To the Editor: During the coronavirus disease 2019 (Covid-19) pandemic, the emergence of the BA.1, BA.2, and BA.3 sublineages of the B.1.1.529 (omicron) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has generated serious concern over the durability of vaccine- and infection-induced immunity in the face of continuing evolution of the virus. This worrisome viral evolution has been exacerbated by recombination of the omicron variant with the B.1.617.2 (delta) variant.1-3 The ability of such “deltacron” variants to evade immunity induced by either vaccination or previous infection remains unclear.
Here, we used a pseudotyped virus neutralization assay4 to examine neutralizing-antibody titers in serum samples obtained from vaccinated health care workers at the Wexner Medical Center at Ohio State University as well as from patients with confirmed Covid-19 during the delta and omicron waves in the Columbus, Ohio, area. We evaluated neutralizing-antibody titers against the ancestral SARS-CoV-2 strain bearing the D614G mutation, along with the understudied BA.3 and deltacron variants, and compared the responses with previously reported results for the BA.1, BA.2, and delta variants (Fig. S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org).5
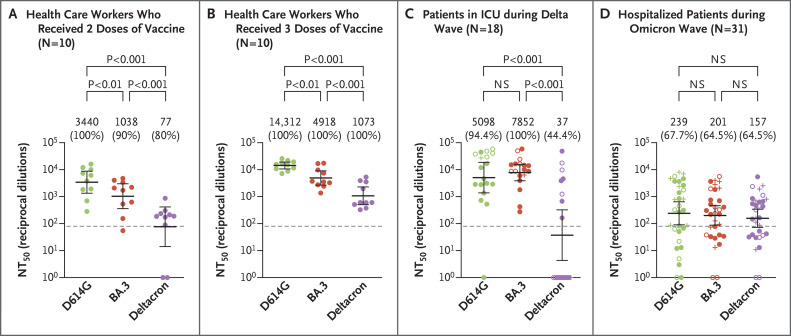
We first examined neutralizing-antibody titers in serum samples that had been obtained from 10 health care workers 3 to 4 weeks after they had received the second dose of the mRNA-1273 vaccine (Moderna) (in 3 workers) or the BNT162b2 vaccine (Pfizer–BioNTech) (in 7 workers) (Table S1). As compared with the response against the D614G variant, neutralizing-antibody titers were 3.3 times as low against the BA.3 variant and 44.7 times as low against the deltacron variant (P<0.001 for both comparisons) (Figure 1A). However, after the same health care workers had received a booster dose of the same vaccine used in the two-dose series, neutralizing-antibody titers were 2.9 times as low against the BA.3 variant and 13.3 times as low against the deltacron variant as against the D614G variant (P<0.001 for both comparisons) (Figure 1B). As compared with the major omicron sublineages, the deltacron variant showed similar neutralizing-antibody resistance to the BA.1 and BA.2 variants, whereas the BA.3 variant (which does not contain many critical mutations in the receptor-binding domain, as seen in other omicron variants) was more sensitive to both two-dose and boosted samples obtained from the health care workers (Fig. S2A, S2B, and S2C).
Figure 1. Neutralization of the BA.3 Omicron and Deltacron Variants.
Shown are neutralizing-antibody titers against virus pseudotyped with spike protein from ancestral severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) bearing the D614G mutation, along with the BA.3 and deltacron variants, in 10 serum samples obtained from health care workers who had received two doses of a messenger RNA (mRNA) vaccine (Panel A) and from the same workers after they had received three mRNA vaccine doses (Panel B). Also shown are neutralizing-antibody titers in samples obtained from 18 patients admitted to the intensive care unit (ICU) during the delta wave of the pandemic, including 12 unvaccinated patients (closed circles), 5 fully vaccinated patients (open circles), and 1 vaccinated and boosted patient (plus signs) (Panel C). In addition, neutralizing-antibody titers are shown for 31 patients who were hospitalized with Covid-19 but were not admitted to an ICU during the omicron wave of the pandemic, including 15 unvaccinated patients (closed circles), 8 fully vaccinated patients (open circles), and 8 vaccinated and boosted patients (plus signs) (Panel D). In all cases, geometric mean values for the 50% neutralization titers (NT50) are provided at the top of the plots along with the percentage of patients with neutralizing-antibody titers above the limit of detection (value of 80), as indicated by the dashed lines. 𝙸 bars represent 95% confidence intervals. Statistical significance was determined by one-way repeated-measures analysis of variance with Bonferroni’s multiple testing correction. NS denotes not significant.
We next examined neutralizing-antibody titers in serum samples that had been obtained from 18 patients 3 days after admission to an intensive care unit (ICU) during the delta wave of the pandemic (Table S1). Among these patients, 12 were unvaccinated, 5 were fully vaccinated, and 1 was vaccinated and boosted. These patients had similar neutralizing-antibody titers against the D614G and BA.3 variants. However, the titers against the deltacron variant were 137.8 times as low as the titers against the D614G variant, with only 44.4% of the patients having neutralizing-antibody titers against the deltacron variant that were above the limit of detection (Figure 1C). Again, neutralization escape of the deltacron variant paralleled that of the BA.1 and BA.2 variants, whereas the BA.3 variant remained largely sensitive to neutralization (Fig. S3A, S3B, and S3C). The 6 patients who had been vaccinated had substantially higher titers against the D614G and BA.3 variants than the patients who were unvaccinated, whereas the deltacron variant largely escaped neutralization.
Finally, we examined serum samples that had been obtained from 31 patients who were hospitalized during the omicron wave but were not admitted to the ICU (Table S1). We observed that neutralization of the deltacron and BA.3 variants was similar to that of the D614G variant, for which titers were much lower than those in samples obtained during the delta wave (Figure 1D). Neutralization of both the deltacron and BA.3 variants was similar to that of the BA.1 and BA.2 variants; the delta variant had the most resistance to serum obtained during the omicron wave (Fig. S3D, S3E, and S3F). This similar neutralization of omicron sublineages occurred regardless of vaccination status, with 8 patients having received two doses and 8 patients having received three doses. It is critical to note that the patients who were hospitalized during the omicron wave had broader neutralization of all the tested omicron variants than did those hospitalized during the delta wave (Fig. S3A and S3D). On average, the health care workers who had received three doses of vaccine had stronger and broader immunity than the patients who had been evaluated during the omicron wave regardless of vaccination status, with neutralizing-antibody titers against the D614G variant that were 59.9 times as high as those in patients during the omicron wave. Furthermore, the boosted health care workers had neutralizing-antibody titers against the D614G variant that were 4.2 times as high as those among health care workers who had received two doses of vaccine and 2.8 times as high as those among patients who had been assessed during the delta wave.
Overall, our results indicate that BA.3 is not a substantial immune-escape variant, a finding that is likely due to its reduced number of mutations in the receptor-binding domain as compared with the BA.1 and BA.2 variants. However, the deltacron variant retains the strong resistance of other omicron sublineages and has no enhanced sensitivity to serum obtained during the delta wave. Although the effect of the delta-derived spike mutations in the N-terminal domain on virus replication and pathogenesis remains unclear, these mutations do not appear to impair neutralization resistance. Recombination of SARS-CoV-2 variants and the potential emergence of a more virulent variant with strong immune escape remains a critical concern and requires ongoing monitoring.
Supplementary Appendix
Disclosure Forms
The views expressed in this letter are those of the authors and do not necessarily reflect the official views of the National Institutes of Health.
This letter was published on May 18, 2022, at NEJM.org.
Footnotes
Supported by a fund provided by a private donor to Ohio State University (to Dr. Liu); by an award (U54CA260582, to Drs. Liu, Lozanski, Gumina, Saif, and Oltz) from the National Cancer Institute of the National Institutes of Health (NIH); a grant (R01 AI150473, to Dr. Liu) from the NIH; a Glenn Barber Fellowship (to Mr. Evans) from the Ohio State University College of Veterinary Medicine; grants (to Dr. Gumina) from the Robert J. Anthony Fund for Cardiovascular Research and the JB Cardiovascular Research Fund; by a grant (R01 HD095881, to Dr. Saif) from the NIH; and by grants (UL1TR002733 and KL2TR002734, to Dr. Bednash) from the National Center for Advancing Translational Sciences.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Ou J, Lan W, Wu X, et al. Tracking SARS-CoV-2 omicron diverse spike gene mutations identifies multiple inter-variant recombination events. Signal Transduct Target Ther 2022;7:138-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacek KA, Rambo-Martin B, Batra D, et al. Identification of a novel SARS-CoV-2 delta-omicron recombinant virus in the United States. March 21, 2022. (https://www.biorxiv.org/content/10.1101/2022.03.19.484981v1). preprint. [DOI] [PMC free article] [PubMed]
- 3.Bolze A, White S, Basler T, et al. Evidence for SARS-CoV-2 delta and omicron co-infections and recombination. March 12, 2022. (https://www.medrxiv.org/content/10.1101/2022.03.09.22272113v1). preprint. [DOI] [PMC free article] [PubMed]
- 4.Zeng C, Evans JP, Pearson R, et al. Neutralizing antibody against SARS-CoV-2 spike in COVID-19 patients, health care workers, and convalescent plasma donors. JCI Insight 2020;5(22):e143213-e143213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans JP, Zeng C, Qu P, et al. Neutralization of SARS-CoV-2 omicron sub-lineages BA.1, BA.1.1, and BA.2. Cell Host Microbe. April 24, 2022. (https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(22)00220-7). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.