To the Editor: For persons who received a single dose of the Ad26.COV2.S vaccine (Johnson & Johnson–Janssen) against coronavirus disease 2019 (Covid-19), a booster dose of a messenger RNA (mRNA) vaccine at least 2 months after the primary dose is recommended. Recipients of Ad26.COV2.S for both the primary and booster doses may receive a second booster dose of an mRNA Covid-19 vaccine at least 4 months after the first Ad26.COV2.S booster dose.1 Immunogenicity data from a phase 1–2 clinical trial conducted before B.1.1.529 and the BA sublineages of omicron emerged showed that increases in the titers of binding and neutralizing antibodies with heterologous boosting were similar to or greater than the increases with homologous boosting.2 In a study involving U.S. veterans, data that were obtained during a period in which omicron was the predominant circulating variant also showed that among Ad26.COV2.S recipients, vaccine effectiveness against omicron infection was higher with heterologous boosting than with homologous boosting3; however, data from the general adult population and on vaccine effectiveness over time are lacking. More than 18 million doses of the Ad26.COV2.S vaccine have been administered in the United States alone4; therefore, data are needed on boosting strategies that are effective over time.
We performed a test-negative, case–control analysis to assess the effectiveness of four vaccination regimens against symptomatic infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during a period when omicron was the predominant circulating variant: a single priming dose of Ad26.COV2.S, a single priming dose of Ad26.COV2.S plus a booster dose of Ad26.COV2.S (Ad26.COV2.S/Ad26.COV2.S), a single priming dose of Ad26.COV2.S plus a booster dose of mRNA vaccine (Ad26.COV2.S/mRNA), and two priming doses of an mRNA vaccine plus a booster dose of mRNA vaccine (mRNA/mRNA/mRNA). In the regimens that included an mRNA vaccine, either the BNT162b2 vaccine (Pfizer–BioNTech) or the mRNA-1273 vaccine (Moderna) was used. The methods have been published previously5 and are described in the Supplementary Appendix, available with the full text of this letter at NEJM.org.
A total of 512,928 rapid and laboratory-based nucleic acid amplification tests (NAATs) were used in the current study; the tests were obtained between January 2 and March 23, 2022, from the Increasing Community Access to Testing (ICATT) platform, which facilitates no-cost, drive-through SARS-CoV-2 testing at pharmacies.5 In our analysis, we included tests from adults 18 years of age or older who reported vaccination status (product and month and year of receipt of each dose) and at least one Covid-19–like symptom, which most likely reflected mild disease. The included tests were from 7036 testing sites across 49 states, Washington, D.C., and Puerto Rico. All sites belonged to a single pharmacy chain that obtained booster-dose information during this period. This chain had widespread national coverage. During the period when this study was conducted, 93.5% of all tests in adults provided through the ICATT platform, including those that did not meet our inclusion criteria, were conducted at this one chain. Swab samples were collected by the participants on-site, and the results were processed either on-site by the pharmacy (rapid polymerase-chain-reaction tests) or were sent out for processing at contracted laboratories (laboratory-based NAATs). All positive tests were assumed to involve the omicron variant, because more than 90% of the infections detected nationally during this period were caused by this variant.4 Tests from persons who reported previous SARS-CoV-2 infection were excluded. A sensitivity analysis that included tests regardless of previous SARS-CoV-2 infection was performed (see the Supplementary Appendix).
Vaccine effectiveness (calculated as [1−the odds ratio]×100) of each of the four vaccination regimens, as compared with no vaccination, against symptomatic infection during the periods of 14 days to 1 month and of 2 to 4 months since receipt of the last vaccine dose was estimated by means of logistic regression. The number of months since receipt of the last dose was calculated as the difference between the month and year of testing and the month and year of the last vaccine dose. For doses received in the month of or month preceding test registration, persons were asked whether the most recent dose was received at least 2 weeks before the test date, and tests from persons with less than 2 weeks between the date of the last dose and the date of testing were excluded. Models were adjusted for calendar day of testing, age group, sex, race, ethnic group, testing site location (the Department of Health and Human Services region where the test was performed), Social Vulnerability Index of the U.S. census tract containing the testing site, and number of underlying chronic conditions (Tables S1 and S2 in the Supplementary Appendix).
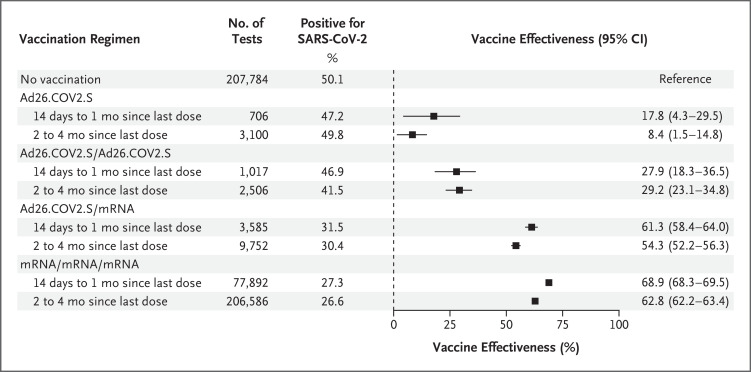
The vaccine effectiveness of the Ad26.COV2.S regimen, as compared with no vaccination, against symptomatic infection was 17.8% (95% confidence interval [CI], 4.3 to 29.5) during the period of 14 days to 1 month since receipt of the last dose and 8.4% (95% CI, 1.5 to 14.8) during the period of 2 to 4 months since receipt of the last dose. The corresponding values for the Ad26.COV2.S/Ad26.COV2.S regimen were 27.9% (95% CI, 18.3 to 36.5) and 29.2% (95% CI, 23.1 to 34.8); for the Ad26.COV2.S/mRNA regimen, 61.3% (95% CI, 58.4 to 64.0) and 54.3% (95% CI, 52.2 to 56.3); and for the mRNA/mRNA/mRNA regimen, 68.9% (95% CI, 68.3 to 69.5) and 62.8% (95% CI, 62.2 to 63.4) (Figure 1).
Figure 1. Effectiveness of Four Vaccination Regimens against Symptomatic Infection with the Omicron Variant among Adults.
Vaccine effectiveness was assessed among adults 18 years of age or older who were tested in the Increasing Community Access to Testing platform during the period from January 2 to March 23, 2022. The vaccine effectiveness (calculated as [1−the odds ratio]×100) of each of the four regimens, as compared with no vaccination, against symptomatic infection with the omicron variant of severe acute respiratory syndrome coronavirus 2 was estimated by means of logistic regression. Regression models were adjusted for the number of days between the start of the analysis period and the test date (as a continuous variable with linear and quadratic terms), age group (18 to 24, 25 to 34, 35 to 44, 45 to 54, 55 to 64, and ≥65 years), sex, race, ethnic group, testing site location (the Department of Health and Human Services region where the test was performed), Social Vulnerability Index of the U.S. census tract containing the testing site (dichotomized as 0 to <0.5 and ≥0.5 to 1.0; the Social Vulnerability Index ranges from 0 to 1.0, with higher scores indicating greater social vulnerability), and number of underlying chronic conditions (0, 1, or ≥2) to control for possible confounding (Table S1). No adjustment was made for multiplicity. The number of months since receipt of the last dose was calculated as the difference between the month and year of testing and the month and year of the last vaccine dose. Tests from participants with less than 2 weeks between the date of the last dose and the date of testing were excluded. For the three regimens that included a booster dose (Ad26.COV2.S/Ad26.COV2.S, Ad26.COV2.S/messenger RNA (mRNA) vaccine, and mRNA/mRNA/mRNA), tests were excluded if a booster dose was administered less than 2 months after an Ad26.COV2.S primary series or less than 5 months after an mRNA vaccine primary series. In estimating the vaccine effectiveness of the single-dose Ad26.COV2.S regimen over time, tests were not excluded from evaluation on the basis of months since primary vaccination. Additional details of the methods are provided in the Supplementary Methods section in the Supplementary Appendix.
Our results show that all the regimens that included a booster dose, as compared with no vaccination, offered protection against symptomatic omicron infection (the 95% confidence intervals did not include 0), although vaccine effectiveness was highest for the regimens that included a booster dose of an mRNA vaccine and was lowest for the homologous Ad26.COV2.S/Ad26.COV2.S regimen. The vaccine effectiveness of the three-dose mRNA regimen and the Ad26.COV2.S/mRNA regimen was lower during the period of 2 to 4 months since receipt of the booster dose than during the period of 14 days to 1 month since receipt of the booster dose; for the Ad26.COV2.S/Ad26.COV2.S regimen, the 95% confidence intervals of the estimates for the two time periods overlapped, but sample sizes were smaller and confidence intervals wider than for other boosted regimens. The limitations of our study have been described previously5 and include residual or unmeasured confounding related to unmeasured differences according to vaccination status and test-seeking behavior. Nonetheless, the results of this study suggest that a single booster dose of an mRNA Covid-19 vaccine in persons who received primary vaccination with single-dose Ad26.COV2.S provided protection close to that of the three-dose mRNA vaccine regimen and support the current recommendation of a booster dose of mRNA vaccine at least 2 months after primary vaccination with single-dose Ad26.COV2.S or at least 4 months after an Ad26.COV2.S booster dose.
Supplementary Appendix
Disclosure Forms
The views expressed in this letter are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
This article was published on May 25, 2022, at NEJM.org.
Footnotes
Supported by the CDC. Funding for the Increasing Community Access to Testing platform was provided by the Department of Health and Human Services.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Centers for Disease Control and Prevention. Use of COVID-19 vaccines in the United States: interim clinical considerations. 2022. (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html).
- 2.Atmar RL, Lyke KE, Deming ME, et al. Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med 2022;386:1046-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayr FB, Talisa VB, Shaikh O, Yende S, Butt AA. Effectiveness of homologous or heterologous Covid-19 boosters in veterans. N Engl J Med 2022;386:1375-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. COVID data tracker. (https://covid.cdc.gov/covid-data-tracker/#datatracker-home).
- 5.Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA 2022;327:639-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.