To the Editor: The incidence of the omicron BA.1/1.1 variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which rapidly spread worldwide even among vaccinated persons, is incompletely defined.1 We quantified the incidence of SARS-CoV-2 infection during the initial omicron BA.1/1.1 variant wave among Canadian adults2 and the contribution of previous infection and concurrent vaccination to age-specific active immunity (Fig. S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org).3
From May 2020 through March 2022, in the Action to Beat Coronavirus (Ab-C) study, we conducted four serial assessments of SARS-CoV-2 seropositivity, each involving 5000 to 9000 adults, using the Angus Reid Forum, a nationally representative online polling platform (Fig. S2). Dried-blood-spot samples that had been obtained by the participants were tested with highly sensitive and specific chemiluminescence-based enzyme-linked immunosorbent assays targeting the spike protein, receptor-binding domain, and nucleocapsid (N) protein.2,4 Vaccines that have been used in Canada contain only spike protein and thus should not elicit N protein positivity.5 Approval for the study was obtained from the institutional review board at Unity Health Toronto.
The 5031 adults who were surveyed in phase 4 of the Ab-C study and whose dried-blood-spot samples were received between January 24 and March 15, 2022, were broadly representative of Canadian adults. The study population had similar prevalences of obesity, smoking, diabetes, and vaccination against SARS-CoV-2 as the general Canadian adult population, but the study population included a lower percentage of lower-educated adults (Table S1). More women than men and more vaccinated adults than unvaccinated adults provided dried-blood-spot samples in phase 4 of the study.
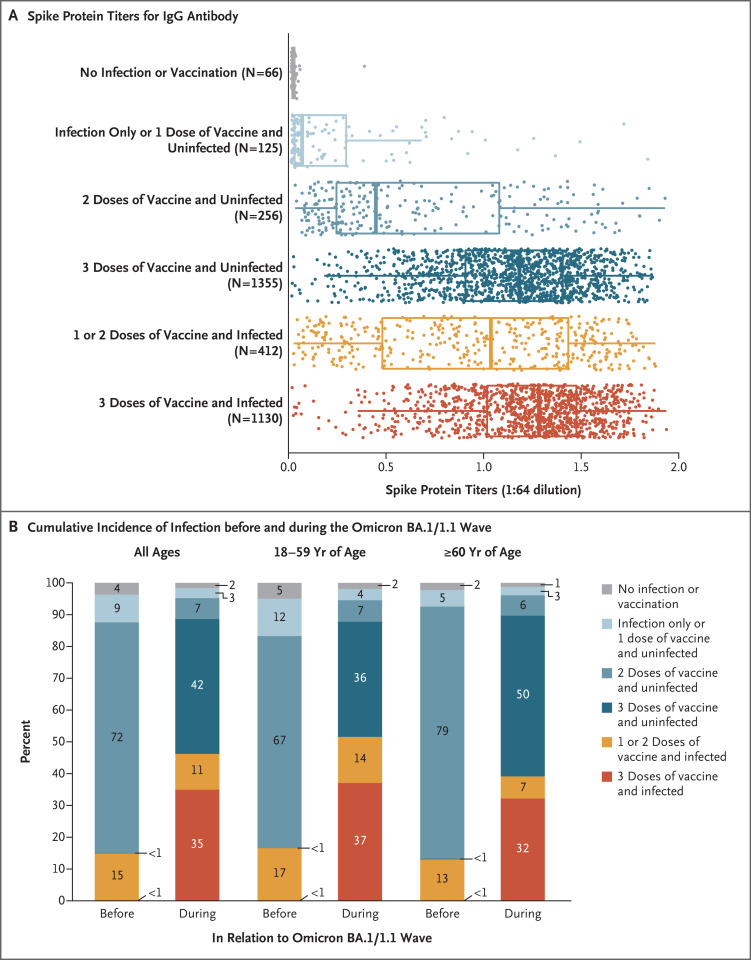
Among 3468 adults who tested negative for the N protein in phase 3 of the study (August through October 2021), 1040 had positive results for the N protein in phase 4 (education-weighted between-phase incidence, 30%; 95% confidence interval [CI], 26 to 33). Among 91 unvaccinated, uninfected participants in phase 3 of the study, 36 had a positive result in phase 4 (education-weighted between-phase incidence, 40%; 95% CI, 25 to 54). After the exclusion of participants who had been vaccinated less than 1 month before the dried-blood-spot samples were obtained, spike protein titers were negligible among participants who were uninfected or unvaccinated. Spike protein titers were lower among participants who had received only one vaccine dose than among those who had received multiple vaccine doses (Figure 1A). The spike protein titers were highest among participants who had received three vaccine doses and had been infected.
Figure 1. Spike Protein Titers for IgG Antibody in Canadian Adults and the Age-Specific Cumulative Incidence of Infection before and during the Omicron BA.1/1.1 Variant Wave.
Shown are spike titers, which represent the relative ratios of IgG antibody values against the spike protein to the control samples (Section S1 in the Supplementary Appendix), with stratification according to infection and vaccination status in Canadian adults (Panel A) and the age-specific cumulative incidence of infection in each stratum of previous infection and vaccination before and during the omicron BA.1/1.1 variant wave (Panel B). Dots represent participants who received their last dose of vaccine (or were unvaccinated) at least 1 month before dried-blood-spot samples were obtained. (A total of 3344 participants had complete information available as of the time of analyses after the exclusion of 21 low-quality samples.) In the box-and-whisker plots, the solid line represents the median, the box represents the interquartile range, and the whiskers represent 1.5 times the interquartile range. Results with the use of the receptor-binding domain antigen were similar to those with the spike protein (Fig. S3). The cumulative incidences of Canadian adults in each category of vaccination and infection (assessed on the basis of nucleocapsid protein positivity or molecular or antigen rapid testing) were drawn from the groups of 3481 participants in phase 3 (before the omicron BA.1/1.1 wave) and 4032 participants in phase 4 (during the omicron BA.1/1.1 wave, with a reference period starting December 1, 2021) for whom complete testing, vaccination, and antibody data were available by the time of the analyses. The first column in the overall and age-specific sets represents the antibody and viral test positivity for the entire period before the omicron BA.1/1.1 wave, and the second column represents the values during the omicron BA.1/1.1 wave. Percentages may not total 100 because of rounding.
When we examined phases 3 and 4 separately, we found that the cumulative incidence of N protein positivity before the omicron BA.1/1.1 variant wave was 11% (95% CI, 10 to 12; of 5155 participants tested, 571 had a positive result) but increased during the omicron BA.1/1.1 wave to an education-weighted cumulative incidence of 37% (95% CI, 35 to 39; of 5031 participants tested, 1869 had a positive result). When we applied the between-phase incidence among vaccinated or unvaccinated participants to the 29.7 million adults in Canada, we found that an estimated 9.0 million adults (95% CI, 7.9 to 10.2 million) had been newly infected during the omicron BA.1/1.1 wave, including 0.9 million infections (95% CI, 0.6 to 1.2 million) among 2.3 million unvaccinated adults. Figure 1B shows the age-specific patterns of cumulative infection (defined as N protein positivity or positivity on viral testing) or cumulative vaccination among participants before and during the omicron BA.1/1.1 wave. The incidence of infection with the omicron BA.1/1.1 variant increased to a lesser extent among older adults than among younger adults.
The use of N protein positivity may have resulted in an underestimation of the actual incidence of infection with the omicron BA.1/1.1 variant because an antibody response was not mounted in vaccinated adults with mild cases or because participants did not have seroconversion during the sampling period.1 Section S1 provides further details of the laboratory methods and analyses.
Despite the finding of widespread infection, the age-specific patterns caution against the notion that the omicron BA.1/1.1 variant will immunize everyone. In contrast to younger adults, persons 60 years of age or older face the highest rates of hospitalization and death but have the lowest rates of combined infection and vaccination. Strategies to build an immunity wall will continue to depend on high coverage levels of vaccination, especially among persons who have yet to receive any vaccination, including those who have recovered from infection.3 Ongoing assessment of the incidence of infection by the omicron BA.2 variant and future SARS-CoV-2 variants is essential.
Supplementary Appendix
Disclosure Forms
This letter was published on May 18, 2022, at NEJM.org.
Footnotes
Supported by the Covid-19 Immunity Task Force, the Canadian Institutes of Health Research, Pfizer Global Medical Grants, and St. Michael’s Hospital Foundation.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our World In Data, 2022. (https://ourworldindata.org/coronavirus).
- 2.Tang X, Sharma A, Pasic M, et al. Assessment of SARS-CoV-2 seropositivity during the first and second viral waves in 2020 and 2021 among Canadian adults. JAMA Netw Open 2022;5(2):e2146798-e2146798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammerman A, Sergienko R, Friger M, et al. Effectiveness of the BNT162b2 vaccine after recovery from Covid-19. N Engl J Med 2022;386:1221-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colwill K, Galipeau Y, Stuible M, et al. A “made-in-Canada” serology solution for profiling humoral immune responses to SARS-CoV-2 infection and vaccination. October 26, 2021. (https://www.medrxiv.org/content/10.1101/2021.10.25.21265476v1). preprint. [DOI] [PMC free article] [PubMed]
- 5.Duarte N, Yanes-Lane M, Arora RK, et al. Adapting serosurveys for the SARS-CoV-2 vaccine era. Open Forum Infect Dis 2022;9:ofab632-ofab632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.