Abstract
A major breakthrough in cancer treatment was ushered in by the development of immune checkpoint blockade therapy such as anti-CTLA4 antibody and anti-PD-1 and anti-programmed cell death-ligand 1 antibodies that are now approved for use in an increasing number of malignancies. Despite the relative success of immune checkpoint inhibitors with certain tumor types, many patients still fail to respond to such therapies, and the field is actively trying to understand the mechanisms of resistance, intrinsic or acquired, to immune checkpoint blockade. Herein, we discuss the roles that somatic genomic mutations in oncogenic pathways play in immune editing, as well as some of the current approaches toward improving response to immunotherapy.
Keywords: immunotherapy, PTEN, EGFR, angiogenesis
INTRODUCTION
Immune checkpoint inhibitors such as anti-PD-1, anti-programmed cell death-ligand 1 (PD-L1), and anti-CTLA4 antibodies, work by activating dormant T cells directed at cancer cells and, since their development, have revolutionized cancer treatment in the past decade for multiple tumor types.[1,2] However, it remains uncertain why only a small percentage of patients develop long responses to these treatments.[3] There are likely several factors that could influence cancer immune responsiveness, including tumor immune microenvironment, somatic alterations, germline variants, and transcriptional changes.[4] An example of direct relation between tumor genomic alterations and response to immunotherapy are the somatic mutations in mismatch repair genes (MMR) and high microsatellite instability (MSI-H), leading to a particular immunophenotype with increased responsiveness to immune checkpoint inhibitors.[5] Increased tumor mutation burden (TMB), usually defined as the number of nonsynonymous mutations per megabase of DNA sequenced, is also a predictive biomarker for better response to PD-1 blockade and improved clinical outcomes.[6,7]
With the integration of next-generation sequencing in the clinic for tumor molecular profiling for personalized cancer treatment, there is an increasing knowledge about the somatic alterations that could influence the response to immunotherapy.[8]
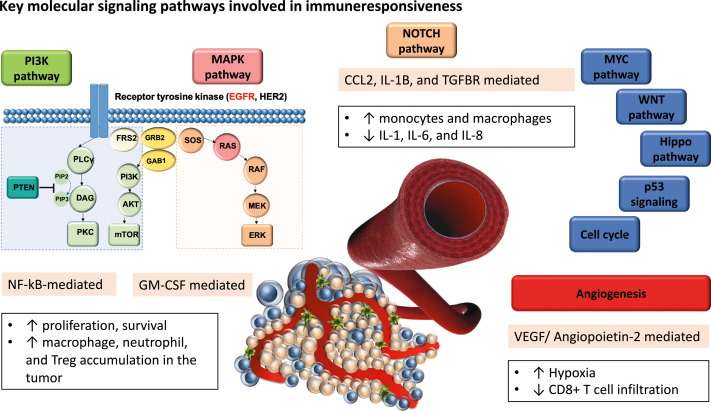
Here we review how somatic genomic alterations in key oncogenic pathways and angiogenesis are shaping immune editing, and we discuss current approaches to improve immune responsiveness and to personalize immunotherapy treatments for cancer patients (Fig. 1 and Table 1). We performed a literature search on PubMed to identify key biomarkers contributing to immune checkpoint inhibitor resistance. We limited our search results to recent data, after 2015, and to actionable alterations for which targeted therapies are either approved or are currently in clinical trials.
Figure 1.
Key oncogenic and angiogenic pathways involved in immune editing. PI3K: phosphoinositide 3-kinase; EGFR:Epidermal Growth Factor Receptor; HER2: human epidermal growth factor receptor 2; PIP2: phosphatidylinositol 4,5-bisphosphate; PIP3: phosphatidylinositol (3,4,5)-trisphosphate; PLCy: phospholipase gamma; DAG: diacylglycerol; PKC: protein kinase C; FRS2: fibroblast growth factor receptor substrate 2; AKT: Ak strain transforming (AKT) serine/threonine kinase, or protein kinase B; mTOR: mammalian target of rapamycin; GRB2: growth factor receptor-bound protein 2; GAB1: GRB2-associated-binding protein 1; SOS: son of sevenless; RAS: rat sarcoma; RAF: rapidly accelerated fibrosarcoma; MEK: MAPK/ERK kinase; ERK: extracellular signal-regulated kinases; NF-kB: nuclear factor kappa-light-chain-enhancer of activated B cells; GM-CSF: granulocyte-macrophage colony-stimulating factor; IL-1β: interleukin-1β; TGFβR: transforming growth factor beta receptor; MYC: myelocytoma proto-oncogene; WNT: wingless-type MMTV integration site family; p53: tumor protein p53.
Table 1.
Summary of angiogenic and oncogenic pathways implicated in immune editing
|
Gene
|
Mechanism
|
References
|
| Angiogenesis | Expression of FasL allows selective killing of effector T cells through tumor-derived VEGF, IL-10, and prostaglandin action. | Motz et al.[20] Carman et al.[21] |
| Expression of molecules such as IDO and STING by endothelial cells is associated with response or overcoming resistance to immunotherapy. | Seeber et al.[27] Chevolet et al.[28]; Krähenbühl et al.[29] Meireson et al.[30] Yang et al.[33] | |
| EGFR Mutation/ Overexpression | Aberrant expression leads to decreased PD-L1 expression in NSCLC and is associated with decreased tumor mutational burden and immunogenicity. | Dong et al.[45]; Soo et al.[46]; Zhang et al.[49]; Gainor et al.[52] |
| Activating mutations associated with increased PD-L1 and immunosuppressive cytokine expression, and reduced T-cell viability. | Chen et al.[39]; Akbay et al.[40]; Azuma et al.[41] | |
| PTEN Loss | Loss leads to increased PD-L1 expression, increased autologous T-cell apoptosis, and overall decreased tumor immunogenicity. | Parsa et al.[65]; Waldron et al.[66] |
| PTEN loss leads to increased immunosuppressive cytokine expression (including VEGF), decreased T-cell trafficking, and inhibition of autophagy and cytolytic activity. | Peng et al.[67]; George et al.[69] | |
| NOTCH Amplification | Promotes an immunosuppressive microenvironment by increasing myeloid-derived suppressor cells, tumor-associated macrophages and regulatory T cells, and decreasing cytotoxic T cells. | Shen et al.[78] Balli et al.[79] Qiu et al.[80] |
| Activation of the NOTCH pathway promotes TH1 and inhibits TH2 differentiation. | Tindemans et al.[81] | |
| Promotes TGFβ signaling-mediated tumor growth through upregulation of proinflammatory IL1B and CCL2 cytokines and recruitment of tumor-associated macrophages. | Shen et al.[78] |
FasL: Fas ligand; VEGF: vascular endothelial growth factor; IL-10: interleukin-10; IDO: indoleamine 2,3-dioxygenase; STING: stimulator of interferon genes; PD-L1: programmed death-ligand 1; NSCLC: non-small cell lung cancer; PTEN: phosphatase and tensin homolog; TH1: T helper type 1; TH2: T helper type 2; TGFβ: transforming growth factor beta; IL1β: interleukin 1 beta; CCL2: C-C motif chemokine ligand 2.
Angiogenesis
One of the hallmarks of tumor growth and progression is new vessel formation.[9,10] Once it reaches a critical size, the metabolic needs of the growing tumor can no longer be sustained by the existing vasculature, thereby triggering the “angiogenic switch.”[11] During this process, pro-angiogenic factors such as vascular endothelial growth factor (VEGF), platelet-derived growth factor, angiopoietins (ANGPT), and interleukin 8 (IL-8) overcome antiangiogenic factors such as angiostatin and endostatin, tipping the imbalance toward tumor neovascularization.[12,13] The resulting tumor vasculature is leaky and disorganized, further exacerbating the problem by oversupplying certain portions of the tumor with nutrients and oxygen while undersupplying others. Furthermore, this dysfunctional vascular network impacts the efficiency of delivery of therapeutic agents to cancer cells.[14]
How the immune system regulates angiogenesis is still not completely understood; however, there is increasing evidence that endothelial cells lining the tumor vasculature can assist in the escape of cancer cells from immunosurveillance.[15] Inhibitory molecules, including PD-L1, PD-L2, indoleamine 2,3-dioxygenase (IDO1), the T-cell immunoglobulin domain, and mucin domain protein 3 (TIM3), are expressed by the endothelium and contribute to the regulation of immune cell recruitment, adhesion, and function.[16–19] In several tumor types, including breast, prostate, and colon cancers, endothelial cells also selectively express the Fas ligand (FasL).[20] This so called “tumor endothelial death barrier” allows the killing of effector T cells, but not of regulatory T cells (Treg) through tumor-derived VEGF, IL-10, and prostaglandin action.[21] Tumor endothelial cells also present tumor antigens to T cells, resulting in their activation.[22] Expression of costimulatory molecules CD80 and CD86, which are required for activation of naïve T cells, has also been reported for endothelial cells.[23]
In an analysis of biopsies from melanoma patients obtained prior to receiving immune checkpoint blockade therapies, treatment-resistant tumors displayed a transcriptional signature that indicated the upregulated expression of angiogenic genes (VEGFA, VEGFC, FLT1, and ANGPT2). Certain subsets of T cells also produce transforming growth factor-β (TGFβ), which stimulates regulatory T cells and further contributes to angiogenesis and immunosuppression.[24] Tumor-associated macrophages have also been found to promote angiogenesis and influence responses to immunotherapy.[25]
A preclinical study using prostate and melanoma mouse models has demonstrated that activation of the tumor endothelium with tumor necrosis factor (TNF) has promoted intratumoral T-cell infiltration, depletion of regulatory T cells, and expansion of cytotoxic T lymphocytes, all of which contribute to the overall therapeutic activity of immune checkpoint inhibitors.[26]
It has also been shown that endothelial cells, rather than the metastatic renal tumor cells themselves, are responsible for increased IDO expression and that IDO-1 expression levels in the endothelium could also be predictive of response to immunotherapy.[27–30]
In a preclinical study, immune checkpoint inhibition increased tumor vessel perfusion by promoting CD8+ T-cell accumulation and interferon (IFN)-γ production in treatment-sensitive breast and colon tumor cell lines, but not in treatment-resistant models.[31,32] The stimulator of IFN genes (STING) has been shown to have antitumor activity, and it is expressed also on endothelial cell vasculatures, suggesting that the combination of STING agonists with anti-PD1 or anti-PD-L1 antibodies and antiangiogenics could overcome primary or secondary resistance to immunotherapy.[33]
Numerous studies have already explored efforts to normalize the tumor vasculature to improve responses to immune checkpoint blockade.[34–36]
The coadministration of antiangiogenic agents can allow for the transient improvement of the tumor vasculature and result in enhanced drug delivery and immune cell infiltration, as well as improved response to immunotherapy. This hypothesis was clinically proven by the recent Keynote-426 trial, showing improved overall and progression-free survival (PFS) in patients with metastatic renal cell carcinoma treated with axitinib and pembrolizumab when compared to the sunitinib group.[37] Furthermore, the combination of lenvatinib (a multityrosine kinase inhibitor targeting VEGF receptors 1-3 [VEGFR-1-3], fibroblast growth factor receptors 1-4 [FGFR-1-4], RET, c-kit) and pembrolizumab showed 39% objective responses in endometrial cancer and was recently FDA approved for this indication.[38]
Epidermal Growth Factor Receptor (EGFR) Mutations and Activation of the Mitogen-Activated Protein Kinase (MAPK) Pathway Signaling
Activating mutations in EGFR lead to downstream signaling through the MAPK pathway and has been associated with increased expression of PD-L1.[39] Upregulation of PD-L1 in EGFR-mutant non-small cell lung cancer (NSCLC) cells cocultured with human peripheral blood mononuclear cells has led to reduced T-cell viability, increased expression of immune checkpoint molecules, and immunosuppressive cytokines.[40] Preclinical studies have shown that PD-1 inhibition improved survival of mice with EGFR-driven adenocarcinomas by enhancing effector T-cell function and also reduced viability of NSCLC tumors with aberrant EGFR expression.[41] Taken together, these studies imply that EGFR-activating mutations contribute to an immunosuppressive tumor microenvironment and that patients with EGFR mutations may not respond favorably to anti-PD-1 or anti-PD-L1 therapy.
In a randomized, open-label phase 3 study, NSCLC patients receiving nivolumab had improved overall survival (OS) and PFS compared to those who received docetaxel, with the exception of those who had never smoked or had EGFR mutations.[42] In another phase 2/3 study, patients with NSCLC with at least 50% of tumor cells expressing PD-L1 had significantly longer OS and PFS with pembrolizumab than with docetaxel.[43] This includes those with EGFR-mutant status, although the authors do acknowledge the lower incidence of EGFR mutations encountered in this study than was expected in the general NSCLC population. Two other studies that performed meta-analyses of multiple immunotherapy trials compared to standard chemotherapy demonstrated that only EGFR-wild-type patients benefit from anti-PD1 or anti-PD-L1 antibodies and that patients with EGFR-mutated tumors did not achieve improved OS or longer PFS while on immune checkpoint inhibitor therapy.[44]
In contrast to the studies described earlier, epidemiologic studies suggest an inverse relationship between oncogenic EGFR mutations and PD-L1 expression.[45] A pooled analysis of close to 4000 patients from 19 peer-reviewed studies demonstrated that aberrant oncogenic EGFR expression in NSCLC was less likely to be PD-L1-positive compared to wild-type tumors.[46] Two other epidemiologic reports show that patients with activating EGFR mutations have a higher likelihood of decreased PD-L1 expression and that increased PD-L1 expression was actually associated with wild-type EGFR.[47,48]
Although there is mechanistic evidence supporting increased PD-L1 expression associated with EGFR-activating mutations, the concept of adaptive immune resistance renders support to an inverse relationship between EGFR and PD-L1 that could be mediated by the IL-6/JAK/STAT3[49] or NFκB[50] pathways. Cancer cells are capable of increasing PD-L1 expression in response to a robust immune attack that is usually mounted by tumor antigen-specific T cells. This process is largely dependent on effective immune recognition, which itself is dependent on increased somatic mutational and neoantigen burden. EGFR-driven tumors have been reported to possess lower mutational burden.[51]
Quantitative pooled analysis of the TCGA (THe Cancer Genome Atlas) and Broad Institute dataset has shown that tumors with oncogenic EGFR mutations, specifically those with EGFR-activating mutations, have a significantly reduced tumor mutational burden and lower immunogenicity compared to those that are wild type.[45] This is consistent with a study by Gainor and colleagues,[52] which not only reported that EGFR-mutant NSCLCs have lower PD-L1 expression compared to their wild-type counterparts but also that the lower overall response to PD-1/PD-L1 inhibition may be due to the reduced infiltration of CD8+ lymphocytes in this tumor microenvironment.
In addition to EGFR, some alterations that activate the MAPK pathway (Fig. 1), such as KRAS G12D, can confer immune resistance.[53–55] On the other hand, recent findings have also suggested that KRAS G12C in these tumor types might likely have a different immune profile and may respond better to immune checkpoint inhibitors.[56,57] This pathway, in addition to the known oncogenic roles, also has important functions in CD4 T-cell differentiation, IL-4 receptor function, and IL-10 and IL-12 production regulation.[58,59]
Phosphatase and Tensin Homolog (PTEN) Deletions and Inactivating Mutations
PTEN is a lipid phosphatase that acts as the primary negative regulator of intracellular phosphoinositide 3-kinase (PI3K), a major downstream effector of receptor tyrosine kinases and G protein-coupled receptors. In response to receptor binding by growth factors, PI3K generates phospholipids, which in turn activate Ak strain transforming (AKT) and other downstream effectors involved in cellular processes such as cell survival, proliferation, and differentiation.[60] Components of the PI3K pathway (such as PIK3CA and PTEN) are frequently mutated in cancer; PTEN itself is considered a tumor suppressor since loss of PTEN expression due to deletions or inactivating mutations results in increased tumor cell survival and growth.[61]
PTEN alterations are frequently observed in many tumor types, including melanoma, glioblastoma, prostate, endometrial, and breast cancers.[62,63] Frequent loss of at least one copy of PTEN has been reported in human pancreatic ductal adenocarcinoma (PDAC).[64] Experiments in mice revealed activation of the PI3K pathway resulting from PTEN loss not only leads to PDAC development but also influences the tumor microenvironment by promoting NF-κB activation, thereby favoring tumor growth. PTEN loss and subsequent activation of the PI3K pathway in human glioblastoma tumors have also been reported to increase PD-L1 expression. Moreover, human glioma cells expressing wild-type PTEN were demonstrated to be more sensitive to T-cell-mediated lysis in vitro compared to PTEN mutant gliomas.[65] Tumors with loss of PTEN expression thus are poorly immunogenic, with immune resistance mediated in part by PD-L1 expression. In a follow-up study, PTEN wild-type gliomas elicited minimal T-cell apoptosis compared to tumors deficient in PTEN when cocultured with autologous T cells. Inhibition of the PI3K/AKT pathway in tissues lacking PTEN expression restored tumor-induced T-cell apoptosis to wild-type levels, providing mechanistic in vitro evidence for the involvement of this pathway to immune resistance following PTEN loss.[66]
Peng et al.[67] demonstrated that silencing of PTEN in melanoma cells results in reduced T-cell-mediated cytolytic activity. Clinical specimens from melanoma patients likewise demonstrated correlation of PTEN loss with decreased T-cell tumor infiltration and reduced expansion of tumor-derived T cells. Moreover, metastatic melanoma patients with PTEN-expressing tumors displayed better responses to FDA-approved checkpoint inhibitors than did their PTEN-deficient counterparts. Loss of PTEN expression was also shown to contribute to increased immunosuppressive cytokine production and subsequent inhibition of autophagy. Although the exact mechanism needs to be further defined, taken together, these suggest that PTEN loss leads to resistance to immunotherapy in melanoma by decreasing both T-cell trafficking into tumors and T-cell-mediated cell death in a PD-L1 independent manner.[68] A study of an exceptional uterine leiomyosarcoma responder on anti-PD-1 (pembrolizumab) monotherapy also reported that biallelic PTEN loss, along with reduced expression of two neoantigens, are potential mediators of resistance to immune checkpoint inhibitors. This study also reported increased VEGF expression with decreased T-cell infiltration in the treatment-resistant, PTEN-deficient tumor.[69]
NOTCH Signaling Pathway
NOTCH signaling, highly conserved through evolution, has important functions in regulating developmental processes and tissue homeostasis.[70] Activation of the NOTCH pathway occurs after a NOTCH receptor (NOTCH 1-4) is bound to one of the Delta-like ligands (DLL 1-4) or Jagged ligands (Jag 1,2),[71] and subsequently the activated NOTCH intracellular domain translocates in the nucleus where it activates the transcription of genes involved in tumor growth and proliferation.[72]
NOTCH signaling an important role in T-cell acute lymphoblastic leukemia (T-ALL), where NOTCH1-activating mutations are one of most frequent alterations in this disease.[73] In addition to T-ALL, NOTCH signaling also has oncogenic functions in other tumor types such as breast, colorectal, and ovarian cancers, NSCLC, medulloblastoma, and melanoma.[71,74,75] Furthermore, depending on the cell type, alterations in NOTCH1 can act as a tumor suppressor.[76,77]
NOTCH1 signaling has been shown to increase the myeloid-derived suppressor cells, tumor-associated macrophages, and regulatory T cells, and to decreased cytotoxic T cells with a role in promoting an immunosuppressive microenvironment in several tumor types such as breast cancer, melanoma, and pancreatic cancer.[78–80] Signaling through the NOTCH pathway in CD4+ T cells has an important role in promoting T helper type 1 (TH1) and inhibiting TH2 differentiation.[81]
In basal-like breast cancer, Notch activation promotes tumor growth through TGFβ signaling by proinflammatory cytokines such as IL-1β and chemokine ligand 2 (CCL2) and recruitment of tumor-associated macrophages.[78]
Targeting the NOTCH pathway has been done mostly by gamma-secretase inhibitors, but other antibodies targeting NOTCH receptors or ligands are currently in development.
Because of NOTCH signaling implications in TH population differentiation and its promotion of immunosuppressive tumor microenvironment, it provides a rationale for possible combination studies of NOTCH pathway-targeted therapies with immune checkpoint inhibitors or other T-cell therapies.[82]
However, a major concern in treatments involving gamma-secretase inhibitors (GSI) with other agents has been gastrointestinal toxicities, warranting alternate therapeutic strategies to overcome this.[83]
CONCLUSIONS
Dysregulation of key signaling pathways that promote tumorigenesis can also influence the immunosurveillance. The influence of tumor genomics on the activation of the tumor immune microenvironment can explain the heterogenous response to immunocheckpoint inhibitors among the same tumor type. There is an acute need to identify biomarkers that could help personalize immunotherapy and identify patients who are more or less likely to benefit for immunocheckpoint inhibitors. Although high microsatellite instability by mutations in MMR genes and high TMB are becoming validated biomarkers for responsiveness to immunocheckpoint inhibitors, these do not explain all responses to immunocheckpoint inhibitors. Identifying actionable alterations in key signaling molecular pathways that have immunomodulatory effect could help the development of genomically matched treatments and help personalize immunocheckpoint inhibitors.
Funding Statement
Source of Support: None.
Footnotes
Conflict of Interest: None.
REFERENCES
- 1.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol . 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang J, Yu JX, Hubbard-Lucey VM, Neftelinov ST, Hodge JP, Lin Y. The clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug Discov . 2018;17:854–855. doi: 10.1038/nrd.2018.210. [DOI] [PubMed] [Google Scholar]
- 3.Bedognetti D, Ceccarelli M, Galluzzi L, Lu R, Palucka K, Samayoa J, et al. Toward a comprehensive view of cancer immune responsiveness: a synopsis from the SITC workshop. J Immunother Cancer . 2019;7:167. doi: 10.1186/s40425-019-0640-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wellenstein MD, de Visser KE. Cancer-cell-intrinsic mechanisms shaping the tumor immune landscape. Immunity . 2018;48:399–416. doi: 10.1016/j.immuni.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med . 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan TA, Yarchoan M, Jaffee E, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol . 2019;30:44–56. doi: 10.1093/annonc/mdy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ros J, Baraibar I, Vivancos A, Rodon J. Review of immunogenomics and the role of tumor mutational burden as a biomarker for immunotherapy response. J Immunother Precis Oncol . 2019;2:144–151. [Google Scholar]
- 8.Miao D, Margolis CA, Vokes NI, et al. Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Nat Genet . 2018;50:1271–1281. doi: 10.1038/s41588-018-0200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell . 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell . 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 11.Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Semin Cancer Biol . 2009;19:329–337. doi: 10.1016/j.semcancer.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 Directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol . 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 13.Katayama Y, Uchino J, Chihara Y, et al. Tumor neovascularization and developments in therapeutics. Cancers (Basel) . 2019;11(3) doi: 10.3390/cancers11030316. pii: E316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamzah J, Jugold M, Kiessling F, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature . 2008;453:410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 15.Schadler KL, Crosby EJ, Zhou AY, et al. Immunosurveillance by antiangiogenesis: tumor growth arrest by T cell-derived thrombospondin-1. Cancer Res . 2014;74:2171–2181. doi: 10.1158/0008-5472.CAN-13-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang X, Bai X, Cao Y, et al. Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion. J Exp Med . 2010;207:505–520. doi: 10.1084/jem.20090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bansal R, Tomar T, Östman A, Poelstra K, Prakash J. Selective targeting of interferon γ to stromal fibroblasts and pericytes as a novel therapeutic approach to inhibit angiogenesis and tumor growth. Mol Cancer Ther . 2012;11:2419–2428. doi: 10.1158/1535-7163.MCT-11-0758. [DOI] [PubMed] [Google Scholar]
- 18.Riesenberg R, Weiler C, Spring O, et al. Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. Clin Cancer Res . 2007;13:6993–7002. doi: 10.1158/1078-0432.CCR-07-0942. [DOI] [PubMed] [Google Scholar]
- 19.Rodig N, Ryan T, Allen JA, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8 + T cell activation and cytolysis. Eur J Immunol . 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 20.Motz GT, Santoro SP, Wang L-P, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med . 2014;20:607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carman CV, Martinelli R. T lymphocyte–endothelial interactions: emerging understanding of trafficking and antigen-specific immunity. Front Immunol . 2015;6:603. doi: 10.3389/fimmu.2015.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wheway J, Latham SL, Combes V, Grau GER. Endothelial microparticles interact with and support the proliferation of T cells. J Immunol . 2014;193:3378–3387. doi: 10.4049/jimmunol.1303431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pober JS, Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol . 2012;33:49–57. doi: 10.1016/j.it.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hugo W, Zaretsky JM, Sun L, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell . 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petty AJ, Yang Y. Tumor-associated macrophages: implications in cancer immunotherapy. Immunotherapy . 2017;9:289–302. doi: 10.2217/imt-2016-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kühl AA, Pawlowski NN, Grollich K, et al. Human peripheral γδ T cells possess regulatory potential. Immunology . 2009;128:580–588. doi: 10.1111/j.1365-2567.2009.03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seeber A, Klinglmair G, Fritz J, et al. High IDO-1 expression in tumor endothelial cells is associated with response to immunotherapy in metastatic renal cell carcinoma. Cancer Sci . 2018;109:1583–1591. doi: 10.1111/cas.13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chevolet I, Speeckaert R, Haspeslagh M, et al. Peritumoral indoleamine 2,3-dioxygenase expression in melanoma: an early marker of resistance to immune control? Br J Dermatol . 2014;171:987–995. doi: 10.1111/bjd.13100. [DOI] [PubMed] [Google Scholar]
- 29.Krähenbühl L, Goldinger SM, Mangana J, et al. A longitudinal analysis of IDO and PDL1 expression during immune- or targeted therapy in advanced melanoma. Neoplasia . 2018;20:218–225. doi: 10.1016/j.neo.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meireson A, Chevolet I, Hulstaert E, et al. Peritumoral endothelial indoleamine 2, 3-dioxygenase expression is an early independent marker of disease relapse in colorectal cancer and is influenced by DNA mismatch repair profile. Oncotarget . 2018;9:25216–2524. doi: 10.18632/oncotarget.25393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng X, Fang Z, Liu X, et al. Increased vessel perfusion predicts the efficacy of immune checkpoint blockade. J Clin Invest . 2018;128:2104–2115. doi: 10.1172/JCI96582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson A, Hamzah J, Payne CJ, Ganss R. Tumor-targeted TNFα stabilizes tumor vessels and enhances active immunotherapy. Proc Natl Acad Sci . 2012;109:7841–7846. doi: 10.1073/pnas.1118296109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H, Lee WS, Kong SJ, et al. STING activation reprograms tumor vasculatures and synergizes with VEGFR2 blockade. J Clin Invest . 2019. p. 129. [DOI] [PMC free article] [PubMed]
- 34.Huang Y, Yuan J, Righi E, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci . 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian L, Goldstein A, Wang H, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature . 2017;544:250–254. doi: 10.1038/nature21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Georganaki M, van Hooren L, Dimberg A. Vascular targeting to increase the efficiency of immune checkpoint blockade in cancer. Front Immunol . 2018;9:3081. doi: 10.3389/fimmu.2018.03081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med . 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 38.Makker V, Rasco D, Vogelzang NJ, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol . 2019;20:711–718. doi: 10.1016/S1470-2045(19)30020-8. [DOI] [PubMed] [Google Scholar]
- 39.Chen N, Fang W, Zhan J, et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol . 2015;10:910–923. doi: 10.1097/JTO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 40.Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov . 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol . 2014;25:1935–1940. doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- 42.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med . 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet . 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 44.Lee CK, Man J, Lord S, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non–small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol . 2018;4:210–216. doi: 10.1001/jamaoncol.2017.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong Z-Y, Zhang J-T, Liu S-Y, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology . 2017;6:e1356145. doi: 10.1080/2162402X.2017.1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soo RA, Lim S-M, Syn N, Teng R, Soong R, Mok TSK, et al. Immune checkpoint inhibitors in epidermal growth factor receptor mutant non-small cell lung cancer: current controversies and future directions. Lung Cancer . 2018;115:12–20. doi: 10.1016/j.lungcan.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Zhang M, Li G, Wang Y, et al. PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci Rep . 2017;7:10255. doi: 10.1038/s41598-017-10925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang A, Wang HY, Liu Y, et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol . 2015;41:450–456. doi: 10.1016/j.ejso.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 49.Zhang N, Zeng Y, Du W, et al. The EGFR pathway is involved in the regulation of PDL1 expression via the IL-6/JAK/STAT3 signaling pathway in EGFR-mutated non-small cell lung cancer. Int J Oncol . 2016;49:1360–1368. doi: 10.3892/ijo.2016.3632. [DOI] [PubMed] [Google Scholar]
- 50.Lin K, Cheng J, Yang T, Li Y, Zhu B. EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through inhibiting NF-κB. Biochem Biophys Res Commun . 2015;463:95–101. doi: 10.1016/j.bbrc.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 51.Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science . 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gainor JF, Shaw AT, Sequist LV, Fu X, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non–small cell lung cancer: a retrospective analysis. Clin Cancer Res . 2016;22:4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bedognetti D, Roelands J, Decock J, Wang E, Hendrickx W. The MAPK hypothesis: immune-regulatory effects of MAPK-pathway genetic dysregulations and implications for breast cancer immunotherapy. Emerg Top Life Sci . 2017;1:429–445. doi: 10.1042/ETLS20170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liao W, Overman MJ, Boutin AT, et al. KRAS-IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell . 2019;35(4):559–572.e7. doi: 10.1016/j.ccell.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hänggi K, Ruffell B. Oncogenic KRAS drives immune suppression in colorectal cancer. Cancer Cell . 2019;35(4):535–537. doi: 10.1016/j.ccell.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 56.Herbst RS, Lopes G, Kowalski DM, et al. LBA4-association of KRAS mutational status with response to pembrolizumab monotherapy given as first-line therapy for PD-L1-positive advanced non-squamous NSCLC in keynote-042. Ann Oncol . 2019;30(suppl 11) [Google Scholar]
- 57.Gadgeel S, Rodriguez-Abreu D, Felip E, et al. LBA5-KRAS mutational status and efficacy in KEYNOTE-189: pembrolizumab (pembro) plus chemotherapy (chemo) vs placebo plus chemo as first-line therapy for metastatic non-squamous NSCLC. Ann Oncol . 2019;30(suppl 11) [Google Scholar]
- 58.Yamashita M, Kimura M, Kubo M, et al. T cell antigen receptor-mediated activation of the Ras/mitogen-activated protein kinase pathway controls interleukin 4 receptor function and type-2 helper T cell differentiation. Proc Natl Acad Sci . 1999;96:1024–1029. doi: 10.1073/pnas.96.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arthur JSC, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol . 2013;13:679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 60.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov . 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee Y-R, Chen M, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol . 2018;19:547–562. doi: 10.1038/s41580-018-0015-0. [DOI] [PubMed] [Google Scholar]
- 62.Chen C-Y, Chen J, He L, Stiles BL. PTEN: tumor suppressor and metabolic regulator. Front Endocrinol (Lausanne) . 2018;9:338. doi: 10.3389/fendo.2018.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Álvarez-Garcia V, Tawil Y, Wise HM, Leslie NR. Mechanisms of PTEN loss in cancer: it's all about diversity. Semin Cancer Biol . 2019;59:66–79. doi: 10.1016/j.semcancer.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 64.Ying H, Elpek KG, Vinjamoori A, et al. PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-κB–cytokine network. Cancer Discov . 2011;1:158–169. doi: 10.1158/2159-8290.CD-11-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med . 2006;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 66.Waldron JS, Yang I, Han S, et al. Implications for immunotherapy of tumor-mediated T-cell apoptosis associated with loss of the tumor suppressor PTEN in glioblastoma. J Clin Neurosci . 2010;17:1543–1547. doi: 10.1016/j.jocn.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peng W, Chen JQ, Liu C, et al. Loss of PTEN promotes resistance to T cell–mediated immunotherapy. Cancer Discov . 2016;6:202–216. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rieth J, Subramanian S. Mechanisms of intrinsic tumor resistance to immunotherapy. Int J Mol Sci . 2018;19(5):E1340. doi: 10.3390/ijms19051340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.George S, Miao D, Demetri GD, et al. Loss of PTEN is associated with resistance to Anti-PD-1 checkpoint blockade therapy in metastatic uterine leiomyosarcoma. Immunity . 2017;46:197–204. doi: 10.1016/j.immuni.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sato C, Zhao G, Ilagan MXG. An overview of notch signaling in adult tissue renewal and maintenance. Curr Alzheimer Res . 2012;9:227–240. doi: 10.2174/156720512799361600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer . 2011;11:338–351. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- 72.Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development . 2011;138:3593–3512. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 73.Weng AP, Ferrando AA, Lee W, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science . 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 74.Lobry C, Oh P, Aifantis I. Oncogenic and tumor suppressor functions of Notch in cancer: it's NOTCH what you think. J Exp Med . 2011;208:1931–1935. doi: 10.1084/jem.20111855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park JT, Li M, Nakayama K, et al. NOTCH3 gene amplification in ovarian cancer. Cancer Res . 2006;66:6312–6318. doi: 10.1158/0008-5472.CAN-05-3610. [DOI] [PubMed] [Google Scholar]
- 76.Nowell CS, Radtke F. Notch as a tumour suppressor. Nat Rev Cancer . 2017;17:145. doi: 10.1038/nrc.2016.145. [DOI] [PubMed] [Google Scholar]
- 77.Aster JC, Pear WS, Blacklow SC. The varied roles of Notch in cancer. Annu Rev Pathol . 2017;12:245–275. doi: 10.1146/annurev-pathol-052016-100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen Q, Cohen B, Zheng W, et al. Notch shapes the innate immunophenotype in breast cancer. Cancer Discov . 2017;7:1320–1335. doi: 10.1158/2159-8290.CD-17-0037. [DOI] [PubMed] [Google Scholar]
- 79.Balli D, Rech AJ, Stanger BZ, Vonderheide RH. Immune cytolytic activity stratifies molecular subsets of human pancreatic cancer. Clin Cancer Res . 2017;23:3129–3138. doi: 10.1158/1078-0432.CCR-16-2128. [DOI] [PubMed] [Google Scholar]
- 80.Qiu H, Zmina PM, Huang AY, Askew D, Bedogni B. Inhibiting Notch1 enhances immunotherapy efficacy in melanoma by preventing Notch1 dependent immune suppressive properties. Cancer Lett . 2018;434:144–151. doi: 10.1016/j.canlet.2018.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tindemans I, Peeters MJW, Hendriks RW. Notch signaling in T helper cell subsets: instructor or unbiased amplifier? Front Immunol . 2017;8:419. doi: 10.3389/fimmu.2017.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ileana Dumbrava EE, Mills GB, Yap TA. Targeting gamma secretase: has progress moved up a Notch? Ann Oncol . 2018;29:1889–1891. doi: 10.1093/annonc/mdy307. [DOI] [PubMed] [Google Scholar]
- 83.Milano J, McKay J, Dagenais C, et al. Modulation of Notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci . 2004;82(1):341–358. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]