly assist in validating findings that relate to the effects of aging on neurogenesis and cognition (Disouky and Lazarov, 2021). Furthermore, the use of human cellular models from AD patients in 3D systems or in co-cultures could be a complementary addition to other model systems already in use, as these cell lines recapitulate the genetic background of the disease, and can help identify relevant regulatory molecules including secreted molecules, signaling pathways and cellular defects specific to impaired adult neurogenesis in AD. Using neuronal and non-neuronal cell types to model environmental factors acting on the neurogenic niche, such as neuroinflammation associated with AD, will undoubtedly give a fuller picture of how the disease affects AHN. Importantly, caution must be exercised when interpreting results using patient-derived induced pluripotent stem cells as age-associated signatures are lost during reprogramming, with age being a major factor in both AHN and AD (Mertens et al., 2021). To bypass this, the use of directly reprogrammed neurons from aged or AD differentiated cells, which retain markers of aging, could be added as an extra tool to assess specific molecular mechanisms, especially those related to transcriptional and epigenetic signatures. Finally, it is imperative to demonstrate that altering a putative neurogenesis target has a functional outcome on cognition in the disease. Combining these approaches will drive advancements in our understanding of these two processes at the molecular level and could lead to potential therapies in the future.
Acknowledgments:
The authors would like to thank James Duce, Jill Richardson and Jason M Uslaner (MSD) for providing feedback on the manuscript. We apologize to the many authors whose work we could not discuss and cite because of lack of space.
Footnotes
Conflicts of interest: The authors are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA known as MSD outside of the US and Canada. The authors are shareholders of Merck & Co., Inc., Kenilworth, NJ, USA.
C-Editors: Zhao M, Liu WJ, Wang Lu; T-Editor: Jia Y
References
- 1.Arber C, Lovejoy C, Harris L, Willumsen N, Alatza A, Casey JM, Lines G, Kerins C, Mueller AK, Zetterberg H, Hardy J, Ryan NS, Fox NC, Lashley T, Wray S. Familial Alzheimer's disease mutations in PSEN1 lead to premature human stem cell neurogenesis. Cell Rep. 2021;34:108615. doi: 10.1016/j.celrep.2020.108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018;22:589–599. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demars M, Hu YS, Gadadhar A, Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer's disease in transgenic mice. J Neurosci Res. 2010;88:2103–2117. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Disouky A, Lazarov O. Adult hippocampal neurogenesis in Alzheimer's disease. Prog Mol Biol Transl Sci. 2021;177:137–156. doi: 10.1016/bs.pmbts.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 6.Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer's disease. J Comp Neurol. 2006;495:70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- 7.Ermini FV, Grathwohl S, Radde R, Yamaguchi M, Staufenbiel M, Palmer TD, Jucker M. Neurogenesis and alterations of neural stem cells in mouse models of cerebral amyloidosis. Am J Pathol. 2008;172:1520–1528. doi: 10.2353/ajpath.2008.060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faure A, Verret L, Bozon B, El Tannir El Tayara N, Ly M, Kober F, Dhenain M, Rampon C, Delatour B. Impaired neurogenesis, neuronal loss, and brain functional deficits in the APPxPS1-Ki mouse model of Alzheimer's disease. Neurobiol Aging. 2011;32:407–418. doi: 10.1016/j.neurobiolaging.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Gan L, Qiao S, Lan X, Chi L, Luo C, Lien L, Yan Liu Q, Liu R. Neurogenic responses to amyloid-beta plaques in the brain of Alzheimer's disease-like transgenic (pPDGF-APPSw,Ind) mice. Neurobiol Dis. 2008;29:71–80. doi: 10.1016/j.nbd.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillotin S, Sahni V, Lepko T, Hanspal MA, Swartz JE, Alexopoulou Z, Marshall FH. Targeting impaired adult hippocampal neurogenesis in ageing by leveraging intrinsic mechanisms regulating neural stem cell activity. Ageing Res Rev. 2021;71:101447. doi: 10.1016/j.arr.2021.101447. [DOI] [PubMed] [Google Scholar]
- 11.Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 12.Hollands C, Tobin MK, Hsu M, Musaraca K, Yu TS, Mishra R, Kernie SG, Lazarov O. Depletion of adult neurogenesis exacerbates cognitive deficits in Alzheimer's disease by compromising hippocampal inhibition. Mol Neurodegener. 2017;12:64. doi: 10.1186/s13024-017-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin K, Galvan V, Xie L, Mao XO, Gorostiza OF, Bredesen DE, Greenberg DA. Enhanced neurogenesis in Alzheimer's disease transgenic (PDGF-APPSw,Ind) mice. Proc Natl Acad Sci U S A. 2004;101:13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-Toledano MA, Shelanski ML. Increased neurogenesis in young transgenic mice overexpressing human APP(Sw, Ind) J Alzheimers Dis. 2007;12:229–240. doi: 10.3233/jad-2007-12304. [DOI] [PubMed] [Google Scholar]
- 15.Mertens J, Herdy JR, Traxler L, Schafer ST, Schlachetzki JCM, Böhnke L, Reid DA, Lee H, Zangwill D, Fernandes DP, Agarwal RK, Lucciola R, Zhou-Yang L, Karbacher L, Edenhofer F, Stern S, Horvath S, Paquola ACM, Glass CK, Yuan SH, et al. Age-dependent instability of mature neuronal fate in induced neurons from Alzheimer's patients. Cell Stem Cell. 2021;28:1533–1548. doi: 10.1016/j.stem.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer K, Feldman HM, Lu T, Drake D, Lim ET, Ling KH, Bishop NA, Pan Y, Seo J, Lin YT, Su SC, Church GM, Tsai LH, Yankner BA. REST and neural gene network dysregulation in iPSC models of Alzheimer's disease. Cell Rep. 2019;26:1112–1127. doi: 10.1016/j.celrep.2019.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon M, Cha MY, Mook-Jung I. Impaired hippocampal neurogenesis and its enhancement with ghrelin in 5XFAD mice. J Alzheimers Dis. 2014;41:233–241. doi: 10.3233/JAD-132417. [DOI] [PubMed] [Google Scholar]
- 18.Moreno-Jiménez EP, Flor-García M, Terreros-Roncal J, Rábano A, Cafini F, Pallas-Bazarra N, Ávila J, Llorens-Martín M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- 19.Phan T, Gupta M, Mishra R, Kumar P, Disouky A, Stephen TKL, Rakowiecki K, Lazarov O. Questioning the evidence for a Janus-faced nature of adult neurogenesis in Alzheimer's disease. Stem Cell Reports. 2021;16:1646–1648. doi: 10.1016/j.stemcr.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodríguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, LaFerla FM, Oddo S, Verkhratsky A. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease. PLoS One. 2008;3:e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S, Chang J, Auguste KI, Chang EF, Gutierrez AJ, Kriegstein AR, Mathern GW, Oldham MC, Huang EJ, Garcia-Verdugo JM, Yang Z, Alvarez-Buylla A. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018;555:377–381. doi: 10.1038/nature25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taniuchi N, Niidome T, Goto Y, Akaike A, Kihara T, Sugimoto H. Decreased proliferation of hippocampal progenitor cells in APPswe/PS1dE9 transgenic mice. Neuroreport. 2007;18:1801–1805. doi: 10.1097/WNR.0b013e3282f1c9e9. [DOI] [PubMed] [Google Scholar]
- 23.Tobin MK, Musaraca K, Disouky A, Shetti A, Bheri A, Honer WG, Kim N, Dawe RJ, Bennett DA, Arfanakis K, Lazarov O. Human hippocampal neurogenesis persists in aged adults and Alzheimer's disease patients. Cell Stem Cell. 2019;24:974–982. doi: 10.1016/j.stem.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walgrave H, Balusu S, Snoeck S, Vanden Eynden E, Craessaerts K, Thrupp N, Wolfs L, Horré K, Fourne Y, Ronisz A, Silajdžić E, Penning A, Tosoni G, Callaerts-Vegh Z, D'Hooge R, Thal DR, Zetterberg H, Thuret S, Fiers M, Frigerio CS, et al. Restoring miR-132 expression rescues adult hippocampal neurogenesis and memory deficits in Alzheimer's disease. Cell Stem Cell. 2021;28:1805–1821. doi: 10.1016/j.stem.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Wang R, Dineley KT, Sweatt JD, Zheng H. Presenilin 1 familial Alzheimer's disease mutation leads to defective associative learning and impaired adult neurogenesis. Neuroscience. 2004;126:305–312. doi: 10.1016/j.neuroscience.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 26.Zaletel I, Schwirtlich M, Perović M, Jovanović M, Stevanović M, Kanazir S, Puškaš N. Early impairments of hippocampal neurogenesis in 5xFAD mouse model of Alzheimer's disease are associated with altered expression of SOXB transcription factors. J Alzheimers Dis. 2018;65:963–976. doi: 10.3233/JAD-180277. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Mei Y, He Y, Wang D, Wang J, Wei X, Yang E, Zhou D, Shen H, Peng G, Shu Q, Li X, Luo B, Zhou Y, Sun B. Ablating adult neural stem cells improves synaptic and cognitive functions in Alzheimer models. Stem Cell Reports. 2021a;16:89–105. doi: 10.1016/j.stemcr.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Mei Y, Wang J, Wei X, Sun B. Response to questioning the evidence for a Janus-faced nature of adult neurogenesis in Alzheimer's disease. Stem Cell Reports. 2021b;16:1649–1651. doi: 10.1016/j.stemcr.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]