Abstract
The population of ammonia-oxidizing bacteria in a temperate oligotrophic freshwater lake was analyzed by recovering 16S ribosomal DNA (rDNA) from lakewater and sediment samples taken throughout a seasonal cycle. Nitrosospira and Nitrosomonas 16S rRNA genes were amplified in a nested PCR, and the identity of the products was confirmed by oligonucleotide hybridization. Nitrosospira DNA was readily identified in all samples, and nitrosomonad DNA of the Nitrosomonas europaea-Nitrosomonas eutropha lineage was also directly detected, but during the summer months only. Phylogenetic delineation with partial (345 bp) 16S rRNA gene sequences of clones obtained from sediments confirmed the fidelity of the amplified nitrosomonad DNA and identified two sequence clusters closely related to either N. europaea or N. eutropha that were equated with the littoral and profundal sediment sites, respectively. Determination of 701-bp sequences for 16S rDNA clones representing each cluster confirmed this delineation. A PCR-restriction fragment length polymorphism (RFLP) system was developed that enabled identification of clones containing N. europaea and N. eutropha 16S rDNA sequences, including subclasses therein. It proved possible to analyze 16S rDNA amplified directly from sediment samples to determine the relative abundance of each species compared with that of the other. N. europaea and N. eutropha are very closely related, and direct evidence for their presence in lake systems is limited. The correlation of each species with a distinct spatial location in sediment is an unusual example of niche adaptation by two genotypically similar bacteria. Their occurrence and relative distribution can now be routinely monitored in relation to environmental variation by the application of PCR-RFLP analysis.
Autotrophic ammonia-oxidizing bacteria are largely responsible for the oxidation of ammonia to nitrite and are important in the global cycling of nitrogen in terrestrial, aquatic, and marine ecosystems (8, 29). Difficulties in isolating and cultivating these slow-growing chemoautotrophs have encouraged the application of molecular biological methods to the study of their ecology. The existence of two phylogenetic groups, the nitrosospiras and nitrosomonads, within the β-subdivision of the class Proteobacteria was revealed by 16S ribosomal DNA (rDNA) sequencing (11), and further studies have considerably expanded the sequence database (15, 28, 37, 41, 42). Although most information on the physiology and biochemistry of ammonia oxidation has been obtained from experiments with the nitrosomonad Nitrosomonas europaea, studies of the molecular ecology of ammonia oxidizers have commonly recovered nitrosospira 16S rDNA sequences from natural environments (12, 37, 39). N. europaea and its close relative, Nitrosomonas eutropha, comprise a lineage described as Nitrosomonas “cluster 7” (15, 35, 37), that has been associated with enrichment cultures (17, 46), fertilized soils (9), and sewage treatment systems (13, 20, 44). More recently, sequence information on the functional ammonia monooxygenase gene (amo) has provided an alternative target for molecular ecological studies of both nitrosospiras and nitrosomonads (19, 23, 30, 34, 39).
Temperate freshwater lakes undergo stratification in summer to the extent that populations of ammonia-oxidizing bacteria become concentrated at the oxythermocline, where oxygen and ammonia gradients combine to produce conditions favorable for their proliferation (8). There is some evidence that differential ammonia concentrations in activated sludge, lakewater, and sediments have driven the adaptive evolution of different subpopulations (9, 10, 36, 40). Whether lakes of different nutrient statuses (i.e., hypereutrophic, eutrophic, and oligotrophic) also show differences in ammonia oxidizer community structure that can be equated with environmental variation is also worthy of investigation. Hastings et al. (10) studied the relative distribution of nitrosospiras and nitrosomonads in the water column and sediment of a eutrophic lake as it progressed through a seasonal stratification cycle. They confirmed the ubiquity of nitrosospiras and the failure to obtain direct 16S rDNA evidence for nitrosomonads without enrichment. It has been suggested that this is due to the targeting of the N. europaea-N. eutropha cluster 7 lineage rather than sequences from other nitrosomonads, such as Nitrosomonas ureae in Nitrosomonas cluster 6 (15, 35, 37), that are more relevant to the freshwater environment (28, 35). Ward et al. (46) used a range of 16S rDNA primers to study the phylogenetic diversity of ammonia oxidizers in lake samples and found variation in species composition with depth and between different aquatic environments. More recently, Phillips et al. (27) provided evidence for segregation at the genus level between two distinct environmental niches in seawater. In this paper, we describe the direct recovery of ammonia oxidizer 16S rDNA from sites within an oligotrophic lake environment throughout a seasonal cycle. Evidence for the presence of nitrosomonads of the N. europaea-N. eutropha lineage is presented, and we attempt to relate genotypic population composition to site location on the basis of both sequence and restriction fragment length polymorphism (RFLP) information.
MATERIALS AND METHODS
Bacterial strains.
The ammonia-oxidizing bacteria cultures used in this study were those maintained in the culture collection of the School of Biological Sciences; the origins and accession numbers of the strains are given by Head et al. (11). Cultures were maintained in the medium described by Watson and Mandel (47). Nucleic acids were extracted from these cultures as described previously (11) and were used as controls for the specificity of 16S rDNA PCR primers and oligonucleotide probes throughout.
Environmental sampling.
Sediment cores and lakewater samples were collected periodically throughout the year 1996 to 1997 from Buttermere, an oligotrophic freshwater lake in the English Lake District, Cumbria, United Kingdom. Both profundal (25-m depth) and littoral (10-m depth) sediments were sampled by using a modified Jenkin corer as described by Ohnstadt and Jones (24). Lake temperature and oxygen profiles were measured with a combined oxygen electrode and thermistor (model 57; Yellow Springs Instruments, Yellow Springs, Ohio). Lakewater (60 liters) taken at the oxycline (14-m depth) was concentrated to 1 liter by using a Pellicon tangential flow filtration apparatus (Millipore) equipped with three 0.45-μm-pore-size Durapore cassettes (5 ft2 per filter).
Extraction and PCR amplification of DNA from environmental samples.
For DNA isolation, the protocol of Bruce et al. (2) was applied to sediments, and the protocol of Schmidt et al. (33) was used for lakewater. The sequences of the PCR amplification primers and oligonucleotide probe (AAO258) are detailed by Hiorns et al. (12). A 100-μl reaction mixture was prepared that contained the following: 100 mM deoxynucleotide mix (Pharmacia Biotech), 80 μl of sterile Hypersolv water (BDH), 10 pM each forward and reverse primer, 10 μl of 10× buffer, template DNA equivalent to 4 to 10 ng of environmental DNA (or 1 ng of control pure culture DNA), and 2 U of Super Taq polymerase (HT Biotechnologies). The reaction mixture was overlaid with 2 to 3 drops of sterile mineral oil. PCR cycling was performed in a Perkin-Elmer 480 thermal cycler. The reaction parameters for eubacterial primers pAf and pHr (5) were 26 cycles of 94°C for 1 min, 55°C for 1 min, 72°C for 2 min, and 72°C for 5 min. Nested amplifications with the Nitrosospira-specific primer pair Ns85f and Ns1009r and Nitrosomonas-specific primer pair Nm75f and Nm1007r were annealed at 62 and 63°C, respectively. To enhance product yield for environmental samples, a hot start PCR protocol was adopted. Template DNA was added to the standard PCR mixture, and the reaction mixture was heated to 95°C for 6 min and then at 80°C for the addition of 2 U of Super Taq polymerase (HT Biotechnologies) through the overlay of mineral oil. Amplification products were resolved by electrophoresis of 10-μl aliquots of the reaction mixtures on a 0.8% (wt/vol) agarose gel run in 1× TAE buffer (40 mM Tris-acetate, 1 mM EDTA) (32).
16S rDNA oligonucleotide probe hybridization.
Electrophoresed PCR products were transferred from agarose gels to Positive (Appligene) nylon membranes by capillary action according to the manufacturer’s recommendations. In all experiments, 0.4 M NaOH was used for alkaline transfer of nucleic acids. Probes were end labelled with [γ-32P]dATP (ICN Supplies) by using 10 pM oligonucleotide, 1 μl of 10× kinase buffer (Tris-HCl, 50 mM; MgCl2, 10 mM; EDTA, 0.1 mM; dithiothreitol, 5 mM; spermidine, 0.1 mM [pH 8.2]), 6 μl of sterile Hypersolv water (BDH), 1 μl of T4 phage polynucleotide kinase (Boehringer-Mannheim), and 1 μl of [γ-32P]dATP (370 kbq) incubated at 37°C for 1 h. The prehybridization solution contained the following: blocking reagent (Boehringer; 2% [wt/vol]), 5× SSPE (20× SSPE is 3.6 M NaCl, 0.2 M Na2HPO4, and 20 mM EDTA, adjusted to pH 7.4), 20% (vol/vol) deionized formamide, 0.02% (wt/vol) sodium dodecyl sulfate and 0.1% (wt/vol) N-laurylsarcosine prepared in sterile deionized water. Prehybridization was for 1 h at 45°C. Hybridization with oligonucleotide probe (AAO258) was at 10 pM in 20 ml of hybridization solution (prehybridization solution with blocking reagent omitted) and was performed at 55°C overnight. Following hybridization, the membranes were washed three times for 5 min in fresh hybridization solution at the hybridization temperature. The filters were double wrapped in cling film and X-ray film (Fuji-film X-O-graphic) exposed for an appropriate period.
Cloning and sequencing of PCR products.
N. europaea-N. eutropha PCR amplicons recovered from profundal and littoral sediment samples were ligated into the pGEM-T vector (Promega) in accordance with the manufacturer’s instructions. Recombinant plasmids were used to transform 50 μl of high-efficiency competent Escherichia coli cells (Promega). Transformed cells were plated onto agar containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) and ampicillin as recommended by the manufacturer. White colonies were selected and checked for inserts by PCR amplification with the primers Nm75f and Nm1007r and by probing with oligonucleotide AAO258. Sequences were obtained with an automated laser ABI 373A DNA sequencer and analyzed by using the Genetics Computer Group (GCG) suite of programs (4). 16S rDNA sequences derived from environmental samples were aligned in conjunction with representative sequences deposited in the National Science Foundation Ribosomal Database Project (RDP) (25). Data analysis and manipulation were performed with the Genetic Data Environment (GDE) software (6). Phenograms were generated by using either the Jukes-Cantor (14) correction in the DNADIST program and the neighbor-joining method (31) or by maximum parsimony analysis from the PHYLIP 3.4. program. The robustness of the inferred phylogenies was determined by bootstrap analysis based on 100 resamplings of the data performed with SEQBOOT (PHYLIP 3.4). A consensus phenogram was generated by using the program CONSENSE (PHYLIP 3.4).
RFLP analysis of cloned 16S rDNA.
16S rDNA sequences of ammonia oxidizers in the GenBank and RDP databases were examined by using the MAPSORT program (GCG) to identify potential restriction sites. Restriction enzymes which theoretically distinguished between species were applied to 16S rDNA amplified from pure cultures of ammonia oxidizers, and restriction patterns were examined by agarose gel electrophoresis as described above. Restriction digestions were performed with enzymes supplied by Boehringer-Mannheim in accordance with the manufacturer’s instructions. 16S rDNA amplified from sediment samples by using Nitrosomonas-specific nested PCR amplification as described above was subjected to this restriction analysis.
Nucleotide sequence accession number.
The 16S rDNA sequences obtained from the environmental samples have been deposited in GenBank under accession no. AF134441 to AF134470.
RESULTS
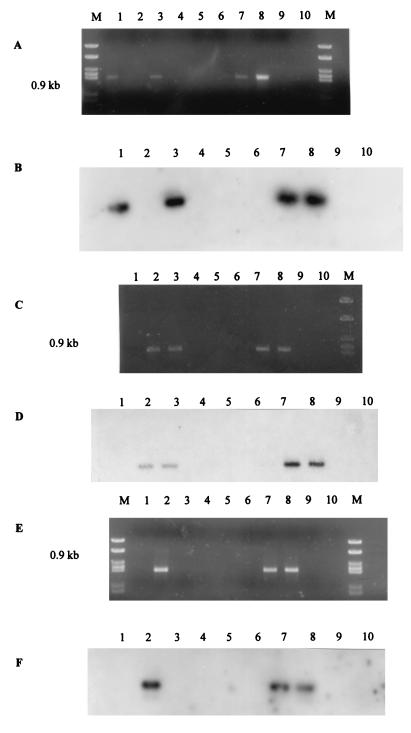
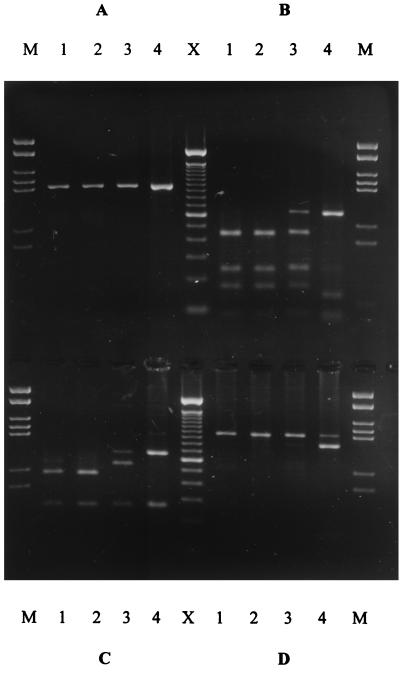
Buttermere has an area of 0.94 km2, a maximum depth of 28.6 m, and a mean depth of 16.6 m. The pH of the lakewater was constant at pH 6.6 to 6.9 throughout the year. Lake stratification became established in July and persisted until overturned in November. The oxythermocline was at a 14-m depth, which was used as the lakewater sampling point. Water column and sediment samples were analyzed by a nested PCR technique. Products obtained from the initial eubacterial PCR amplification by using the pAf-pHr primer pair were diluted appropriately and used as template DNA for PCRs with either the Nitrosospira-specific or N. europaea-N. eutropha-specific primers designed by Head et al. (11). Amplification of a 0.93-kb region of the 16S rRNA gene was achieved in all sediment and lakewater samples by using the Nitrosospira-specific primer pair Ns85f and Ns1009r as confirmed by hybridization to the oligonucleotide probe AAO258 (11). Nitrosospira spp. were therefore detected in Buttermere sediment and lakewater throughout the seasonal cycle (1995 to 1996). PCR amplification with the N. europaea-N. eutropha-specific primers (Nm75f and Nm1007r) (11) generated products of the correct size (0.9 kb) from a number of sediment and lakewater samples collected during the summer months (Fig. 1). The amplicons were confirmed as ammonia oxidizer rrn genes by Southern hybridization with the radioactively labelled oligonucleotide probe AAO258 (Fig. 1). Nitrosomonad DNA could not be detected in any of the sediment or lakewater samples taken during the winter months. The Nitrosomonas primer pair (Nm75f and Nm1007r) is highly specific for the N. europaea-N. eutropha lineage, but does not amplify 16S rDNA from other Nitrosomonas spp. (43). The N. europaea-N. eutropha environmental 16S rDNA obtained from this oligotrophic lake was subjected to further analysis.
FIG. 1.
Nitrosomonas-specific 16S rDNA amplification products from lakewater and sediment samples. (A and B) Littoral sediment. (A) Agarose gel. (B) Southern blot with AAO258. (C and D) Profundal sediment. (C) Agarose gel. (D) Southern blot with AAO258. (E and F) Lakewater. (E) Agarose gel. (F) Southern blot with AAO258. Lanes: 1, July 1995; 2, August 1995; 3, September 1995; 4, November 1995; 5, March 1996; 6, May 1996; 7, N. europaea (Nm50); 8, N. eutropha (Nm57); 9, Nitrosospira sp. (NV141); 10, sterile distilled water; M, MBI molecular weight marker, 21.
Ammonia-oxidizing bacterium 16S rDNA sequence diversity.
The N. europaea-N. eutropha amplification products were ligated into the pGEM-T cloning vector, and high transformation efficiencies were obtained (up to 5.86 × 109 CFU μg−1). A total of 73 clones were recovered, all of which hybridized to the ammonia oxidizer oligonucleotide probe AAO258. This further confirmed the widespread occurrence of the AAO258 target sequence in β-Proteobacteria ammonia oxidizers (37). A total of 27 of these clones (14 from littoral sediment samples and 13 from profundal sediment samples) were selected for partial sequence analysis (345 bp of the V3 region of the 16S rRNA gene). The inserts in each of the clones were sequenced in both directions with corroboration by a third analysis by using three primers: Nm75f, Nm1007r, and pDr. The primer sequences and E. coli positions for Nm75 and Nm1007r are described by Hiorns et al. (12), and the sequence and E. coli position for the third primer (pDr) are given by Edwards et al. (5). Data obtained from FASTA and BLAST searches of the GenBank database confirmed that the 14 clones from littoral sediment DNA (L) showed >95% homology to the 16S rDNA sequence of the N. europaea type strain. The 13 clones from profundal sediment DNA (P) were >95% homologous with the 16S rDNA sequence of the N. eutropha type strain. In all cases, the next 10 best alignments were also sequences from ammonia-oxidizing bacteria. These data suggest that the Nitrosomonas cluster 7 (15, 35, 37) population at each site is characteristic, with either N. europaea or N. eutropha-like organisms predominating. Furthermore, the specificity of the primer-probe combination used to obtain cloned DNA from environmental samples was confirmed by the sequence alignments without exception.
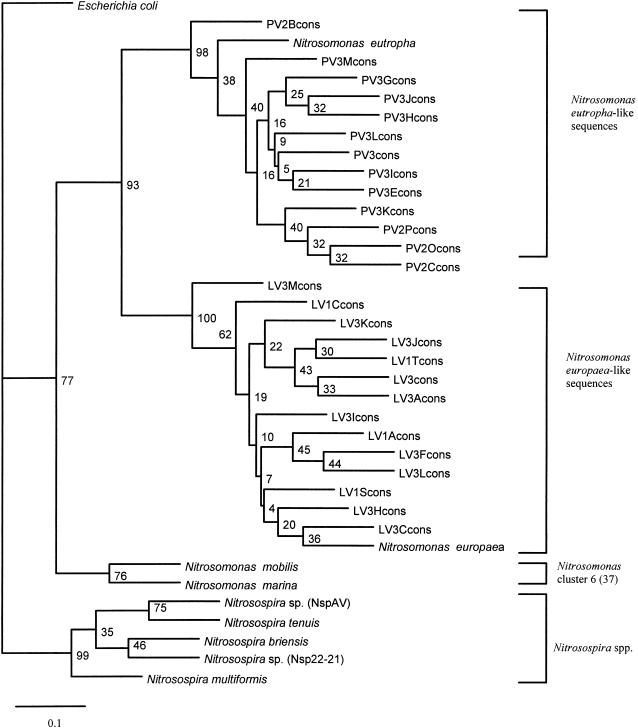
A phylogenetic tree constructed by the analysis of alignments of 27 unambiguous environmental DNA sequences and generated by the Jukes-Cantor DNA distance method (14) is presented in Fig. 2. The topology of the trees was confirmed by using maximum parsimony analysis of the bootstrapped sequence data. The branching order of the tree (Fig. 2) demonstrated that the sequences of 16S rDNA amplified by the N. europaea-N. eutropha primers from Buttermere sediments formed two clusters that corresponded to the location of the sampling site (i.e., profundal versus littoral sediment). The sequences (345 bp) from the littoral sediment samples (L) grouped with the N. europaea type strain, and the sequences (345 bp) derived from profundal sediment (P) grouped with N. eutropha. The relatively high bootstrap values confirmed the distinction between these two clusters. Bootstrap values within each group were low due to the high intracluster sequence homology and the relatively short length of sequence analyzed. Two clones (designated PV3 and LV3) from profundal and littoral sediments, respectively, were selected from each cluster, and extended sequences (701 bp) were determined. The phenogram generated was analogous to that previously obtained from the shorter sequences (345 bp) with comparable bootstrap values (data not shown). This more detailed sequence analysis of the representative clones resulted in greater definition of the two Nitrosomonas clusters and their linkage to the N. eutropha and N. europaea type strains, with bootstrap values of 100%. This is the first reported recovery of a spatial distinction between N. europaea and N. eutropha sequence homologues directly from the environment.
FIG. 2.
Phylogenetic tree showing the position within members of the class β-Proteobacteria of partial 16S rDNA sequences recovered from Buttermere lake sediments by using primers designed to amplify sequences from the β-subgroup ammonia-oxidizing bacteria. Sequences from the strains named were obtained from the RDP (25). Sequence analysis was performed for 345 base positions. E. coli was used as an outgroup. Bootstrap values represent percentages from 100 resamplings of the data (14, 31). The scale bar indicates 0.1 substitutions per nucleotide base. Environmental clones are referred to with reference to the sample site from which they were recovered, with PV3 indicating profundal sediment (September), PV2 indicating profundal sediment (August), LV1 indicating littoral sediment (July), and LV3 indicating littoral sediment (September). cons, consensus.
Restriction analysis of 16S rRNA genes of ammonia-oxidizing bacteria.
PCR-RFLP analysis can be used to analyze genotypes within complex microbial populations and was developed here on the basis of published sequence information. The objective was to provide a system for rapidly screening environmental clones (and ultimately amplified environmental DNA preparations) to make cluster assignments. Analysis of suitable restriction sites within a 0.93-kb region of the 16S rRNA gene from selected strains of ammonia-oxidizing bacteria was undertaken with the MAPSORT program in the GCG package (4). Three restriction enzymes (EagI, EaeI, and HinfI) could in theory be applied to differentiate N. europaea and N. eutropha 16S rDNA. In addition, EaeI discriminates between the Nitrosospira group and the N. europaea-N. eutropha lineage. A combination of the three restriction enzymes HaeIII, RsaI, and TaqI revealed theoretical RFLP patterns that further delineated six published Nitrosospira sequences from one another and from the two Nitrosomonas spp. (N. europaea and N. eutropha).
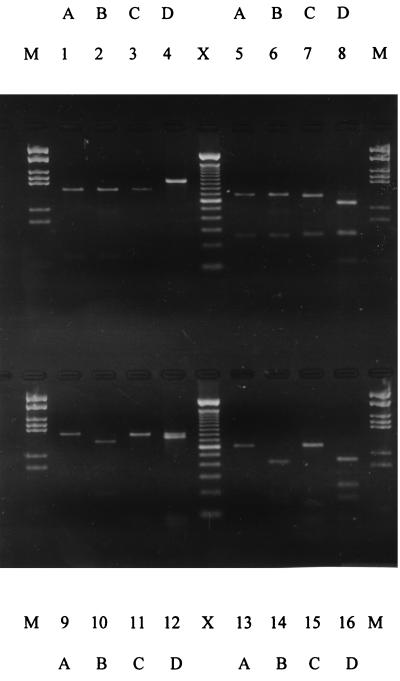
The 0.93-kb regions of 16S rDNA amplified from laboratory cultures of N. europaea, N. eutropha, and two strains of Nitrosospira (Nv141 and Nsp22) were digested with EagI, HinfI, TaqI, and HaeIII. The banding patterns (Fig. 3) gave the restriction profiles predicted by the MAPSORT analysis described above. The RFLP patterns with either HaeIII or TaqI differentiated the two Nitrosospira strains from one another, suggesting that this RFLP analysis has the potential to discriminate genotypes within the Nitrosospira group. EagI, HinfI, and HaeIII digestion of N. europaea and N. eutropha 16S rDNA gave RFLP patterns that discriminated between these two species (Fig. 3). Clones of 16S rDNA PCR amplified from Buttermere littoral and profundal sediment samples by using N. europaea-N. eutropha lineage primer pairs were similarly analyzed. Initially, the restriction analysis was applied to four sequenced clones of N. europaea and N. eutropha to confirm the validity of species identification based on the RFLP system. An example of the restriction profiles obtained is presented in Fig. 4. As expected from the data in Fig. 2 and 4, by RFLP, the LV3L and LV3K clones were identified as N. europaea sequences, and the PV3 and PV2P clones were identified as N. eutropha sequences. A total of 19 clones from amplified sediment 16S rDNA which had not been sequenced were then selected at random for similar analysis, and the RFLP data are presented in Table 1. Each of the clones was identified accordingly as either N. europaea or N. eutropha, and the distribution of these two Nitrosomonas spp. was again segregated between the two sediment sampling locations in complete agreement with the sequence data (Fig. 2). Further analysis of the RFLP banding patterns obtained with the three restriction enzymes enabled the recognition of five RFLP classes (Table 1). Two of the classes (A and B) were recovered from profundal sediment (N. eutropha), and three (C, D, and E) were recovered from littoral sediment (N. europaea). It is noteworthy that the N. europaea type strain was located in the RFLP class (E) that clearly predominated in littoral sediment (Table 1).
FIG. 3.
Agarose gel electrophoresis demonstrating the restriction patterns of a selection of ammonia oxidizer pure culture 16S rDNA sequences digested with EagI (lanes 1 to 4), HinfI (lanes 5 to 8), TaqI (lanes 9 to 12), or HaeIII (lanes 13 to 16). Lanes: A, Nitrosospira sp. (NV141); B, Nitrosospira sp. (Nsp.22); C, N. europaea (Nm50); D, N. eutropha (Nm57); M, MBI molecular weight marker, 21; X, 100-bp ladder (Gibco).
FIG. 4.
Agarose gel electrophoresis demonstrating the restriction patterns of cloned 16S rDNA from Buttermere sediments digested with HaeIII (lanes 5 to 8), HinfI (lanes 9 to 12), EagI (lanes 13 to 16), or uncut DNA (lanes 1 to 4). Lanes: A, clone LV3K; B, clone LV3; C, clone PV2P; D, clone PV3K; M, MBI molecular weight marker, 21; X, 100-bp ladder (Gibco).
TABLE 1.
Distribution of RFLP classes amongst Nitrosomonas 16S rDNA clones from sediment samples and pure cultures
| Sediment or culture | No. of clones within PCR-RFLP classa:
|
|||||
|---|---|---|---|---|---|---|
| Total | A | B | C | D | E | |
| Sediments | ||||||
| Profundal | 7 | 5 | 2 | |||
| Littoral | 12 | 1 | 1 | 10 | ||
| Cultures | ||||||
| N. eutropha (Nm57) | 1 | 1 | ||||
| N. europaea (Nm50) | 1 | 1 | ||||
RFLP classes A to E correspond to DNA banding patterns on agarose gels after restriction digestion with enzymes EaeI, HinfI, and HaeIII. A and B, N. eutropha-like; C, D, and E, N. europaea-like.
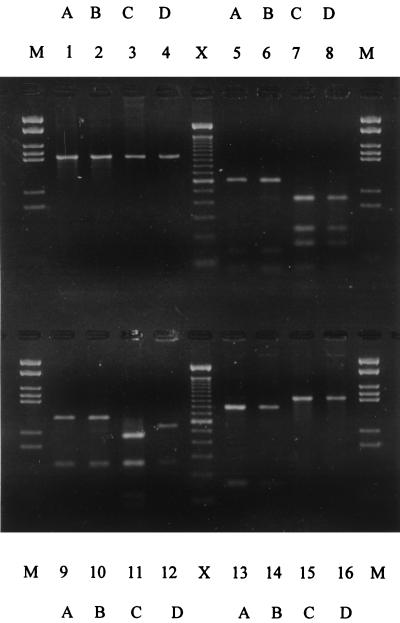
The amplified Nitrosomonas 16S rDNA products from the two profundal and two littoral sediment samples cloned and sequenced to produce the phylogenetic tree (Fig. 2) were analyzed, and the RFLP patterns are presented in Fig. 5. Each of the three enzymes generated RFLP patterns that were in agreement in their classification of each 16S rDNA sample. The two profundal 16S rDNAs identified as showing the relative predominance of N. eutropha over N. europaea by cloning and sequencing (Fig. 2) were also classified as such by the RFLP analysis (Fig. 5, lanes 1 and 2). Similarly, one of the littoral 16S rDNAs demonstrated a relative predominance of N. europaea over N. eutropha (Fig. 2 and Fig. 5, lane 4). The other littoral 16S rDNA (Fig. 5, lane 3) gave a pattern that suggested a mixture of the two species, although all 11 of the clones sequenced from this sample had been identified as N. europaea (Fig. 2).
FIG. 5.
Agarose gel electrophoresis of 16S rDNA amplified from profundal and littoral sediment samples with N. europaea-N. eutropha lineage primers and restriction digested with EaeI, HinfI, and HaeIII. (A) Uncut DNA. (B) HaeIII. (C) HinfI. (D) EaeI. Lanes: 1, profundal sediment (September); 2, profundal sediment (August); 3, littoral sediment (September); 4, littoral sediment (July); M, MBI molecular weight marker, 21; X, 100-bp DNA mass ladder (Gibco).
DISCUSSION
Analysis of bacterial DNA amplified directly from environmental samples has become a well-established approach to the determination of community structure in an ecological context. Ammonia-oxidizing bacteria are functionally important in terrestrial and aquatic ecosystems. In the study reported here, the presence of autotrophic ammonia-oxidizing bacteria in sediment and lakewater was detected by nested PCR amplification and subsequent oligonucleotide probing. While direct amplification of ammonia oxidizer 16S rDNA from the environment in a single step has been demonstrated (15, 18, 35, 38), the nested approach has the advantage that species of limited abundance may also be recovered. The application of oligonucleotide probe hybridization to PCR products throughout also ensured the fidelity of the amplified DNA, confirmed by the observation that all sequenced clones gave close matches with ammonia oxidizer 16S rDNA sequences in the GenBank database. The data for the oligotrophic Buttermere environment reported here corroborate those reported previously for the eutrophic Esthwaite Water environment (12) and indeed elsewhere (15, 37) in demonstrating the ubiquity of nitrosospiras. There is accordingly no shortage of phylogenetic analyses of environmental Nitrosospira sequences, while direct detection of Nitrosomonas 16S rDNA is less frequently reported and is usually associated with nutrient-rich environments (9, 13, 20). Nitrosomonas 16S rDNA can readily be detected in enrichment cultures inoculated with either freshwater (10, 12) or marine (17) samples from which nitrosomonad 16S rDNA could not be recovered directly. A number of subclusters within the nitrosomonad group of β-Proteobacteria have been described in recent publications, with the N. europaea-N. eutropha lineage designated as cluster 7 (15, 35, 38). It has been suggested that failure to detect nitrosomonads directly has been due to the use of primers that target only the closely related species N. europaea and N. eutropha, which have a limited environmental distribution that does not include the natural freshwater environment (28). The data presented here clearly show direct detection of the N. europaea-N. eutropha lineage in both freshwater and sediment samples from Buttermere, but only in the summer months, perhaps suggesting that these organisms are not predominant within the freshwater ammonia-oxidizing community. It may be significant that the only other report of direct detection of N. europaea-N. eutropha 16S rDNA in freshwater was also from an oligotrophic lake (35).
The direct amplification of Nitrosomonas spp. from Buttermere was only achieved with samples taken during the summer months (July to September). This seasonal occurrence corresponds to a slight increase in ammonia concentration from 5 μg liter−1 (January) to 12 μg liter−1 (July) at a 5-m depth (7), which may have led to an increase in Nitrosomonas cell numbers. This relationship between Nitrosomonas population size and nutrient concentration would explain the direct molecular biological detection of N. europaea in sewage treatment systems (20, 45), enrichment cultures (12), and amended soils (9), but not the failure to detect these species in eutrophic lakes (10, 12). The relative distribution of species within the ammonia-oxidizing community of complex environments such as freshwater lakes is, however, likely to be influenced by many parameters. Ward et al. (46) reported that oxygen concentration, temperature, and inorganic nutrient concentrations across the oxic-anoxic interface of the Plussee, a eutrophic environment, all affected the distribution of ammonia oxidizers.
Due to the relative paucity of 16S rDNA sequence information about nitrosomonad DNA recovered directly from the environment, we concentrated our cloning and sequencing study on these samples. The high specificity of the primer pair used to amplify DNA from members of the N. europaea-N. eutropha lineage was confirmed by the alignment of all environmental sequences obtained with this group (Fig. 2). In fact, the sequences formed two clusters centered on each of the type strains, demonstrating the occurrence of both species in Buttermere sediment. N. europaea and N. eutropha are very closely related on the basis of full 16S rDNA sequence alignments (11), to the extent that separate species status is doubtful. The recovery of distinct clusters here, with bootstrap support (93% [Fig. 2]), at least confirms that there are two centers of variation within this lineage. The complete segregation of clones from profundal and littoral sediment into two Nitrosomonas clusters most closely related to each of these named species is surprising, given their close 16S rDNA sequence similarity. The data suggest that the genotypes are location specific and have adapted to the conditions present at each site. Unlike higher organisms, examples of the relationship between environmental niche and speciation are relatively rare in environmental microbiology, where speciation is less well defined and the structure of the bacterial community is heterogeneous and complex. In the marine environment, Phillips et al. (27) recently used 16S rDNA data to demonstrate the predominance of N. eutropha in particle-associated samples in contrast to planktonic samples that were dominated by Nitrosospira spp. In terrestrial environments, niches are isolated and distinct ammonia oxidizer species compositions have been described and related to environmental parameters (15, 18, 39). Oxygen tension is the most likely significant difference between the littoral (10 m) and profundal (25 m) sediment sites studied, because the oxycline in summer was recorded at a 14-m depth. Enrichment studies of Esthwaite Water provided evidence of distinct ammonia oxidizer communities on the basis of ammonia sensitivity (10), and we have obtained similar data for Buttermere (not shown). However, the phylogenetic analysis presented in Fig. 2 actually demonstrates that the nitrosomonad 16S rDNA sequences recovered from two distinct sites are related to two species. It is worth noting that this kind of fine-scale observation of the relationship between ammonia oxidizer species composition and habitats within an environment can only be made by the application of molecular biological techniques.
Descriptions of the genotypic composition of natural microbial communities is best achieved by cloning and sequencing 16S rDNA recovered from environmental samples. This is time-consuming and laborious, and methods based on DNA fingerprinting are therefore attractive. Denaturing or temperature gradient gel electrophoresis is emerging as a powerful technique in this respect (21) and has been applied to ammonia-oxidizing bacterial communities (15, 16, 38). Sequence information and the phylogenies produced can also be used to develop protocols for direct detection of well-defined genotypes or species by RFLP, and this was the approach adopted here for N. europaea and N. eutropha. When using 16S rDNA amplified with N. europaea-N. eutropha-specific primers, restriction enzymes can be selected which differentiate pure cultures of each species and clones that have been identified by sequence analysis (Fig. 3 and 4). The utility of this RFLP method was demonstrated by identifying clones with one or the other of the two species without the need to sequence the 16S rDNA, and the RFLP data further confirmed the segregation of N. europaea and N. eutropha-like 16S rDNA sequences between two sediment sites. We extended this analysis to 16S rDNA amplified from each site and were able to classify the samples in relation to the relative abundance of N. europaea and N. eutropha 16S rDNA sequences, one against the other (Fig. 5), in accordance with the detailed phylogenetic analysis. In one of the four samples analyzed, the data suggested that both species were present, but the protocol can at least be applied to screen samples for further analysis. We used four restriction enzymes and suggest that environmental samples can be processed in this way to rapidly determine both the presence and relative predominance of these two ammonia-oxidizing species and monitor shifts between them in relation to environmental variation. Again, this information on the occurrence and distribution of N. europaea and N. eutropha would be virtually impossible to obtain without the application of molecular biological techniques and would be cumbersome by conventional cloning and sequence analysis. In addition to the differentiation of environmental 16S rDNA sequences which form clades with either N. europaea or N. eutropha, our RFLP data on classes within this group and on nitrosospiras (Table 1) show that the technique could be further developed to refine genotypic analysis of ammonia-oxidizing communities in general. Previous studies have applied PCR-RFLP analysis to analyze the sequence diversity of bacterial populations in the environment (3, 22, 26, 45). Ward (45) identified intra- and intersite diversity of sequences from isolates of aquatic denitrifying bacteria by RFLP analysis. In a similar study, Navarro et al. (22) characterized populations of Nitrobacter from soils and a freshwater lake. They applied PCR-RFLP analysis to DNA sequences from the intergenic spacer region (IGS) and suggested that the Nitrobacter populations which coexisted in the same niches were genetically divergent. For ammonia-oxidizing bacteria, RFLP identification based on PCR-amplified 16S rDNA should be unequivocal, because ribotyping of the 16S and 23S rDNA from 12 isolates of ammonia-oxidizing bacteria revealed the presence of only one rrn operon per genome (1), an observation that is probably correlated with the slow growth rate of these chemoautotrophic bacteria.
ACKNOWLEDGMENTS
We are grateful to Glenn Rhodes and Paul Loughnane for assistance with lakewater and sediment sampling and Angela Rosin for technical assistance with DNA sequencing. This work was funded by the Natural Environment Research Council (NERC) of the United Kingdom.
REFERENCES
- 1.Aakra A, Utåker J B, Nes I F. Restriction fragment length polymorphism of rRNA genes and sequencing of the 16S-23S rDNA intergenic spacer region of ammonia-oxidising bacteria: a phylogenetic approach. Int J Syst Bacteriol. 1999;49:123–130. doi: 10.1099/00207713-49-1-123. [DOI] [PubMed] [Google Scholar]
- 2.Bruce K D, Hiorns W D, Hobman J L, Osborn A M, Strike P, Ritchie D A. Amplification of DNA from native populations of soil bacteria by using the polymerase chain reaction. Appl Environ Microbiol. 1992;58:3413–3416. doi: 10.1128/aem.58.10.3413-3416.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruce K D. Analysis of mer gene subclasses within bacterial communities in soils and sediments resolved by fluorescent-PCR–restriction fragment length polymorphism profiling. Appl Environ Microbiol. 1997;63:4914–4919. doi: 10.1128/aem.63.12.4914-4919.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence-analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards U, Rogall T, Blöcker H, Emde M, Böttger E C. Isolation and direct complete nucleotide determination of entire genes. Characterisation of a gene encoding 16S ribosomal RNA. Nucleic Acids Res. 1989;17:7843–7853. doi: 10.1093/nar/17.19.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felsenstein J. PHYLIP (Phylogeny Inference Package) version 3.5c. Seattle: Department of Genetics, University of Washington; 1993. [Google Scholar]
- 7.Freshwater Biological Association. Annual reports 1938–1998. Ambleside, United Kingdom: Freshwater Biological Association; 1998. [Google Scholar]
- 8.Hall G H. Nitrification in lakes. In: Prosser J I, editor. Nitrification. Oxford, United Kingdom: IRL Press; 1986. pp. 127–156. [Google Scholar]
- 9.Hastings R C, Ceccherini M T, Miclaus N, Saunders J R, Bazzicalupo M, McCarthy A J. Direct molecular biological analysis of ammonia-oxidising bacteria populations in cultivated soil plots treated with swine manure. FEMS Microbiol Ecol. 1997;23:45–54. [Google Scholar]
- 10.Hastings R C, Saunders J R, Hall G H, Pickup R W, McCarthy A J. Application of molecular biological techniques to a seasonal study of ammonia oxidation in a eutrophic freshwater lake. Appl Environ Microbiol. 1998;64:3674–3682. doi: 10.1128/aem.64.10.3674-3682.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Head I M, Hiorns W D, Embley T M, McCarthy A J, Saunders J R. The phylogeny of autotrophic ammonia-oxidising bacteria as determined by analysis of 16S ribosomal RNA gene sequences. J Gen Microbiol. 1993;139:1147–1153. doi: 10.1099/00221287-139-6-1147. [DOI] [PubMed] [Google Scholar]
- 12.Hiorns W D, Hastings R C, Head I M, McCarthy A J, Saunders J R, Pickup R W, Hall G H. Amplification of 16S ribosomal RNA genes of the autotrophic ammonia-oxidizing bacteria demonstrates the ubiquity of Nitrosospiras in the environment. Microbiology. 1995;141:2793–2800. doi: 10.1099/13500872-141-11-2793. [DOI] [PubMed] [Google Scholar]
- 13.Hovanec T A, DeLong E F. Comparative analysis of nitrifying bacteria associated with freshwater and marine aquaria. Appl Environ Microbiol. 1996;62:2888–2896. doi: 10.1128/aem.62.8.2888-2896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jukes T H, Cantor C R. Evolution of protein molecules. In: Munro H N, editor. Mammalian protein metabolism. New York, N.Y: Academic Press; 1969. pp. 21–132. [Google Scholar]
- 15.Kowalchuk G A, Stephen J R, De Boer W, Prosser J I, Embley T M, Woldendorp J W. Analysis of ammonia-oxidizing bacteria of the β subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl Environ Microbiol. 1997;63:1489–1497. doi: 10.1128/aem.63.4.1489-1497.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kowalchuk G A, Naoumenko Z S, Derikx P, Felske A, Stephen J R, Arkhipchenko I A. Molecular analysis of ammonia-oxidizing bacteria of the β subdivision of the class Proteobacteria in compost and composted materials. Appl Environ Microbiol. 1999;65:396–403. doi: 10.1128/aem.65.2.396-403.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCaig A E, Embley T M, Prosser J I. Molecular analysis of enrichment cultures of marine ammonia-oxidisers. FEMS Microbiol Lett. 1994;120:363–368. doi: 10.1111/j.1574-6968.1994.tb07059.x. [DOI] [PubMed] [Google Scholar]
- 18.McCaig A E, Phillips C J, Stephen J R, Kowalchuk G A, Harvey S M, Herbert R A, Embley T M, Prosser J I. Nitrogen cycling and community structure of proteobacterial β-subgroup ammonia-oxidizing bacteria within polluted marine fish farm sediments. Appl Environ Microbiol. 1999;65:213–220. doi: 10.1128/aem.65.1.213-220.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McTavish H, Fuchs J A, Hooper A B. Sequence of the gene coding for ammonia monooxygenase in Nitrosomonas europaea. J Bacteriol. 1993;175:2436–2444. doi: 10.1128/jb.175.8.2436-2444.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mobarry B K, Wagner M, Urbain V, Rittmann B E, Stahl D A. Phylogenetic probes for analyzing abundance and spatial organization of nitrifying bacteria. Appl Environ Microbiol. 1996;62:2156–2162. doi: 10.1128/aem.62.6.2156-2162.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muyzer G, De Waal E C, Uitterlinden A G. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarro E, Simonet P, Norman P, Bardin R. Characterization of natural populations of Nitrobacter spp. using PCR/RFLP analysis of the ribosomal intergenic spacer. Arch Microbiol. 1992;157:107–115. doi: 10.1007/BF00245277. [DOI] [PubMed] [Google Scholar]
- 23.Norton J M, Low J M, Klotz M G. The gene encoding ammonia-monooxygenase subunit A exists in three nearly identical copies in Nitrosospira sp. NpAV. FEMS Microbiol Lett. 1996;139:181–188. doi: 10.1111/j.1574-6968.1996.tb08200.x. [DOI] [PubMed] [Google Scholar]
- 24.Ohnstad F R, Jones J G. The Jenkin surface mud sampler. User manual. Freshwater Biological Association annual report no. 15. Ambleside, United Kingdom: Freshwater Biological Association; 1982. . (Occasional publication.) [Google Scholar]
- 25.Olsen G J, Larsen N, Woese C R. The ribosomal RNA database project (RDP) Nucleic Acids Res. 1991;19(Suppl.):2017–2021. doi: 10.1093/nar/19.suppl.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osborn A M, Bruce K D, Strike P, Ritchie D A. Polymerase chain reaction-restriction length polymorphism analysis shows divergence among mer determinants from gram-negative soil bacteria indistinguishable by DNA-DNA hybridization. Appl Environ Microbiol. 1993;59:4024–4030. doi: 10.1128/aem.59.12.4024-4030.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips C J, Smith Z, Embley T M, Prosser J I. Phylogenetic differences between particle-associated and planktonic ammonia-oxidizing bacteria of the β subdivision of the class Proteobacteria in the northwestern Mediterranean Sea. Appl Environ Microbiol. 1999;65:779–786. doi: 10.1128/aem.65.2.779-786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pommerening-Röser A, Rath G, Koops H-P. Phylogenetic diversity within the genus Nitrosomonas. Syst Appl Microbiol. 1996;19:344–351. [Google Scholar]
- 29.Prosser J I. Autotrophic nitrification in bacteria. Adv Microb Physiol. 1989;30:125–181. doi: 10.1016/s0065-2911(08)60112-5. [DOI] [PubMed] [Google Scholar]
- 30.Rotthauwe J-H, Witzel K-P, Liesack W. The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of the natural ammonia-oxidizing populations. Appl Environ Microbiol. 1997;63:4704–4712. doi: 10.1128/aem.63.12.4704-4712.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saitou N, Nei M. The neighbour joining method; a new method for constructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Schmidt T M, DeLong E F, Pace N R. Analysis of a marine picoplankton community by 16S rRNA gene cloning and sequencing. J Bacteriol. 1991;173:4371–4378. doi: 10.1128/jb.173.14.4371-4378.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinigalliano C D, Kuhn D N, Jones R D. Amplification of the amoA gene from diverse species of ammonia-oxidizing bacteria and from an indigenous bacterial population from seawater. Appl Environ Microbiol. 1995;61:2702–2706. doi: 10.1128/aem.61.7.2702-2706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Speksnijder A, Kowalchuk G A, Roest K, Laanbroek H. Recovery of a Nitrosomonas-like 16S rDNA sequence group from freshwater habitats. Syst Appl Microbiol. 1998;21:321–330. doi: 10.1016/S0723-2020(98)80040-4. [DOI] [PubMed] [Google Scholar]
- 36.Stehr G, Bottcher B, Dittberner P, Rath G, Koops H-P. The ammonia-oxidizing nitrifying populations of the River Elbe estuary. FEMS Microbiol Ecol. 1995;17:177–186. [Google Scholar]
- 37.Stephen J R, McCaig A E, Smith Z, Prosser J I, Embley T M. Molecular diversity of soil and marine 16S rRNA gene sequences related to β-subgroup ammonia-oxidizing bacteria. Appl Environ Microbiol. 1996;62:4147–4154. doi: 10.1128/aem.62.11.4147-4154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephen J R, Kowalchuk G A, Bruns M-A V, McCaig A E, Phillips C J, Embley T M, Prosser J I. Analysis of β-subgroup proteobacterial ammonia oxidizer populations in soil by denaturing gradient gel electrophoresis analysis and hierarchical phylogenetic probing. Appl Environ Microbiol. 1998;64:2958–2965. doi: 10.1128/aem.64.8.2958-2965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephen J R, Chang Y-J, Macnaughton S J, Kowalchuk G A, Leung K T, Flemming C A, White D C. Effect of toxic metals on indigenous soil β-subgroup proteobacterium ammonia oxidizer community structure and protection against toxicity by inoculated metal-resistant bacteria. Appl Environ Microbiol. 1999;65:95–101. doi: 10.1128/aem.65.1.95-101.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suwa Y, Sumino T, Noto K. Phylogenetic relationships of activated sludge isolates of ammonia-oxidisers with different sensitivities to ammonium sulphate. J Gen Appl Microbiol. 1997;43:373–379. doi: 10.2323/jgam.43.373. [DOI] [PubMed] [Google Scholar]
- 41.Teske A, Alm E, Regan J M, Toze S, Rittmann B E, Stahl D A. Evolutionary relationships among ammonia- and nitrite-oxidizing bacteria. J Bacteriol. 1994;176:6623–6630. doi: 10.1128/jb.176.21.6623-6630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Utåker J B, Bakken L R, Jiang Q Q, Nes I F. Phylogenetic analysis of seven new isolates of the highly related ammonia-oxidising bacteria based on 16S rRNA gene sequences. Syst Appl Microbiol. 1995;18:549–559. [Google Scholar]
- 43.Utåker J B, Nes I F. A qualitative evaluation of the published oligonucleotide specific for the 16S rRNA gene sequences of the ammonia-oxidising bacteria. Syst Appl Microbiol. 1998;21:1–17. doi: 10.1016/S0723-2020(98)80010-6. [DOI] [PubMed] [Google Scholar]
- 44.Wagner M, Rath G, Amann R, Koops H-P, Schleifer K-H. In situ identification of ammonia-oxidising bacteria. Syst Appl Microbiol. 1995;18:251–264. [Google Scholar]
- 45.Ward B B. Diversity of culturable denitrifying bacteria-limits of rDNA RFLP analysis and probes for the functional gene, nitrite reductase. Arch Microbiol. 1995;163:167–175. [Google Scholar]
- 46.Ward B B, Voytek M A, Witzel K-P. Phylogenetic diversity of natural populations of ammonia-oxidisers investigated by specific PCR amplification. Microb Ecol. 1997;33:87–96. doi: 10.1007/s002489900011. [DOI] [PubMed] [Google Scholar]
- 47.Watson S W, Mandel M. Comparisons of the morphology and deoxyribonucleic acid components of 27 strains of nitrifying bacteria. J Bacteriol. 1971;107:563–569. doi: 10.1128/jb.107.2.563-569.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]