Abstract
Background
Flare is a poorly defined term used by patients and clinicians to indicate inflammatory bowel disease (IBD) status. This study aimed to evaluate the validity of a single-item 7-point flare indicator relative to other measures of disease flare.
Methods
The longitudinal Manitoba Living with IBD Study followed persons with IBD for 1 year; they completed biweekly online surveys and provided 3 stool samples. Disease flare on a single-item flare indicator with 7 possible responses developed for the study was defined by report of symptoms as “moderately” or “much” worse. The flare indicator was evaluated against 5 measures of disease activity: fecal calprotectin score (FCAL), a 2-point disease status indicator, a 4-point flare certainty indicator, the IBD Symptom Index short form (SIBDSI), and the short form IBD Questionnaire (SIBDQ). Participants in a flare, based on the 7-point measure, were matched to a nonflaring participant, and a stool sample was collected.
Results
Of the 155 IBD participants, almost half (n = 74) experienced a flare. Of those who flared, 97.0% endorsed active IBD on the 2-point indicator (controls 42.5%; P < .001); 91.9% endorsed active IBD on the 4-point certainty indicator (controls 32.9%; P < .001); 90.5% endorsed active disease on the SIBDSI (controls 34.2%; P < .001); and 48.5% had an elevated FCAL (controls 34.3%; P < .05). The mean SIBDQ was lower for the flare group compared with controls (43.9 [SD 11.1] vs 58.3 [SD 8.5]; P < .001), indicating worse disease.
Conclusions
The 7-point flare indicator robustly identified symptomatic flares. This patient self-report indicator reflected meaningful changes in more complex clinical indices and had only weak concordance with the presence of inflammation.
Keywords: disease flare, disease activity, symptoms, fecal calprotectin, IBD symptom inventory
Introduction
Inflammatory bowel diseases (IBDs), which include Crohn’s disease (CD) and ulcerative colitis (UC), are chronic and incurable. The unpredictable course of IBD, with relapses and remissions along with a diverse set of symptoms, makes it challenging to define disease exacerbations in terms that communicate disease status consistently between patients and clinicians.1 Patients and providers commonly use the term “flare” to reference an increase in an individual’s gastrointestinal and extraintestinal burden of symptoms, which combined may be ascribed to an increase in their overall IBD activity.2 Additionally, providers use blood and stool testing to query inflammatory activity and radiological imaging and endoscopy as objective evidence of increased disease activity.3 Although endoscopic and radiologic procedures, which display evidence of mucosal healing, are the optimal and preferred methods for measuring disease activity, they are not suitable for repeated measures and they fail to incorporate the patient experience.4 It is the patient experience of a flare, for example escalating diarrhea and pain, which often drives clinic visits and medication changes.5 Understanding the patient’s experience of their disease is critical for optimal patient care, and as such, the patient is an essential partner in disease monitoring and management.6,7
Although patient-reported outcomes (PROs) are one of the cornerstones of clinical trial assessments, there is a surprising lack of validated self-report instruments that can reliably identify an IBD flare.8 Currently, multiple clinical indices varying in length, scoring, and content are being used to assess disease activity in CD, but the responsiveness of these instruments to detect a patient-defined flare is not well-defined.9–11 Not only does this lead to a lack of understanding between patients and their providers but it also results in limitations for conducting IBD research. With the potential to influence the development of a more patient-centered clinical indices, the purpose of this case-control study is to establish a better understanding of the symptoms and health-related quality of life measures that are important in predicting the transition from an inactive to an active disease stat—or what patients and providers often term “an IBD flare.”
The Manitoba Living with IBD Study, which obtained biweekly measurement from persons with IBD for a year, was designed to examine and evaluate flare indicators.12 We wanted to gain a full understanding of what patients are mostly reporting when they say they are in a flare and to determine how often these subjective flares were associated with an objective measure of active disease. Hence, the study aimed to evaluate the validity of a single-item 7-point flare indicator, relative to the fecal calprotectin (FCAL) and a number of PROs, including the Short form of the validated IBD Symptom Index (SIBDSI)13 and the Short form IBD Questionnaire (SIBDQ).14 To evaluate the symptom profile characterizing the self-reported flare, the relationship was examined between the 7-point flare indicator and 3 symptom subscores derived from the SIBDSI: (1) bowel symptoms score (BS), (2) abdominal and body discomfort score (ABD), and a (3) fatigue and general well-being score (FGW).
Methods
The Manitoba Living With IBD Study Protocol
Persons age 18 to 75 years, living in Manitoba, with a confirmed IBD diagnosis were recruited from a previous longitudinal cohort study, our provincial population-based IBD research registry, regional gastroenterology clinics, posters in hospitals and gastroenterologists’ offices, and information posted on our center’s website (ibdmanitoba.org). Recruitment took place between May 2015 and May 2017. Individuals with active disease within the past 2 years were invited to participate in a prospective, longitudinal study utilizing online biweekly surveys to regularly gather information about the experience of living with IBD. Participants completed surveys every 2 weeks for 1 year (totaling 26 surveys), hosted on the RedCap software platform.15 They provided a stool sample at week 0, week 26, and week 52. Additional stool samples were collected from participants at the time they experienced a flare during the year study period or if they were matched as a control for another participant who was experiencing a flare, allowing for case-control analysis. Additional stool samples were collected within 28 days of the reported flare. The only fecal calprotectin results used in this study were the ones collected around the time of the flare. Individuals with a known underlying irritable bowel syndrome (IBS) or other functional diagnoses were not included in this protocol. The study had a participant retention rate of 98.7%. The entire protocol has been previously published.12
The Health Research Ethics Board (HREB) located at the University of Manitoba’s Bannatyne Campus approved the Manitoba Living with IBD Study. Study identification numbers were used during all secondary analysis to protect the privacy of all study participants.
Participant demographic characteristics were collected at week 0. Health care use measures including hospitalizations, emergency department (ED) visits, and current medications (IBD/non-IBD) were collected at every measurement occasion.
Ethics approval was granted by the University of Manitoba Health Research Ethics Board. All patients gave informed consent to participate in this study.
Identification of Persons Reporting a Flare and Matched Controls
An individual was identified as a flare case by the study team using a 7-point flare indicator tool. The indicator was created specifically for the Manitoba Living with IBD protocol to determine if it may be useful in predicting an individual’s transition to a worse disease state. Specifically, an individual was considered a flare case if they had a score >5 on the indicator: “Compared with 2 weeks ago my IBD symptoms are: (1) much improved, (2) moderately improved, (3) minimally improved, (4) no change, (5) minimally worse, (6) moderately worse, or (7) much worse.” The indicator was used in every survey except the week 0 survey. Controls were identified as individuals who had not previously experienced a flare during the study period and were not currently experiencing a flare. Controls were matched on age (within 5 years), disease type (CD with CD; UC with UC), sex, and study entry date (to control for season). Controls were notified via email that they had been identified as a comparator for a study participant who may be experiencing a worsening of IBD symptoms and were invited to participate in this additional data collection. The email included a link to a supplemental survey gathering additional information on potential disease triggers; the same survey was automatically generated via RedCap for flare cases. Flare cases and controls were sent an additional stool collection kit to complete.
Fecal Calprotectin Testing
Fecal calprotectin (FCAL) is a biochemical marker for inflammation in the intestine as calprotectin is released from mucosal neutrophils. The FCAL score highly correlates with intestinal inflammation.16,17 Fecal calprotectin levels can remain stable for up to 7 days at room temperature.18 Stool collection kits were delivered and returned by courier to the University of Manitoba IBD Clinical and Research Centre, Winnipeg, Canada. Once the kit was received at the study center, it was kept at −80ºC until analyzed with a calprotectin enzyme-linked immunosorbent assay (ALPCO, Salem, NH, USA). We obtained 2 measurements from each sample, and the average FCAL level was used for analysis. The upper limit of measurement for FCAL was 1888 μg/g stool. Participants were considered to have active intestinal inflammation if FCAL exceeded 250 μg/g stool.19,20 Batch testing for FCAL was performed.
Self-reported Flare Indicators
At every measurement occasion except week 0, individuals were asked to respond to the 2-point disease status indicator categorizing their IBD as (1) inactive or (2) active. Additionally, a 4-point flare certainty indicator was used: “Do you consider yourself to be in an IBD disease flare?” Response options included, “I am not in an IBD flare (0); I am possibly in an IBD flare (1); I am probably in an IBD flare (2); I am definitely in an IBD flare (3).” This scale was created by our research group for this study.
Short-form Inflammatory Bowel Disease Symptom Inventory
The SIBDSI, a 25-item self-report measure assessing 3 symptom clusters, bowel symptoms, abdominal and body discomfort, and fatigue,13 was collected at every measurement occasion. It was derived from the validated longer form IBD Symptom Inventory, which contains both the Harvey-Bradshaw Index (HBI) for CD21 and the Powell Tuck Index (PTI) for UC,22 allowing them to be accurately derived. Factor 1, bowel symptoms (BS), includes frequency of bowel movements, blood in stool, urgency, difficulties with soiling, and fistulas. Factor 2, abdominal and body discomfort (ABD), includes abdominal pain, abdominal mass, difficulties with gas/bloating, nausea or vomiting, and arthralgias. Factor 3, fatigue and general well-being (FGW), includes feeling tired, trouble getting things done, and perceived health. The SIBDSI and IBDSI have strong convergent validity.13 Additionally, both are strongly correlated with clinician-administered HBI, PTI, and Manitoba IBD Index, which is a patient-reported outcome measure of symptom activity for monitoring disease status over time.23 The SIBDSI total score ranges from 0 to 95, with a higher score indicating greater symptoms; individuals with a score >14 in CD and >13 in UC (cutoff value) on the SIBDSI were considered to have active symptomatic disease.
Inflammatory Bowel Disease Quality of Life Measure
At every measurement occasion, the SIBDQ was also collected. Use of the Inflammatory Bowel Disease Questionnaire (IBDQ), was licensed from McMaster University, Hamilton, Canada. The SIBDQ is based on the original 32-item IBDQ and is a 10-item scale measuring bowel, systemic, social, and emotional aspects of quality of life with IBD.14 Scores range from 7 to 70, with higher scores indicating greater quality of life. It is responsive to changes in symptomatic disease activity.
When the grant from the Canadian Institutes of Health Research was awarded for this project, an IBD Patient Advisory Committee was created. This Committee advised on issues that were of importance to patients’ experiences with the disease and what types of questions they would want to be asked serially. Study results will be posted on our website (ibdmanitoba.org).
Analysis
We used frequencies, percentages, and means (standard deviations) to describe the demographic and disease characteristics for both flare cases and the control cases. Differences between the 2 groups were tested using χ 2 tests of independence for categorical variables and t-tests for continuous variables. A Shapiro-Wilk test for normality was used to test for normal distribution within groups. To achieve the first objective, we tested the association between a flare as defined by the 7-point flare indicator and 4 measures of disease activity: (1) FCAL, (2) 2-point disease status indicator, (3) 4-point flare certainty indicator, and (4) SIBDSI scores using a χ 2 test of association. A nominal alpha (α = 0.05) was used to reduce the risk of Type I error. Health care use including reported hospitalizations, emergency department visits, and medication use at the time of the recorded flare were also assessed among both flares and controls using frequencies, percentages, and means (standard deviations). Differences between the 2 groups were tested using χ 2 tests of independence for categorical variables and t tests for continuous variables.
Next, we calculated the prevalence of IBD symptoms in both flare cases and control cases based on their SIBDSI and SIBDQ total scores. We then calculated the ΔSIBDSI and ΔSIBDQ in cases and controls, the difference being between the values of these scores during the week of the flare and the point in time 2 weeks before the flare. To assess the relationship between a flare on the 7-point indicator and a change in symptom activity (or a transition to a worse disease state), we used a general linear model (GLM) applied to ΔSIBDSI and ΔSIBDQ to test for differences in the change in these variables between cases and controls. We similarly applied a GLM to explore the relationship between case control status and a change in any of the SIBDSI subscores for bowel symptoms (BS), abdominal and body discomfort (ABD), and fatigue and general well-being (FGW) at the time of the flare for cases and the matched time for controls (ΔSIBDSI and ΔSIBDQ).
Finally, a multivariable logistic regression model was used to test the association between each of the SIBDSI symptom subscores (BS, ABD, and FGW) and transition to a flare. For this analysis, the dependent variable was the binomial flare case vs control case, and the change score (change in symptom subscores) was the independent variable. Therefore, the individual symptom subscores both at the time of the flare and 2 weeks prior to the flare were added to the model for both cases and controls. The C statistic assessed the goodness of fit of the overall model.
Results
In total, 155 individuals were enrolled in the study. The mean age of participants was 42.6 years (SD, 12.6), with 69.7% being female. Crohn’s disease was diagnosed in 65.8% of patients. The number of participants with active disease by IBDSI at baseline was 74 (47.7%), and 71 (45.8%) had active disease by FCAL. Seventy-four (47.7%) participants experienced at least 1 flare during the 1-year study period based on the 7-point flare indicator tool. Each of these 74 flare cases was systematically matched to 74 controls at the time of the flare (Table 1). Medication characteristics of flare and indexed control cases were analyzed (Table 2). Sixty-six flare cases (89.2%) and 67 controls (90.5%) provided stool samples. One matched control did not complete their biweekly assessments.
Table 1.
Baseline demographic and disease characteristics of flare and matched control cases.
| Flares (n = 74) | Controls (n = 74) | P value | |
|---|---|---|---|
| Age mean (Standard deviation, SD) | 42.9 (12.2) | 42.8 (13.1) | 0.954 |
| Sex % male | 24.3% | 24.3% | 1.000 |
| Disease type % CD | 71.6% | 71.6% | 0.856 |
| Experienced ≥ 1 IBD-related surgery | 39.2% | 40.5% | 0.867 |
| Current smoker | 20.3% | 14.9% | 0.287 |
| Current alcohol use | 83.8% | 74.3% | 0.157 |
Table 2.
Medication characteristics of flare and control cases being used at time of flare for flare cases and their matched controls.
| Flares (n = 74) | Controls (n = 74) | P value | |
|---|---|---|---|
| Taking ≥ 1 IBD medication | 81.1% | 85.1% | 0.510 |
| No. IBD medications mean (SD) | 1.30 (1.17) | 1.18 (0.82) | 0.464 |
| Immunosuppressant (Ex: Methotrexate) | 27.0% | 21.6% | 0.443 |
| 5-ASA | 23.0% | 25.7% | 0.702 |
| ustekinumab | 5.4% | 8.1% | 0.512 |
| Anti-TNF agent | 27.0% | 33.8% | 0.372 |
| vedolizumab | 0.0% | 1.4% | 0.316 |
| Other IBD medication | 28.4% | 18.9% | 0.161 |
| Changed medication/dosage ± 4 weeks of flare | 39.2% | 9.5% | 0.000 |
| Introduced oral corticosteroid ± 4 weeks of flare | 21.6% | 6.8% | 0.010 |
In terms of health care utilization, of the 74 flare cases, there were 6 hospitalizations and 7 emergency department visits; whereas of the 74 control cases, there were no hospitalizations or emergency department visits in the study period (the 2-week period at the time of reported flare). There were evident medication changes at the time of the flare for 39% the flare cases compared with only 9% of the control cases (Table 2).
FCAL Results
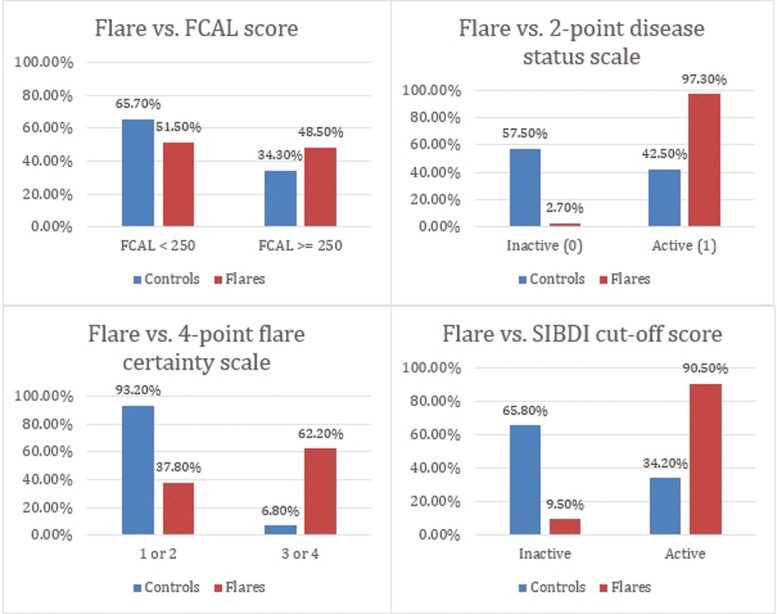
Almost half, 48.5% (32 of 66), of individuals who reported a flare had an elevated FCAL score ≥250 μg/g at the time of the flare compared with 34.3% (23 of 67) of controls who had an elevated FCAL score (P < .05). This meant just over half (51.5%, 34 of 66) of individuals who reported a flare did not have evident active inflammation, and one-third who did not feel they were experiencing a flare nevertheless had active inflammation. Closer examination of the 23 control cases with an elevated FCAL score ≥250 μg/g indicated that 6 of 23 reported being minimally worse on the flare indicator (mean FCAL, 1296.67 ug/g ± 624.90); 10 reported no change (mean FCAL, 880.40 ug/g ± 578.68); and the remaining 7 reported improvement (minimally or much improvement; mean FCAL, 801.14 ug/g ± 746.35).
Comparison of 7-point Flare Indicator With Other PROs
There was high concordance of the flare indicator with the disease status indicator, as 97% (72 of 74) of individuals who flared also endorsed their IBD as active on the 2-point indicator compared with 42.5% (31 of 73) of controls (P < .001). Similarly, the 4-point flare certainty indicator revealed that 91.9% (68 of 74) of flare cases reported being possibly, probably, or definitely in an IBD flare compared with only 32.9% (24 of 73) of control cases (P < .001). Of controls who reported a score greater than 0 on the 4-point flare certainty indicator, the majority (19 of 24) reported being “possibly” in an IBD flare as opposed to “probably” or “definitely.” A very high proportion of flare cases (90.5%, 67 of 74) had a SIBDSI scored above the cutoff value for active disease on the SIBDSI compared with 34.2% (25 of 73) of controls (P < .001; Figure 1).
Figure 1.
Percentage of flare cases and matched control cases with active disease as defined by clinical and patient report measures.
Symptoms of IBD
The mean SIBDSI score for flare cases (32.3; standard deviation, 14.0) was significantly higher than controls (12.5; SD, 9.4; P < .001; Table 3) and well above the active disease cutoff. Moreover, the mean SIBDQ score for flare cases was 43.9 (SD, 11.1), which was significantly lower than for control cases (58.3; SD, 8.5; P < .000). Flare cases reported a higher volume of IBD symptoms and poorer health-related quality of life. Additionally, there was a significant change in SIBDSI and SIBDQ scores over time for this group from 2 weeks before the flare to the time of the flare, demonstrating an increase in symptoms and a decrease in quality of life (Table 3).
Table 3.
Change in SIBDSI and SIBDQ scores from the time of the flare and from 2 weeks prior to the flare for flare and matched control cases.
| Measure | Flares | Δ | P value | |
|---|---|---|---|---|
| Two Weeks prior to Reported Flare | Time of Reported Flare | |||
| SIBDSI | 20.7 (95% CI,18.1–23.4) | 32.3 (95% CI, 28.9–34.4) | 11.0 | 0.000 |
| SIBDQ | 51.1 (95% CI, 48.7–53.5) | 43.8(95% CI, 41.5–46.1) | 7.3 | 0.000 |
| Controls | ||||
| Matched Time of 2 Weeks Prior to Reported Flare in Case | Matched Time of Reported Flare in Case | |||
| SIBDSI | 12.2 (95% CI, 9.6–14.9) | 12.5 (95% CI, 9.8–15.2) | 0.3 | |
| SIBDQ | 57.6 (95% CI, 55.3–60.0) | 58.3 (95% CI, 56.1–60.5) | 0.7 |
*P < .05 for change in score between 2 time points for the flare cases
Flare cases experienced a significant increase in the SIBDI symptom subscores between the assessment occurring 2 weeks before the reported flare to the time of reported flare in bowel symptoms score, abdominal and body discomfort score, and fatigue and general wellbeing score; however, there were no significant changes in symptoms over time for the control cases (Table 4).
Table 4.
Change in SIBDSI symptom subscale scores from the time of the flare and from 2 weeks prior to the flare for flare and matched control cases.
| Measure | Flares | δ | |
|---|---|---|---|
| Two Weeks prior to Reported Flare | Time of Reported Flare | ||
| Bowel Symptoms | 7.4 (95% CI, 6.1–8.7) | 11.2 (95% CI, 9.8–12.6) | 3.8* |
| Abdominal & Body Discomfort | 8.6 (95% CI,7.3–9.9) | 12.8 (95% CI, 11.4–14.3) | 4.2* |
| Fatigue & General Well-being | 3.6 (95% CI, 3.1–4.1) | 5.7 (95% CI, 5.2–6.3) | 2.1* |
| Controls | |||
| Matched Time of 2 Weeks Prior to Reported Flare in Case | Matched Time of Reported Flare in Case | ||
| Bowel Symptoms | 4.2 (95% CI, 2.9–5.4) | 4.3 (95% CI, 2.9–5.7) | 0.1 |
| Abdominal & Body Discomfort | 5.8 (95% CI, 4.5–7.1) | 5.8 (95% CI, 4.4–7.3) | 0.0 |
| Fatigue & General Well-being | 2.3 (95% CI, 1.7–2.8) | 2.4 (95% CI, 1.8–2.9) | 0.1 |
*P < .05 for change in score between 2 time points for the flare cases
The results from the logistic regression model identified that the SIBDSI symptom subscales of bowel symptoms (odds ratio [OR], 1.3; 95% CI, 1.1–1.5) and fatigue and general well-being (OR, 1.5; 95% CI, 1.1–2.1) were significantly contributory in predicting the transition to a flare based on the 7-point flare indicator. The first model predicted only 12% of the variation between flare cases and controls. After symptom subscores at flare time were added to the model, it was determined that persons who transitioned to a flare were more likely to report an increase in bowel symptoms and fatigue symptoms. The second model predicted 45% of the variation between flares and controls. When the logistic regression is run separately for CD, the model identified that the SIBDSI symptom subscales for bowel symptoms (OR, 1.3; 95% CI,1.2–1.6) and fatigue and general well-being (OR, 1.9; 95% CI, 1.2–2.8) were significantly contributory in predicting the transition to a flare based on the 7-point flare indicator. In UC, the logistic regression identified that only the bowel symptom subscale (OR, 1.8; 95% CI,1.1–2.9) was significantly contributory in predicting the transition to a flare. Fatigue and general well-being were not contributory to the transition.
Discussion
We found that a single-item 7-point indicator used to identify an IBD flare based on patient experience of magnitude of recent symptom change was significantly associated with other relevant indicators of a flare, including a validated clinical index of symptomatic active disease, IBD-specific quality of life, changes in medication, and elevated fecal calprotectin. Further, levels of these indicators for flare cases were significantly different in the expected direction compared with levels for matched case controls. The correspondence of the patient-reported flare indicator to significant increases in symptoms from the prior 2-week period as measured by the SIBDSI and decreases in health-related quality of life as measured by the SIBDQ provides further support for the utility of this single-item indicator.
The increase in symptoms for the flare cases was seen across all 3 symptom clusters, which included bowel symptoms, abdominal and body discomfort, and fatigue and general well-being—bowel symptoms and fatigue predicted much of the variation between flare and control cases. Although bowel symptoms are commonly considered, this suggests that these symptoms may also be particularly important in signaling active disease for persons with IBD.24,25 In fact, many of these symptoms, especially fatigue, are also reported by persons with inactive disease.26
There was reasonable concordance of the single-item flare indicator with other simple and complex patient-reported disease activity measures for the flare and control cases; however, it was notable that only half of those identifying a flare had evidence of intestinal inflammation. Further, one-third of those who were controls and not reporting a flare also had elevated FCAL indicating intestinal inflammation. This discrepancy between symptoms and active inflammation, especially in Crohn’s disease,27 is important when considering care plans for patients.
The findings demonstrate the central role of patients in identifying a disease flare. Their observation of relative symptom change serves as an important signal that is associated with increased disease burden. Even when symptoms are not directly associated with inflammation, changes in symptoms that are significant enough to be perceived as a flare may still require clinical intervention. However, as there was not full concordance noted between patient-reported disease flare and FCAL levels, these findings further suggest that if mucosal healing is the goal of therapeutic interventions, relying on self-reported symptoms, while relevant to care, is an insufficient indicator of underlying inflammation. Certainly, when biologics are being introduced or dosing changes are under consideration, direct evidence of inflammation may be necessary. In a previous study by our group, we found that increases in dosing of antibodies to tumor necrosis factor was most often done without documented evidence of inflammation.28
There are several strengths to this study. The study included validated patient-reported disease activity measures and an activity biomarker. Participants were prospectively followed for a year with frequent and regular measurement of GI symptoms and other relevant variables. This allowed for more granular data regarding changes in symptoms than other studies to date. It also facilitated timely stool sampling at the first report of a flare in order to evaluate correspondence with intestinal inflammation. The study has a substantial sample size, and participants were well-engaged with high data completion levels and minimal attrition over the year. In addition, the matched case control design allowed for flare case comparison to control for age, sex, disease type, and even potential seasonal impact. Finally, we were able to prove the robustness of our simple 7-item flare questionnaire with other more complex and detailed measures of symptoms and quality of life.
Limitations to this study should also be noted. The case-control design could match on participant demographics but was not able to match on other clinical disease features that may have been relevant such as location of disease or disease duration, the latter potentially impacting perception of symptoms. Also, the repeated administration of the measures every 2 weeks, while providing timely data on disease experience, may have increased attentiveness to symptoms or a tendency for automatic rather than thoughtful completion. Further, the sample size was not sufficiently large to allow for robust assessments of Crohn’s disease and ulcerative colitis separately.
In summary, use of a simple 7-point item can reliably identify a flare as defined by more extensive validated patient-reported disease activity measures. This flare tool meaningfully maps important indicators of the patient’s disease experience such as their symptom burden and their disease-related quality of life. As such, the tool provides a straightforward framework for patients to identify a flare and can be used readily for patient monitoring. Asking patients to report moderate (or worse) symptom changes using this tool merits clinical attention. However, the findings also identified that elevations in intestinal inflammation may not be sufficiently sensitive to capture or explain the important changes in symptoms that patients experience and, conversely, that patient-reported flares do not fully align with evident intestinal inflammation. Hence, when a patient states they are flaring on a simple measure, it is reflected by meaningful changes in more complex disease scores. However, a patient stating they are flaring is not completely reflected by the presence of inflammation.
Funding
This study was supported by a grant from the Canadian Institutes of Health Research.
Conflicts of Interest
C.B. is supported by the Bingham Chair in Gastroenterology; he has served on advisory Boards for AbbVie Canada, Amgen Canada, Bristol Myers Squibb Canada, Roche Canada, Janssen Canada, Sandoz Canada, Takeda Canada, and Pfizer Canada and is a consultant for Mylan Pharmaceuticals and Takeda; he has received educational grants from Abbvie Canada, Pfizer Canada, Takeda Canada, and Janssen Canada and is on the speaker’s panel for Abbvie Canada, Janssen Canada, Medtronic Canada, and Takeda Canada; he has received research funding from Abbvie Canada and Pfizer Canada. L.T. has received investigator-initiated funding from Janssen Canada and served on advisory boards for AbbVie Canada, Takeda Canada, Merck Canada, Pfizer Canada, Janssen Canada, and Roche Canada. L.G. has consulted for Roche Canada. All other authors have no potential conflicts to report.
Data Availability
The data underlying this article are available in the article.
Author Contribution
K.W. contributed to study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; technical or material support.
K.S. contributed to study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis; technical or material support.
L.G. contributed to study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis; technical or material support.
L.T. contributed to study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content.
L.L. contributed to study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis; technical or material support.
C.H. contributed to study concept and design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis; technical or material support.
J.S. contributed to critical revision of the manuscript for important intellectual content; technical or material support.
L.A.S. contributed to analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis; technical or material support.
K.V. contributed to study concept and design and critical revision of the manuscript for important intellectual content.
C.B. contributed to study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; statistical analysis; technical or material support; study supervision.
References
- 1. Liverani E, Scaioli E, Digby RJ, et al. . How to predict clinical relapse in inflammatory bowel disease patients. World J Gastroenterol. 2016;22:1017–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bernstein CN, Singh S, Graff LA, et al. . A prospective population-based study of triggers of symptomatic flares in IBD. Am J Gastroenterol. 2010;105:1994–2002. [DOI] [PubMed] [Google Scholar]
- 3. Matsuoka K, Kobayashi T, Ueno F, et al. . Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018;53:305–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Falvey JD, Hoskin T, Meijer B, et al. . Disease activity assessment in IBD: clinical indices and biomarkers fail to predict endoscopic remission. Inflamm Bowel Dis. 2015;21:824–831. [DOI] [PubMed] [Google Scholar]
- 5. Docherty MJ, Jones RC 3rd, Wallace MS. Managing pain in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011;7:592–601. [PMC free article] [PubMed] [Google Scholar]
- 6. Waljee AK, Joyce JC, Wren PA, et al. . Patient reported symptoms during an ulcerative colitis flare: a Qualitative Focus Group Study. Eur J Gastroenterol Hepatol. 2009;21:558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Devlen J, Beusterien K, Yen L, et al. . The burden of inflammatory bowel disease: a patient-reported qualitative analysis and development of a conceptual model. Inflamm Bowel Dis. 2014;20:545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bojic D, Bodger K, Travis S. Patient reported outcome measures (PROMs) in inflammatory bowel disease: new data. J Crohns Colitis. 2017;11:S576–S585. [DOI] [PubMed] [Google Scholar]
- 9. Travis SP, Higgins PD, Orchard T, et al. . Review article: defining remission in ulcerative colitis. Aliment Pharmacol Ther. 2011;34:113–124. [DOI] [PubMed] [Google Scholar]
- 10. Sandborn WJ, Feagan BG, Hanauer SB, et al. . A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology. 2002;122:512–530. [DOI] [PubMed] [Google Scholar]
- 11. D’Haens G, Sandborn WJ, Feagan BG, et al. . A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–786. [DOI] [PubMed] [Google Scholar]
- 12. Witges K, Targownik LE, Haviva C, et al. . Living with inflammatory bowel disease: protocol for longitudinal study of factors associated with symptom exacerbations. JMIR Protocols. 2018;7:e11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sexton KA, Walker JR, Targownik LE, et al. . The inflammatory bowel disease symptom inventory: a patient-report scale for research and clinical application. Inflamm Bowel Dis. 2019;25:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol. 1996;91:1571–1578. [PubMed] [Google Scholar]
- 15. Harris PA, Taylor R, Thielke R, et al. . Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schoepfer AM, Beglinger C, Straumann A, et al. . Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162–169. [DOI] [PubMed] [Google Scholar]
- 17. Abraham BP, Kane S. Fecal markers: calprotectin and lactoferrin. Gastroenterol Clin North Am. 2012;41:483–495. [DOI] [PubMed] [Google Scholar]
- 18. Naess-Andresen CF, Egelandsdal B, Fagerhol MK. Calcium binding and concomitant changes in the structure and heat stability of calprotectin (L1 protein). Clin Mol Pathol. 1995;48:M278–M284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin JF, Chen JM, Zuo JH, et al. . Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis. 2014;20:1407–1415. [DOI] [PubMed] [Google Scholar]
- 20. Meuwis MA, Vernier-Massouille G, Grimaud JC, et al. ; GETAID (Groupe d’Étude Thérapeutique Des Affections Inflammatoires Digestives) . Serum calprotectin as a biomarker for Crohn’s disease. J Crohns Colitis. 2013;7:e678–e683. [DOI] [PubMed] [Google Scholar]
- 21. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 22. Powell-Tuck J, Bown RL, Lennard-Jones JE. A comparison of oral prednisolone given as single or multiple daily doses for active proctocolitis. Scand J Gastroenterol. 1978;13:833–837. [DOI] [PubMed] [Google Scholar]
- 23. Clara I, Lix LM, Walker JR, et al. . The Manitoba IBD index: evidence for a new and simple indicator of IBD activity. Am J Gastroenterol. 2009;104:1754–1763. [DOI] [PubMed] [Google Scholar]
- 24. Villoria A, García V, Dosal A, et al. . Fatigue in out-patients with inflammatory bowel disease: prevalence and predictive factors. PLoS One. 2017;12:e0181435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Romberg-Camps MJ, Bol Y, Dagnelie PC, et al. . Fatigue and health-related quality of life in inflammatory bowel disease: results from a population-based study in the Netherlands: the IBD-South Limburg cohort. Inflamm Bowel Dis. 2010;16:2137–2147. [DOI] [PubMed] [Google Scholar]
- 26. Singh S, Blanchard A, Walker JR, et al. . Common symptoms and stressors among individuals with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2011;9:769–775. [DOI] [PubMed] [Google Scholar]
- 27. Targownik LE, Sexton KA, Bernstein MT, et al. . The relationship among perceived stress, symptoms, and inflammation in persons with inflammatory bowel disease. Am J Gastroenterol. 2015;110:1001–1012; quiz 1013. [DOI] [PubMed] [Google Scholar]
- 28. Elias ED, Bernstein CN, Singh H, et al. . Dose augmentation of tumor necrosis factor inhibitors is frequently performed in persons with inflammatory bowel disease in the absence of objective evidence of active inflammation. J Clin Gastroenterol. 2021;55: 602–608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article.