Abstract
The influence of grazing by the bacterivorous nanoflagellate Ochromonas sp. strain DS on the taxonomic and morphological structures of a complex bacterial community was studied in one-stage chemostat experiments. A bacterial community, consisting of at least 30 different strains, was fed with a complex carbon source under conditions of low growth rate (0.5 day−1 when nongrazed) and low substrate concentration (9 mg liter−1). Before and after the introduction of the predator, the bacterial community composition was studied by in situ techniques (immunofluorescence microscopy and fluorescent in situ hybridization), as well as by cultivation on agar media. The cell sizes of nonspecifically stained and immunofluorescently labeled bacteria were measured by image analysis. Grazing by the flagellate caused a bidirectional change in the morphological structure of the community. Medium-size bacterial cells, which dominated the nongrazed community, were largely replaced by smaller cells, as well as by cells contained in large multicellular flocs. Cell morphological changes were combined with community taxonomic changes. After introduction of the flagellate, the dominating strains with medium-size cells were largely replaced by single-celled strains with smaller cells on the one hand and, on the other hand, by Pseudomonas sp. strain MWH1, which formed the large, floc-like forms. We assume that size-selective grazing was the major force controlling both the morphological and the taxonomic structures of the model community.
Predation by bacterivorous protists, particularly by the ubiquitous bacterivorous nanoflagellates, is a major mortality factor for aquatic bacteria (28, 46, 47). Several field studies and laboratory investigations have demonstrated that flagellate grazing potentially influences both the size distribution of bacterial communities (2, 11, 18, 29, 40) and their taxonomic composition (11, 20, 30, 31, 40, 41).
Grazing by direct-interception-feeding flagellates takes place in two steps, encounter and ingestion. The probability of a bacterial cell and a particular flagellate encountering one another depends on bacterial cell size and may be influenced by bacterial motility and bacterial surface characteristics (25). Jürgens and DeMott demonstrated that the ingestion step is influenced by the physiological state of the flagellate cells (19). In two flagellate species, they found significantly lower food selectivity by flagellates grown under food-limited conditions than by food-saturated flagellates.
The influence of flagellate grazing on the composition of bacterial communities is based on a complex interplay of several factors. Probably the most important factors are the above-mentioned selectivity of flagellate grazing (by size or other qualities) (9, 21, 24, 39), differences in cell size distribution of bacterial species (11, 12), and different abilities of bacterial species in grazing defense (11, 30). Furthermore, grazing may influence the growth conditions of individual bacterial species through the regeneration of substrates or by reduction of competitors.
We studied the influence of grazing by the flagellate Ochromonas sp. strain DS on a bacterial model community under defined experimental conditions to obtain further insights into this complex interplay. We used chemostats for our experiments in order to minimize the influences on bacterial composition of changes in substrate supply. In order to involve a high number of bacterial strains, as well as a high number of bacterial defense strategies, in the studies, we used a complex and nondefined bacterial community obtained from Lake Constance in Germany. The bacteria were fed with a defined mixture of three different complex media to enable the permanent coexistence of a high number of strains or species. In the first step of the experiment, we tried to establish, in two parallel reactors, nongrazed communities, stabilized in terms of abundance and morphological and taxonomic structures. In only one of the two reactors were all three criteria fulfilled; in the second reactor only numerical and morphological stabilities were observed. In the second step of the experiments, the bacterivorous flagellate Ochromonas sp. strain DS was introduced into both reactors and the influence on the morphological and taxonomic structure of the community, as well as on the specific cell sizes of selected strains, was investigated. Due to the lack of taxonomic stability in one reactor before inoculation with the predator, in respect to the influence on taxonomic composition, the paper will focus primarily on results from the other reactor. To exclude the possibility that the changes observed for this single reactor were caused by chance, we temporarily eliminated the flagellates, using specific inhibitors, and studied the subsequent community changes. For further confirmation, we performed separate experiments on the influence of grazing on the major players isolated from the experiments presented here (11, 13).
MATERIALS AND METHODS
Chemostat experiments.
The experiments were carried out with a one-stage chemostat system (11). This consisted of two parallel reactors (designated reactors I and II) with working volumes of 2 liters. The two reactors were fed from one reservoir and mixed by aeration with sterile air. Sampling and inoculation of the reactors, as well as all other chemostat work, were done under sterile conditions. Bacteria were cultured at 15 ± 1°C, in the dark, with NSY medium containing 9 mg of a complex substrate liter−1 (11). The dilution rate was maintained at 0.5 day−1 (the bacterial growth rate in the flagellate-free phase of the experiments was 0.5 day−1).
Chemostat reactors were inoculated with a complex bacterial community obtained from a nonaxenic culture of the bacterivorous flagellate Ochromonas sp. strain DS. The flagellate and the accompanying bacteria were isolated from Lake Constance in Germany by D. Springmann. All subsequent culture work was done under sterile conditions. The flagellate culture was treated with inhibitors specific for eukaryotic cells (200 mg each of colchicine and cycloheximide liter−1) to separate the bacteria from the flagellates. The chemostat reactors were initially inoculated with the mixed bacterial community plus two bacterial strains, Comamonas acidovorans PX54 (8, 11) and Aeromonas hydrophila PU7718 (8), both isolated from Lake Plußsee, Germany.
Chemostat experiments consisted of several phases (reactor I, four phases, and reactor II, two phases) (Table 1). In the first phase, the mixed bacterial community was cultured in the absence of any predator, and the second phase started, in both reactors, with the inoculation of the flagellate Ochromonas sp. strain DS. Only in the case of reactor I was the flagellate eliminated by injection of inhibitors (200 mg each of colchicine and cycloheximide liter−1) specific for eukaryotic organisms (phase 3).
TABLE 1.
Durations of different experimental phases in the two reactorsa
| Phase | Duration (days)
|
Flagellate control | |
|---|---|---|---|
| Reactor I | Reactor II | ||
| 1 | 1–75 | 1–145 | − |
| 2 | 83–231 | 154–261 | + |
| 3 | 234–249 | − | |
| 4 | 251–261 | + | |
During phases 2 and 4 (only reactor I) the bacterial communities were controlled by flagellate grazing. Periods of transient stages were not included in the comparisons of phases with (+) and without (−) flagellate control, which are presented in Results.
Microbial abundance.
Determination of total bacterial and flagellate abundance was done by staining formaldehyde-fixed samples with 0.1% (wt/vol) DAPI (4′,6-diamidino-2-phenylindole), filtration on 0.2-μm-pore-size polycarbonate filters, and enumeration by epifluorescence microscopy (11).
Characterization of bacterial community composition by immunofluorescence microscopy.
Two polyclonal rabbit antisera and two monoclonal antibodies were used to determine the abundance and specific cell sizes of four different bacterial strains. For the detection of the Vibrio sp., C. acidovorans PX54, and A. hydrophila PU7718, an antiserum and antibodies described previously were used (8, 11). Those antibodies and the polyclonal antiserum showed no cross-reactivities with other strains of the investigated mixed bacterial community. For detection of Pseudomonas sp. strain MWH1, a polyclonal rabbit antiserum was developed and tested for specificity by immunofluorescence microscopy. Twenty-six reference strains isolated from the investigated mixed bacterial community and 14 strains of species of the genus Pseudomonas were tested. The antiserum showed weak cross-reactivity with four strains obtained from the investigated community and with Pseudomonas aeruginosa DSM 50071T, Pseudomonas alcaligenes DSM 50342T, Pseudomonas fluorescens DMS 50090T, Pseudomonas pseudoalcaligenes DSM 50188T, Pseudomonas putida DSM 50222, and Pseudomonas viridifalva DSM 50338. Determination of the specific abundance of bacterial strains by immunofluorescence microscopy was performed as described previously (11).
FISH.
The group-specific composition of the mixed bacterial community was determined by in situ cell hybridization with fluorescent oligonucleotide probes (fluorescent in situ hybridization [FISH]). The group-specific probes used were as follows: EUB338 (domain Bacteria) (1); BET42a (beta-proteobacteria) (22); GAM42a (gamma-proteobacteria) (22); and PS (genus Pseudomonas) (38). Hybridization and counterstaining with DAPI were carried out according to the method of Amann et al. (1) and Manz et al. (23). Total bacterial cell numbers (DAPI) and positively probe-hybridized cells were enumerated by analyzing slides, which were taken of the same section of a double-stained sample (Zeiss Axiophot).
Characterization of bacterial community composition by cultivation on agar plates.
Dilution and plating of chemostat samples on NSY agar (four replicates) were carried out as described previously (11). Based on several morphological features (size, color, shape, and surface and border characteristics), 18 different colony types (I to XVIII) were distinguished. Very rare colony types or irregularly grown colonies (due to contact with other colonies or the plate border) were pooled in a separate group (type Z). The relative abundances of the 19 colony types on agar plates were determined by counting the total numbers of colonies (CFU) and the type-specific numbers.
Taxonomic analysis of colony types and single strains.
The morphologically defined colony types were tested for taxonomic homogeneity by comparison of 5 to 10 isolates by FISH, by using the phenotypic test system BIOLOG (BIOLOG Inc., Hayward, Calif.) and by performing low-molecular-weight (LMW) RNA profiles (15, 16). Isolates of types II, III, and XVI were characterized by clearly distinct colony morphologies but showed no differences in BIOLOG tests, LMW RNA profiles, cell morphologies, or reaction with the anti-Vibrio sp. strain CB5 serum (CB5 belongs to the colony type III group). Therefore, those three colony types were tested additionally for taxonomic homogeneity by amplified ribosomal DNA (rDNA) restriction analysis (ARDRA) (44). Four isolates each of colony types II, III, and XVI were compared.
Identifications of the strains Pseudomonas sp. strain MWH1 (13), Vibrio sp. strain CB5 (colony type III) (11), Vibrio sp. strain CB2 (colony type II), C. acidovorans PX54 (8), and A. hydrophila PU7718 (8) were carried out by analysis of the 16S rDNA sequences (12).
Determination of nonspecific and specific cell size.
The cell sizes of DAPI-stained (nonspecific-size) and antibody-labeled (specific-size) cells were determined by image cytometry as described previously (11). Specific size data were corrected for overestimation, as described previously (11).
Assay for size-selective grazing.
The uptake of fluorescent latex beads of different diameters (Polysciences Inc.) was followed for estimation of size-selective grazing by Ochromonas sp. strain DS. Chemostat samples from reactor I (phase 2) received a small volume of a suspension of beads and were incubated at 15°C. The suspension contained an equal mixture of beads with diameters of 0.5, 0.75, 1.0, and 2.0 μm in NSY medium. Samples were taken five times at 10-min intervals and were fixed immediately in an equal volume of 4% ice-cold glutaraldehyde (36). The number of ingested beads of the different diameters was counted for 100 randomly selected flagellates. Size-specific grazing rates were calculated, using data on mean numbers of beads taken up per flagellate from the first four samplings (no saturation of uptake). The highest grazing rate was set at 100% for calculation of size-specific relative grazing efficiency. The experiment was carried out in triplicate.
Statistical analysis.
The data were checked for significance of differences by t test.
RESULTS
Influence of flagellate grazing on bacterial abundance, cell size, and morphology.
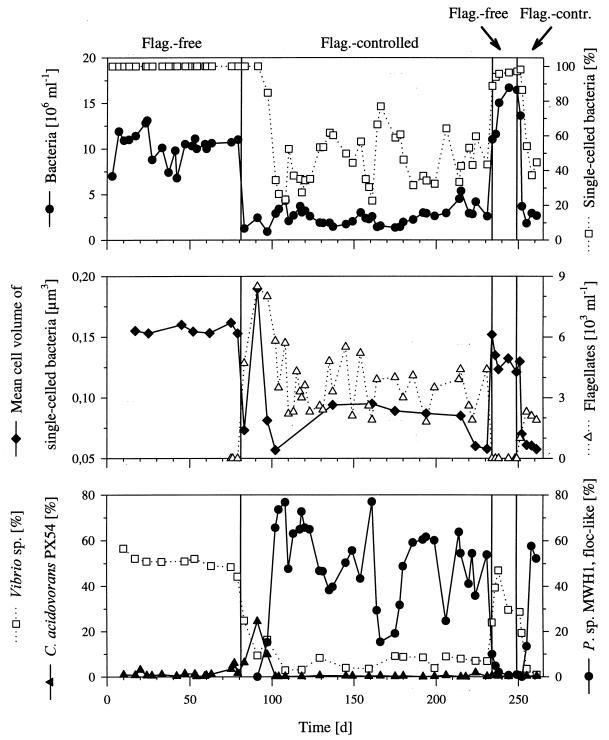
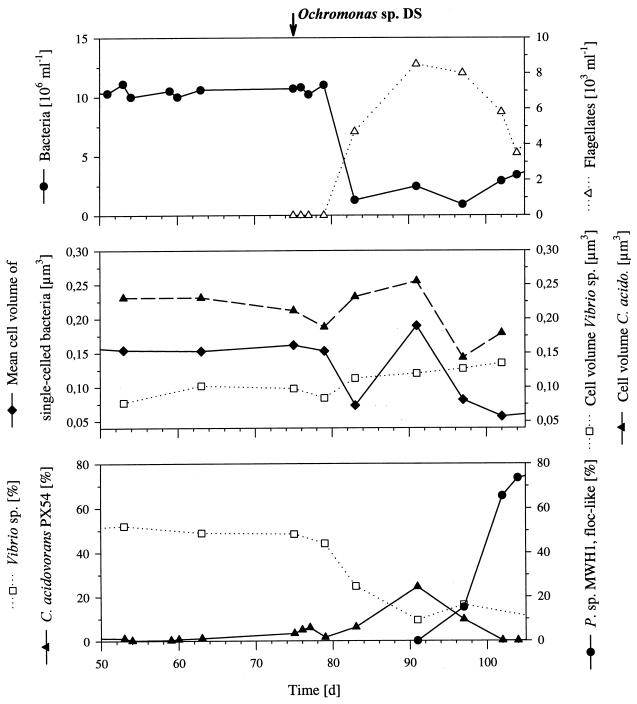
In terms of bacterial abundance, cell size, and bacterial morphology, the nongrazed communities (phase 1), cultured in the two parallel reactors, showed relatively stable patterns with only minor differences observed between the two communities. Total bacterial numbers were (10.3 ± 1.6) × 106 ml−1 (reactor I) and (12.5 ± 1.3) × 106 ml−1 (reactor II), and the two communities consisted of only single-celled bacteria (Fig. 1) with mean cell volumes of 0.156 ± 0.003 μm3 (reactor I) (Fig. 2) and 0.125 ± 0.050 μm3 (reactor II). After introduction of the flagellate, the bacterial abundance and the mean bacterial cell size decreased with increasing flagellate abundance (Fig. 2). During the grazing-controlled phase 2, which lasted for 148 (reactor I) and 107 (reactor II) days, the abundance of single-celled bacteria was stable at (1.1 ± 0.4) × 106 ml−1 (reactor I) and (0.9 ± 0.2) × 106 ml−1 (reactor II). Besides the reduction in numbers of single-celled bacteria, the two communities showed different reactions to flagellate grazing. In reactor I the initial decrease in mean bacterial cell volume was followed by a short increase to 0.190 μm3 and thus to a value higher than those observed for the nongrazed community (Fig. 2). This maximum in mean cell size was correlated with a maximum in the relative abundance of mdit.C. acidovorans PX54 (see below) and the maximal flagellate abundance (Fig. 2). Afterwards, the mean cell volume of single-celled bacteria decreased again and, simultaneously, a population of floc-like bacteria was established (Fig. 1 and 2). The bacterial flocs consisted of as many as 900 rod-shaped cells of similar size and morphology. For the following 137 days, the flagellate-controlled bacterial community of reactor I was divided morphologically into two populations; one consisting of small, single cells with a mean volume of 0.078 ± 0.016 μm3 and one consisting of the large bacterial flocs with diameters of 5 to more than 50 μm. The average cell length of single-celled bacteria decreased from 0.87 μm (phase 1) to 0.65 μm (the mean value for the period of phase 2 after the peak).
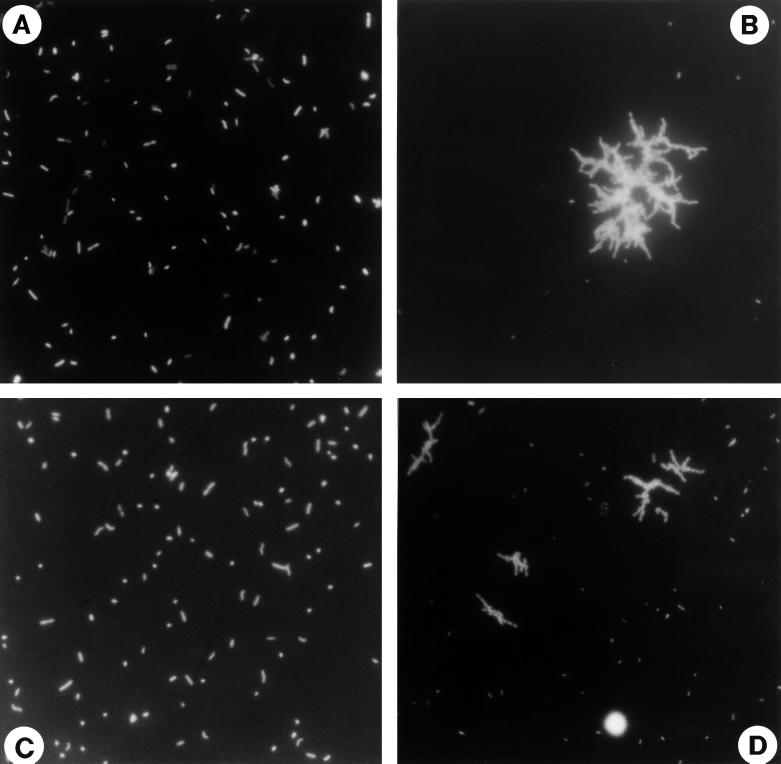
FIG. 1.
Photomicrographs of bacteria from reactor I stained with DAPI. (A) Before introduction of the flagellate (phase 1). (B) After introduction of the flagellate (phase 2). (C) After inhibition of the flagellate population (phase 3). (D) After reestablishment of the flagellate population (phase 4). A single cell of the bacterivorous flagellate Ochromonas sp. strain DS is shown in panel D. The images represent different sample volumes filtered onto membrane filters.
FIG. 2.
Influence of flagellate grazing on the complex bacterial community cultured in reactor I. The upper panel shows the total bacterial cell numbers and the percentage of single-celled bacteria. The lower panel shows the relative abundances (percentages of total cell numbers) of the three species detected by immunofluorescence microscopy. The four experimental phases are indicated by vertical lines. Flag., flagellate; contr., controlled; P. sp. MWH1, Pseudomonas sp. strain MWH1.
Also, in reactor II, the mean bacterial cell volume decreased after introduction of the flagellate (to a mean value of 0.050 ± 0.008 μm3), but no intermediate increase in cell size or occurrence of floc-like bacteria was observed in the 81 days after introduction of the flagellate. Therefore, the reactor was inoculated with a sample (10 ml) from reactor I which contained floc-like bacteria. This resulted in the establishment of a population of large, floc-like bacteria in reactor II, rather similar to the events observed in reactor I.
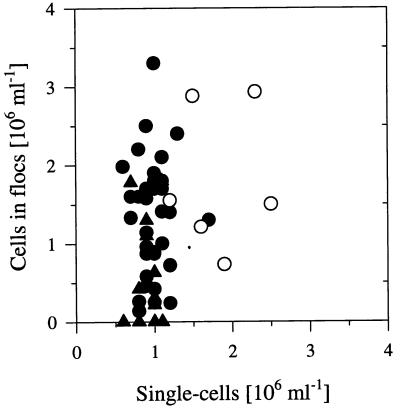
During flagellate-controlled phases, the numbers of single-celled bacteria fluctuated in both reactors within a narrow range of 0.6 × 106 to 1.3 × 106 ml−1 (Fig. 3) and thus on a level approximately 10-fold lower than the level during flagellate-free phases. In the case of reactor I, a period covering approximately 10% of the total flagellate-controlled phases showed higher abundances of single cells (1.2 × 106 to 2.5 × 106 ml−1). The cell numbers of the floc-like bacteria (Pseudomonas sp. strain MWH1) fluctuated (0.2 × 106 to 3.3 × 106 ml−1) much more than those of single cells (Fig. 3). During the period when the floc-like bacteria were present, the total numbers of cells building these flocs made up an average of 55% (reactor I) and 44% (reactor II) of the total bacterial cell numbers. The mean cell size of bacteria in the flocs (0.206 μm3) was larger than that of the single-celled bacteria, and thus, these bacteria dominated the total bacterial biomass, with mean values of 72% (reactor I) and 51% (reactor II).
FIG. 3.
Plot of the number of single-celled bacteria against the number of cells in flocs. The data set represents only samples from the flagellate-controlled phases of reactor I (circles) as well as from reactor II (triangles). The open circles represent data from a 20-day period (about 10% of the total flagellate-controlled phases), and the solid circles represent data from the other periods.
Elimination of the flagellate population in reactor I by specific inhibitors resulted in (i) an increase of bacterial numbers, (ii) an increase in the mean bacterial cell size, and (iii) a decrease in the floc-like bacteria to levels below the detection limit (Fig. 1 and 2). Thus, the bacterial community restored the morphological structure typical for phase 1 of both reactors. The subsequent reestablishment of the flagellate population resulted, again, in a decrease of bacterial numbers and in bidirectional changes in the morphological structure of the bacterial community (Fig. 1 and 2).
Characterization of the taxonomic structure of the bacterial communities.
The nondefined community was characterized taxonomically by plating samples on NSY agar, by immunofluorescence microscopy, and by FISH. The quantitative characterization of the bacterial communities by culturing was handicapped less by the general culturability of the bacteria than by the lack of formation of separate colonies by cells stuck together in the bacterial flocs. During periods with an absence of floc-like bacteria, the mean values of total cell counts and total CFU showed no significant (P > 0.01) differences. During other periods, the total cell numbers were higher than the total CFU, although no significant difference was observed between the numbers of single cells and total CFU (P > 0.01).
On the agar plates, more than 25 morphologically distinguishable colony types were observed over the total period of the experiment. Of these 25 types, 18 (designated I to XVIII) were found regularly, with percentages of more than 5% of the total colonies. For these 18 colony types, the respective relative CFU were determined, and only these types were tested for taxonomic homogeneity. Only two (IV and V) of the 18 colony types were found to consist of morphologically indistinguishable but taxonomically different colonies. On the other hand, colony types II, III, and XVI demonstrated clear differences in colony morphology, but no taxonomic difference was found (by LMW RNA analysis, ARDRA, or reaction with anti-Vibrio sp. strain CB5 serum). Sequencing of the 16S rDNA genes revealed virtually identical sequences for two tested isolates (CB2 and CB5) of different colony morphologies (II and III). Comparisons of 16S rDNA gene sequences demonstrated that these isolates were most closely related to Vibrio pelagius (sequence similarity, 99.6% [26]).
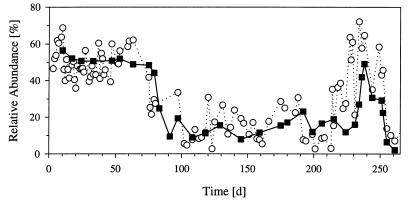
During the absence of bacterial flocs the summarized relative abundance of the Vibrio sp. colonies (types II, III, and XVI) in both reactors showed no significant differences (P > 0.1) from the relative abundance of the Vibrio sp. determined by immunofluorescence microscopy (Fig. 4, Table 2). During periods when floc-like bacteria were present, the values differed significantly.
FIG. 4.
Comparison of the relative abundance of the Vibrio sp. determined by the plating approach (open circles) and by immunofluorescence microscopy (solid squares). The plating data represent the percentage of Vibrio sp. colonies (colony types II, III, and XVI) among total CFU, and the microscopically determined data represent the percentage of Vibrio sp. cells among the total number of single-celled bacteria. The plating data are based on 100 to 250 counted colonies (total CFU), and the microscopy data are based on at least 500 counted cells (total single cells).
TABLE 2.
Taxa of particular interest and their detection, type of colony morphology, and range of relative abundance observed during the experiment
| Taxon | Detection methoda | Colony type | Relative abundanced (%) | Permanent presenceh |
|---|---|---|---|---|
| Vibrio sp. | PAb, Colc | II, III, XVI | 5–60e | + |
| Pseudomonas sp. strain MWH1 | PAb | IV | <0.1–80e | +g |
| C. acidovorans PX54 | MAb | IV | <0.1–25e | + |
| A. hydrophila PU7718 | MAb, Col | XXII | <1e | − |
| Colony type IVb | Col | IV | <1–80f | + |
| Colony type Vb | Col | V | <1–60f | + |
PAb, polyclonal antibody; MAb, monoclonal antibody; Col, detection and enumeration by the plating approach.
Taxonomically heterogeneous.
For comparison of the two methods, see Fig. 4.
Range observed for both reactors throughout the experiment.
Percentage of total cell numbers.
Percentage of total CFU.
Due to cross-reactivity of PAb not being ensured.
+, detected in all investigated samples; −, not detected in all of the investigated samples.
By immunofluorescence microscopy, 10 to 80% of the bacterial cells in samples from the two reactors could be identified by using the four antisera and antibodies. While, in reactor I, an average of 49.6% ± 15.9% of the total cell numbers were detectable, the other reactor had a lower average detectability of 37.8% ± 17.8%.
The bacterial flocs were formed exclusively by Pseudomonas sp. strain MWH1. This was demonstrated by immunofluorescence microscopy, by FISH, and by studies with the isolated strain (13).
Stability of the nongrazed communities.
During the first 10 days after inoculation of the two reactors, the same succession pattern was observed in the two communities. Initially, type I colonies dominated, making up approximately 50% of the total CFU, but they were replaced extensively by the Vibrio sp. and strains of colony type V during the following days. For the next 20 days, the community compositions were very similar as well as stable in the two reactors. During this period, the Vibrio sp. (approximately 50% of cells and colonies) and strains of colony type V (25 to 30% of colonies) dominated in both reactors.
In the further course of the experiment, the communities cultured in the two reactors showed different types of development. The community of reactor I was more or less stable until the inoculation with the flagellate (Fig. 2), while the community of reactor II showed a slow but steady change in composition (data not shown). In this reactor, the percentage of Vibrio sp. cells and colonies declined over a period of 115 days from 50 to 25% and type IV colonies increased from less than 10% to fluctuating percentages of 25 to 60%. C. acidovorans PX54 established stable populations in both reactors, with a mean relative abundance of 0.9% ± 1.7% (reactor I) and 2.7% ± 1.1% (reactor II). In both reactors A. hydrophila PU7718 was only found (<1% of total cell numbers) during the first days after inoculation.
Influence of flagellate grazing on the taxonomic composition of the bacterial communities.
In reactor I the composition of the stable community changed with the establishment of the flagellate population. The following succession was observed (Fig. 2): (i) a decrease in the Vibrio sp. from 51.3% ± 2.5% to 7.1% ± 3.7% of the total cell numbers; (ii) an increase in C. acidovorans PX54 from 0.9% ± 1.7% to a maximum of 24.5%; (iii) a decrease in C. acidovorans PX54 to mean values of 0.4% ± 0.5%; (iv) a simultaneous occurrence and a numerical increase in bacterial flocs (Pseudomonas sp. strain MWH1 [see below]); (v) simultaneous with the increase in flocs, an increase in the percentage of type IV colonies from 3.7% ± 6.5% to a maximum of 80%; (vi) a steady decrease in the relative abundance of type IV colonies; and (vii) a simultaneous increase in type V colonies.
The maximum in the absolute abundance of C. acidovorans PX54, observed after the introduction of the flagellate (Fig. 2 and 5), was 7-fold higher than the mean abundance and 3.5-fold higher than the maximum abundance observed during the flagellate-free phase of the experiment.
FIG. 5.
Changes in the cell size of the total single-celled bacteria, of C. acidovorans PX54, and of the Vibrio sp. after introduction of the flagellate Ochromonas sp. strain DS into reactor I (center panel). The upper panel gives the total bacterial and flagellate abundances, and the lower panel gives the relative abundances of three investigated bacteria (percentage of total cell numbers).
The elimination of flagellates by using specific inhibitors resulted in the recovery of the morphological structure, as well as the recovery of a taxonomic structure similar to that of the nongrazed community (Fig. 1 and 2). The reestablishment of the flagellate population was followed by a succession similar to that observed after the introduction of the flagellate (Fig. 2). The main difference was the lack of a maximum of C. acidovorans PX54.
In reactor II, no significant influence of flagellate grazing on the taxonomic structure was detectable by the methods used. Before inoculation with the flagellate, the relative abundance of the Vibrio sp. was already low and those of the taxonomically heterogenous colony types IV and V were high. Possible changes within these two heterogenous groups cannot be excluded. After inoculation with a sample from reactor I, the bacterial composition changed simultaneously with the establishment of bacterial flocs consisting of Pseudomonas sp. strain MWH1 cells.
FISH.
Three samples each from phase 1 and phase 2 of reactor I were hybridized with four oligonucleotide probes. On average, 80.5% ± 8.2% of the DAPI-labeled cells hybridized positively with the EUB338 probe targeted against all taxa belonging to the domain Bacteria. Samples of the two phases showed no differences in their reactions to hybridization. Introduction of the flagellate caused no significant change in bacterial composition detectable by the probes GAM42a and BET42a. The γ-proteobacteria dominated among the EUB338-hybridizable cells with 77% (phase 1) and 71% (phase 2), and 5% (phase 1) and 10% (phase 2) reacted positively with the BET42a probe. The PS probe always hybridized positively with cells in bacterial flocs (Pseudomonas sp. strain MWH1), but signals from single cells were always very weak.
Specific cell size of Vibrio sp. and C. acidovorans PX54 in reactor I.
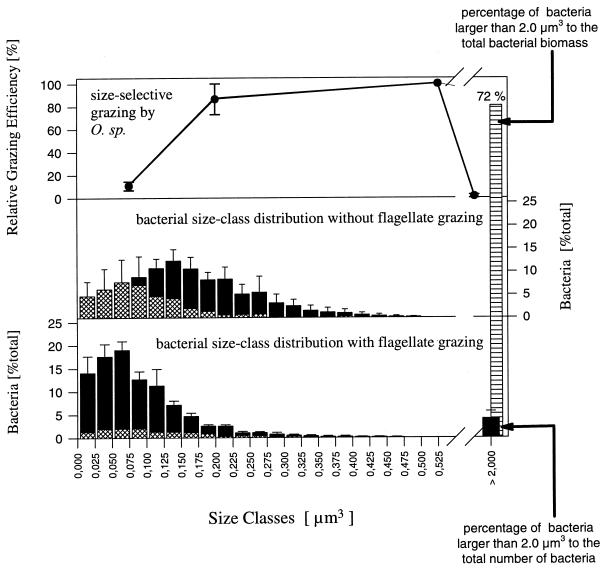
The mean cell volume of the Vibrio sp. increased slightly after introduction of the flagellate from 0.090 ± 0.012 μm3 (phase 1) to 0.124 ± 0.009 μm3 (phase 2) (Fig. 5). A comparison of the size class distribution of the Vibrio sp. with the distribution of the total community revealed that the Vibrio population completely covered the lower-size classes during phase 1 (Fig. 6). The percentages for the Vibrio sp. found for the three classes from 0 to 0.075 μm3 were slightly higher than those for the total community, which may indicate an underestimation of the sizes of the Vibrio sp., but the differences were not significant (P > 0.1). During the flagellate-controlled phases, the mean cell size of the total population of single-celled bacteria was smaller than the mean cell size of the Vibrio sp. (Fig. 5). This resulted in the dominance of other strains in the lower-size classes (Fig. 6), indicating that the Vibrio population was strongly replaced by populations of other strains which had smaller cells under the given growth conditions.
FIG. 6.
Influence of size-specific grazing of the flagellate Ochromonas sp. strain DS (upper graph) on the size distribution of the bacterial community of reactor I. The bars of the graph in the middle represent the size class distribution of the community during phase 1 (mean values of five samples). The hatched parts of the bars indicate the proportion of Vibrio sp. cells. The lower graph represents the distribution after introduction of the predator (five samples from phase 2). The size class >2.0 μm3 is exclusively formed by the Pseudomonas sp. strain MWH1 flocs (the bars represent mean values for the whole microcolony population). This size class contained 4% of the bacterial particles (single-celled bacteria and flocs) but 72% of the bacterial biomass.
The mean cell size of C. acidovorans PX54 increased with increasing grazing pressure, although afterwards, the cell size decreased with the increase of the Pseudomonas sp. strain MWH1 population (Fig. 5). The maximal cell size of C. acidovorans PX54 occurred with the maximal cell numbers and relative abundance of the strain (Fig. 5).
Size-selective grazing of Ochromonas sp. strain DS.
The flagellate showed a marked size-selective grazing. Grazing rates on beads with diameters of 0.5 and 2.0 μm were 10- and 200-fold lower, respectively, than the rate determined for 1.0-μm-diameter beads (Fig. 6). Based on the uptake of beads, we calculated size-dependent grazing rates of 19.9 ± 5.5 bacteria flagellate−1 h−1 (1.0-μm-diameter beads) and 1.9 ± 0.05 bacteria flagellate−1 h−1 (0.5-μm-diameter beads).
DISCUSSION
The experimental conditions used in our experiments mimicked the growth rates and abundances of bacteria (12), the growth rates and abundances of bacterivorous flagellates (3, 35, 46), and the mean cell size of bacteria (7, 29, 42) reported for mesotrophic to eutrophic lakes. On the other hand, experimental studies like the one presented here are not able to copy a natural bacterial community. The number of relevant bacterial and flagellate species is limited, and the presence of species dominating in natural assemblages is rather unlikely. However, on the level of morphological community structure, we observed responses to flagellate grazing (Fig. 6), as predicted for natural bacterial communities by the empirical model of Güde (10), as well as observed several times in field studies (10, 29). These general similarities indicate that our system is, to some extent, suitable for general investigations of the interplay of morphological and taxonomic changes caused by flagellate grazing.
Bacterial successions after introduction of the flagellate. (i) Reactor I.
The bacterial community of reactor I was dominated by the Vibrio sp., C. acidovorans PX54, and Pseudomonas sp. strain MWH1 over almost the whole experiment (Fig. 2). These major players responded to the introduction of the flagellate and to the reestablishment of the inhibited flagellate population with marked changes in relative abundance and size. These responses were reproduced in separate chemostat experiments under the same experimental conditions but involving only one or two of the major players, as well as the flagellate Ochromonas sp. strain DS (11, 13). In a chemostat experiment with Vibrio sp. strain CB5 (one of the Vibrio sp. strains) and C. acidovorans PX54 (11), we also observed, after introduction of the flagellate, a decrease in the relative abundance of the Vibrio sp. and a simultaneous increase of C. acidovorans PX54, as well as similar trends in changes in the cell sizes of the two strains (11) (Fig. 5). In contrast to this previous study, the increase in cell size of C. acidovorans PX54 stopped in the present study before the formation of filaments (>10 μm) occurred. An increase in the cell size and the formation of filaments by C. acidovorans PX54 is controlled by the growth rate (12). We assume that in the present study the lack of further cell elongation was a result of competition, which influenced the growth rate of the strain. A stronger limitation of C. acidovorans PX54 by competition on substrates in the present study is indicated by the 80%-lower (in comparison to the nongrazed part of the reference experiment) biomass of the population. After the introduction of the flagellate, C. acidovorans PX54 may have been released from this limitation through a reduction of competitors as a result of grazing. The later decrease in cell size may have been influenced by the simultaneous increase of the potential competitor, Pseudomonas sp. strain MWH1 (Fig. 5).
Formation of flocs by single-celled Pseudomonas sp. strain MWH1, after establishment of the flagellate population (Fig. 2), was also observed in separate chemostat experiments (13). These flocs differed markedly in size from all single-celled bacteria and should, in comparison to those, receive protection against flagellate grazing. However, differences in fluctuations of the total abundance of cells in flocs (Pseudomonas sp. strain MWH1) and in the total abundance of single-celled bacteria (Fig. 2 and 3) demonstrate that these two groups were controlled by different mechanisms. We can largely exclude the possibility that phages were responsible for the observed fluctuations of the floc-like Pseudomonas sp. strain MWH1 population. An investigation of several chemostat samples by transmission electron microscopy revealed a very low abundance of <104 phages ml−1 (45). No differences in phage abundance were found for samples with low, medium, and high numbers of bacterial flocs. We assume that the fluctuations of the floc-like Pseudomonas sp. strain MWH1 were a result of competition with single-celled bacteria and/or a consequence of changes in the proportion of floc-forming to single-celled Pseudomonas sp. strain MWH1 (13).
Based on the comparisons with the reference experiments (11, 13), we conclude that the total bacterial succession observed in reactor I was mainly the result of flagellate grazing.
(ii) Reactor II.
The bacterial community of reactor II responded to flagellate grazing through changes in morphology and cell size, similar to the reaction of the community of reactor I (Table 3), although the methods used to follow the taxonomic composition were not able to reveal whether a taxonomic response to the introduction of the flagellate also occurred. However, several factors may have been responsible for the taxonomic changes observed during the experimental phase before the introduction of the flagellate. Firstly, minimal differences in the taxonomic composition of the inoculi used for the two reactors may be the reason. Secondly, different physiological adaptations of the populations present in the reactors may have caused the different developments. Such changes in the physiological features of bacteria cultured in chemostats have been reported previously (14, 33).
TABLE 3.
Observed changes in morphological and taxonomic structure of bacterial communities after introduction of the flagellate Ochromonas sp. strain DS
| Parameter | Observed ina:
|
|
|---|---|---|
| Reactor I | Reactor II | |
| Morphological structure | ||
| Stable during phase 1 | + | + |
| Decrease in cell size of single-celled bacteria | + | + |
| Floc-like bacteria | + | +b |
| Bidirectional change | + | +b |
| Taxonomic structure | ||
| Stable composition during phase 1 | + | − |
| Change due to flagellate grazing | + | +/−c |
| Dominance of Vibrio sp. in absence of flagellates | + | +/−d |
| Dominance of Pseudomonas sp. strain MWH1 in presence of flagellates | + | +b |
| Maximum of C. acidovorans PX54 after introduction of flagellates | + | − |
+, observed; −, not observed.
After inoculation with a 10-ml sample (0.5% of working volume) from reactor I.
+/−, not detectable by the methods used.
+/−, no dominance for the entire flagellate-free phase.
Interplay of morphological and taxonomic structures.
The bacterial communities of both reactors responded to flagellate grazing with a marked bidirectional shift in their size distributions (Fig. 2 and 6 and Table 3). These shifts could be easily explained by size selectivity of the flagellate grazing (2, 9, 29, 39). However, in the assay used for estimating the size selectivity of the flagellate grazing, we used fluorescent latex beads. Because these particles were different in shape, surface qualities, and other traits from the bacteria cultured in the chemostat, the assay can give only a rough idea about the size selectivity of the flagellates grazing upon bacteria. Additionally, a size-independent selectivity of flagellate grazing was demonstrated (5, 21, 24), as well as a dependence of selectivity on the physiological state of the flagellate cells (19). On the other hand, the flagellate population grew in the chemostat under strongly food-limited conditions. The flagellate growth rate was 0.5 day−1 and thus only approximately 30% of the maximum growth rate observed under similar but food-saturated growth conditions. Jürgens and DeMott found only a weak size-independent selectivity by food-limited flagellates (19). Therefore, and due to the clear trends in bidirectional shifts of bacterial size distribution, we assume that size selectivity of flagellate grazing was the major structuring force controlling the morphological structure of the bacterial community. However, we cannot rule out the possibility that size-independent selectivity played a minor role in the influence of grazing on the bacterial community structure.
In terms of changes in the mean size of single-celled bacteria, the complex bacterial communities of both reactors responded to flagellate grazing in a totally different way than five bacterial species investigated in separate experiments (11–13). While the single-celled part of the complex communities showed a marked decrease in mean cell size, each of the five populations responded to flagellate grazing by a slight-to-marked increase in cell size. We previously demonstrated that the increases in cell size were not a direct reaction to flagellate grazing but a reaction to an increase in bacterial growth rates due to grazing (11, 12). Such a dependence of cell size on growth rate was also found for other bacterial species (4, 6, 37) and may be a result of an increased need for space for the ribosomes, which increase in number as growth rate increases (4, 32, 34). In the current experiment, the responses in cell size of selected taxa were followed (Fig. 5). These populations showed the expected increases in cell size after the reduction of total bacterial biomass by the predator. The opposite responses of mean cell size of single species and mean cell size of the whole single-celled part of the total bacterial community indicate that strains with larger cells were partly replaced by strains with smaller cells (Fig. 5 and 6). However, we cannot rule out the possibility that some of the minor players actively decreased their cell sizes despite the expected increase in bacterial growth rate. Whether such a bacterial grazing defense mechanism exists is unknown.
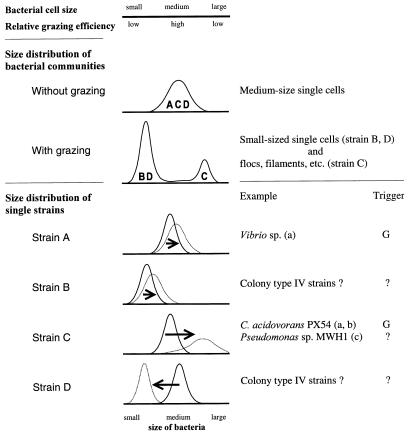
We suggest that the observed morphological and taxonomic responses of the investigated bacterial community to the flagellate grazing were mainly a result of the interplay of size-selective grazing, differences in cell size distribution of single bacterial strains, and differences in the specific responses of bacterial cell size distribution to grazing and growth rate (Fig. 7).
FIG. 7.
Schematic model of the interplay of size-selective grazing, specific bacterial cell size, and morphological as well as taxonomic structure of the bacterial communities investigated in the chemostat experiments. The upper part of the model depicts the size selectivity of protist grazing. The middle part shows the size distribution of bacterial communities in situations with and without grazing pressure, as well as their possible strain composition (indicated by letters). The lower part depicts the size distribution of single bacterial strains (A to D), as well as grazing-influenced changes in their size distributions (dotted curves). The arrows indicate the direction and strength of induced changes in the size distributions of the strains. Examples for some of the distribution patterns are shown, and the triggers of changes are given. The key result is that differences in the distribution patterns of single bacterial strains produced different grazing mortalities of the respective populations and thus resulted in changes in the morphological and taxonomic structures of the bacterial community. The possible influence of grazing on bacterial competition for substrates or on changes in substrate supply, as well as size-independent grazing defense strategies, is not considered. (a), reference 11; (b), reference 12; (c), reference 13; G, growth rate; ?, unknown.
In the present study, and in other experiments, we observed the domination of medium-size single cells in the absence of grazers (Fig. 6) (11–13). This corresponds with the model of Güde (10) and with several other observations (2, 10, 17, 29), but the reason for the superiority of medium-size strains to smaller and larger strains is unknown. One may speculate that larger cells have disadvantages in competition for substrates due to a reduced surface-to-volume ratio. For the smaller cells, this argument does not hold true. However, Norland and coworkers (27), as well as Simon and Azam (43), demonstrated in size-fractionated bacterial communities that smaller bacterial cells tend to have a higher dry-weight-to-volume ratio than larger ones. The water content of the cells decreases with cell size, but the percentage of DNA, proteins, and cell wall and other cellular components in the cellular dry mass increases. This indicates that the evolutionary process of development of smaller bacteria requires a compromise between a general reduction in cell size and conservation of essential cellular functions, which cannot be decreased below a minimum level (e.g., the genome size). We assume that this compromise results in additional metabolic costs for small bacterial cells and thus leads to the superiority of medium-size cells. However, in situations without predation, the medium-size cells may have competitive advantages, although in the opposite situation these cells should have higher grazing losses due to size-selective predation. This results in advantages for smaller and larger cells and, thus, in changes in the taxonomic and morphological compositions of bacterial communities (Fig. 7).
Because we found no hints of size-independent grazing defense strategies of bacteria in our experiments, and due to the general lack of knowledge about such mechanisms, we only considered size-dependent defense mechanisms (Fig. 7). However, in numerous field studies, marked morphological shifts of bacterial communities were reported as responses to increases in protist grazing pressure (10, 18, 29, 41). This may indicate that size-independent grazing defense mechanisms play only a minor role in natural bacterial communities.
ACKNOWLEDGMENTS
We thank M. G. Weinbauer for determination of phage abundances, F. Ziemke for help with the ARDRA, E. R. B. Moore for 16S rDNA analysis and for linguistic improvements, and K. Seikowsky and K. Dominik for performing LMW RNA profiles, as well as two anonymous reviewers for constructive criticism on the manuscript.
This study was supported by funds from the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (grant BEO-0319433B).
REFERENCES
- 1.Amann R I, Binder B J, Olson R J, Chisholm S W, Devereux R, Stahl D A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson A, Larsson U, Hagström Å. Size-selective grazing by a microflagellate on pelagic bacteria. Mar Ecol Prog Ser. 1986;33:51–57. [Google Scholar]
- 3.Berninger U-G, Finlay B J, Kuuppo-Leinikki P. Protozoan control of bacterial abundances in freshwater. Limnol Oceanogr. 1991;36:139–147. [Google Scholar]
- 4.Bremer H, Dennis P P. Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt F C, Curtiss III R, Ingraham J P, Lin E C C, Low K B, Magasanik B, Rezikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1533–1569. [Google Scholar]
- 5.Christoffersen K, Nybroe O, Jürgens K, Hansen M. Measurement of bacterivory by heterotrophic nanoflagellates using immunofluorescence labelling of ingested cells. Aquat Microb Ecol. 1997;13:127–134. [Google Scholar]
- 6.Chrzanowski T H, Crotty R D, Hubbard G J. Seasonal variation in cell volume of epilimnetic bacteria. Microb Ecol. 1988;16:155–163. doi: 10.1007/BF02018911. [DOI] [PubMed] [Google Scholar]
- 7.Cole J J, Pace M L, Caraco N F, Steinhart G S. Bacterial biomass and cell size distributions in lakes: more and larger cells in anoxic waters. Limnol Oceanogr. 1993;38:1627–1632. [Google Scholar]
- 8.Faude U C, Höfle M G. Development and application of monoclonal antibodies for in situ detection of indigenous bacterial strains in aquatic ecosystems. Appl Environ Microbiol. 1997;63:4534–4542. doi: 10.1128/aem.63.11.4534-4542.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.González J M, Sherr E B, Sherr B B. Size-selective grazing on bacteria by natural assemblages of estuarine flagellates and ciliates. Appl Environ Microbiol. 1990;56:583–589. doi: 10.1128/aem.56.3.583-589.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Güde H. The role of grazing on bacteria in plankton succession. In: Sommer U, editor. Plankton ecology. Succession in plankton communities. Berlin, Germany: Springer; 1989. pp. 337–369. [Google Scholar]
- 11.Hahn M W, Höfle M G. Grazing pressure by a bacterivorous flagellate reverses the relative abundance of Comamonas acidovorans PX54 and Vibrio sp. strain CB5 in chemostat cocultures. Appl Environ Microbiol. 1998;64:1910–1918. doi: 10.1128/aem.64.5.1910-1918.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn M W, Moore E R B, Höfle M G. Bacterial filament formation, a defense mechanism against flagellate grazing, is growth rate controlled in bacteria of different phyla. Appl Environ Microbiol. 1999;65:25–35. doi: 10.1128/aem.65.1.25-35.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn, M. W., E. R. B. Moore, and M. G. Höfle. Role of morphological plasticity in the grazing defense strategy of the aquatic bacterium Pseudomonas sp. MWH1. Submitted for publication.
- 14.Höfle M G. Long-term changes in chemostat cultures of Cytophaga johnsonae. Appl Environ Microbiol. 1983;46:1045–1053. doi: 10.1128/aem.46.5.1045-1053.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Höfle M G. Identification of bacteria by low molecular weight RNA profiles: a new chemotaxonomic approach. J Microbiol Methods. 1988;8:235–248. [Google Scholar]
- 16.Höfle M G. Transfer RNAs as genotypic fingerprints of eubacteria. Arch Microbiol. 1990;153:299–304. [Google Scholar]
- 17.Jürgens K. Is there plenty of food for bacterivorous flagellates in eutrophic waters? Arch Hydrobiol Beih Ergeb Limnol. 1992;37:195–205. [Google Scholar]
- 18.Jürgens K, Arndt H, Rothhaupt K O. Zooplankton-mediated changes of bacterial community structure. Microb Ecol. 1994;27:27–42. doi: 10.1007/BF00170112. [DOI] [PubMed] [Google Scholar]
- 19.Jürgens K, DeMott W R. Behavioral flexibility in prey selection by bacterivorous nanoflagellates. Limnol Oceanogr. 1995;40:1503–1507. [Google Scholar]
- 20.Jürgens K, Pernthaler J, Schalla S, Amann R. Morphological and compositional changes in a planktonic bacterial community in response to enhanced protozoan grazing. Appl Environ Microbiol. 1999;65:1241–1250. doi: 10.1128/aem.65.3.1241-1250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landry M R, Lehner-Fournier J M, Sundstrom J A, Fagerness V L, Selph K E. Discrimination between living and heat-killed prey by a marine zooflagellate, Paraphysomonas vestita (Stokes) J Exp Mar Biol Ecol. 1991;146:139–151. [Google Scholar]
- 22.Manz W, Amann R, Ludwig W, Wagner M, Schleifer K-H. Phylogenetic oligodeoxynucleotide probes for the major subclasses of Proteobacteria: problems and solutions. Syst Appl Microbiol. 1992;15:593–600. [Google Scholar]
- 23.Manz W, Szewzyk U, Ericsson P, Amann R, Schleifer K-H, Stenström T-A. In situ identification of bacteria in drinking water and adjoining biofilms by hybridization with 16S and 23S rRNA-directed fluorescent oligonucleotide probes. Appl Environ Microbiol. 1993;59:2293–2298. doi: 10.1128/aem.59.7.2293-2298.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell G C, Baker J H, Sleigh M A. Feeding of a freshwater flagellate, Bodo saltans, on diverse bacteria. J Protozool. 1988;35:219–222. [Google Scholar]
- 25.Monger B C, Landry M R. Size-selective grazing by heterotrophic nanoflagellates: an analysis using live-stained bacteria and dual-beam flow cytometry. Arch Hydrobiol Beih Ergeb Limnol. 1992;37:173–185. [Google Scholar]
- 26.Moore, E. R. B. Unpublished data.
- 27.Norland S, Heldal M, Tumyr O. On the relation between dry matter and volume of bacteria. Microb Ecol. 1987;13:95. doi: 10.1007/BF02011246. [DOI] [PubMed] [Google Scholar]
- 28.Pace M L. Bacterial mortality and the fate of bacterial production. Hydrobiologia. 1988;159:41–49. [Google Scholar]
- 29.Pernthaler J, Sattler B, Šimek K, Schwarzenbacher A, Psenner R. Top-down effects on the size-biomass distribution of a freshwater bacterioplankton community. Aquat Microb Ecol. 1996;10:255–263. [Google Scholar]
- 30.Pernthaler J, Posch T, Šimek K, Vrba J, Amann R, Psenner R. Contrasting bacterial strategies to coexist with a flagellate predator in an experimental microbial assemblage. Appl Environ Microbiol. 1997;63:596–601. doi: 10.1128/aem.63.2.596-601.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Posch T, Šimek K, Vrba J, Pernthaler J, Nedoma J, Sattler B, Sonntag B, Psenner R. Predator-induced changes of bacterial size-structure and productivity studied on an experimental microbial community. Aquat Microb Ecol. 1999;18:235–246. [Google Scholar]
- 32.Poulsen L K, Ballard G, Stahl D A. Use of rRNA fluorescence in situ hybridization for measuring the activity of single cells in young and established biofilms. Appl Environ Microbiol. 1993;59:1354–1360. doi: 10.1128/aem.59.5.1354-1360.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenzweig R F, Sharp R R, Treves D S, Adams J. Microbial evolution in a simple unstructured environment: genetic differentiation in Escherichia coli. Genetics. 1994;137:903–917. doi: 10.1093/genetics/137.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosset R, Julien J, Monier R. Ribonucleic acid composition of bacteria as a function of growth rate. J Mol Biol. 1966;18:308–320. doi: 10.1016/s0022-2836(66)80248-6. [DOI] [PubMed] [Google Scholar]
- 35.Sanders R W, Caron D A, Berninger U-G. Relationships between bacteria and heterotrophic nanoplankton in marine and fresh waters: an inter-ecosystem comparison. Mar Ecol Prog Ser. 1992;86:1–14. [Google Scholar]
- 36.Sanders R W, Porter K G, Bennett S J, DeBiase A D. Seasonal patterns of bacterivory by flagellates, ciliates, rotifers and cladocerans in a freshwater planktonic community. Limnol Oceanogr. 1989;34:673–687. [Google Scholar]
- 37.Schaechter M, Maaløe O, Kjeldgaard N O. Dependency on medium and temperature of cell size and chemical composition during balanced growth of Salmonella typhimurium. J Gen Microbiol. 1958;19:592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- 38.Schleifer K H, Amann R, Ludwig W, Rothemund C, Springer N, Dorn S. Nucleic acid probes for the identification and in situ detection of pseudomonads. In: Galli E, Silver S, Witholt B, editors. Pseudomonas: molecular biology and biotechnology. Washington, D.C.: American Society for Microbiology; 1992. pp. 127–134. [Google Scholar]
- 39.Šimek K, Chrzanowski T H. Direct and indirect evidence of size-selective grazing on pelagic bacteria by freshwater nanoflagellates. Appl Environ Microbiol. 1992;58:3715–3720. doi: 10.1128/aem.58.11.3715-3720.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Šimek K, Vrba J, Pernthaler J, Posch T, Hartman P, Nedoma J, Psenner R. Morphological and compositional shifts in an experimental bacterial community influenced by protists with contrasting feeding modes. Appl Environ Microbiol. 1997;63:587–595. doi: 10.1128/aem.63.2.587-595.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Šimek, K., P. Kojecka, J. Nedoma, P. Hartman, J. Vrba, and J. R. Dolan. Shifts in the bacterial community composition associated with different microzooplankton size fractions in a eutrophic reservoir. Limnol. Oceanogr., in press.
- 42.Simon M. Biomass and production of small and large free-living and attached bacteria in Lake Constance. Limnol Oceanogr. 1987;32:591–607. [Google Scholar]
- 43.Simon M, Azam F. Protein content and protein synthesis rates of planktonic marine bacteria. Mar Ecol Prog Ser. 1989;51:201–213. [Google Scholar]
- 44.Vaneechoutte M, Rossau R, De Vos P, Gillis M, Janssens D, Paepe N, De Rouck A, Fiers T, Claeys G, Kersters K. Rapid identification of bacteria of the Comamonadaceae with amplified ribosomal DNA-restriction analysis (ARDRA) FEMS Microbiol Lett. 1992;93:227–234. doi: 10.1111/j.1574-6968.1992.tb05102.x. [DOI] [PubMed] [Google Scholar]
- 45.Weinbauer, M. G. Unpublished data.
- 46.Weisse T. Growth and production of heterotrophic nanoflagellates in a meso-eutrophic lake. J Plankton Res. 1997;19:703–722. [Google Scholar]
- 47.Weisse T, Müller H. Planktonic protozoa and the microbial food web in Lake Constance. Arch Hydrobiol Spec Issues Adv Limnol. 1998;53:223–254. [Google Scholar]