Abstract
This paper provides a description of the MyCap data collection platform, utilization metrics, and vignettes associated with use from diverse research institutions. MyCap is a participant-facing mobile application for survey data collection and the automated administration of active tasks (activities performed by participants using mobile device sensors under semi-controlled conditions). Launched in 2018, MyCap is a no-code solution for research teams conducting longitudinal studies, integrates tightly with REDCap and is available at no cost to research teams at academic, nonprofit, or government organizations. MyCap has been deployed at multiple research institutions with application usage logged across 135 countries in 2021. Vignettes demonstrate that MyCap empowered research teams to explore and implement novel methods of information collection and use. MyCap’s integration with REDCap provides a comprehensive data collection ecosystem and is best suited for longitudinal studies with frequent requests for information from participants.
Keywords: clinical trials, database management systems, medical informatics, mobile applications, translational medical research
INTRODUCTION
In recent years, clinical research has expanded beyond the physical walls of the clinical research center and hospital. Mobile electronic data capture (mEDC) and computer-assisted self-interview (CASI) methods are evolving for data collection and offer “unprecedented geographic freedom to participate in research”.1,2 While mEDC and CASI provide solutions for researchers, they can be expensive and time-consuming to develop.3,4 Researchers and software developers often require months to deploy a mobile application for a single research study. This approach is inefficient and leads to fragmented solutions.4,5
Our team at Vanderbilt University Medical Center (VUMC) develops and disseminates REDCap, a secure web application designed to support data management for clinical and translational research.6,7 We developed the REDCap Mobile Application in 2014 to better equip coordinators collecting field data in areas with scarce internet connectivity.8 While the REDCap Mobile Application has served an important purpose, the platform was designed for research team use rather than direct data capture from participants.
OBJECTIVES
Our objective in developing MyCap was to create a no-code solution for research teams conducting longitudinal studies where a participant-facing mobile application was desired for survey collection and the administration of active tasks (activities performed by participants using smart device sensors under semi-controlled conditions).9 A secondary objective was tight integration with REDCap to ensure researchers had a reliable central repository for collected mobile data.
MATERIALS AND METHODS
Defining specifications for a minimum viable product
We conducted informal assessment exercises with VUMC research personnel to determine the need for a no-code mobile application data collection platform. Given positive responses, we then performed a scoping exercise reviewing features from single-study mobile applications (eg, Health eHeart, mPower, GlucoSuccess, MyHeart Counts, Asthma Health, and Share the Journey).10–15 These efforts led to defining a minimum viable product with specifications below.
Compatibility specifications
The platform should: (1) support native mobile application data collection on Android and iOS devices; (2) support use in online or offline modalities; (3) be downloadable at no cost from Apple and Google Play Stores; (4) allow a single mobile device to participate in multiple concurrent or sequential research studies; and (5) offer seamless integration with institutional REDCap data management services.
Data security specifications
The platform should: (1) encrypt data stored on local mobile devices; (2) encrypt transmitted information during project configuration, authentication, and data synchronization processes; (3) remove participant data after synchronization to a REDCap server; and (4) protect access using password or biometric authentication.
Data capture and study authoring specifications
The platform should: (1) provide a user-friendly mobile application interface clearly detailing questionnaires or tasks to be completed by the participant (eg, “what do I need to do today?”); (2) enable project-level customization options (eg, study logo, details, contacts, color schemes) through an intuitive no-code study authoring tool; (3) automate synchronization of data to a specific REDCap server; and (4) allow study modifications over time without requiring versioning of the mobile application.
Choosing a technical framework
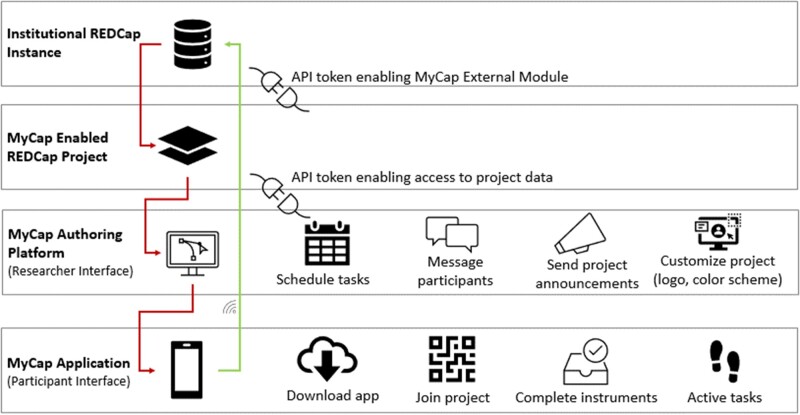
We chose a native technology approach for the development of MyCap applications for iOS (Objective-C/Swift) and Android (Java/Kotlin).16,17 For iOS devices, we leveraged Apple’s open-source ResearchKit framework to administer surveys and active tasks.9,18 For Android devices, we used the open-source ResearchStack framework.19 We created a REDCap application programming interface dedicated to MyCap for registering and configuring study projects, distributing participant survey details, customizing the mobile application interface, and exchanging data between mobile devices using MyCap and a REDCap server.6Figure 1 provides an architectural view of connectivity and information exchange between the MyCap mobile application and a researcher’s local REDCap environment.
Figure 1.
Connectivity and information exchange between MyCap and REDCap.
MyCap project authoring
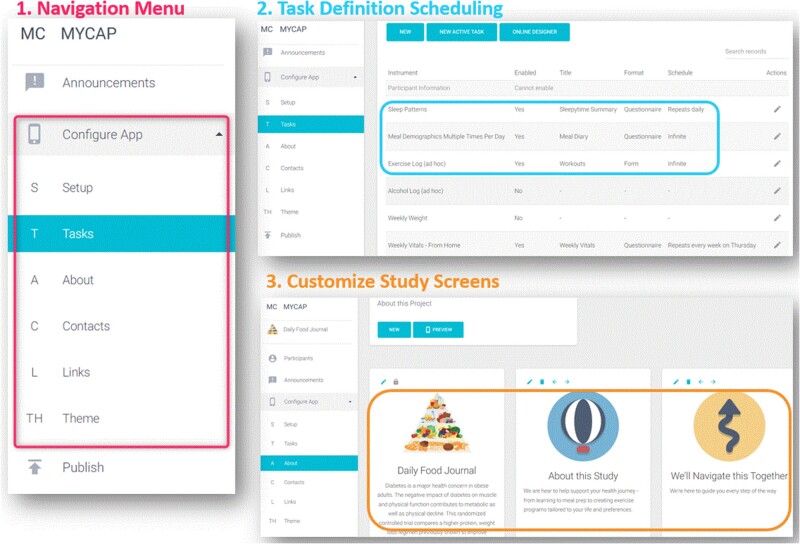
Research teams desiring to use MyCap must have a functioning REDCap server and must enable the MyCap Authoring Platform. The authoring platform is provided as a REDCap External Module and is distributed at no cost within the REDCap Consortium.20Figure 2 shows authoring screens (navigation, study customization, and data collection task definition and scheduling) in context of a research team member defining a MyCap project.
Figure 2.
MyCap Authoring Platform screens, including: (1) navigation menu; (2) project-specific survey and active task specification and scheduling options; and (3) customizable study screens, including logos and images.
Disseminating MyCap to research teams
We launched MyCap at VUMC in March 2018. MyCap installation instructions, FAQs, version changelogs, video tutorials, and a monitored Q/A forum were made available on the REDCap Consortium Intranet site in 2019.7 We established weekly “clinics” to provide project-specific support to research teams wishing to deploy MyCap for studies. Test projects allowing research teams to download MyCap from the iOS or Android application stores and join a “demo” study were posted on a MyCap public facing descriptor website.21
Evolving the platform based on local and consortium feedback
As researchers at VUMC and across the REDCap Consortium use MyCap to support individual research studies, enhancement requests are communicated to our development team. New features are prioritized for development based on applicability breadth, end-user consensus, and potential impact. Examples of MyCap platform features based on user feedback since initial launch include: (1) secure bi-directional messaging between research coordinators and individual research participants (2018); (2) survey skip logic (2019); (3) enhanced synchronization features (2019); (4) MyCap reporting tools for REDCap Administrators (2019); (5) enhanced authoring platform options for study task definition and scheduling (2020); (6) barcode scanning for complex workflow support (2021); (7) refined active task registration and setup processes (2021); and (8) application-wide language rendering options for English, Spanish, Brazilian Portuguese, and French (2020–2021).
RESULTS
The first MyCap project at VUMC in 2018 served as a daily pain diary for participants having undergone transcatheter aortic valve replacement.22 As of December 31, 2021, there were 106 VUMC projects in development or actively collecting data using MyCap for research (n = 34), healthcare operations (61), and quality improvement (11).
Shortly after deployment at VUMC, we began sharing MyCap with the REDCap Consortium. The required MyCap External Module has been installed by REDCap institutions over 830 times. Due to privacy and confidentiality policies, we are unable to access discrete MyCap use cases or usage statistics from other REDCap installations, but Table 1 provides selected vignettes gathered through bibliometric and internet searches.
Table 1.
Selected MyCap vignettes
| Project description | MyCap utility | Participant AUDIENCE |
|---|---|---|
| VUMC conducted a home-based mobile health exercise intervention for patients recovering from transcatheter aortic valve replacements to track exercise adherence and progress.22 | Participants were assigned exercises, answered daily and weekly questionnaires, and tracked their progress on MyCap. | Older adults |
| University of Washington and Sammamish TruMedicines assessed the efficacy of mobile apps in measuring or encouraging medication adherence.23 | Participants photographed a pill before daily consumption and answered accompanying health-related questions. | Adults |
| Mt. Sinai healthcare system launched the COVID-19 Precision Recovery Program, using MyCap for remote patient monitoring.24 | Patients logged daily symptoms and physiologic data in MyCap, which were monitored by clinical staff to identify when triage or in-person care was needed. | Adults |
| VUMC conducted a COVID-19 surveillance study at a local high school and used MyCap to connect participant samples with test results.25 | Participants submitted weekly saliva samples and symptom surveys. MyCap’s barcode scanning feature linked participants’ sample test tubes to their results, which were pushed to participants’ MyCap app. | High school students and teachers |
| UC Denver is evaluating the efficacy of mobile app usage in a suicide prevention intervention.26 | Participants complete biweekly surveys for 3 months. MyCap also provides mental-health and self-help resources. | Active-duty Naval personnel |
| University of Windsor is using MyCap to track participant saliva samples and return test results to individuals as a part of a COVID-19 Detection Platform.27 | MyCap’s barcode scanning feature linked participants’ sample test tubes to test results, which were pushed to participants’ MyCap app. | College faculty and students |
| VUMC is assessing the safety and effectiveness of a drug in reducing Crohn’s disease-associated symptoms.28 | Participants log daily symptoms in MyCap. | Adults |
| VUMC is assessing the efficacy of a drug for treating neurogenic orthostatic hypotension.29 | Participants complete daily activity diary forms and weekly symptom questionnaires in MyCap. | 40–80 years old w/neurogenic ortho hypotension |
| VUMC uses MyCap to report the use of behavior intervention strategies in schools for students with disabilities and problem behaviors.30 | Participants log the presence and type of behavioral problems, treatment dosage, trainee engagement with professional development programs, and contacts with school-based teams. | Student-facing consultants/coordinators |
| Weill Cornell identified Parkinson’s Disease patients from Healthy Controls utilizing MyCap’s Tapping active task.31 | Participants completed the active tapping task 2× about 1 h apart, 3 days per week, for 5 weeks on MyCap. | Adults |
Using Firebase and Google Analytics, we measured MyCap use by participants across all REDCap installations during calendar year 2021.32,33 During this time period, we observed activity from approximately 320 unique users per day, 750 unique users per week, and 1800 unique users per month. Utilization was geographically diverse with device installations across 135 countries. Approximately 63% of MyCap use was from iOS platform users and 37% from use of the Android platform. Measurable gender representation was nearly equal between males and females.
DISCUSSION
Our model of creating and supporting a single customizable mobile application with no per-project coding requirements has been successfully implemented and is supporting diverse research studies and operational use cases around the world. MyCap is not unique as a mobile application supporting direct data capture from participants in research studies. Examples of similar or related products include Open Data Kit (ODK), Computerized Intervention Authoring System (CIAS), Thread, Medable, MyStudies, and Eforms.34–38 These applications share many of MyCap’s capabilities, such as offline data collection, customizable surveys, and compatibility with both Android and iOS devices. However, MyCap is unique in its seamless integration with REDCap and support through the REDCap Consortium.
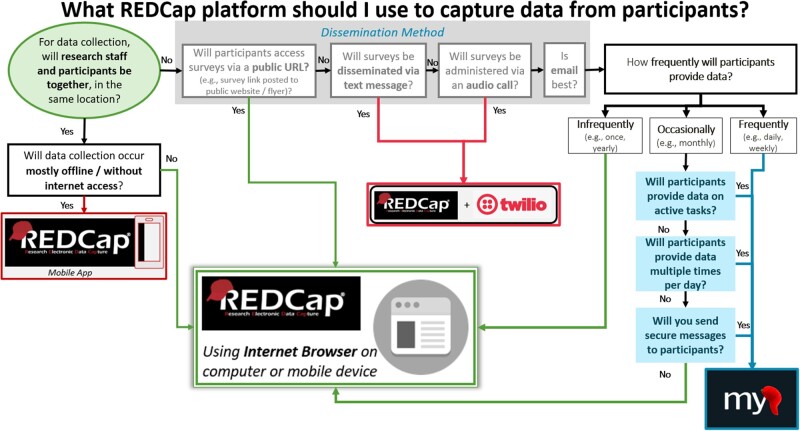
In the process of deploying MyCap at VUMC, we encountered numerous questions from researchers about when to use MyCap versus other REDCap methods designed to collect data directly from participants. In response, we developed a decision tool (Figure 3) clarifying that: (1) the REDCap Mobile application is designed primarily for research teams sending research coordinators into the field for in-person data collection; (2) traditional REDCap web surveys work well on phones and tablets whenever sustainable internet connectivity is available; (3) third-party integrations (eg, Twilio) allow SMS collection or invitations to complete web-based surveys when singular reminders are helpful39; and (4) MyCap is best suited for longitudinal studies with frequent requests (surveys or active tasks) for information from participants.
Figure 3.
Decision tool for research teams using the REDCap + MyCap ecosystem for direct participant data capture.
Limitations
MyCap use is limited to individuals who possess an iOS (versions ≥ 11.0) or Android (versions ≥ 8.0) device with at least periodic access to the internet. While the digital divide for mobile device ownership is narrowing, mobile research apps are still utilized less in low-income countries versus high-income countries and equity issues persist.40 Regular use of MyCap for longitudinal studies is also dependent on individuals actually opening the application on a regular basis. Many applications on smart devices are installed and not used on a regular basis.41,42 This can pose a challenge in setting realistic expectations with researchers in terms of how often and how long a participant will keep a nonessential mobile application installed on their mobile device.
CONCLUSION
MyCap has been successfully deployed at research institutions around the world through the REDCap Consortium. Uptake by research teams has been measured, but steady and MyCap has opened new possibilities for mobilizing the participant voice via device-based data collection for clinical research trials that use REDCap. Given early successes, we are investing in additional researcher-driven features, converting to a multi-platform codebase (Flutter) to streamline technical development, and launching awareness campaigns to increase use by research teams.
FUNDING
This work has been funded by the National Institutes of Health’s National Center for Clinical and Translational Science (U24TR001608 and U24TR001579) and Office of the Director (U24OD023319).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception or design of the work; drafted and/or revised the content; approved the final version to be published; and agree to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
We would like to thank member organizations and operational leaders from the REDCap Consortium for continuous engagement contributing to the development, deployment, and evolution of the MyCap platform. We would also like to thank the Trial Innovation Network project leads and the Recruitment Innovation Center’s Community Advisory Board for applied use cases for MyCap and helping inform participant-centered usability.
CONFLICT OF INTEREST STATEMENT
The authors have no competing interests to declare.
DATA AVAILABILITY
No new data were generated or analyzed in support of this research.
Contributor Information
Paul A Harris, Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Department of Biomedical Engineering, Vanderbilt University, Nashville, Tennessee, USA; Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Jonathan Swafford, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Emily S Serdoz, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Jessica Eidenmuller, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Giovanni Delacqua, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Vaishali Jagtap, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Robert J Taylor, Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Alexander Gelbard, Department of Otolaryngology, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Alex C Cheng, Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Stephany N Duda, Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
REFERENCES
- 1. Dorsey ER, Yvonne Chan Y-F, McConnell MV, et al. The use of smartphones for health research. Acad Med 2017; 92 (2): 157–60. [DOI] [PubMed] [Google Scholar]
- 2. Brown JL, Vanable PA, Eriksen MD.. Computer-assisted self-interviews: a cost effectiveness analysis. Behav Res Methods 2008; 40 (1): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. von Niederhäusern B, Saccilotto R, Schädelin S, et al. Validity of mobile electronic data capture in clinical studies: a pilot study in a pediatric population. BMC Med Res Methodol 2017; 17 (1): 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang J, Sun L, Liu Y, et al. Mobile device-based electronic data capture system used in a clinical randomized controlled trial: advantages and challenges. J Med Internet Res 2017; 19 (3): e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zens M, Grotejohann B, Tassoni A, et al. Development of a modular research platform to create medical observational studies for mobile devices. JMIR Res Protoc 2017; 6 (5): e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42 (2): 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019; 95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harris PA, Delacqua G, Taylor R, et al. The REDCap Mobile Application: a data collection platform for research in regions or situations with internet scarcity. JAMIA Open 2021; 4 (3): ooab078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ActiveTasks Document. http://researchkit.org/docs/docs/ActiveTasks/ActiveTasks.html Accessed April 14, 2021.
- 10.Health eHeart Study. https://www.health-eheartstudy.org/ Accessed December 21, 2021.
- 11. Bot BM, Suver C, Neto EC, et al. The mPower study, Parkinson disease mobile data collected using ResearchKit. Sci Data 2016; 3: 160011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mass Gen ResearchKit app GlucoSuccess shows impact of data insights on behavior in diabetes patients. 2016. https://www.mobihealthnews.com/content/mass-gen-researchkit-app-glucosuccess-shows-impact-data-insights-behavior-diabetes-patients Accessed December 28, 2021.
- 13. McConnell MV, Shcherbina A, Pavlovic A, et al. Feasibility of obtaining measures of lifestyle from a smartphone app: the MyHeart Counts Cardiovascular Health Study. JAMA Cardiol 2017; 2 (1): 67–76. [DOI] [PubMed] [Google Scholar]
- 14. Chan Y-FY, Wang P, Rogers L, et al. The Asthma Mobile Health Study, a large-scale clinical observational study using ResearchKit. Nat Biotechnol 2017; 35 (4): 354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sage Bionetworks Launches ‘Share the Journey,’ A Mobile App-Based Research Study for the Breast Cancer Community—Sage Bionetworks. 2015. https://sagebionetworks.org/in-the-news/sage-bionetworks-launches-share-the-journey-a-mobile-app-based-research-study-for-the-breast-cancer-community/ Accessed December 28, 2021.
- 16.Apple Inc. Swift—Apple Developer. https://developer.apple.com/swift/ Accessed December 28, 2021.
- 17.Kotlin and Android. https://developer.android.com/kotlin Accessed December 28, 2021.
- 18.Apple Inc. ResearchKit—Apple Developer. https://developer.apple.com/researchkit/ Accessed April 14, 2021.
- 19.ResearchStack. http://researchstack.org/ Accessed April 14, 2021.
- 20.REDCap External Module Code Repository. https://redcap.vanderbilt.edu/consortium/modules/index.php Accessed April 19, 2021.
- 21.MyCap. https://projectmycap.org/ Accessed April 19, 2021.
- 22. Lindman BR, Gillam LD, Coylewright M, et al. Effect of a pragmatic home-based mobile health exercise intervention after transcatheter aortic valve replacement: a randomized pilot trial. Eur Heart J Digit Health 2021; 2 (1): 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prokop D, Babigumira J, Lewis A. Pill identification using a mobile phone app for assessing medication adherence and post-market drug surveillance. arXiv [cs.CY] 2020. http://arxiv.org/abs/2004.11479.
- 24. Tabacof L, Kellner C, Breyman E, et al. Remote patient monitoring for home management of coronavirus disease 2019 in New York: A cross-sectional observational study. Telemed J E Health 2021; 27 (6): 641–8. [DOI] [PubMed] [Google Scholar]
- 25. Govern P. VUMC team screens high school for SARS-CoV-2 asymptomatic infections. https://news.vumc.org/2021/04/29/vumc-team-screens-high-school-for-sars-cov-2-asymptomatic-infections/ Accessed June 4, 2021.
- 26.Facilitating Assessment of At-Risk Sailors Using Technology—Full Text View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04159480 Accessed January 7, 2022.
- 27.Researchers set to screen for COVID-19 on campus. https://www.uwindsor.ca/dailynews/2021-03-12/researchers-set-screen-covid-19-campus Accessed January 7, 2022.
- 28.Reduce Crohn’s-Associated Diarrhea With Sodium Channel Therapy—Full Text View—ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04456517?term=ranolazine&cond=Crohn+Disease&cntry=US&draw=2&rank=1 Accessed January 7, 2022.
- 29.Use of accelerometer for quantification of neurogenic orthostatic hypotension symptoms. https://clinicaltrials.gov/ct2/show/NCT04782830 Accessed January 7, 2022.
- 30. Staubitz JE. Intensive partnership for behavior intervention: training educators to plan, implement, and evaluate behavior change strategies. 2019. https://www.abainternational.org/events/program-details/event-detail.aspx?&sid=59177&by=Area. Accessed January 5, 2022.
- 31. Tripathi S, Barkan S, Hou Y, et al. Is the MyCap finger tapping smartphone application effective in differentiating healthy controls from Parkinson’s disease patients? Preliminary pilot study data (2807). Neurology 2021; 96https://n.neurology.org/content/96/15_Supplement/2807.abstract Accessed January 7, 2022. [Google Scholar]
- 32.Firebase. https://firebase.google.com/ Accessed January 7, 2022.
- 33.Google Analytics. https://analytics.google.com/ Accessed January 7, 2022.
- 34. Christle JW, Hershman SG, Torres Soto J, et al. Mobile health monitoring of cardiac status. Annu Rev Biomed Data Sci 2020; 3 (1): 243–63. [Google Scholar]
- 35. Maduka O, Akpan G, Maleghemi S.. Using android and open data kit technology in data management for research in resource-limited settings in the Niger Delta Region of Nigeria: cross-sectional household survey. JMIR Mhealth Uhealth 2017; 5 (11): e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mooney JS, Cappelli T, Byrne-Davis L, et al. How we developed eForms: an electronic form and data capture tool to support assessment in mobile medical education. Med Teach 2014; 36 (12): 1032–7. [DOI] [PubMed] [Google Scholar]
- 37. Ondersma SJ, Svikis DS, Schuster CR.. Computer-based brief intervention a randomized trial with postpartum women. Am J Prev Med 2007; 32 (3): 231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wyner Z, Dublin S, Chambers C, et al. The FDA MyStudies app: a reusable platform for distributed clinical trials and real-world evidence studies. JAMIA Open 2020; 3 (4): 500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Minor B. How digital health surveying is critical to flattening the COVID-19 curve. 2020. https://www.twilio.com/blog/how-digital-health-surveying-critical-flattening-covid-19-curve AccessedDecember 27, 2021.
- 40. Rosenberg S. Smartphone Ownership Is Growing Rapidly Around the World, but Not Always Equally. 2019. https://www.pewresearch.org/global/2019/02/05/smartphone-ownership-is-growing-rapidly-around-the-world-but-not-always-equally/ Accessed December 27, 2021.
- 41. Perez S. Report: Smartphone owners are using 9 apps per day, 30 per month. TechCrunch2017. http://techcrunch.com/2017/05/04/report-smartphone-owners-are-using-9-apps-per-day-30-per-month/ Accessed June 3, 2021.
- 42. Perez S. Consumers spend 85% of time on smartphones in apps, but only 5 apps see heavy use. TechCrunch2015. http://techcrunch.com/2015/06/22/consumers-spend-85-of-time-on-smartphones-in-apps-but-only-5-apps-see-heavy-use/ Accessed June 3, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.