Abstract
Random noise stimulation technique involves applying any form of energy (for instance, light, mechanical, electrical, sound) with unpredictable intensities through time to the brain or sensory receptors to enhance sensory, motor, or cognitive functions. Random noise stimulation initially employed mechanical noise in auditory and cutaneous stimuli, but electrical energies applied to the brain or the skin are becoming more frequent, with a series of clinical applications. Indeed, recent evidence shows that transcranial random noise stimulation can increase corticospinal excitability, improve cognitive/motor performance, and produce beneficial aftereffects at the behavioral and psychological levels. Here, we present a narrative review about the potential uses of random noise stimulation to treat neurological disorders, including attention deficit hyperactivity disorder, schizophrenia, amblyopia, myopia, tinnitus, multiple sclerosis, post-stroke, vestibular-postural disorders, and sensitivity loss. Many of the reviewed studies reveal that the optimal way to deliver random noise stimulation-based therapies is with the concomitant use of neurological and neuropsychological assessments to validate the beneficial aftereffects. In addition, we highlight the requirement of more randomized controlled trials and more physiological studies of random noise stimulation to discover another optimal way to perform the random noise stimulation interventions.
Key Words: auditory noise, mechanical noise, neurological disorders, neuronal noise, noise galvanic vestibular stimulation, non-invasive brain stimulation, transcranial electrical stimulation, transcranial random noise stimulation
Introduction
Random noise stimulation (RNS) is becoming a clinical research technique with potential applications for treating neurological disorders. In this review, we elaborate the idea that, although the results of these investigations are promising, the auditory, tactile, and electrical RNS aftereffects in patients with neurological disorders remain widely unexplored in the context of controlled clinical trials. Hence, this work could serve as an initial framework for future clinical research in this field.
On the other hand, the study of RNS deserves the critical attention of therapists to carefully examine the side effects for extended periods (years) of RNS stimulation. In the second place, though there are several randomized single and double-blinded studies, more systematic evidence is needed to understand these therapies using auditory, electrical, or other types of RNS. Likewise, it is necessary to evaluate the effects of RNS on larger samples, as the ones reported so far are still relatively small (Table 1). Finally, this review could help visualize the common beneficial effects of RNS interventions in different types of neurological diseases.
Table 1.
Summary of the number of participants in the reviewed articles
| Neurological disorder | Number of participants | Noisy stimulation method |
|---|---|---|
| ADHD | 171 | Auditory background noise and binaural noise |
| Schizophrenia | 45 | tRNS and tinnitus control apparatus |
| Myopia and amblyopia | 83 | tRNS |
| Tinnitus | 564 | tRNS |
| Multiple sclerosis | 33 | tRNS |
| Parkinson’s disease | 82 | nGVS and tRNS |
| Balance | 176 | Mechanical or electrical noise over the skin and nGVS |
| Total | 1154 |
The most used noisy stimulation is tRNS. ADHD: Attention deficit hyperactivity disorder; nGVS: noisy galvanic vestibular stimulation; tRNS: transcranial random noise stimulation.
Search Strategy
A narrative review was carried out, including articles from Medline and Web of Science electronic databases updated until November 2021. The terms used for the database search were “RNS OR noise OR transcranial random noise stimulation (tRNS) OR tRNS OR nGVS OR stochastic resonance (SR) AND therapy AND patient AND neuron AND (brain OR peripheral) OR tinnitus OR ADHD OR schizophrenia, OR amblyopia, OR myopia, OR tinnitus, OR multiple sclerosis, OR stroke, OR vestibular-postural disorders, OR sensitivity loss. “ The selected articles focused on auditory, tactile, or electrical RNS in patients previously diagnosed with conditions involving nervous system alterations. At least two independent researchers evaluated the reviewed articles. We filtered the database search to original articles referred to as experimental evidence, randomized controlled trials, controlled clinical trials, observational studies, and case reports published in English. We also explored whether the reviewed publications found beneficial effects of auditory, mechanical, or electrical RNS in various neurological disorders.
General Aspects of Random Noise Stimulation
For several years, non-invasive stimulation techniques using noise have been developed, with the idea of activating sensory receptors and brain regions to improve the quality of life of patients with neuronal dysfunction. In particular, auditory noise stimulation (which we will refer to as “auditory RNS”) was examined to improve visual sensations in the multisensory interaction via a stochastic resonance (SR)-like phenomenon, also known as cross-modal or multisensory SR (Manjarrez et al., 2007; Lugo et al., 2008). SR is a mechanism by which noise enhances the response of a system to an input signal. In 1981, Benzi et al. introduced for the first time this concept, mentioning that “a dynamical system subject to both periodic forcing and random perturbation may show a resonance (peak in the power spectrum) which is absent when either the forcing or the perturbation is absent.”
Auditory RNS was employed to improve the ability of listeners to understand spoken words during the observation of another person performing mouth articulatory movements (Ross et al., 2007; Liu et al., 2013). Furthermore, “mechanical RNS” applied on the finger skin was employed to increase visual evoked potentials via multisensory SR in primary visual cortical areas, but not in regions overlying the somatosensory cortex (Mendez-Balbuena et al., 2015).
In a similar context, Terney et al. (2008) introduced a technique of electrical stimulation known as transcranial random noise stimulation (tRNS), which can increase the amplitude of corticospinal motor evoked potentials elicited by transcranial magnetic stimulation. This technique is a form of transcranial alternating current stimulation (tACS), where a low-intensity current varies randomly with a flat probability density function, similar to white noise. Most studies employed tRNS with a frequency between 0.1 to 640 Hz or with a higher frequency range from 101 to 640 Hz (Antal and Herrmann, 2016; Ghin et al., 2018).
In another study, Van der Groen and Wenderoth (2016) applied tRNS in participants who were asked to detect Gabor patches in two trials visually. These authors found that stimulating the visual cortex with tRNS at an intensity of approximately 1 to 2 mA improved the performance in a visual alternative forced-choice task. Furthermore, they suggested that tRNS improved the detection of a subthreshold signal by increasing cortical excitability and lowering the response threshold (Potok et al., 2021). These results were consistent with an SR-like phenomenon and with other reports showing that high-frequency RNS (hf-tRNS) of 1.5 mA to the extrastriate MT+ visual area produced maximal enhancement of performance (Guin et al., 2018; Pavan et al., 2019). In the study by Pavan et al. (2019), there was an optimal intensity for hf-tRNS, but it also confirmed the detrimental effect when using higher noise intensities.
The utility of tRNS is beneficial for perceptual learning (Fertonani et al., 2011), memory performance (Penton et al., 2018), auditory gap discrimination (Rufener et al., 2017), visual motion adaptation (Campana et al., 2016), and the induction of long-lasting effects on execution speed (i.e., reduction of reaction times) in Go-Nogo tasks in healthy subjects (Brevet-Aeby et al., 2019). In the same context, hf-tRNS reduced the task-related activity in the prefrontal cortex, the precuneus, and the anterior cingulate cortex during a visuomotor learning task (Saiote et al., 2013).
Another modality of non-invasive noisy stimulation recently developed is noise galvanic vestibular stimulation (nGVS), which employs electrodes bilaterally placed caudal to the ear on the mastoid, close to the tensor tympani muscle (for instance, Mulavara et al., 2011; Dlugaiczyk et al., 2019). The nGVS usually uses imperceptible zero-mean Gaussian-white Galvanic-noise. There is evidence that nGVS improves balance and locomotor stability in healthy persons (Mulavara et al., 2011, 2015; Goel et al., 2015; Galvan-Garza et al., 2018; Temple et al., 2018).
Regarding the mechanisms of tRNS, there are only a few physiological studies in the brain (Chaieb et al., 2015; Remedios et al., 2019) and dorsal root ganglia (Onorato et al., 2016) evidencing the participation of sodium (Na+) channels in these tRNS amplifying aftereffects. As a potential mechanism, it was speculated that due to the nonlinearity of voltage-gated ion channels, the repeated pulses generated by tRNS could induce multiple ionic influxes, amplifying membrane voltage fluctuations. In this context, more physiological studies about the mechanisms of action of tRNS, or other forms of RNS, are required.
The studies mentioned in this section reveal that RNS of different energies could be a research technique with potential clinical applications. In the following paragraphs, we will describe the effects of RNS in the treatment of particular neurological disorders.
Attention Deficit Hyperactivity Disorder
The effect of auditory RNS under several experimental paradigms has demonstrated its alleged role as a therapeutic option for patients with attention deficit hyperactivity disorder (ADHD) (Figure 1). It has shown its usefulness in improving language, working memory (Pickens et al., 2019), and reducing impulsivity (Cook et al., 2014). One of the first studies exploring the effects of auditory RNS on memory tasks originated partly from the observation that background music could improve performance on arithmetic tasks, which is interesting considering it is a task-irrelevant stimulus (Abikoff et al., 1996). Another relevant study involved a controlled clinical trial in children performing a memory task (Söderlund et al., 2007). Auditory RNS exerted beneficial effects on cognitive performance for the ADHD group but a deteriorated performance for the control group, indicating that ADHD subjects need more noise than controls for optimal cognitive function. In other words, auditory RNS was beneficial for the cognitive performance of ADHD children, while it was detrimental for the healthy ones.
Figure 1.

Scheme of noisy stimulation applied to attention deficit hyperactivity disorder patients.
(A) Auditory noise is added to the verbal instructions to be remembered by children performing a memory task. (B) Auditory white noise is binaurally delivered while children perform cognitive tasks. Figure is created with BioRender software based on information from the following references: Söderlund et al. (2007, 2016).
Subsequently, Söderlund et al. (2010) performed an observational study in which auditory RNS (acoustic background noise in the environment) was played in a classroom with attentive and inattentive children performing episodic verbal free recall tests. The authors found statistically significant proof that auditory RNS only improved the attention of the inattentive-children (Söderlund et al., 2010). Similarly, binaural auditory RNS has been shown to improve cognitive performance in ADHD children, as reported in an experimental study involving 29 diagnosed children who underwent neuropsychological assessments (Baijot et al., 2016). These children performed a computerized attentional performance battery, a counting Stroop task, and a Go-Nogo task. However, the observed benefit was mainly related to vigilance, as reflected in the computerized attentional performance, without a generalized improvement in other functions (Baijot et al., 2016). Also, when performing a Go-Nogo task, the electroencephalogram P300 amplitude was increased in the group with auditory RNS. However, this effect was not reflected in the Go-Nogo task, suggesting that the auditory RNS influences inhibitory processes not observed at the behavioral level (Baijot et al., 2016).
In a pilot study, Söderlund et al. (2016) also revealed the benefits of auditory RNS on executive and non-executive memory tasks of ADHD children and inattentive children who have undergone methylphenidate medication. In addition, the authors showed that the performance of verbal episodic memory tasks was better under the auditory RNS condition, suggesting that auditory RNS could work as a complementary therapy. In the same context, a recent randomized controlled trial by Berger et al. (2021) supports this observation, finding that tRNS yielded a clinical improvement, as indicated by the reduced ADHD rating-scale score from baseline compared to the changes produced by transcranial direct current stimulation (tDCS). Finally, Cook et al. (2014) reported that auditory RNS delivered through headphones reduced passive off-task behavior in three ADHD children undergoing stimulant medication. These authors recommended auditory RNS in individuals with ADHD due to its easy application in classrooms and homes and no side effects.
In contrast, Metin et al. (2016) examined the impulsive choice in children with ADHD, finding that auditory RNS in the environment did not reduce impulsive choice in ADHD. These controversial findings indicate that the hypothesis that auditory RNS is beneficial in children with ADHD requires further examination regarding the types of acoustic noise employed and their biological processes.
We can also speculate regarding the theories behind the observed behavioral aspects of ADHD improved by the auditory RNS. For instance, ADHD could be due to abnormally low tonic Dopamine levels, which are compensated by phasic or stimulus-dependent dopamine release (Sikström and Söderlund, 2007). In this scenario, the stimulation with moderately continuous auditory RNS in environments could benefit cognitive performance and motor learning, possibly due to the facilitation of information transmission or the constant promoting of phasic dopamine release. Consequently, this increase in tonic dopamine release could conduct the brain towards moderate arousal, benefiting cognitive performance. However, several other theories about the effects of auditory RNS in the treatments of ADHD could be incorporated, as the “optimal stimulation theory,” which establishes that every individual has its optimal level of arousal, an idea which has been supported by empirical studies (Abikoff et al., 1996; Baijot et al., 2016). Finally, we could speculate that other theories and methods employing neurofeedback with an optimal auditory RNS, or tRNS, based on chaotic resonance (Nobukawa et al., 2021), could also be helpful in the treatment of ADHD.
Schizophrenia
The effects of electrical noise (i.e., tRNS) and auditory RNS on schizophrenic patients have been tested based on empirical methods in some studies. For instance, Palm et al. (2013) reported the case of a 29-year-old patient diagnosed with paranoid schizophrenia who received an experimental treatment with tRNS. The patient was also medicated with clozapine, haloperidol, pregabalin, and lamotrigine. In this study, tRNS was delivered on twenty occasions to the dorsolateral prefrontal cortex. The authors found that the tRNS produced a modest improvement as reflected by the positive and negative symptom scale (PANSS) and the scale for assessing negative symptoms. However, the authors recognized that this effect could be associated with the medication (Palm et al., 2013).
Later, the effects of tRNS were tested in a female patient with paranoid schizophrenia, whose auditory verbal hallucinations did not seem to improve with medication, as severe suicidal conduct eventually developed. In this case report, tRNS at high frequencies was applied to the dorsolateral prefrontal cortex twice a day for five days, after which a global clinical improvement was reported. The analysis included a significant reduction in the PANSS score and the scale for assessing positive symptoms, producing effects that remained a month after the sessions (Haesebaert et al., 2014).
More recently, a randomized, double-blind pilot study evaluated patients with schizophrenia through hf-tRNS and various psychiatric medications (Chang et al., 2021). Here hf-tRNS was applied on AF3 (Figure 2) twice a day for five weeks to 35 patients with adequate antipsychotic medication and who exhibited negative symptoms, assessed through the PANSS factor score for negative symptoms (PANSS-FSNS). Consistent with prior studies, the negative symptoms of schizophrenia decreased in the group treated with hf-tRNS, maintaining this effect up to one month from the first hf-tRNS session. The authors suggested that the underlying mechanisms could be associated with increased cortical excitability in the brain, probably involving an SR-like phenomenon.
Figure 2.

A schizophrenic patient receives white noise through either transcranial random noise stimulation or tinnitus control apparatus.
In transcranial random noise stimulation, the cathode is placed on the AF3 position, aiming towards the dorsolateral prefrontal cortex. Figure is created with BioRender.com based on information from the following references: Chang et al. (2021) and Kaneko et al. (2013).
Finally, Kaneko et al. (2013) showed that auditory RNS delivered through a tinnitus control apparatus improved behavioral and psychological symptoms of dementia in six elder patients exhibiting dementia with schizophrenia (Figure 2). Here, the auditory RNS intervention applied once a day for four weeks significantly reduced the Neuropsychiatric Inventory scores in these patients compared to patients diagnosed with dementia. Furthermore, these authors did not find changes in the mini-mental state and Barthel tests in these patients with dementia and schizophrenia (Kaneko et al., 2013).
Although the etiology of schizophrenia has not yet been completely elucidated, there is work suggesting the possible involvement of neuronal noise as one of the factors in the symptoms observed (Braver et al., 1999). Therefore, we can speculate that the tRNS intervention could modulate such neuronal noise in patients with schizophrenia. Furthermore, this possibility supports the suggestion that dopamine serves as a gating neuromodulator, regulating access to context representations into active memory (Braver et al., 1999). Thus, the combined use of tRNS with the Braver et al. 1999 theory could be helpful to understand the benefits of tRNS in schizophrenia.
Moreover, increased neuronal noise in prefrontal cortical information processing in schizophrenia has been described (Winterer and Weinberger, 2004), reinforcing the proposal that tRNS could modulate such neuronal noise in these patients. Similarly, the increased cortical noise during cognitive tasks relates to a small local field potential synchronization in cortical microcircuits, translating into a decreased signal-to-noise ratio in cortical computations (Winterer and Weinberger, 2004). This is consistent with studies claiming that increased internal noise is responsible for facial recognition and speed discrimination (Christensen et al., 2013). Therefore, it is tempting to speculate that the application of tRNS could impact the reestablishment of these altered internal noise levels in schizophrenia disease. A recent review by Haller et al. (2022) discusses the promising therapeutic options of using tRNS in the treatment of schizophrenia.
Myopia, Amblyopia, and Visual Learning
Camilleri et al. (2014b) showed that a combination of behavioral training and tRNS can be fast and efficacious in improving sight in individuals with mild myopia. These authors examined whether 2 weeks of behavioral training with a Gabor patch procedure combined with online tRNS improved visual functions in participants with mild myopia compared to a 2-month behavioral training regime without tRNS (Figure 3). Perceptual learning acquired through a forced-choice task with a Gabor patch of two intervals combined with tRNS improved visual acuity (VA) and, more subtlety, the contrast sensitivity (CS) in patients with mild myopia (Camilleri et al., 2014b).
Figure 3.

An amblyopic/myopic patient undergoes a perceptual learning task, consisting of a two-interval forced-choice, where a single Gabor patch detection changed the contrast according to the performance.
During perceptual learning, transcranial random noise stimulation (tRNS) is delivered through an electrode positioned above the inion. After the task, visual acuity (VA) and contrast sensitivity (CS) are evaluated. Figure is created with BioRender.com based on information from the following reference: Camilleri et al. (2016).
Another experimental study using behavioral training illustrated in Figure 3 was conducted in eight sessions for two weeks. However, eight of the sixteen participants with mild myopia received hf-tRNS on the occipital cortex during the training. The control group, which only performed behavioral training, did not change VA and CS after the test. In contrast, the group that received concurrent hf-tRNS improved VA and CS as shown in the uncorrected VA and CS tests, with the progress being more pronounced at intermediate spatial frequencies for the latter (Camilleriet al., 2014a).
Subsequently, a randomized controlled trial carried out in thirty patients with mild myopia was developed to show that the improvement effects previously observed appeared in the following three conditions: perceptual learning alone, perceptual learning combined with hf-tRNS, and hf-tRNS alone (Camilleri et al., 2016; Campana et al., 2016). However, as mentioned above, a combination of behavioral and tRNS can be fast and efficacious in improving sight in individuals with mild myopia.
Later, a similar sham-controlled study recreated the behavioral training regime and the VA and CS assessment and hf-tRNS procedure, although it consisted of a larger sample (n = 20) (Moret et al., 2018). In this experimental design, two groups were formed, one which carried out the behavioral training with hf-tRNS and another with the same behavioral training with sham stimulation. In this study, CS did not exhibit a significant difference between groups, thus suggesting that hf-tRNS is not crucial for improving CS. However, a considerable improvement was only observed in the hf-tRNS group for VA. These results prove that the hf-tRNS is selective, producing differential effects in VA and CS in adults with amblyopia.
The abovementioned results are supported by recent evidence that tRNS produces a long-lasting improvement in VA in a 28-day follow-up. Still, it induces short-term CS improvements in adult amblyopic eyes (Donkor et al., 2021). However, further experiments will be necessary to understand the physiological mechanisms of these differential effects produced by hf-tRNS in these patients.
Tinnitus
The effect of electrical tRNS in tinnitus has shown exciting results. In a preliminary study, Claes et al. (2014) compared the impact of tRNS and tACS applied on T3 and T4 positions in 226 patients with chronic non-pulsatile tinnitus. These authors used both stimulation modalities either in a single session or in 8 sessions distributed in a four-week interval (Claes et al., 2014). Participants were asked to rank loudness and annoyance on a scale from 1 to 10, revealing that while tACS had no impact on reducing these symptoms, tRNS appeared to improve both.
This result of the beneficial effects of tRNS obtained by Claes et al. (2014) is consistent with previous reports that evaluated tinnitus loudness, distress, and annoyance after non-invasive brain stimulation consisting of tDCS, tACS, or tRNS, where all three aspects of the conditions were improved (Vanneste et al., 2013). However, after performing univariate analysis, it was shown that tRNS was responsible for most of the observed results in loudness and distress. These results open the question of whether tRNS acts through different mechanisms apart from tDCS and tACS, desynchronizing the over-synchronized network in the auditory cortex of tinnitus patients.
In a randomized controlled trial, other authors evaluated the effects of low-frequency tRNS (lf-tRNS), hf-tRNS, and whole spectrum tRNS in 154 chronic non-pulsatile tinnitus patients who underwent a single session of stimulation (Joos et al., 2015). The results indicated that both lf-tRNS and hf-tRNS positively impacted tinnitus loudness and distress reflected on a numerical scale report. However, these authors assumed that hf-tRNS only influenced pure tone tinnitus, while lf-tRNS affected pure tone and narrow bandwidth noise tinnitus. They also suggested that the effects observed after tRNS may be due to a non-focal effect, pointing towards modulation of areas involved in the distress network, such as the parahippocampal-subgenual anterior cingulate cortex (Figure 4).
Figure 4.
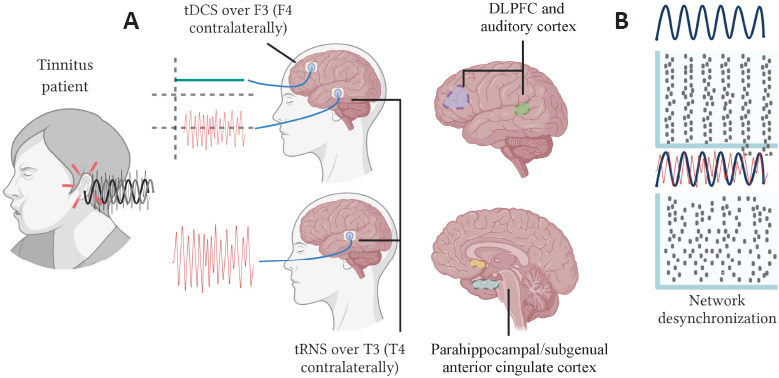
Different stimulation protocols are applied to a non-pulsatile tinnitus patient.
(A) Upper panel, tDCS is delivered over F3/F4 targeting the DLPFC and over T3/T4, aiming towards the auditory cortex to provide more substantial relief. Lower panel, the electrodes are only positioned on T3/T4, close to the parahippocampal/subgenual anterior cingulate cortex. (B) The effect of noise desynchronizing neural networks is shown, which could also influence tinnitus symptoms. Figure is created with BioRender.com based on information from the following references: Joos et al. (2015) and To et al. (2017). DLPFC: Dorsolateral prefrontal cortex; tDCS: transcranial direct current stimulation; tRNS: transcranial random noise stimulation.
Other authors employed the Tinnitus Questionnaire and numerical rating for annoyance, unpleasantness, and depression to explore the effects of tRNS in tinnitus patients who had previously received repetitive transcranial magnetic stimulation. This pilot study applied hf-tRNS at the T7/T8 electroencephalogram position in ten consecutive sessions, reducing tinnitus loudness after the tRNS even though some patients reported a temporary increase in tinnitus loudness (Kreuzer et al., 2019). Nevertheless, the effectiveness of the intervention (31%) was comparable to that obtained in repetitive transcranial magnetic stimulation in the center where the study took place. Furthermore, these results agree with a previous case report of a woman suffering from red ear syndrome in combination with tinnitus, in which tRNS given in 2-3 day sessions alleviated pain intensity and prolonged the interval between the pain episodes (Kreuzer et al., 2017).
Other procedures have shown a positive effect of tRNS on alleviating negative tinnitus symptoms, such as the paradigm developed by To et al. (2017). In this randomized controlled trial, patients received tDCS at F3 and F4, or tRNS delivered at T3 and T4 after tDCS (To et al., 2017). The added value of this combined tDCS and tRNS showed the most significant relief, although tDCS alone also reduced the Tinnitus Questionnaire score and the score of a visual analog scale for tinnitus. The authors claimed that tRNS inhibits the auditory cortex activity facilitating the prefrontal cortex output by tDCS, providing more potent relief (Figure 4).
Multiple Sclerosis and Post-Stroke
Another neurological disorder where the effect of tRNS has been addressed is multiple sclerosis (MS) (left panel of Figure 5). Mainly, two studies have explored this disease in the context of tRNS. First, a randomized controlled trial was carried out in patients with relapsing or remitting MS, in which tRNS was administered over the primary motor cortex (M1) of the most affected limb, at a frequency of 640 Hz, for 2 consecutive weeks (Salemi et al., 2019). The authors evaluated the patients’ fatigue through the modified fatigue impact scale. When compared to the sham control group, Salemi et al. (2019) found that the patients significantly improved after a week of tRNS. However, in a previous report, tRNS applied towards the dorsolateral prefrontal cortex did not produce significant changes in attention and mood in MS patients even though tRNS tended to diminish pain, as reflected in the decreased amplitude of pain-related evoked potentials (Palm et al., 2016). The lack of effects by tRNS in attention and mood could be possibly due to the short duration of the intervention, which consisted of two blocks of three consecutive sessions separated by three weeks. Consistently, in a recent single-blind, randomized controlled trial, the intervention with nGVS did not change the dizziness and imbalance symptoms in MS patients (Lotfi et al., 2021).
Figure 5.

Multiple sclerosis and post-stroke patients receive transcranial random noise stimulation through an electrode positioned over the primary motor cortex.
Note that post-stroke (PS) patients undergo GRASP therapy along with noisy stimulation. GRASP means graded repetitive arm supplementary program on motor rehabilitation. Figure is created with BioRender.com based on information from the following references: Palm et al. (2016) and Salemi et al. (2019).
Regarding limb disability secondary to subacute ischemic stroke, noisy electrical stimulation (tRNS) has also shown complementary benefits for rehabilitation in post-stroke patients (right panel of Figure 5). The application of tRNS in the corresponding motor cortex of the affected limb for five days, combined with a graded repetitive arm supplementary program, improved patients’ condition, as evaluated through the Fugl-Meyer assessment-upper extremity (FMA-UE). However, more work is necessary to know whether these beneficial effects can last more than one month after tRNS in subacute ischemic stroke patients.
Parkinson’s Disease
The uses of RNS in Parkinsonians revealed that this technique could change the electrical activity of their central nervous system and produce improvements in several motor disorders. For example, Stephani et al. (2011) demonstrated that hf-tRNS could decrease motor cortex excitability in Parkinson’s disease. Moreover, the stochastic whole-body vibration (mechanical RNS) improves bradykinesia and postural stability in Parkinson’s disease patients (Kaut et al., 2011). In the same way, Kaut et al. (2014) demonstrated that mechanical RNS improves postural stability in some clinical scores for patients with spinocerebellar ataxia. Furthermore, more recent studies confirmed that mechanical RNS applied to idiopathic Parkinson’s disease patients improves postural stability (Kaut et al., 2016).
Similarly, a crossover, double-blind, randomized study reported improvements in motor performance after tRNS applied over M1 in patients with multidimensional Parkinson’s disease with mild cognitive impairment (Monastero et al., 2020). In addition, this research demonstrated a reduction in the unified Parkinson’s disease rating scale values. Moreover, applying 24-hour flickering or pink-noise nGVS during a clinical trial in patients with multisystem atrophy presenting parkinsonian symptoms showed decreased response times during a continuous Go-Nogo task (Yamamoto et al., 2005) (Figure 6). Consistently, this effect of nGVS reflects the symptom severity in parkinsonian patients, occurring in akinetic and ataxic patients, in which the occurrence of an SR-like event produces an anti-akinetic effect through the vestibulo-cerebellar connections (Pan et al., 2008).
Figure 6.

Parkinson’s disease patients receive pink noise through electrodes placed over the primary motor cortex (M1) or the mastoids (noisy galvanic vestibular stimulation, nGVS).
The latter developed a continuous performance task during the stimulation. Figure is created with BioRender.com software based on information from the following references: Monastero et al. (2020) and Pan et al. (2008).
Vestibular-Postural Disorders and Sensitivity Loss
The effects of auditory or electrical noisy stimulation aiming towards central structures have been described in previous sections. However, there is also exciting work on the peripheral nervous system in skin mechanoreceptors. Many of these results have been reviewed by White et al. (2019) in the context of SR or SR-like phenomena. Thus suggesting that sub sensory mechanical-noise approaches are plausible options for preventing falls, especially in older people (White et al., 2019) and in treatments to enhance balance control in patients with diabetes and stroke symptoms (Liu et al., 2002; Priplata et al., 2006).
Among some of the most recent results reviewed by White et al. (2019) are the positive effects of vibratory and electrical noise stimuli (tactile RNS) on fingertips or feet for body sway (Magalhães and Kohn, 2011, 2014) in healthy persons, as well as for balance and hand sensitivity in patients with variable conditions, such as post-stroke motor impairment and diabetic neuropathy (Bagherzadeh et al., 2016). Other reviewed reports include the sub-plantar mechanical or transcutaneous electrical noise stimulation (transcutaneous RNS) that improves balance sensation and gait in diabetic and older adults (Bagherzadeh et al., 2016). We consider that future experiments about the beneficial effects of mechanical or transcutaneous RNS in the control of balance in these patients will require the simultaneous activation of skin receptors and spinal reflexes, given that subthreshold stimulus to the skin or Ia afferents activate feedback circuits that affect posture.
On the other hand, nGVS has been demonstrated to enhance postural stability and head rotation rhythm in patients with bilateral vestibular hypofunction (Ko et al., 2020). In such a study, the patients were asked to perform seventy trials of five seconds of walking with horizontal head movements. Then, the patients stopped and stood for another five seconds, and a computer-assisted system captured the motion (Figure 7). It was suggested that the nGVS exerted these effects by promoting neural plasticity in the vestibular cortex, as indicated by electroencephalogram signal changes in the bilateral precentral gyrus and parietal lobe. This is an example of the overall observation of this kind of nGVS improving balance control, postural sway, and dynamic gait stability in patients with bilateral vestibulopathy. In the same context, the nGVS also is capable of improving vestibular motion perception in healthy subjects (Wuehr et al., 2016, 2017; Dlugaiczyk et al., 2019). However, it is essential to note that some degree of neural activity is needed to produce these beneficial effects. For instance, a patient with bilateral vestibular loss did not appear to respond to these interventions (Wuehr et al., 2016, 2017; Dlugaiczyk et al., 2019).
Figure 7.

Noise improving balance is evaluated through motion capture and force plates in older people and patients with bilateral vestibulopathy receiving noise through noisy galvanic vestibular stimulation (nGVS) or vibrating insoles.
In addition, sensitivity is enhanced in diabetic patients with neuropathy receiving vibratory mechanical noise on the plantar surface or in the fingertips. Figure is created with BioRender.com based on information from the following references: Cloutier et al. (2009), Magalhães and Kohn (2011) and Priplata et al. (2006).
As happens with other noisy stimulation interventions, the mechanisms underlying the effects of nGVS in balance and locomotor stability are unknown, or they are attributed to SR-like phenomena (Mulavara et al., 2011, 2015; Goel et al., 2015; Galvan-Garza et al., 2018; Temple et al., 2018). Moreover, although nGVS has been proven safe, it still requires further experimentation in patients with postural disorders and sensitivity loss to evaluate its impact on the overall quality of those who receive it.
Conclusions and Future Directions of Random Noise Stimulation
We described several studies where noisy stimulation has benefited certain aspects of particular neurological and psychiatric disorders. As may have been noted, tRNS and nGVS are those modalities of stimulation that have been reported more widely. Nevertheless, perhaps because of the relatively recent development of such techniques, well-defined mechanisms of action are missing at the neurobiological level, and more systematic and controlled studies are required.
There are three common observations derived from this review. The first is that the optimal way to deliver RNS based therapies is with the concomitant use of neurological and neuropsychological assessments to validate the beneficial aftereffects. The second is that the tRNS produces beneficial aftereffects only in particular aspects of each neurological disorder. The third is that most authors correlate the observed effects to an SR-like phenomenon, which is exciting due to the novel paradigms that this theoretical approach implies and the extensive evidence of its presence in several neural systems. Therefore, it is possible that integrating the beneficial effect of RNS and the nonlinear behavior of the nervous system could offer new possibilities, not only for explaining the noisy stimulation but also for elucidating the pathophysiology of the diseases studied.
As proposed for tinnitus, noise in the RNS intervention could be working by desynchronizing a network whose over-synchronization accounts for the pathology observed. An over-synchronized network has already been associated with several psychiatric diseases such as schizophrenia and Parkinson’s disease (Uhlhaas and Singer, 2006). Therefore, it is tempting to speculate that the RNS intervention in these pathologies could also be working by desynchronizing over-synchronized neuronal networks. In this context, the use of new computational simulations showing the ability of external noise and the power of population heterogeneity to promote neural desynchronization (Hunsberger et al., 2014) could be helpful to understand the impact of tRNS in tinnitus, schizophrenia, and Parkinson’s disease.
Exploring other energies for noise stimulation beyond tRNS, nGVS, or acoustic noise could give more information about the differences in the nervous system’s response to each RNS intervention. Furthermore, experimental procedures involving no invasive interventions in humans combined with computational modeling could open more opportunities to investigate the effects of RNS in neurological disorders. Finally, previous results utilizing other types of non-invasive stimulation techniques such as tDCS and tACS (Liu et al., 2018) could help select appropriate targets in various disorders when different noisy energies could be employed as a therapeutic tool.
On the other hand, transcranial brain stimulation devices with different stimulation profiles have been used to treat epilepsy, probably much earlier than other neurological disorders. For instance, in drug-resistant epilepsy, tDCS can reduce seizure frequencies, lasting even up to two months, with a favorable safety profile (Sudbrack-Oliveira et al., 2021). Similarly, other studies have shown the efficacy of repetitive transcranial magnetic stimulation to reduce seizure frequency as well as epileptiform discharges (Cooper et al., 2017; Walton et al., 2021). Therefore, given the dynamic nature of epilepsy, studies of tRNS on epilepsy models or patients may shed more light on the physiological mechanisms of tRNS.
With the increasing evidence of noise being beneficial for neuronal function, RNS stimulation in neurological disorders could provide new information about how this benefit happens and the importance of the RNS interventions for health. We believe this is an attractive avenue for investigation, as noise has acquired a new position on our understanding of neural function. Hence, novel techniques of RNS with diverse energies offer promising approaches.
Additional file: Open peer review reports 1 (80.6KB, pdf) and 2 (80.7KB, pdf) .
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open peer reviewers: Ramkinker Mishra, Baylor College of Medicine, USA; Jiaxin Du, The University of Queensland, Australia.
P-Reviewers: Mishra R, Du J; C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
Funding: This work was supported by Cátedra Marcos Moshinsky (to EM), CONACyT Fronteras de la Ciencia #536 (to EM), VIEP-PIFI-FOMES-PROMEP-BUAP-Puebla (to EM), and Comité de Internacionalización de la Investigación (to EM), México.
References
- 1.Abikoff H, Courtney ME, Szeibel PJ, Koplewicz HS. The effects of auditory stimulation on the arithmetic performance of children with ADHD and nondisabled children. J Learn Disabil. 1996;29:238–246. doi: 10.1177/002221949602900302. [DOI] [PubMed] [Google Scholar]
- 2.Antal A, Herrmann CS. Transcranial alternating current and random noise stimulation:possible mechanisms. Neural Plast. 2016;2016:3616807. doi: 10.1155/2016/3616807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagherzadeh Cham M, Mohseni-Bandpei MA, Bahramizadeh M, Kalbasi S, Biglarian A. The clinical and biomechanical effects of subthreshold random noise on the plantar surface of the foot in diabetic patients and elder people:a systematic review. Prosthet Orthot Int. 2016;40:658–667. doi: 10.1177/0309364616631351. [DOI] [PubMed] [Google Scholar]
- 4.Baijot S, Slama H, Söderlund G, Dan B, Deltenre P, Colin C, Deconinck N. Neuropsychological and neurophysiological benefits from white noise in children with and without ADHD. Behav Brain Funct. 2016;12:11. doi: 10.1186/s12993-016-0095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benzi R, Sutera A, Vulpiani A. The mechanism of stochastic resonance. J Phys A Math Gen. 1981;14:L453–457. [Google Scholar]
- 6.Berger I, Dakwar-Kawar O, Grossman ES, Nahum M, Cohen Kadosh R. Scaffolding the attention-deficit/hyperactivity disorder brain using transcranial direct current and random noise stimulation:a randomized controlled trial. Clin Neurophysiol. 2021;132:699–707. doi: 10.1016/j.clinph.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Braver TS, Barch DM, Cohen JD. Cognition and control in schizophrenia:a computational model of dopamine and prefrontal function. Biol Psychiatry. 1999;46:312–328. doi: 10.1016/s0006-3223(99)00116-x. [DOI] [PubMed] [Google Scholar]
- 8.Brevet-Aeby C, Mondino M, Poulet E, Brunelin J. Three repeated sessions of transcranial random noise stimulation (tRNS) leads to long-term effects on reaction time in the Go/No Go task. Neurophysiol Clin. 2019;49:27–32. doi: 10.1016/j.neucli.2018.10.066. [DOI] [PubMed] [Google Scholar]
- 9.Camilleri R, Pavan A, Campana G. The application of online transcranial random noise stimulation and perceptual learning in the improvement of visual functions in mild myopia. Neuropsychologia. 2016;89:225–231. doi: 10.1016/j.neuropsychologia.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri R, Pavan A, Ghin F, Battaglini L, Campana G. Improvement of uncorrected visual acuity (UCVA) and contrast sensitivity (UCCS) with perceptual learning and transcranial random noise stimulation (tRNS) in individuals with mild myopia. Front Psychol. 2014a;5:1234. doi: 10.3389/fpsyg.2014.01234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camilleri R, Pavan A, Ghin F, Campana G. Improving myopia via perceptual learning:is training with lateral masking the only (or the most) efficacious technique? Atten Percept Psychophys. 2014b;76:2485–2494. doi: 10.3758/s13414-014-0738-8. [DOI] [PubMed] [Google Scholar]
- 12.Campana G, Camilleri R, Moret B, Ghin F, Pavan A. Opposite effects of high-and low-frequency transcranial random noise stimulation probed with visual motion adaptation. Sci Rep. 2016;6:38919. doi: 10.1038/srep38919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaieb L, Antal A, Paulus W. Transcranial random noise stimulation-induced plasticity is NMDA-receptor independent but sodium-channel blocker and benzodiazepines sensitive. Front Neurosci. 2015;9:125. doi: 10.3389/fnins.2015.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang CC, Lin YY, Tzeng NS, Kao YC, Chang HA. Adjunct high-frequency transcranial random noise stimulation over the lateral prefrontal cortex improves negative symptoms of schizophrenia:a randomized, double-blind, sham-controlled pilot study. J Psychiatr Res. 2021;132:151–160. doi: 10.1016/j.jpsychires.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Christensen BK, Spencer JMY, King JP, Sekuler AB, Bennett PJ. Noise as a mechanism of anomalous face processing among persons with schizophrenia. Front Psychol. 2013;4:401. doi: 10.3389/fpsyg.2013.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claes L, Stamberger H, Van De Heyning P, De Ridder D, Vanneste S. Auditory cortex tACS and tRNS for tinnitus:single versus multiple sessions. Neural Plast. 2014;2014:436713. doi: 10.1155/2014/436713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cloutier R, Horr S, Niemi JB, D'Andrea S, Lima C, Harry JD, Veves A. Prolonged mechanical noise restores tactile sense in diabetic neuropathic patients. Int J Low Extrem Wounds. 2009;8:6–10. doi: 10.1177/1534734608330522. [DOI] [PubMed] [Google Scholar]
- 18.Cook A, Bradley-Johnson S, Johnson CM. Effects of white noise on off-task behavior and academic responding for children with ADHD. J Appl Behav Anal. 2014;47:160–164. doi: 10.1002/jaba.79. [DOI] [PubMed] [Google Scholar]
- 19.Cooper YA, Pianka ST, Alotaibi NM, Babayan D, Salavati B, Weil AG, Ibrahim GM, Wang AC, Fallah A. Repetitive transcranial magnetic stimulation for the treatment of drug-resistant epilepsy:a systematic review and individual participant data meta-analysis of real-world evidence. Epilepsia Open. 2017;3:55–65. doi: 10.1002/epi4.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dlugaiczyk J, Gensberger KD, Straka H. Galvanic vestibular stimulation:from basic concepts to clinical applications. J Neurophysiol. 2019;121:2237–2255. doi: 10.1152/jn.00035.2019. [DOI] [PubMed] [Google Scholar]
- 21.Donkor R, Silva AE, Teske C, Wallis-Duffy M, Johnson AP, Thompson B. Repetitive visual cortex transcranial random noise stimulation in adults with amblyopia. Sci Rep. 2021;11:3029. doi: 10.1038/s41598-020-80843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fertonani A, Pirulli C, Miniussi C. Random noise stimulation improves neuroplasticity in perceptual learning. J Neurosci. 2011;31:15416–15423. doi: 10.1523/JNEUROSCI.2002-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galvan-Garza RC, Clark TK, Mulavara AP, Oman CM. Exhibition of stochastic resonance in vestibular tilt motion perception. Brain Stimul. 2018;11:716–722. doi: 10.1016/j.brs.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Ghin F, Pavan A, Contillo A, Mather G. The effects of high-frequency transcranial random noise stimulation (hf-tRNS) on global motion processing:an equivalent noise approach. Brain Stimul. 2018;11:1263–1275. doi: 10.1016/j.brs.2018.07.048. [DOI] [PubMed] [Google Scholar]
- 25.Goel R, Kofman I, Jeevarajan J, De Dios Y, Cohen HS, Bloomberg JJ, Mulavara AP. Using low levels of stochastic vestibular stimulation to improve balance function. PLoS One. 2015;10:e0136335. doi: 10.1371/journal.pone.0136335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haesebaert F, Mondino M, Saoud M, Poulet E, Brunelin J. Efficacy and safety of fronto-temporal transcranial random noise stimulation (tRNS) in drug-free patients with schizophrenia:a case study. Schizophr Res. 2014;159:251–252. doi: 10.1016/j.schres.2014.07.043. [DOI] [PubMed] [Google Scholar]
- 27.Haller N, Hasan A, Padberg F, Strube W, da Costa Lane Valiengo L, Brunoni AR, Brunelin J, Palm U. Transkranielle elektrische Hirnstimulationsverfahren zur Behandlung der Negativsymptomatik bei Schizophrenie [Transcranial electrical brain stimulation methods for treatment of negative symptoms in schizophrenia] Nervenarzt. 2022;93:41–50. doi: 10.1007/s00115-021-01065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunsberger E, Scott M, Eliasmith C. The competing benefits of noise and heterogeneity in neural coding. Neural Comput. 2014;26:1600–1623. doi: 10.1162/NECO_a_00621. [DOI] [PubMed] [Google Scholar]
- 29.Joos K, De Ridder D, Vanneste S. The differential effect of low- versus high-frequency random noise stimulation in the treatment of tinnitus. Exp Brain Res. 2015;233:1433–1440. doi: 10.1007/s00221-015-4217-9. [DOI] [PubMed] [Google Scholar]
- 30.Kaneko Y, Butler JP, Saitoh E, Horie T, Fujii M, Sasaki H. Efficacy of white noise therapy for dementia patients with schizophrenia. Geriatr Gerontol Int. 2013;13:808–810. doi: 10.1111/ggi.12028. [DOI] [PubMed] [Google Scholar]
- 31.Kaut O, Allert N, Coch C, Paus S, Grzeska A, Minnerop M, Wüllner U. Stochastic resonance therapy in Parkinson's disease. NeuroRehabilitation. 2011;28:353–358. doi: 10.3233/NRE-2011-0663. [DOI] [PubMed] [Google Scholar]
- 32.Kaut O, Brenig D, Marek M, Allert N, Wüllner U. Postural stability in Parkinson's disease patients is improved after stochastic resonance therapy. Parkinsons Dis. 2016;28:353–358. doi: 10.1155/2016/7948721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaut O, Jacobi H, Coch C, Prochnicki A, Minnerop M, Klockgether T, Wüllner U. A randomized pilot study of stochastic vibration therapy in spinocerebellar ataxia. Cerebellum. 2014;13:237–242. doi: 10.1007/s12311-013-0532-5. [DOI] [PubMed] [Google Scholar]
- 34.Ko LW, Chikara RK, Chen PY, Jheng YC, Wang CC, Yang YC, Li LPH, Liao KK, Chou LW, Kao CL. Noisy galvanic vestibular stimulation (Stochastic resonance) changes electroencephalography activities and postural control in patients with bilateral vestibular hypofunction. Brain Sci. 2020;10:1–15. doi: 10.3390/brainsci10100740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreuzer PM, Poeppl TB, Rupprecht R, Vielsmeier V, Lehner A, Langguth B, Schecklmann M. Daily high-frequency transcranial random noise stimulation of bilateral temporal cortex in chronic tinnitus –a pilot study. Sci Rep. 2019;9:12274. doi: 10.1038/s41598-019-48686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreuzer PM, Vielsmeier V, Poeppl TB, Langguth B. A case report on red ear syndrome with tinnitus successfully treated with transcranial random noise stimulation. Pain Physician. 2017;20:199–205. [PubMed] [Google Scholar]
- 37.Liu B, Lin Y, Gao X, Dang J. Correlation between audio-visual enhancement of speech in different noise environments and SNR:a combined behavioral and electrophysiological study. Neuroscience. 2013;247:145–151. doi: 10.1016/j.neuroscience.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Liu W, Lipsitz LA, Montero-Odasso M, Bean J, Kerrigan DC, Collins JJ. Noise-enhanced vibrotactile sensitivity in older adults, patients with stroke, and patients with diabetic neuropathy. Arch Phys Med Rehabil. 2002;83:171–176. doi: 10.1053/apmr.2002.28025. [DOI] [PubMed] [Google Scholar]
- 39.Liu A, Vöröslakos M, Kronberg G, Henin S, Krause MR, Huang Y, Opitz A, Mehta A, Pack CC, Krekelberg B, Berényi A, Parra LC, Melloni L, Devinsky O, Buzsáki G. Immediate neurophysiological effects of transcranial electrical stimulation. Nat Commun. 2018;9:5092. doi: 10.1038/s41467-018-07233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lotfi Y, Farahani A, Azimiyan M, Moossavi A, Bakhshi E. Comparison of efficacy of vestibular rehabilitation and noisy galvanic vestibular stimulation to improve dizziness and balance in patients with multiple sclerosis. J Vestib Res. 2021;31:541–551. doi: 10.3233/VES-201609. [DOI] [PubMed] [Google Scholar]
- 41.Magalhães FH, Kohn AF. Vibratory noise to the fingertip enhances balance improvement associated with light touch. Exp Brain Res. 2011;209:139–151. doi: 10.1007/s00221-010-2529-3. [DOI] [PubMed] [Google Scholar]
- 42.Magalhães FH, Kohn AF. Effectiveness of electrical noise in reducing postural sway:a comparison between imperceptible stimulation applied to the anterior and to the posterior leg muscles. Eur J Appl Physiol. 2014;114:1129–41. doi: 10.1007/s00421-014-2846-5. [DOI] [PubMed] [Google Scholar]
- 43.McDonnell MD, Ward LM. The benefits of noise in neural systems:Bridging theory and experiment. Nat Rev Neurosci. 2011;12:415–425. doi: 10.1038/nrn3061. [DOI] [PubMed] [Google Scholar]
- 44.Manjarrez E, Mendez I, Martinez L, Flores A, Mirasso CR. Effects of auditory noise on the psychophysical detection of visual signals:cross-modal stochastic resonance. Neurosci Lett. 2007;415:231–236. doi: 10.1016/j.neulet.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 45.Méndez-Balbuena I, Huidobro N, Silva M, Flores A, Trenado C, Quintanar L, Arias-Carrión O, Kristeva R, Manjarrez E. Effect of mechanical tactile noise on amplitude of visual evoked potentials:multisensory stochastic resonance. J Neurophysiol. 2015;114:2132–2143. doi: 10.1152/jn.00457.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metin B, Roeyers H, Wiersema JR, van der Meere JJ, Gasthuys R, Sonuga-Barke E. Environmental stimulation does not reduce impulsive choice in ADHD:a “pink noise”study. J Atten Disord. 2016;20:63–70. doi: 10.1177/1087054713479667. [DOI] [PubMed] [Google Scholar]
- 47.Monastero R, Baschi R, Nicoletti A, Pilati L, Pagano L, Cicero CE, Zappia M, Brighina F. Transcranial random noise stimulation over the primary motor cortex in PD-MCI patients:a crossover, randomized, sham-controlled study. J Neural Transm. 2020;127:1589–1597. doi: 10.1007/s00702-020-02255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moret B, Camilleri R, Pavan A, Lo Giudice G, Veronese A, Rizzo R, Campana G. Differential effects of high-frequency transcranial random noise stimulation (hf-tRNS) on contrast sensitivity and visual acuity when combined with a short perceptual training in adults with amblyopia. Neuropsychologia. 2018;114:125–133. doi: 10.1016/j.neuropsychologia.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 49.Mulavara AP, Fiedler MJ, Kofman IS, Wood SJ, Serrador JM, Peters B, Cohen HS, Reschke MF, Bloomberg JJ. Improving balance function using vestibular stochastic resonance:optimizing stimulus characteristics. Exp Brain Res. 2011;210:303–312. doi: 10.1007/s00221-011-2633-z. [DOI] [PubMed] [Google Scholar]
- 50.Mulavara AP, Kofman IS, De Dios YE, Miller C, Peters BT, Goel R, Galvan-Garza R, Bloomberg JJ. Using low levels of stochastic vestibular stimulation to improve locomotor stability. Front Syst Neurosci. 2015;9:117. doi: 10.3389/fnsys.2015.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nobukawa S, Wagatsuma N, Nishimura H, Doho H, Takahashi T. An Approach for stabilizing abnormal neural activity in ADHD using chaotic resonance. Front Comput Neurosci. 2021;15:726641. doi: 10.3389/fncom.2021.726641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Onorato I, D'Alessandro G, Di Castro MA, Renzi M, Dobrowolny G, Musarò A, Salvetti M, Limatola C, Crisanti A, Grassi F. Noise enhances action potential generation in mouse sensory neurons via stochastic resonance. PLoS One. 2016;11:e0160950. doi: 10.1371/journal.pone.0160950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palm U, Chalah MA, Padberg F, Al-Ani T, Abdellaoui M, Sorel M, Dimitri D, Créange A, Lefaucheur JP, Ayache SS. Effects of transcranial random noise stimulation (tRNS) on affect, pain and attention in multiple sclerosis. Restor Neurol Neurosci. 2016;34:189–199. doi: 10.3233/RNN-150557. [DOI] [PubMed] [Google Scholar]
- 54.Palm U, Hasan A, Keeser D, Falkai P, Padberg F. Transcranial random noise stimulation for the treatment of negative symptoms in schizophrenia. Schizophr Res. 2013;146:372–373. doi: 10.1016/j.schres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Pan W, Soma R, Kwak S, Yamamoto Y. Improvement of motor functions by noisy vestibular stimulation in central neurodegenerative disorders. J Neurol. 2008;255:1657–1661. doi: 10.1007/s00415-008-0950-3. [DOI] [PubMed] [Google Scholar]
- 56.Pavan A, Ghin F, Contillo A, Milesi C, Campana G, Mather G. Modulatory mechanisms underlying high-frequency transcranial random noise stimulation (hf-tRNS):a combined stochastic resonance and equivalent noise approach. Brain Stimul. 2019;12:967–977. doi: 10.1016/j.brs.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 57.Penton T, Bate S, Dalrymple KA, Reed T, Kelly M, Godovich S, Tamm M, Duchaine B, Banissy MJ. Using high frequency transcranial random noise stimulation to modulate face memory performance in younger and older adults:lessons learnt from mixed findings. Front Neurosci. 2018;12:863. doi: 10.3389/fnins.2018.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pickens TA, Khan SP, Berlau DJ. White noise as a possible therapeutic option for children with ADHD. Complement Ther Med. 2019;42:151–155. doi: 10.1016/j.ctim.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 59.Potok W, Bächinger M, van der Groen O, Cretu AL, Wenderoth N. Transcranial random noise stimulation acutely lowers the response threshold of human motor circuits. J Neurosci. 2021;41:3842–3853. doi: 10.1523/JNEUROSCI.2961-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Priplata AA, Patritti BL, Niemi JB, Hughes R, Gravelle DC, Lipsitz LA, Veves A, Stein J, Bonato P, Collins JJ. Noise-enhanced balance control in patients with diabetes and patients with stroke. Ann Neurol. 2006;59:4–12. doi: 10.1002/ana.20670. [DOI] [PubMed] [Google Scholar]
- 61.Remedios L, Mabil P, Flores-Hernández J, Torres-Ramírez O, Huidobro N, Castro G, Cervantes L, Tapia JA, De la Torre Valdovinos B, Manjarrez E. Effects of short-term random noise electrical stimulation on dissociated pyramidal neurons from the cerebral cortex. Neuroscience. 2019;404:371–386. doi: 10.1016/j.neuroscience.2019.01.035. [DOI] [PubMed] [Google Scholar]
- 62.Ross LA, Saint-Amour D, Leavitt VM, Javitt DC, Foxe JJ. Do you see what I am saying?Exploring visual enhancement of speech comprehension in noisy environments. Cereb Cortex. 2007;17:1147–1153. doi: 10.1093/cercor/bhl024. [DOI] [PubMed] [Google Scholar]
- 63.Rufener KS, Ruhnau P, Heinze H-J, Zaehle T. Transcranial random noise stimulation (tRNS) shapes the processing of rapidly changing auditory information. Front Cell Neurosci. 2017;11:162. doi: 10.3389/fncel.2017.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saiote C, Polanía R, Rosenberger K, Paulus W, Antal A. High-frequency TRNS reduces BOLD activity during visuomotor learning. PLoS One. 2013;8:59669. doi: 10.1371/journal.pone.0059669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salemi G, Vazzoler G, Ragonese P, Bianchi A, Cosentino G, Croce G, Gangitano M, Portera E, Realmuto S, Fierro B, Brighina F. Application of tRNS to improve multiple sclerosis fatigue:a pilot, single-blind, sham-controlled study. J Neural Transm. 2019;126:795–799. doi: 10.1007/s00702-019-02006-y. [DOI] [PubMed] [Google Scholar]
- 66.Sikström S, Söderlund G. Stimulus-dependent dopamine release in attention-deficit/hyperactivity disorder. Psychol Rev. 2007;114:1047–1075. doi: 10.1037/0033-295X.114.4.1047. [DOI] [PubMed] [Google Scholar]
- 67.Stephani C, Nitsche MA, Sommer M, Paulus W. Impairment of motor cortex plasticity in Parkinson's disease, as revealed by theta-burst-transcranial magnetic stimulation and transcranial random noise stimulation. Parkinsonism Relat Disord. 2011;17:297–298. doi: 10.1016/j.parkreldis.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 68.Söderlund G, Björk C, Gustafsson P. Comparing auditory noise treatment with stimulant medication on cognitive task performance in children with attention deficit hyperactivity disorder:Results from a pilot study. Front Psychol. 2016;7:1331. doi: 10.3389/fpsyg.2016.01331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Söderlund G, Sikström S, Loftesnes JM, Sonuga-Barke EJ. The effects of background white noise on memory performance in inattentive school children. Behav Brain Funct. 2010;6:55. doi: 10.1186/1744-9081-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Söderlund G, Sikström S, Smart A. Listen to the noise:noise is beneficial for cognitive performance in ADHD. J Child Psychol Psychiatry Allied Discip. 2007;48:840–847. doi: 10.1111/j.1469-7610.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- 71.Sudbrack-Oliveira P, Barbosa MZ, Thome-Souza S, Razza LB, Gallucci-Neto J, da Costa Lane Valiengo L, Brunoni AR. Transcranial direct current stimulation (tDCS) in the management of epilepsy:a systematic review. Seizure. 2021;86:85–95. doi: 10.1016/j.seizure.2021.01.020. [DOI] [PubMed] [Google Scholar]
- 72.Temple DR, De Dios YE, Layne CS, Bloomberg JJ, Mulavara AP. Efficacy of stochastic vestibular stimulation to improve locomotor performance during adaptation to visuomotor and somatosensory distortion. Front Physiol. 2018;9:301. doi: 10.3389/fphys.2018.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Terney D, Chaieb L, Moliadze V, Antal A, Paulus W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J Neurosci. 2008;28:14147–14155. doi: 10.1523/JNEUROSCI.4248-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.To WT, Ost J, Hart J, De Ridder D, Vanneste S. The added value of auditory cortex transcranial random noise stimulation (tRNS) after bifrontal transcranial direct current stimulation (tDCS) for tinnitus. J Neural Transm. 2017;124:79–88. doi: 10.1007/s00702-016-1634-2. [DOI] [PubMed] [Google Scholar]
- 75.Uhlhaas PJ, Singer W. Neural synchrony in brain disorders:Relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 76.van der Groen O, Wenderoth N. Transcranial random noise stimulation of visual cortex:Stochastic resonance enhances central mechanisms of perception. J Neurosci. 2016;36:5289–5298. doi: 10.1523/JNEUROSCI.4519-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vanneste S, Fregni F, De Ridder D. Head-to-head comparison of transcranial random noise stimulation, transcranial AC stimulation, and transcranial DC stimulation for tinnitus. Front Psychiatry. 2013;4:158. doi: 10.3389/fpsyt.2013.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walton D, Spencer DC, Nevitt SJ, Michael BD. Transcranial magnetic stimulation for the treatment of epilepsy. Cochrane Database Syst Rev. 2021;4:CD011025. doi: 10.1002/14651858.CD011025.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.White O, Babic J, Trenado C, Johannsen L, Goswami N. The promise of stochastic resonance in falls prevention. Front Physiol. 2019;9:1865. doi: 10.3389/fphys.2018.01865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 81.Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, Cohen LG, Fregni F, Herrmann CS, Kappenman ES, Knotkova H, Liebetanz D, Miniussi C, Miranda PC, Paulus W, Priori A, Reato D, Stagg C, Wenderoth N, Nitsche MA. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016;127:1031–1048. doi: 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wuehr M, Decker J, Schniepp R. Noisy galvanic vestibular stimulation:an emerging treatment option for bilateral vestibulopathy. J Neurol. 2017;264:81–86. doi: 10.1007/s00415-017-8481-4. [DOI] [PubMed] [Google Scholar]
- 83.Wuehr M, Nusser E, Decker J, Krafczyk S, Straube A, Brandt T, Jahn K, Schniepp R. Noisy vestibular stimulation improves dynamic walking stability in bilateral vestibulopathy. Neurology. 2016;86:2196–2202. doi: 10.1212/WNL.0000000000002748. [DOI] [PubMed] [Google Scholar]
- 84.Yamamoto Y, Struzik ZR, Soma R, Ohashi K, Kwak S. Noisy vestibular stimulation improves autonomic and motor responsiveness in central neurodegenerative disorders. Ann Neurol. 2005;58:175–181. doi: 10.1002/ana.20574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


