Abstract
Transferring the contralateral C7 nerve root to the median or radial nerve has become an important means of repairing brachial plexus nerve injury. However, outcomes have been disappointing. Electroencephalography (EEG)-based human-machine interfaces have achieved promising results in promoting neurological recovery by controlling a distal exoskeleton to perform functional limb exercises early after nerve injury, which maintains target muscle activity and promotes the neurological rehabilitation effect. This review summarizes the progress of research in EEG-based human-machine interface combined with contralateral C7 transfer repair of brachial plexus nerve injury. Nerve transfer may result in loss of nerve function in the donor area, so only nerves with minimal impact on the donor area, such as the C7 nerve, should be selected as the donor. Single tendon transfer does not fully restore optimal joint function, so multiple functions often need to be reestablished simultaneously. Compared with traditional manual rehabilitation, EEG-based human-machine interfaces have the potential to maximize patient initiative and promote nerve regeneration and cortical remodeling, which facilitates neurological recovery. In the early stages of brachial plexus injury treatment, the use of an EEG-based human-machine interface combined with contralateral C7 transfer can facilitate postoperative neurological recovery by making full use of the brain’s computational capabilities and actively controlling functional exercise with the aid of external machinery. It can also prevent disuse atrophy of muscles and target organs and maintain neuromuscular junction effectiveness. Promoting cortical remodeling is also particularly important for neurological recovery after contralateral C7 transfer. Future studies are needed to investigate the mechanism by which early movement delays neuromuscular junction damage and promotes cortical remodeling. Understanding this mechanism should help guide the development of neurological rehabilitation strategies for patients with brachial plexus injury.
Key Words: arm injuries, brachial plexus, brain-computer interfaces, nerve transfer, nerve regeneration, nerve tissue, neurofeedback, neurological rehabilitation, user-computer interface
Introduction
Traumatic brachial plexus injury (BPI) is a common nerve injury caused mainly by reverse movement of the head, neck, and shoulder. The brachial plexus can also be damaged by surgery or radiation (Yan et al., 2019). BPI is a severe and devastating condition that is observed in up to 4.2% of all multi-trauma patients, usually affects young adults, and has significant socioeconomic implications (Yan et al., 2019; Estrella et al., 2021). Severe BPI, particularly total nerve root avulsion, can lead to partial or complete loss of upper limb function and has poor prognosis. Early nerve repair is essential for functional recovery. Existing repair methods of BPI include nerve repair, nerve grafting, and nerve transfer (Colbert and Mackinnon, 2008; Wehrli et al., 2011; Miller et al., 2021). Other compensatory treatments, such as muscle and tendon transplantation, attempt to restore motor function of the upper limb. Although these repair methods can achieve promising results, motor function is usually not completely restored. Even after successful surgical reconnection of injured peripheral nerves and treatment with different growth factors, satisfactory neurological recovery may not occur (Rocco et al., 2018; Yi et al., 2019).
Peripheral nerve regeneration after BPI is challenging in clinical practice for several reasons (Rui et al., 2018). Because BPI is a proximal nerve injury, it takes a long time for the regenerating nerve to grow and innervate the distal effector. During this extended period, the distal effector can atrophy, which greatly reduces the likelihood of functional recovery (Kemp et al., 2010). In addition, cortical plasticity is a critical factor that affects the success of repair after peripheral nerve injury (PNI) (Wang et al., 2010; Facchini et al., 2021). If the reentrant nerve plays a dominant role, it must form an effective reconnection in the cortex (Sturma et al., 2018; Hou et al., 2020). Therefore, satisfactory peripheral nerve regeneration and cortical remodeling are only possible over the long term.
Since 2013, researchers have been applying electroencephalography (EEG) signal-based upper limb control devices to assist with movement, as shown in Table 1. The primary purpose of the brain-computer interface is to accurately identify and extract EEG signals that control limb movement. The signals are translated and amplified by the computer and then transmitted across the PNI site to a robotic arm exoskeleton or distal electrical stimulation device. Unlike existing purely mechanically driven rehabilitation devices, EEG-based devices can actively control distal limb movement to maintain target muscle activity. Early functional exercise not only promotes peripheral nerve rehabilitation, but also accelerates cerebral cortical remodeling. At the same time, it retransmits peripheral feedback signals, which optimizes the mechanical response to maximize the rehabilitation effect. The timeline of studies of EEG-based human-machine interface combined with contralateral C7 transfer for the treatment of BPI is shown in Figure 1.
Table 1.
A summary of electroencephalography (EEG) signal-based prosthesis in patients with functional limb loss
| Authors | Electrode counts (n) | Features | Task | Application significance |
|---|---|---|---|---|
| Robinson et al., 2013 | 128 | Regularized wavelet-common spatial pattern algorithm | Hand movement | High precision controllable hand-assisted mobility device |
| Yi et al., 2013 | 64 | Multi-class CSP; multi-class stationary Tikhonov regularized CSP; multi-class CSP based on generalized eigenvector | Limb action | Medium-precision controllable upper limb mobility device |
| Woo et al., 2015 | 64 | CSP algorithm | Arm movements | Medium-precision controllable upper limb mobility device |
| Jochumsen et al., 2016 | 25 | Temporal features and spectral features and their combinations | Hand grasping | Low-precision controllable hand mobility device |
| Roy et al., 2017 | 29 | Autoregressive parameter, Hjorth parameter, correlation dimension, Hurst’s exponent | Decoding different grasp types | Low precision controlled upper limb mobility device |
| Iturrate et al., 2018 | 64 | Temporal and spectral domains | R-G actions & variable force | Medium precision upper limb strengthening device |
| Roy et al., 2018 | 29 | Correlation dimension in different bands | Grasp patterns | Low-precision controlled upper limb mobility device |
| Schwarz et al., 2018 | 61 | Low-frequency time domain features from 0.3 to 3 Hz | Reach to grasp actions | Medium-precision prosthetic devices |
CSP: Common spatial patterns.
Figure 1.
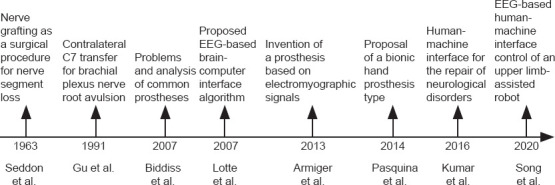
Timeline of studies of electroencephalography-based human-machine interface combined with contralateral C7 transfer for the treatment of brachial plexus injury.
This review summarizes the application of C7 nerve transfer in treating ipsilateral BPI, discusses the possible mechanism by which the brain-computer interface promotes BPI rehabilitation, and proposes a viable procedure for brachial plexus repair that promotes functional recovery.
Search Strategy
This manuscript systematically reviews the rodent, non-human primate, and human studies of nerve transfer treatment of BPI that have been published between 1990 and 2021. The PubMed and EMBASE databases were searched using the following key words: “brachial plexus injury”, “C7 nerve transfer”, and “peripheral nerve regeneration”. The articles generated by the search were further screened based on titles and summaries. The databases were also searched for studies of brain-computer interface use in nerve injury repair using the following key words: “arm injuries”, “brachial plexus”, “brain-computer interface”, “nerve transfer”, “nerve regeneration”, “nerve tissue”, “neurofeedback”, “neurological rehabilitation”, and “user-computer interface”.
Brachial Plexus Injury
Brachial plexus injury treatment
BPI can cause loss of shoulder elevation, abduction, and rotation; elbow flexion and extension; and wrist and finger flexion and extension (Sulaiman et al., 2009; Schessler and McClellan, 2010). As Figure 2 shows, BPI can be classified according to the portion of the arm predominantly affected: upper arm (C5–C6 with or without C7 injury), lower arm (C8–T1 injury), and whole arm (C5–T1 injury). Injury treatment mainly consists of surgery, including nerve repair, nerve grafting, nerve transfer, and functional free muscle transplantation to replace original muscle function (Dubuisson and Kline, 2002; Muhetidier et al., 2011).
Figure 2.
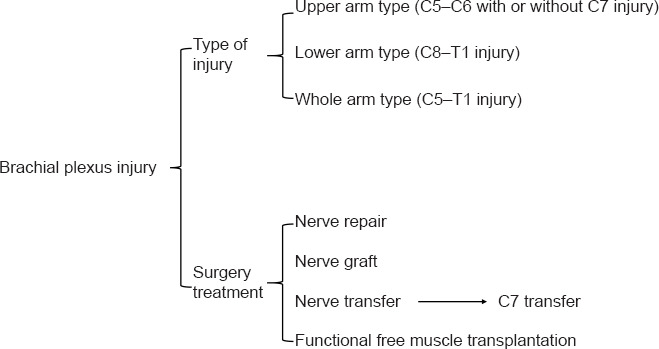
Types of brachial plexus injury and treatment.
Direct suturing of the adventitia/intima is the most common method of nerve repair and is mainly suited for acute localized injury. The proximal and distal nerve stumps can be directly sutured in tension-free injuries, which include sharp nerve transection and penetrating injury. Nerve transplantation can be used when the nerve defect is too large for tension-free direct suturing. The sural nerve is commonly harvested to use for grafting; other options include the medial brachial cutaneous nerve or the medial forearm cutaneous nerve. Although these donor nerves do not have a sufficient number of medullary nerve fibers to repair multiple thicker nerves (Garg et al., 2011; Davidge et al., 2021; Hill et al., 2021; Macêdo et al., 2021; Rasulić et al., 2021), nerve transplantation avoids the need for donor region cortical remodeling and provides a sufficient number of motor bundles. However, nerve donor area damage is inevitable, and it cannot fundamentally solve the problems of neuromuscular dysfunction and muscle atrophy.
Functional muscle and tendon transplantation can also partially restore injured limb function. The trapezius muscle is usually preserved in BPI patients and can be transferred to repair the shoulder. Although the superior trapezius muscle has been used to repair shoulder abduction function, results have been mixed (Terzis and Kostopoulos, 2007; Elhassan et al., 2009). As the function of the infraspinatus muscle is closely related to that of shoulder abduction, the repair method in which the infraspinatus muscle is used as the donor has a success rate of greater than 90% (Elhassan, 2014). However, shoulder abduction and rotation require action of multiple muscles (Elhassan et al., 2012) and single tendon transfer cannot completely restore function; therefore, it is often necessary to rebuild multiple functions. Moreover, the outcome of muscle and tendon transplantation is often not ideal and therefore is generally used when neurosurgery has failed or the patient suffers from forearm and hand motor function limitation.
C7 transfer
The concept of nerve transfer as a nerve injury treatment was introduced in the 1950s. With advances in knowledge and surgical technology, it has become a preferred method for BPI treatment (Feng et al., 2019; Fasce et al., 2021). The spinal accessory, phrenic, suprascapular, axillary, and intercostal nerves as well as the contralateral C7 nerve root may all be used as the donor for nerve transfer (Midha, 2004; Addas and Midha, 2009). A summary of contralateral C7 transfer for various injuries is shown in Table 2. Because isolated C7 nerve root transection does not lead to obvious motor and sensory defects, Gu et al. (1992) proposed using the contralateral C7 nerve root as the donor in repair of traumatic brachial plexus avulsion injury. Anatomically, the muscles innervated by C7 are also usually innervated by C6; C8, C5, and T1 may also play a partial role (Li et al., 2017; Liu et al., 2018). In addition, the C7 nerve root contains more myelinated nerve fibers than other donor nerves, which can provide sufficient power for the transplantation area (Alawieh et al., 2021). In summary, nerve transfer has become a widely used and often successful method to treat BPI. However, surgical repair of complex brachial plexus defects cannot resolve the problems of neuromuscular junction dysfunction and target muscle atrophy.
Table 2.
A summary of contralateral C7 transfer for median, musculocutaneous and radial/triceps nerve injuries
| Recipient nerve | Authors | Number | Injury type | Great motor recovery | Great sensory recovery | Application significance |
|---|---|---|---|---|---|---|
| Median nerve | Gu et al., 1992 | 4 | Total BPAI | 2 | 3 | The earliest report |
| Ei-Gammal et al., 2002 | 7 | Total BPAI | NA | NA | Least effective coverage | |
| Chen et al., 2007 | 3 | Total BPAI | 3 | 3 | Best results reported | |
| Muhetidier et al., 2011 | 16 | Total BPAI | 3 | 11 | Improved transplant effect | |
| Tu et al., 2014 | 40 | Total BPAI | 5 | 21 | Improved transplant effect | |
| Musculocutaneous nerve | Gu et al., 1992 | 3 | Total BPAI | 2 | NA | The earliest report |
| Hierner et al., 2007 | 6 | Total BPAI | 1 | NA | Least effective coverage | |
| Chuang et al., 2012 | 23 | NA | 19 | NA | Best results reported | |
| Wang et al., 2013 | 47 | Total BPAI | 28 | NA | Improved transplant effect | |
| Radial Nerve | Gu et al., 1992 | 2 | Total BPAI | 1 | NA | The earliest report |
| Hattori et al., 2005 | 1 | Total BPI | 0 | NA | Least effective coverage | |
| Terzis et al., 2009 | 10 | NA | 2 | NA | Improved transplant effect | |
| Muhetidier et al., 2011 | 2 | Total BPAI | 0 | NA | Least effective coverage | |
| Triceps nerve | Terzis et al., 2009 | 21 | NA | 7 | NA | The earliest report |
| Terzis et al., 2012 | 20 | NA | 5 | NA | Improved transplant effect | |
| Gao et al., 2013 | 10 | Total BPAI | 0 | NA | Least effective coverage |
BPAI: Brachial plexus avulsion injury; NA: not available.
Realization of Human-Machine Interfaces
Types of human-machine interfaces
The invasive human-machine interface includes skeletal muscle implantation and peripheral nerve implantation (Navarro et al., 2005; Christensen et al., 2014; Gelenitis et al., 2021; Hejazi et al., 2021). As shown in Figure 3A and B, the peripheral nerve-implanted electrode and muscle-implanted electrode can collect the local electrical nerve signals of the corresponding area with high precision. This human-machine interface mainly relies on an invasive guide needle or electrode to obtain signals from skeletal, muscle, or peripheral nerves. Its advantage lies in the stability and high intensity of the signal sources. However, the trauma induced by the needle or electrode limits its clinical application. Although some invasive electrodes are designed to promote peripheral nerve growth, regenerative electrodes can only be used in transected peripheral nerves. Regenerated axons grow slowly through the channel; therefore, the effect cannot be quickly verified by experiments. While it is feasible to use regenerative electrodes to stimulate a small number of regenerated fibers (Spearman et al., 2020), there is no practical way to achieve complete nerve regeneration. The signal source of the non-invasive human-machine interface mainly comes from EEG and electromyography (EMG) (Kuiken et al., 2007; Marchal-Crespo and Reinkensmeyer, 2009).
Figure 3.
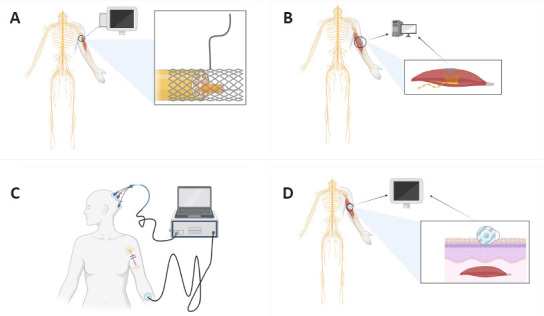
Types of human-machine interface.
(A) Peripheral nerve-implanted electrode. Black line: electric wire; black circle: peripheral nerve injury site; (B) Muscle-implanted electrode. Black circle: peripheral nerve injury site; (C) EEG human-machine interface. Black line: electric wire; (D) EMG human-machine interface. Black circle: Peripheral nerve injury site; black box: magnified view of local damage.
As shown in Figure 3C and D, the EEG and EMG signals of a non-invasive interface are not determined as accurately as invasive electrode signals. Moreover, they are also difficult to distinguish from confounding signals arising from surrounding tissues. Through a series of complex signal processors, functional control signals are extracted, and external flexible exoskeletons or unique robotic arms are directed to assist patients in active recovery. The use of a non-invasive human-machine interface allows complete avoidance of surgical risks. A previous study has shown that a brain-computer interface can perform delicate tasks such as text creation (Marshall and Farah, 2021). Warwick et al. (2003) implanted multielectrode arrays in a healthy subject and achieved bidirectional transmission between the experimental robot arm and the peripheral nervous system. These works have opened many research opportunities in the fields of human-machine interface and activity monitoring (Xiao and Menon, 2019). The signals acquired by the brain-computer interface originate from the cerebral cortex, which is characterized by a complex signal. However, multi-channel high-density electrodes are now available that can accurately acquire signals from different distribution points within a specific area and differentiate and integrate them to obtain the desired signal band. After decoding by a computer, a similar effect to that of an invasive human-machine interface can be achieved. Therefore, compared with an invasive human-machine interface, the non-invasive EEG-based interface ensures recognition accuracy, reduces unnecessary trauma, and has better application prospects in promoting nerve injury rehabilitation.
EEG human-computer interface
Human-machine collaboration helps to improve the recognition of human motion signals. The two main types of non-invasive electrical signals used to recognize movement intention are EEG and EMG (Leeb et al., 2011; Cui et al., 2017). The use of EEG signals allows for faster detection of motor intent than EMG (Haufe et al., 2011). In human-machine collaborative systems, early decoding of human movement intentions allows for better execution of assigned tasks. Considerable research has been conducted regarding brain-computer interface systems over the last few decades. For example, in 1988, Farwell and Donchin (1988) developed an EEG control system with selectable letters. Wolpawant et al. (1991) first investigated an EEG-based computer cursor controller. Edelman et al. (2019) used EEG signals to achieve neural control of robotic devices. Decoding motor parameters directly from EEG signals provides intuitive and natural control compared with brain-computer interfaces based on motor images or visual evoked potentials (Ofner et al., 2019).
Application of a human-machine interface based on EEG
The human-machine interface based on EEG does not directly promote nerve regeneration to achieve improved function. Rather, it connects to a flexible exoskeleton or mechanical arm through the organic coupling of nerves and an electronic system with the help of an external environment to achieve average or superior rehabilitation effects. Several studies have demonstrated the effect of the mechanical exoskeleton on functional rehabilitation. Lo et al. (2010) showed that mechanical exoskeleton adjuvant therapy could be used to achieve better function and task completion ability than conventional treatment and demonstrated how human-machine auxiliary therapy improves the therapeutic effects of traditional therapy. Because of progress in this technology, development of the human-machine interface has gradually moved in the direction of bilateral transmission. With bilateral transmission, the interface can accurately control exoskeleton function by receiving electrical signals from the center and feeding back motor information that prompts the central nervous system to feel the presence of limbs. This feedback system is shown in Figure 4.
Figure 4.
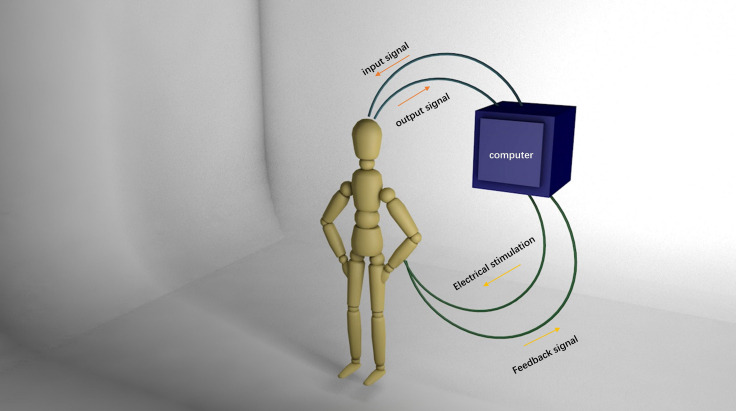
Schematic diagram of an electroencephalography-based human-machine interface.
The computer obtains an electrical signal from the cerebral cortex and directly transmits this signal downward to the distal end of the injury with a certain intensity of electrical stimulation. At the same time, the feedback signal is collected and processed by the computer and transmitted back to the cerebral cortex.
The output of EEG signals can be used to control the movement of prosthetics, orthoses, wheelchairs, robots, and computer mice (Hochberg et al., 2012; Gilja et al., 2015; Jarosiewicz et al., 2015). This signal output can also act directly on muscle in the form of electrical stimulation (Pfurtscheller et al., 2003). The brain response can be fed back as visual (Hochberg et al., 2006; Caria et al., 2012), auditory (Nijboer et al., 2008; Leeb and Pérez-Marcos, 2020), or haptic stimuli that vary according to the measured brain activities (Chatterjee et al., 2007; Lugo et al., 2014). Information about limb position or movement can be used to monitor physical activities or for applications of the human-machine interface (Xiao and Menon, 2019). In addition, a human-machine interface has been used for receiving and translating neural electrical signals in amputation patients to assist with movement (Ortiz-Catalan et al., 2014; Raspopovic et al., 2014; Tan et al., 2014; Oddo et al., 2016; Valle et al., 2018; Petrini et al., 2019) and to feed back touch and proprioceptive sensations from the exoskeleton to the center (Raspopovic et al., 2014; Oddo et al., 2016). Compared with an EMG-based human-machine interface, an EEG-based interface can make full use of the human brain’s computing power without the need for complicated judgment instructions through an external calculator. Such an interface can execute the brain’s motor instructions and transmit feedback signals back to the brain to allow completion of more complex and precise activities. Furthermore, it can receive slow cortical potentials (Kübler et al., 2001), sensorimotor rhythms, P300 event-related potentials (Kübler et al., 2009; Halder et al., 2010), steady-state visual evoked potentials (Lesenfants et al., 2014), and error-related negative evoked potentials (Chavarriaga and Millan Jdel, 2010). To reduce muscle atrophy and maintain muscle function as much as possible, intervention should be performed in the early stage of injury. The influence of the human-machine interface on motor learning offers exciting new prospects for retraining skills after neural injury.
Numerous Mechanisms for Repairing Brachial Plexus Injury
Remodeling skeletal muscle structure and muscle fiber types
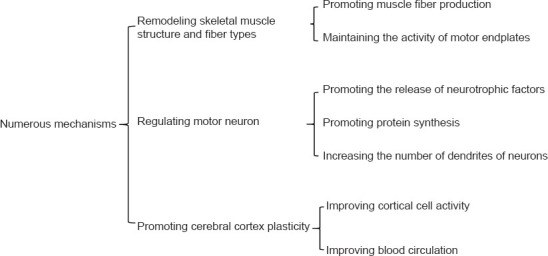
The mechanism of brachial plexus nerve injury repair is shown in Figure 5. Most included studies have shown that denervated muscle mainly manifests as a decrease in wet muscle weight. Reduction in muscle cross-sectional area results from a decline in muscle fiber diameter, loss of cellular nuclei, and cellular apoptosis (Gwam et al., 2021). After treadmill training, the level of IGF-1 protein in muscle cells increases, suggesting that satellite cell activity increased and promoted muscle fiber production (Stevens-Lapsley et al., 2010; Ahuja et al., 2021; Chen et al., 2021). Simultaneously, motor endplates play a vital role in motor function change. Several studies have shown that quantity and quality of motor endplates can significantly improve as a result of long-term exercise. Therefore, the human-machine interface promotes muscle fiber formation and motor endplate maintenance through the early application of exercise training following injury.
Figure 5.
Mechanisms of brachial plexus injury repair.
Motor neuron regulation
After PNI, the speed and number of regenerated axons are disturbed, mainly by neurotrophic factors. Exercise is effective for functional recovery after spinal cord injury in rodents and cats (Alluin et al., 2011), which may be related to neurotrophic factors induced by exercise. Both motor neuron nucleolar area and rate of protein synthesis increase in rats that undergo treadmill training (Kang et al., 1995; Boeltz et al., 2013). Neuronal anterograde and retrograde transport of axonal proteins also increases, which promotes an increase in dendrites (Shin et al., 2014). This positive promoting effect may be realized through the human-machine interface.
Promoting cerebral cortex plasticity
Changes in the cerebral cortex occur after PNI. As nerves regenerate and axons regrow into target organs, the afferent nerves can gradually transmit signals to the center. Afferent stimulation can improve cortical cell activity and induce cerebral cortex reorganization (Hickmott and Merzenich, 2002). As shown in Figure 6, cerebral cortex remodeling after PNI repair can be promoted via the human-computer interface. Mice experiments have shown increased spontaneous neuron generation in the corresponding region of the forelimb cortex after hindlimb PNI, indicating that hindlimb nerve amputation caused cortical remodeling (Kao et al., 2011). Jurkiewicz et al. (2007) found that the degree of functional rehabilitation after functional exercise is related to the degree of cortical activation as measured using fMRI. Bicycle training following repair in a rabbit spinal cord injury model can increase the complexity and density of dendritic spines in the dentate gyrus, which indicates that exercise training can promote axonal regeneration and structural remodeling of the cerebral cortex. In addition, exercise training can affect brain remodeling by improving blood circulation and the status of some neurosecretory factors. Animal PNI studies have shown that plasticity-related neurotrophic factor, adenylate cyclase type 1 (ADCY1), and brain-derived neurotrophic factor secretion are remarkably higher in exercising animals than those that do not exercise. These factors are essential for axonal regeneration and establishment of synapses. Exercise can promote the remodeling of cortical neurons (Graziano et al., 2013). Other studies have shown that exercise stimulates the proliferation of neural endogenous stem cells, which can secrete brain-derived neurotrophic factor, a factor that regulates neural plasticity and can improve motor function.
Figure 6.
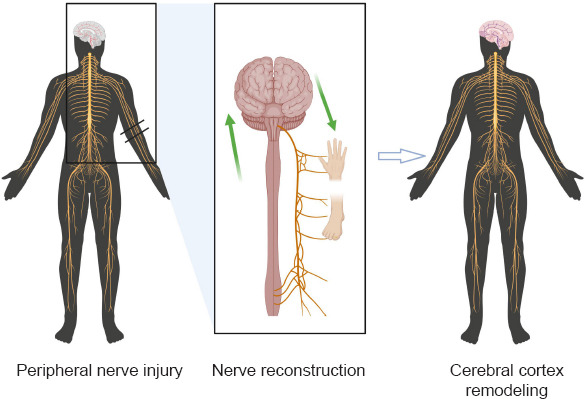
Central remodeling of the cerebral cortex caused by peripheral nerve reconnection.
After the peripheral nerves regenerate and connect, the cerebral cortex that controls the corresponding area undergoes rapid remodeling and forms effective functional control. Black double horizontal line: point of nerve damage; green double arrows: neural signaling and cortical remodeling; black circle: peripheral nerve injury site.
Brachial plexus repair by nerve transfer can provide sufficient motor and sensory fibers to the affected brachial plexus while minimizing impact on the donor area. However, the site of nerve repair is distant from the target muscle. As the nerve regenerates, the target muscle atrophies and becomes nonfunctional, and it is thus difficult for the regenerated nerve to form an effective neuromuscular junction to complete the connection (Ali et al., 2019; Wang et al., 2019; Yin et al., 2019; Gupta et al., 2020; Mole et al., 2020). In contrast, the brain-computer interface can promote peripheral muscle activity in the early stage of injury, slow down neuromuscular junction atrophy, and maintain target muscle activity. Simultaneously, the feedback signal benefits the newly developed nerve in terms of remodeling of the cortex and corresponding brain regions (Alvarez et al., 2010). Studies have shown that the precise control of cortical remodeling may be an important factor in repairing PNI and achieving better rehabilitation results. As illustrated in Figure 7, the human-machine interface can help BPI patients achieve better functional outcome by promoting skeletal muscle fiber regeneration, regulating motor neurons, and promoting cerebral cortex plasticity. In summary, applying an EEG-based human-machine interface in BPI patients may improve the surgical outcome of nerve transfer.
Figure 7.
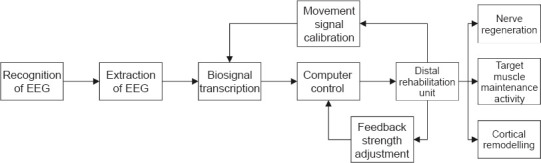
Flowchart for neurological rehabilitation after peripheral nerve injury using an electroencephalography-based human-machine interface.
Conclusions and Future Prospects
Human neural activity is used as input control in human-machine interfaces. Previous studies have demonstrated the advantages of EEG-based interfaces, which are the most common brain-machine interface used today. Song et al. (2020) proposed an EEG-based human-machine interface to enable paralyzed patients to control an assistive robot. EEG can not only identify working signals, but can also distinguish subtle emotional signals (López-Hernández et al., 2019). Assistive robots can help users with motor actions and provide an exploration of broader applications of human-robot interaction. However, integration of an EEG-based human-machine interface with rehabilitation of surgically repaired PNIs has not yet been addressed despite the need for methods to improve rehabilitation outcomes.
Limitations and advantages
Development of an effective human-machine interface for PNI rehabilitation requires in-depth cross-disciplinary collaboration between science, engineering, and medicine. The multi-dimensional combined approach proposed in this study has not yet been implemented in the clinic, and the development and approval cycle for a rehabilitation system can be long. However, brain-computer interface-based prosthetics are a reality and have great potential to improve rehabilitation outcomes after C7 nerve transfer for severe BPI.
Several points deserve particular attention. Future work and reliable replication of previous studies are required to ensure that EEG can be assimilated in automated human-machine interfaces. In addition, engineering quality is equally important. Most existing external brain-machine interface-controlled exoskeleton devices are only in the laboratory stage; eventually, they must be durable enough to allow long-term clinical use. Accuracy of dynamic recognition of EEG signals is another major factor that limits the stable provision of services. The rates of correct recognition by existing algorithms range between 40.5% and 92.7% in previous reports, which should be improved. New density sensors show great potential for use in human-machine interfaces, such as the latest high-density EEG collectors, which have extremely high resolution and the ability to acquire multi-conductor electrical signals in specified areas. Such collectors should provide a basis for precise acquisition and control of human-machine interfaces. In the early stages of rehabilitation after C7 nerve transfer for BPI, full use of the brain’s computing power and active control of external mechanical aids for functional exercise can greatly contribute to better outcomes. In the long term, it can prevent disuse atrophy of muscles and target organs and maintain neuromuscular junction effectiveness. Cortical remodeling also becomes important for recovery as the brain re-establishes effective pathways.
Innovation and comments
This review advocates integrating a human-machine interface into the rehabilitation process after PNI repair and argues for a recovery that does not rely solely on surgery to heal massive injuries. Active, controlled, and safe functional exercise at a later stage is equally important. The unique feedback coordination mechanism of the human-machine interface further enhances controllability of the rehabilitation process. In contrast to conventional manual rehabilitation, the interface has potential to maximize user initiative, regenerate peripheral nerves, and promote cortical remodeling for better and faster recovery.
Conclusion and prospects
The method proposed in this study suggests a possible direction for the future development of human-machine interfaces, as well as a feasible solution to BPI, a difficult clinical problem. In the future, the application of artificial intelligence technology in the field of human-machine interface will make it possible to achieve excellent rehabilitation results with BPI.
Although remarkable progress has been made in BPI repair, in the future, human-machine interfaces will also play a critical role in recovery from nerve injury. The mechanism by which early exercise delays the disintegration of the injured neuromuscular junction and promotes cortical remodeling requires further investigation. Elucidating this mechanism may provide new ideas for functional rehabilitation after nerve injury. Combining various therapeutic methods (new materials, nerve growth factors, electrical stimulation) with C7 nerve transfer may enhance recovery after BPI. An EEG-based human-machine interface shows excellent potential to improve surgical outcomes after brachial plexus repair.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Wetzel M, Song LP; T-Editor: Jia Y
Funding: This work was supported by the National Natural Science Foundation of China, No. 31771322 (to PXZ); the Natural Science Foundation of Beijing, No. 7212121 (to PXZ); Shenzhen Science and Technology Plan Project, No. JCYJ20190806162205278 (to PXZ); Funds for Severe Trauma Standardized Treatment, No. SZSM202011001 (to PXZ); a grant from National Center for Trauma Medicine, Beijing, China, No. BMU2020XY005-01 (to PXZ).
References
- 1.Addas BM, Midha R. Nerve transfers for severe nerve injury. Neurosurg Clin N Am. 2009;20:27–38. doi: 10.1016/j.nec.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Ahuja N, Awad K, Peper S, Brotto M, Varanasi V. Mini review:Biomaterials in repair and regeneration of nerve in a volumetric muscle loss. Neurosci Lett. 2021;762:136145. doi: 10.1016/j.neulet.2021.136145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alawieh AM, Au Yong N, Boulis NM. Contralateral C7 nerve transfer for stroke recovery:new frontier for peripheral nerve surgery. J Clin Med. 2021;10:3344. doi: 10.3390/jcm10153344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali SA, Rosko AJ, Hanks JE, Stebbins AW, Alkhalili O, Hogikyan ND, Feldman EL, Brenner MJ. Effect of motor versus sensory nerve autografts on regeneration and functional outcomes of rat facial nerve reconstruction. Sci Rep. 2019;9:8353. doi: 10.1038/s41598-019-44342-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alluin O, Karimi-Abdolrezaee S, Delivet-Mongrain H, Leblond H, Fehlings MG, Rossignol S. Kinematic study of locomotor recovery after spinal cord clip compression injury in rats. J Neurotrauma. 2011;28:1963–1981. doi: 10.1089/neu.2011.1840. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez FJ, Bullinger KL, Titus HE, Nardelli P, Cope TC. Permanent reorganization of Ia afferent synapses on motoneurons after peripheral nerve injuries. Ann N Y Acad Sci. 2010;1198:231–241. doi: 10.1111/j.1749-6632.2010.05459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armiger RS, Tenore FV, Katyal KD, Johannes MS, Makhlin A, Natter ML, Colgate E, Bensmaia SJ, Vogelstein RJ. Enabling closed-loop control of the modular prosthetic limb through haptic feedback. Johns Hopkins APL Tech Dig. 2013;31:345–353. [Google Scholar]
- 8.Biddiss EA, Chau TT. Upper limb prosthesis use and abandonment:a survey of the last 25 years. Prosthet Orthot Int. 2007;31:236–257. doi: 10.1080/03093640600994581. [DOI] [PubMed] [Google Scholar]
- 9.Boeltz T, Ireland M, Mathis K, Nicolini J, Poplavski K, Rose SJ, Wilson E, English AW. Effects of treadmill training on functional recovery following peripheral nerve injury in rats. J Neurophysiol. 2013;109:2645–2657. doi: 10.1152/jn.00946.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caria A, Sitaram R, Birbaumer N. Real-time fMRI:a tool for local brain regulation. Neuroscientist. 2012;18:487–501. doi: 10.1177/1073858411407205. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee A, Aggarwal V, Ramos A, Acharya S, Thakor NV. A brain-computer interface with vibrotactile biofeedback for haptic information. J Neuroeng Rehabil. 2007;4:40. doi: 10.1186/1743-0003-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chavarriaga R, Millan Jdel R. Learning from EEG error-related potentials in noninvasive brain-computer interfaces. IEEE Trans Neural Syst Rehabil Eng. 2010;18:381–388. doi: 10.1109/TNSRE.2010.2053387. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Gu YD, Hu SN, Xu JG, Xu L, Fu Y. Contralateral C7 transfer for the treatment of brachial plexus root avulsions in children-a report of 12 cases. J Hand Surg. 2007;32:96–103. doi: 10.1016/j.jhsa.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Chen WJ, Lin IH, Lee CW, Chen YF. Aged skeletal muscle retains the ability to remodel extracellular matrix for degradation of collagen deposition after muscle injury. Int J Mol Sci. 2021;22:2123. doi: 10.3390/ijms22042123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen MB, Pearce SM, Ledbetter NM, Warren DJ, Clark GA, Tresco PA. The foreign body response to the utah slant electrode array in the cat sciatic nerve. Acta Biomater. 2014;10:4650–4660. doi: 10.1016/j.actbio.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Chuang DC-C, Hernon C. Minimum 4-year follow-up on contralateral C7 nerve transfers for brachial plexus injuries. J Hand Surg. 2012;37:270–276. doi: 10.1016/j.jhsa.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Colbert SH, Mackinnon SE. Nerve transfers for brachial plexus reconstruction. Hand Clin. 2008;24:341–361. doi: 10.1016/j.hcl.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Cui C, Bian GB, Hou ZG, Zhao J, Zhou H. A multimodal framework based on integration of cortical and muscular activities for decoding human intentions about lower limb motions. IEEE Trans Biomed Circuits Syst. 2017;11:889–899. doi: 10.1109/TBCAS.2017.2699189. [DOI] [PubMed] [Google Scholar]
- 19.Davidge KM, Ho ES, Curtis CG, Clarke HM. Surgical reconstruction of isolated upper trunk brachial plexus birth injuries in the presence of an avulsed C5 or C6 nerve root. J Bone Joint Surg Am. 2021;103:1268–1275. doi: 10.2106/JBJS.20.01379. [DOI] [PubMed] [Google Scholar]
- 20.Dubuisson AS, Kline DG. Brachial plexus injury:a survey of 100 consecutive cases from a single service. Neurosurgery. 2002;51:673–682. [PubMed] [Google Scholar]
- 21.Edelman BJ, Meng J, Suma D, Zurn C, Nagarajan E, Baxter BS, Cline CC, He B. Noninvasive neuroimaging enhances continuous neural tracking for robotic device control. Sci Robot. 2019;4:eaaw6844. doi: 10.1126/scirobotics.aaw6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Gammal TA, Fathi NA. Outcomes of surgical treatment of brachial plexus injuries using nerve grafting and nerve transfers. J Reconstr Microsurg. 2002;18:7–15. doi: 10.1055/s-2002-19703. [DOI] [PubMed] [Google Scholar]
- 23.Elhassan B, Bishop A, Shin A. Trapezius transfer to restore external rotation in a patient with a brachial plexus injury. A case report. J Bone Joint Surg Am. 2009;91:939–944. doi: 10.2106/JBJS.H.00745. [DOI] [PubMed] [Google Scholar]
- 24.Elhassan B, Bishop AT, Hartzler RU, Shin AY, Spinner RJ. Tendon transfer options about the shoulder in patients with brachial plexus injury. J Bone Joint Surg Am. 2012;94:1391–1398. doi: 10.2106/JBJS.J.01913. [DOI] [PubMed] [Google Scholar]
- 25.Elhassan B. Lower trapezius transfer for shoulder external rotation in patients with paralytic shoulder. J Hand Surg Am. 2014;39:556–562. doi: 10.1016/j.jhsa.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Estrella EP, Castillo-Carandang NT, Cordero CP, Juban NR. Quality of life of patients with traumatic brachial plexus injuries. Injury. 2021;52:855–861. doi: 10.1016/j.injury.2020.11.074. [DOI] [PubMed] [Google Scholar]
- 27.Facchini J, Rastoldo G, Xerri C, Péricat D, El Ahmadi A, Tighilet B, Zennou-Azogui Y. Unilateral vestibular neurectomy induces a remodeling of somatosensory cortical maps. Prog Neurobiol. 2021;205:102119. doi: 10.1016/j.pneurobio.2021.102119. [DOI] [PubMed] [Google Scholar]
- 28.Farwell LA, Donchin E. Talking off the top of your head:toward a mental prosthesis utilizing event-related brain potentials. Electroencephalogr Clin Neurophysiol. 1988;70:510–523. doi: 10.1016/0013-4694(88)90149-6. [DOI] [PubMed] [Google Scholar]
- 29.Fasce I, Fiaschi P, Bianconi A, Sacco C, Staffa G, Capone C. Long-term functional recovery in C5-C6 avulsions treated with distal nerve transfers. Neurol Res. 2021 doi: 10.1080/01616412.2021.1942410. doi:10.1080/01616412.2021.1942410. [DOI] [PubMed] [Google Scholar]
- 30.Feng J, Wang T, Luo P. Contralateral C7 transfer to lower trunk via a subcutaneous tunnel across the anterior surface of the chest and neck for total brachial plexus root avulsion:a cadaveric study. J Orthop Surg Res. 2019;14:27. doi: 10.1186/s13018-019-1068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao K, Lao J, Zhao X, Gu Y. Outcome of contralateral C7 transfer to two recipient nerves in 22 patients with the total brachial plexus avulsion injury. Microsurgery. 2013;33:605–611. doi: 10.1002/micr.22137. [DOI] [PubMed] [Google Scholar]
- 32.Garg R, Merrell GA, Hillstrom HJ, Wolfe SW. Comparison of nerve transfers and nerve grafting for traumatic upper plexus palsy:a systematic review and analysis. J Bone Joint Surg Am. 2011;93:819–829. doi: 10.2106/JBJS.I.01602. [DOI] [PubMed] [Google Scholar]
- 33.Gelenitis K, Foglyano K, Lombardo L, Triolo R. Selective neural stimulation methods improve cycling exercise performance after spinal cord injury:a case series. J Neuroeng Rehabil. 2021;18:117. doi: 10.1186/s12984-021-00912-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilja V, Pandarinath C, Blabe CH, Nuyujukian P, Simeral JD, Sarma AA, Sorice BL, Perge JA, Jarosiewicz B, Hochberg LR, Shenoy KV, Henderson JM. Clinical translation of a high-performance neural prosthesis. Nat Med. 2015;21:1142–1145. doi: 10.1038/nm.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graziano A, Foffani G, Knudsen EB, Shumsky J, Moxon KA. Passive exercise of the hind limbs after complete thoracic transection of the spinal cord promotes cortical reorganization. PLoS One. 2013;8:e54350. doi: 10.1371/journal.pone.0054350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu X, Fan Y, Zhou J, Zhu J. Optimized Projection and fisher discriminative dictionary learning for eeg emotion recognition. Front Psychol. 2021;12:705528. doi: 10.3389/fpsyg.2021.705528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu Y, Zhang G, Chen D, Yan J, Cheng X, Chen L. Seventh cervical nerve root transfer from the contralateral healthy side for treatment of brachial plexus root avulsion. J Hand Surg. 1992;17:518–521. doi: 10.1016/s0266-7681(05)80235-9. [DOI] [PubMed] [Google Scholar]
- 38.Gu YD, Zhang GM, Chen DS, Cheng XM, Zhang LY, Yan JG, Cai PQ, Shen LY. Cervical nerve root transfer from contralateral normal side for treatment of brachial plexus root avulsions. Chin Med J (Engl) 1991;104:208–211. [PubMed] [Google Scholar]
- 39.Gu YD, Zhang GM, Chen DS, Yan JG, Cheng XM, Chen L. Seventh cervical nerve root transfer from the contralateral healthy side for treatment of brachial plexus root avulsion. J Hand Surg Br. 1992;17:518–521. doi: 10.1016/s0266-7681(05)80235-9. [DOI] [PubMed] [Google Scholar]
- 40.Gupta R, Chan JP, Uong J, Palispis WA, Wright DJ, Shah SB, Ward SR, Lee TQ, Steward O. Human motor endplate remodeling after traumatic nerve injury. J Neurosurg. 2020 doi: 10.3171/2020.8.JNS201461. doi:10.3171/2020.8.JNS201461. [DOI] [PubMed] [Google Scholar]
- 41.Gwam C, Emara AK, Mohamed N, Chughtai N, Plate J, Ma X. Amniotic stem cell-conditioned media for the treatment of nerve and muscle pathology:a systematic review. Surg Technol Int. 2021;38:407–414.. [PubMed] [Google Scholar]
- 42.Halder S, Rea M, Andreoni R, Nijboer F, Hammer EM, Kleih SC, Birbaumer N, Kübler A. An auditory oddball brain-computer interface for binary choices. Clin Neurophysiol. 2010;121:516–523. doi: 10.1016/j.clinph.2009.11.087. [DOI] [PubMed] [Google Scholar]
- 43.Hattori Y, Doi K, Ikeda K, Pagsaligan JM, Watanabe M. Restoration of prehension using double free muscle technique after complete avulsion of brachial plexus in children:a report of three cases. J Hand Surg. 2005;30:812–819. doi: 10.1016/j.jhsa.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Haufe S, Treder MS, Gugler MF, Sagebaum M, Curio G, Blankertz B. EEG potentials predict upcoming emergency brakings during simulated driving. J Neural Eng. 2011;8:056001. doi: 10.1088/1741-2560/8/5/056001. [DOI] [PubMed] [Google Scholar]
- 45.Hejazi M, Tong W, Ibbotson MR, Prawer S, Garrett DJ. Advances in carbon-based microfiber electrodes for neural interfacing. Front Neurosci. 2021;15:658703. doi: 10.3389/fnins.2021.658703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hickmott PW, Merzenich MM. Local circuit properties underlying cortical reorganization. J Neurophysiol. 2002;88:1288–1301. doi: 10.1152/jn.00994.2001. [DOI] [PubMed] [Google Scholar]
- 47.Hierner R, Berger A. how to improve the results of peripheral nerve surgery. Springer; 2007. Did the partial contralateral C7-transfer fulfil our expectations?Results after 5 year experience; pp. 33–35. [DOI] [PubMed] [Google Scholar]
- 48.Hill JR, Lanier ST, Brogan DM, Dy CJ. Current concepts in the management of adult brachial plexus injuries. J Hand Surg Am. 2021;46:778–788. doi: 10.1016/j.jhsa.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 49.Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, Donoghue JP. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485:372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 51.Hou AL, Zheng MX, Hua XY, Huo BB, Shen J, Xu JG. Electroacupuncture-related metabolic brain connectivity in neuropathic pain due to brachial plexus avulsion injury in rats. Front Neural Circuits. 2020;14:35. doi: 10.3389/fncir.2020.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iturrate I, Chavarriaga R, Pereira M, Zhang H, Corbet T, Leeb R, Millán JdR. Human EEG reveals distinct neural correlates of power and precision grasping types. Neuroimage. 2018;181:635–644. doi: 10.1016/j.neuroimage.2018.07.055. [DOI] [PubMed] [Google Scholar]
- 53.Jarosiewicz B, Sarma AA, Bacher D, Masse NY, Simeral JD, Sorice B, Oakley EM, Blabe C, Pandarinath C, Gilja V, Cash SS, Eskandar EN, Friehs G, Henderson JM, Shenoy KV, Donoghue JP, Hochberg LR. Virtual typing by people with tetraplegia using a self-calibrating intracortical brain-computer interface. Sci Transl Med. 2015;7:313ra179. doi: 10.1126/scitranslmed.aac7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jochumsen M, Niazi IK, Dremstrup K, Kamavuako EN. Detecting and classifying three different hand movement types through electroencephalography recordings for neurorehabilitation. Med Biol Eng Comput. 2016;54:1491–1501. doi: 10.1007/s11517-015-1421-5. [DOI] [PubMed] [Google Scholar]
- 55.Jurkiewicz MT, Mikulis DJ, McIlroy WE, Fehlings MG, Verrier MC. Sensorimotor cortical plasticity during recovery following spinal cord injury:a longitudinal fMRI study. Neurorehabil Neural Repair. 2007;21:527–538. doi: 10.1177/1545968307301872. [DOI] [PubMed] [Google Scholar]
- 56.Kang CM, Lavoie PA, Gardiner PF. Chronic exercise increases SNAP-25 abundance in fast-transported proteins of rat motoneurones. Neuroreport. 1995;6:549–553. doi: 10.1097/00001756-199502000-00035. [DOI] [PubMed] [Google Scholar]
- 57.Kao T, Shumsky JS, Knudsen EB, Murray M, Moxon KA. Functional role of exercise-induced cortical organization of sensorimotor cortex after spinal transection. J Neurophysiol. 2011;106:2662–2674. doi: 10.1152/jn.01017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kemp S, Alant J, Walsh S, Webb A, Midha R. Behavioural and anatomical analysis of selective tibial nerve branch transfer to the deep peroneal nerve in the rat. Eur J Neurosci. 2010;31:1074–1090. doi: 10.1111/j.1460-9568.2010.07130.x. [DOI] [PubMed] [Google Scholar]
- 59.Kübler A, Neumann N, Kaiser J, Kotchoubey B, Hinterberger T, Birbaumer NP. Brain-computer communication:self-regulation of slow cortical potentials for verbal communication. Arch Phys Med Rehabil. 2001;82:1533–1539. doi: 10.1053/apmr.2001.26621. [DOI] [PubMed] [Google Scholar]
- 60.Kübler A, Furdea A, Halder S, Hammer EM, Nijboer F, Kotchoubey B. A brain-computer interface controlled auditory event-related potential (p300) spelling system for locked-in patients. Ann N Y Acad Sci. 2009;1157:90–100. doi: 10.1111/j.1749-6632.2008.04122.x. [DOI] [PubMed] [Google Scholar]
- 61.Kuiken TA, Miller LA, Lipschutz RD, Lock BA, Stubblefield K, Marasco PD, Zhou P, Dumanian GA. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation:a case study. Lancet. 2007;369:371–380. doi: 10.1016/S0140-6736(07)60193-7. [DOI] [PubMed] [Google Scholar]
- 62.Kumar D, Das A, Lahiri U, Dutta A. A human-machine-interface integrating low-cost sensors with a neuromuscular electrical stimulation system for post-stroke balance rehabilitation. J Vis Exp. 2016 doi: 10.3791/52394. doi:10.3791/52394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leeb R, Sagha H, Chavarriaga R, Millán Jdel R. A hybrid brain-computer interface based on the fusion of electroencephalographic and electromyographic activities. J Neural Eng. 2011;8:025011. doi: 10.1088/1741-2560/8/2/025011. [DOI] [PubMed] [Google Scholar]
- 64.Leeb R, Pérez-Marcos D. Brain-computer interfaces and virtual reality for neurorehabilitation. Handb Clin Neurol. 2020;168:183–197. doi: 10.1016/B978-0-444-63934-9.00014-7. [DOI] [PubMed] [Google Scholar]
- 65.Lesenfants D, Habbal D, Lugo Z, Lebeau M, Horki P, Amico E, Pokorny C, Gómez F, Soddu A, Müller-Putz G, Laureys S, Noirhomme Q. An independent SSVEP-based brain-computer interface in locked-in syndrome. J Neural Eng. 2014;11:035002. doi: 10.1088/1741-2560/11/3/035002. [DOI] [PubMed] [Google Scholar]
- 66.Li WJ, He LY, Chen SL, Lyu YW, Wang SF, Yong Y, Tian W, Tian GL, Gu YD. Contralateral C7 nerve root transfer for function recovery in adults:a meta-analysis. Chin Med J (Engl) 2017;130:2960–2968. doi: 10.4103/0366-6999.220316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Yang X, Gao K, Yu H, Xiao F, Zhuang Y, Lao J. Outcome of contralateral C7 transfers to different recipient nerves after global brachial plexus avulsion. Brain Behav. 2018;8:e01174. doi: 10.1002/brb3.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, Ringer RJ, Wagner TH, Krebs HI, Volpe BT, Bever CT, Jr, Bravata DM, Duncan PW, Corn BH, Maffucci AD, Nadeau SE, Conroy SS, Powell JM, Huang GD, Peduzzi P. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362:1772–1783. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lotte F, Congedo M, Lécuyer A, Lamarche F, Arnaldi B. A review of classification algorithms for EEG-based brain-computer interfaces. J Neural Eng. 2007;4:R1–r13. doi: 10.1088/1741-2560/4/2/R01. [DOI] [PubMed] [Google Scholar]
- 70.Lugo ZR, Rodriguez J, Lechner A, Ortner R, Gantner IS, Laureys S, Noirhomme Q, Guger C. A vibrotactile p300-based brain-computer interface for consciousness detection and communication. Clin EEG Neurosci. 2014;45:14–21. doi: 10.1177/1550059413505533. [DOI] [PubMed] [Google Scholar]
- 71.Macêdo LP, Freire Filho JBM, de Souza FHM, Almeida NS, Azevedo-Filho HRC. Transfer of the phrenic nerve to musculocutaneous nerve via sural nerve graft after total brachial plexus injury. Br J Neurosurg. 2021 doi: 10.1080/02688697.2021.1908518. doi:10.1080/02688697.2021.1908518. [DOI] [PubMed] [Google Scholar]
- 72.Marchal-Crespo L, Reinkensmeyer DJ. Review of control strategies for robotic movement training after neurologic injury. J Neuroeng Rehabil. 2009;6:20. doi: 10.1186/1743-0003-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marshall KL, Farah MH. Axonal regeneration and sprouting as a potential therapeutic target for nervous system disorders. Neural Regen Res. 2021;16:1901–1910. doi: 10.4103/1673-5374.308077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Midha R. Nerve transfers for severe brachial plexus injuries:a review. Neurosurg Focus. 2004;16:E5. doi: 10.3171/foc.2004.16.5.6. [DOI] [PubMed] [Google Scholar]
- 75.Miller C, Cross J, O'Sullivan J, Power DM, Kyte D, Jerosch-Herold C. Developing a core outcome set for traumatic brachial plexus injuries:a systematic review of outcomes. BMJ Open. 2021;11:e044797. doi: 10.1136/bmjopen-2020-044797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mole AJ, Bell S, Thomson AK, Dissanayake KN, Ribchester RR, Murray LM. Synaptic withdrawal following nerve injury is influenced by postnatal maturity, muscle-specific properties, and the presence of underlying pathology in mice. J Anat. 2020;237:263–274. doi: 10.1111/joa.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muhetidier A, Yilixiati S, Gulinaer Y, Aihemaitijiang Y. Clinical outcome of contralateral C7 nerve root transposition for treatment of brachial plexus root avulsion injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011;25:1364–1366. [PubMed] [Google Scholar]
- 78.Muhetidier A, Yilixiati S, Gulinaer Y, Aihemaitijiang Y. Clinical outcome of contralateral C7 nerve root transposition for treatment of brachial plexus root avulsion injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011;25:1364–1366. [PubMed] [Google Scholar]
- 79.Navarro X, Krueger TB, Lago N, Micera S, Stieglitz T, Dario P. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J Peripher Nerv Syst. 2005;10:229–258. doi: 10.1111/j.1085-9489.2005.10303.x. [DOI] [PubMed] [Google Scholar]
- 80.Nijboer F, Furdea A, Gunst I, Mellinger J, McFarland DJ, Birbaumer N, Kübler A. An auditory brain-computer interface (BCI) J Neurosci Methods. 2008;167:43–50. doi: 10.1016/j.jneumeth.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oddo CM, Raspopovic S, Artoni F, Mazzoni A, Spigler G, Petrini F, Giambattistelli F, Vecchio F, Miraglia F, Zollo L, Di Pino G, Camboni D, Carrozza MC, Guglielmelli E, Rossini PM, Faraguna U, Micera S. Intraneural stimulation elicits discrimination of textural features by artificial fingertip in intact and amputee humans. Elife. 2016;5:e09148. doi: 10.7554/eLife.09148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ofner P, Schwarz A, Pereira J, Wyss D, Wildburger R, Müller-Putz GR. Attempted arm and hand movements can be decoded from low-frequency EEG from persons with spinal cord injury. Sci Rep. 2019;9:7134. doi: 10.1038/s41598-019-43594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ortiz-Catalan M, Håkansson B, Brånemark R. An osseointegrated human-machine gateway for long-term sensory feedback and motor control of artificial limbs. Sci Transl Med. 2014;6:257re6. doi: 10.1126/scitranslmed.3008933. [DOI] [PubMed] [Google Scholar]
- 84.Pasquina PF, Perry BN, Miller ME, Ling GSF, Tsao JW. Recent advances in bioelectric prostheses. Neurol Clin Pract. 2015;5:164–170. doi: 10.1212/CPJ.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Petrini FM, Valle G, Strauss I, Granata G, Di Iorio R, D'Anna E, Čvančara P, Mueller M, Carpaneto J, Clemente F, Controzzi M, Bisoni L, Carboni C, Barbaro M, Iodice F, Andreu D, Hiairrassary A, Divoux JL, Cipriani C, Guiraud D, et al. Six-month assessment of a hand prosthesis with intraneural tactile feedback. Ann Neurol. 2019;85:137–154. doi: 10.1002/ana.25384. [DOI] [PubMed] [Google Scholar]
- 86.Pfurtscheller G, Müller GR, Pfurtscheller J, Gerner HJ, Rupp R. 'Thought'--control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neurosci Lett. 2003;351:33–36. doi: 10.1016/s0304-3940(03)00947-9. [DOI] [PubMed] [Google Scholar]
- 87.Raspopovic S, Capogrosso M, Petrini FM, Bonizzato M, Rigosa J, Di Pino G, Carpaneto J, Controzzi M, Boretius T, Fernandez E, Granata G, Oddo CM, Citi L, Ciancio AL, Cipriani C, Carrozza MC, Jensen W, Guglielmelli E, Stieglitz T, Rossini PM, et al. Restoring natural sensory feedback in real-time bidirectional hand prostheses. Sci Transl Med. 2014;6:222ra19. doi: 10.1126/scitranslmed.3006820. [DOI] [PubMed] [Google Scholar]
- 88.Rasulić L, Lepić M, Samardžić M. Commentary:nerve graft length and recovery of elbow flexion muscle strength in patients with traumatic brachial plexus injuries:case series. Oper Neurosurg (Hagerstown) 2021;21:E165–166. doi: 10.1093/ons/opab177. [DOI] [PubMed] [Google Scholar]
- 89.Robinson N, Guan C, Vinod A, Ang KK, Tee KP. Multi-class EEG classification of voluntary hand movement directions. J Neural Eng. 2013;10:056018. doi: 10.1088/1741-2560/10/5/056018. [DOI] [PubMed] [Google Scholar]
- 90.Rocco ML, Soligo M, Manni L, Aloe L. Nerve growth factor:early studies and recent clinical trials. Curr Neuropharmacol. 2018;16:1455–1465. doi: 10.2174/1570159X16666180412092859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roy R, Sikdar D, Mahadevappa M, Kumar C. 2017 8th International IEEE/EMBS Conference on Neural Engineering (NER) IEEE; 2017. EEG based motor imagery study of time domain features for classification of power and precision hand grasps; pp. 440–443. [Google Scholar]
- 92.Roy R, Sikdar D, Mahadevappa M, Kumar C. A fingertip force prediction model for grasp patterns characterised from the chaotic behaviour of EEG. Med Biol Eng Comput. 2018;56:2095–2107. doi: 10.1007/s11517-018-1833-0. [DOI] [PubMed] [Google Scholar]
- 93.Rui J, Xu YL, Zhao X, Li JF, Gu YD, Lao J. Phrenic and intercostal nerves with rhythmic discharge can promote early nerve regeneration after brachial plexus repair in rats. Neural Regen Res. 2018;13:862–868. doi: 10.4103/1673-5374.232482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schessler MJ, McClellan WT. The role of nerve transfers for C5-C6 brachial plexus injury in adults. W V Med J. 2010;106:12–17. [PubMed] [Google Scholar]
- 95.Schwarz A, Ofner P, Pereira J, Sburlea AI, Müller-Putz GR. Decoding natural reach-and-grasp actions from human EEG. J Neural Eng. 2017;15:016005. doi: 10.1088/1741-2552/aa8911. [DOI] [PubMed] [Google Scholar]
- 96.Seddon HJ. Nerve grafting. J Bone Joint Surg Br. 1963;45:447–461. [PubMed] [Google Scholar]
- 97.Shin HY, Kim H, Kwon MJ, Hwang DH, Lee K, Kim BG. Molecular and cellular changes in the lumbar spinal cord following thoracic injury:regulation by treadmill locomotor training. PLoS One. 2014;9:e88215. doi: 10.1371/journal.pone.0088215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Song Y, Cai S, Yang L, Li G, Wu W, Xie L. A practical EEG-based human-machine interface to online control an upper-limb assist robot. Front Neurorobot. 2020;14:32–32. doi: 10.3389/fnbot.2020.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Spearman BS, Kuliasha CA, Judy JW, Schmidt CE. Integration of flexible polyimide arrays into soft extracellular matrix-based hydrogel materials for a tissue-engineered electronic nerve interface (TEENI) J Neurosci Methods. 2020;341:108762. doi: 10.1016/j.jneumeth.2020.108762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stevens-Lapsley JE, Ye F, Liu M, Borst SE, Conover C, Yarasheski KE, Walter GA, Sweeney HL, Vandenborne K. Impact of viral-mediated IGF-I gene transfer on skeletal muscle following cast immobilization. Am J Physiol Endocrinol Metab. 2010;299:E730–740. doi: 10.1152/ajpendo.00230.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sturma A, Hruby LA, Prahm C, Mayer JA, Aszmann OC. Rehabilitation of upper extremity nerve injuries using surface emg biofeedback:protocols for clinical application. Front Neurosci. 2018;12:906. doi: 10.3389/fnins.2018.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sulaiman OA, Kim DD, Burkett C, Kline DG. Nerve transfer surgery for adult brachial plexus injury:a 10-year experience at Louisiana State University. Neurosurgery. 2009;65:A55–A62. doi: 10.1227/01.NEU.0000341165.83218.AC. [DOI] [PubMed] [Google Scholar]
- 103.Tan DW, Schiefer MA, Keith MW, Anderson JR, Tyler J, Tyler DJ. A neural interface provides long-term stable natural touch perception. Sci Transl Med. 2014;6:257ra138. doi: 10.1126/scitranslmed.3008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Terzis JK, Kostopoulos VK. The surgical treatment of brachial plexus injuries in adults. Plast Reconstr Surg. 2007;119:73e–92. doi: 10.1097/01.prs.0000254859.51903.97. [DOI] [PubMed] [Google Scholar]
- 105.Terzis JK, Kokkalis ZT. Selective contralateral c7 transfer in posttraumatic brachial plexus injuries:a report of 56 cases. Plast Reconstr Surg. 2009;123:927–938. doi: 10.1097/PRS.0b013e31819ba48a. [DOI] [PubMed] [Google Scholar]
- 106.Terzis JK, Barmpitsioti A. Our experience with triceps nerve reconstruction in patients with brachial plexus injury. J Plast Reconstr Aesthet Surg. 2012;65:590–600. doi: 10.1016/j.bjps.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 107.Tu YK, Tsai YJ, Chang CH, Su FC, Hsiao CK, Tan JSW. Surgical treatment for total root avulsion type brachial plexus injuries by neurotization:a prospective comparison study between total and hemicontralateral C7 nerve root transfer. Microsurgery. 2014;34:91–101. doi: 10.1002/micr.22148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Valle G, Mazzoni A, Iberite F, D'Anna E, Strauss I, Granata G, Controzzi M, Clemente F, Rognini G, Cipriani C, Stieglitz T, Petrini FM, Rossini PM, Micera S. Biomimetic intraneural sensory feedback enhances sensation naturalness, tactile sensitivity, and manual dexterity in a bidirectional prosthesis. Neuron. 2018;100:37–45. doi: 10.1016/j.neuron.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 109.Wang H, Ma J, He X, Xie Q, Fu Y, Wang P. Investigation for the correlation between brain injury and injured ipsilateral sciatic nerve regeneration in a rat model. J Integr Neurosci. 2019;18:467–473. doi: 10.31083/j.jin.2019.04.1155. [DOI] [PubMed] [Google Scholar]
- 110.Wang M, Li ZY, Xu WD, Hua XY, Xu JG, Gu YD. Sensory restoration in cortical level after a contralateral C7 nerve transfer to an injured arm in rats. Neurosurgery. 2010;67:136–143. doi: 10.1227/01.NEU.0000370603.45342.6B. discussion 143. [DOI] [PubMed] [Google Scholar]
- 111.Wang SF, Li PC, Xue YH, Yiu HW, Li YC, Wang HH. Contralateral C7 nerve transfer with direct coaptation to restore lower trunk function after traumatic brachial plexus avulsion. JBJS. 2013;95:821–827. doi: 10.2106/JBJS.L.00039. [DOI] [PubMed] [Google Scholar]
- 112.Warwick K, Gasson M, Hutt B, Goodhew I, Kyberd P, Andrews B, Teddy P, Shad A. The application of implant technology for cybernetic systems. Arch Neurol. 2003;60:1369–1373. doi: 10.1001/archneur.60.10.1369. [DOI] [PubMed] [Google Scholar]
- 113.Wehrli L, Bonnard C, Anastakis DJ. Current status of brachial plexus reconstruction:restoration of hand function. Clin Plast Surg. 2011;38:661–681. doi: 10.1016/j.cps.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 114.Wolpaw JR, McFarland DJ, Neat GW, Forneris CA. An EEG-based brain-computer interface for cursor control. Electroencephalogr Clin Neurophysiol. 1991;78:252–259. doi: 10.1016/0013-4694(91)90040-b. [DOI] [PubMed] [Google Scholar]
- 115.Woo JS, Müller KR, Lee SW. The 3rd International Winter Conference on Brain-Computer Interface. IEEE; 2015. Classifying directions in continuous arm movement from EEG signals; pp. 1–2. [Google Scholar]
- 116.Xiao ZG, Menon C. A Review of force myography research and development. Sensors (Basel) 2019;19:4557. doi: 10.3390/s19204557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yan M, Kong W, Kerr A, Brundage M. The radiation dose tolerance of the brachial plexus:a systematic review and meta-analysis. Clin Transl Radiat Oncol. 2019;18:23–31. doi: 10.1016/j.ctro.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yi S, Xu L, Gu X. Scaffolds for peripheral nerve repair and reconstruction. Exp Neurol. 2019;319:112761. doi: 10.1016/j.expneurol.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 119.Yi W, Qiu S, Qi H, Zhang L, Wan B, Ming D. EEG feature comparison and classification of simple and compound limb motor imagery. J Neuroeng Rehabil. 2013;10:1–12. doi: 10.1186/1743-0003-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yin X, Yu T, Chen B, Xu J, Chen W, Qi Y, Zhang P, Li Y, Kou Y, Ma Y, Han N, Wan P, Luo Q, Zhu D, Jiang B. Spatial distribution of motor endplates and its adaptive change in skeletal muscle. Theranostics. 2019;9:734–746. doi: 10.7150/thno.28729. [DOI] [PMC free article] [PubMed] [Google Scholar]


