Abstract
In recent years, cancer treatment has evolved, and new therapies have been introduced with significant improvement in prognosis. The immunotherapies stand out owing to their efficacy and remission rate. Chimeric antigen receptor (CAR) T-cell therapy is a part of this new era of therapies. Chimeric antigen receptor T-cell therapy is a form of adoptive cellular therapy that uses a genetically encoded CAR in modified human T cells to target specific tumor antigens in a nonconventional, non-major histocompatibility complex (MHC) protein presentation. Chimeric antigen receptor T-cell therapy successfully identifies tumor antigens and through activation of T cells destroys tumoral cells. It has been found to efficiently induce remission in patients who have been previously treated for B-cell malignancies and have persistent disease. As the use of this novel therapy increases, its potential side effects also have become more evident, including major complications like cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). Cytokine release syndrome is a major systemic inflammatory process as a result of massive cytokine production by the proliferating and activated CAR T cells in which multiple interleukins and immune cells contribute to the inflammatory response. Cytokine release syndrome has been associated with cardiovascular life-threatening complications including hypotension, shock, tachycardia, arrhythmias, left ventricular dysfunction, heart failure, and cardiovascular death. Arrhythmias, among its major complications, vary from asymptomatic prolonged corrected QT interval (QTc) to supraventricular tachycardia, atrial fibrillation, flutter, and ventricular arrhythmias like Torsade de pointes. This article focuses on the cardiovascular complications and arrhythmias associated with CRS and CAR T-cell therapy.
Keywords: CAR T-cell therapy, cardiovascular complications, cardiotoxicity, cytokine release syndrome, chimeric antigen receptor, arrhythmias
INTRODUCTION
In the last decade, the mean 5-year survival has increased significantly in many malignancies with improvements in screening and development of novel therapies.[1] Recent advances in therapy have focused on the development of therapies that modulate the immune system, such as immune checkpoint inhibitors, monoclonal antibodies, tyrosine kinase inhibitors, bispecific antibodies, and chimeric antigen receptor (CAR) T-cell therapies.[2] As the use of these novel therapies increases, the undesired secondary effects have also become evident, especially those related to cardiac toxicity. The first CAR T-cell therapy, tisagenlecleucel (Kymriah), was approved by the U.S. Food and Drug Administration (FDA) in August 2017 for patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia (ALL) and relapsed/refractory large B-cell lymphomas after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, high grade B-cell lymphoma, and DLBCL from follicular lymphoma, followed in October 2017 by axicabtagene ciloleucel (Yescarta) for the treatment of adult patients with relapsed or refractory large B-cell lymphoma.[3]
Nonetheless, CAR T-cell therapy has also been found to have potential severe secondary side effects like cytokine release syndrome (CRS), which is associated with systemic effects and potential multi·organ compromise, notably neurotoxicity. This review will focus on CAR T-cell therapy and its cardiovascular toxicity, with emphasis on CRS and associated arrhythmias.
CHIMERIC ANTIGEN RECEPTOR T-CELL THERAPY
Chimeric antigen receptor T-cell therapy is a form of adoptive cellular therapy to treat cancer. It is based on genetically modified human T cells that target specific tumor antigens, not only attacking current malignant cells but also providing ongoing surveillance for new malignant cells.[4]
Normally, tumor antigens are presented to the T cells by the major histocompatibility complex (MHC) proteins, leading to the T-cell activation and destruction of abnormal cells by induction of cytolysis or apoptosis of the malignant cells.[4] The T-cell activation is dependent upon costimulation requiring both major histocompatibility complex-T cell receptor (MHC-TCR) and B7 molecules on the antigen-presenting cells (APCs) binding to CD28 receptors on T cells. The cancer cells activate the immune checkpoint receptors that then inactivate the costimulation and do not allow for T-cell activation.
Therefore, in cancer patients, this T-cell lymphocyte target-recognition function is impaired. This malfunction can lead to an immune evasion by tumor cells resulting in an unrestricted proliferation of tumor cells. Thus, cancer immunotherapy is based on the need to increase recognition of malignant cells by T cells.[4] In CAR T-cell therapy, autologous T cells are collected from the patient usually through leukapheresis, engineered with CAR, expanded ex vivo, and subsequently reinfused after conditioning.[5,6]
Chimeric antigen receptor T-cell therapy uses a genetically encoded CAR that is composed of three parts: an extracellular domain, which is the tumor antigen-binding domain and is a single-chain variable fragment (extracellular ScFv), which comes from the variable heavy and light chains of an antibody that targets specific tumor antigens, a transmembrane, and an intracellular activation domain.[4,6] The antigen-binding domain engages surface-tumor cell antigens in a non-HLA (non-human leukocyte antigen system)-dependent manner, and the intracellular domain fuses with T-cell activation signaling domains like CD3, leading to additional T-cell activation.[4] The CAR is implanted in the patient's T cells using a lentiviral or retroviral vector[7] (Figure 1).
Figure 1.
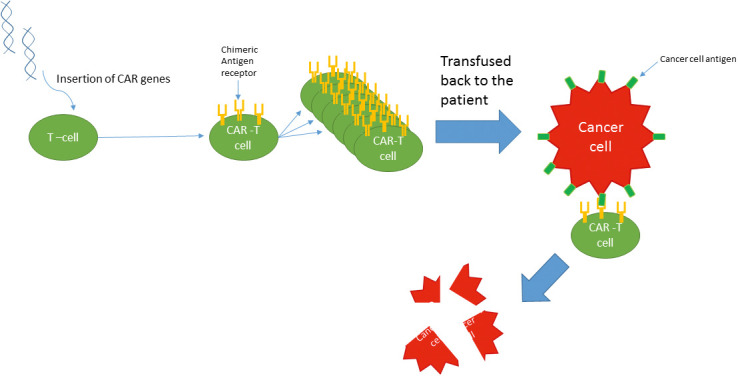
Chimeric antigen receptor (CAR) T-cell therapy mechanism.
First-generation CARs were used without conditioning therapy, which decreased their toxicity but also their half-life and efficacy.[6] The second-generation CARs have two signaling domains, the CD3ζ, which provides the first signal, and the costimulatory domain, which provides the second signal and supports the proliferation, cytotoxic activity, and persistence of the CAR T cells.[6] The use of conditioning therapy with fludarabine and cyclophosphamide prior to the administration of CAR T-cell therapy has been found to decrease the risk of rejection and to prolong its half-life and therefore efficacy; however, there was an increase in the frequency of serious medical complications like CRS and neurotoxicity[8]; similar studies using a less intense conditioning therapy have shown persistent positive results with fewer side effects.[9]
Chimeric antigen receptor T-cell therapy has been found to efficiently induce remission in patients who have been previously treated for B-cell malignancies and have persistent disease.[10] This effect has been associated with an improvement in survival. Of note, it has been found that remissions are more likely to be achieved with higher peak blood levels of the CAR T cells.[10]
CURRENT APPLICATIONS OF CAR T-CELL THERAPY
Currently CAR T-cell therapy is approved by the FDA for use in patients with ALL, chronic lymphocytic leukemia, non-Hodgkin's lymphoma, and relapse/refractory large B-cell lymphomas. However, there is great interest in this therapy, and several studies and trials have addressed its use in other types of tumors including multiple myeloma,[11] mantle cell lymphoma,[12] ovarian cancer, hepatocellular cancer, and other solid tumors,[13–16] with promising results.
CYTOKINE RELEASE SYNDROME (CRS)
One of the most common complications of CAR T-cell therapy is CRS, a systemic inflammatory process that is the result of massive cytokine production by the proliferating activated CAR T cells. Interleukin (IL)-6 is the principal mediator for the development of CRS, but other cytokines including IL-2, IL-8, IL-10, interferon γ, and tumor necrosis factor α are also contributors to the development of the inflammatory response, and their levels correlate with the severity of the syndrome.[17] It is probable that CAR T-cell therapy also activates macrophages and other immune cells that can release more cytokines and further contribute to CRS[7]; these cytokines include IL-5, granulocyte-macrophage colony-stimulating factor (GM-CSF) from activated B-cell lymphocytes, natural killer cells, dendritic cells, monocytes, and endothelial cells.[5]
Cytokine release syndrome is seen more frequently in patients undergoing CAR T-cell therapy than with other immunotherapies. It has been found to have a high incidence, with reports of CRS in up to 100% of the patients in a study of pediatric patients with ALL.[17] Often its presentation is mild to moderate (grade 1–2); however, sometimes patients may develop life-threatening complications (grade 3–4), with severe manifestations found in between 14% and 27% of the patients.[18,19] Fortunately, these complications are potentially reversible when managed appropriately.[7]
Immune Effector Cell-Associated Neurotoxicity Syndrome
The second most common adverse reaction to CAR T-cell therapy is ICANS, which is an encephalopathy presenting with expressive aphasia, mental status changes with the potential to progress to seizure, coma, and cerebral edema.[20] Supportive care measures are provided for mild presentations; however, corticosteroids may be used for severe cases.[20]
CRS Symptoms
Clinical symptoms of CRS may develop within minutes to days, with the highest risk seen within the first 2 weeks.[17] The symptoms associated with CRS are related to the release of cytokines, similar to those symptoms seen during infectious processes. The most common symptoms and signs are fatigue, fever, hypotension, and tachycardia, but almost any organ can be affected. Symptoms and signs related to specific organ toxicities include headaches, tremors, seizures, transaminitis, hyperbilirubinemia, nausea, emesis, diarrhea, anemia, elevated D-dimer, disseminated intravascular coagulation, tachypnea, hypoxemia, acute kidney injury, electrolyte imbalance, myalgias, arrhythmias, decreased left ventricular ejection fraction, and corrected QT interval (QTc) prolongation.[21]
CRS Grading
Given the variable presentation of CRS and in order to create a consensus regarding diagnosis and management, the American Society for Blood and Marrow Transplantation (ASBMT) published a consensus grading system for CRS,[22] with four grades of severity as seen in the Table. However, several grading systems exist, including those from Common Terminology Criteria for Adverse Events (CTCAE), National Comprehensive Cancer Network (NCCN), Chimeric antigen receptor T-cell therapy assessment and management of toxicities (CARTOX), Memorial Sloan Kettering Cancer Center (MSKCC), and Penn criteria, with subtle differences.
Table.
Cytokine release syndrome grading, management, and biomarkers*
| Grade |
Definition |
Management |
Biomarkers |
| I | Fever, T greater than 38 °C No hypotension or hypoxia | Supportive care IVF, antipyretics Evaluation and management to exclude potential infection If neutropenic, may use growth factors and antibiotics | Often unremarkable cardiac biomarkers |
| II | Fever, T greater than 38 °C Hypotension without need for vasopressors, and/or Mild hypoxemia that requires oxygen by low-flow nasal cannula | Supportive care IVF, antipyretics Vasopressor, oxygen May use tocilizumab dose as follows: –For patients less than 30 kg: 12 mg/kg IV × 1 –For patients greater than or equal to 30 kg: 8 mg/kg IV × 1 May repeat every 8 hours up to three times as needed. The total tocilizumab dose should not exceed 800 mg. If no meaningful response, may add dexamethasone or methylprednisolone. | Higher incidence of troponin and pro-BNP assay elevations |
| III | Fever, T greater than 38 °C Hypotension requiring one vasopressor with or without vasopressin, and/or Hypoxemia that requires HFNC or higher oxygen flow system by facemask, NRB, or venturi mask | Supportive care IVF, antipyretics Vasopressor, oxygen Tocilizumab Dexamethasone 10–20 mg IV every 6 hours or equal methylprednisolone dose ICU monitoring recommended | Higher incidence of troponin and pro-BNP assay elevations |
| IV | Fever, T greater than 38 °C Hypotension requiring several vasopressors (excluding vasopressin) Hypoxemia requiring positive pressure like CPAP, BIPAP, or mechanical ventilation | Supportive care IVF, antipyretics Vasopressors Oxygen by positive pressure ventilation, CPAP, BIPAP, mechanical ventilation ICU monitoring Tocilizumab Methylprednisolone 1 mg/kg/day | Higher incidence of troponin and pro-BNP assay elevations |
BIPAP, bilevel positive airway pressure; BNP, b-type natriuretic peptide; CPAP, continuous positive airway pressure; HFNC, high-flow nasal cannula; ICU, intensive care unit; IV, intravenous; IVF, intravenous fluids; NRB. non-rebreather mask.
Grading and management source: ASBMT.[22]
CRS Risk Factors
Proposed risk factors for CRS are T-cell therapy targeting CD19 or CD22,[23] high disease burden, severe thrombocytopenia, higher infused CAR T-cell doses, higher-intensity lymphodepletion doses, addition of fludarabine to cyclophosphamide during lymphodepletion, use of unselected bulk CD8+ T cells, high levels of CTL019+ CD8 and CD3 cells, higher peak of C reactive protein (CRP), inflammatory markers, and patient factors, like older age.[5,24]
Burstein et al[25] found, in a study of pediatric patients with ALL, that personal history of systolic or diastolic dysfunction, abnormal electrocardiogram findings, and having more than 25% blasts in the bone marrow were associated with a higher incidence of hypotension needing vasopressors. Although there is no available clinical data with regard to how cardiovascular risk factors and presence of cardiomyopathy could influence adverse cardiovascular outcomes during CAR T-cell treatment, it is reasonable to obtain a thorough cardiovascular risk assessment and baseline echocardiogram in those with significant cardiovascular risk burden prior to treatment with CAR, particularly in those patients who have previously received anthracycline chemotherapy.[26] It is recommended that all patients receive routine vital signs and cardiac monitoring during initiation of treatment. Recent data per Alvi et al[27] suggest that 95% of cardiovascular events during CAR T-cell treatment occur with a preceding serum troponin elevation. The study included 137 adult patients who received CAR T-cell therapy for DLBCL (61% of the patients), transformed follicular lymphoma (27% of the patients), and multiple myeloma (8% of the patients); these patients had a mean age of 62 years. Based on this study it appears that CAR T-cell therapy cardiovascular complications occur more frequently in elderly patients, patients with history of prior therapy with anthracyclines and radiotherapy, and patients with cardiovascular risk factors. However, earlier initiation of CRS treatment with tocilizumab was associated with a lower rate of cardiovascular events independent of the presence of cardiovascular risk factors.[27] In terms of the relationship between age and the development of CRS the data are limited, but in a study of CAR T-cell therapy in patients with refractory large B-cell lymphoma there was not a significant difference in secondary effects between patients older and younger than 65 years.[28]
CARDIOTOXICITY RELATED TO CAR T-CELL THERAPY AND CRS
Chimeric antigen receptor T-cell therapy and CRS can cause several cardiovascular toxicities. Direct and indirect mechanisms have been proposed to explain these toxicities. First, there is a direct mechanism mediated by the release of cytokines and its effects on the cardiovascular system, leading to hypotension and tachycardia, which are the most common side effects, with progression to instability and shock in the most severe cases.[21] Hypotension has been reported to be present in up to one-third of patients with CRS[18,19,29,30] and usually improves with supportive care. Second, an indirect mechanism is caused by cross-reactivity against the cardiac protein titin, a protein that is active during the contraction and relaxation of the heart. In this case, affinity-enhanced T-cell receptors recognize an off-target peptide from the protein titin, causing cardiac muscle toxicity that often is fatal.[31,32]
Other reported cardiac toxicities include an increase in serum troponins and a decrease in left ventricular systolic function. The resulting ventricular dysfunction may be related to a release of tumor necrosis factor α, which may cause a decrease in myocyte shortening and mitochondrial dysfunction.[33] In a study by Kochenderfer et al,[34] in patients who received CAR T-cell therapy for persistent B-cell malignancies after allogenic hematopoietic cell transplantation, a decrease in the mean left ventricular ejection fraction after an infusion of the CAR T cells was seen in 12 days, and lasted for 4 months before recovering to normal levels. While in the recently reported JULIET trial[35] the authors did not find any major cardiovascular events, other studies have shown various degrees of cardiovascular events, like the ZUMA 1 trial,[36] in which one patient died of cardiac arrest, and the ELIANA trial,[37] in which three patients had cardiac arrest with no cardiovascular deaths noted, and two patients were reported to have heart failure. In the latest study by Alvi et al,[27] decreased left ventricular function was found in 28% of the patients who had an echocardiogram before and after CAR T-cell therapy (n = 8); they also had a CRS grade greater than or equal to 2. This study reported 17 cardiovascular events including six cardiovascular deaths (12%), six patients with decompensated heart failure (12%), and five patients who had new-onset arrhythmias.
ARRHYTMYAS RELATED TO CAR T-CELL THERAPY
Arrhythmias, among the most common complications from CAR T-cell therapy, have been reported mostly in the context of the CRS. The most common abnormalities found in these patients are asymptomatic prolonged QTc and atrial fibrillation.[5,21] In the study by Alvi et al,[27] five patients had new-onset cardiac arrhythmias; two patients developed supraventricular tachycardia, and three had new-onset atrial fibrillation. Three patients in this study died from complications related to new-onset heart failure and/or tachyarrhythmias complicated by shock.
Although the mechanisms for the development of arrhythmias in these patients are not completely understood, it is possible that the arrhythmias occur secondary to a direct effect of the cytokines on the electrical system, similar to what has been found in patients with inflammatory diseases like rheumatoid arthritis[38] or myocarditis, or in patients after cardiac surgery[39] (Figure 2).
Figure 2.
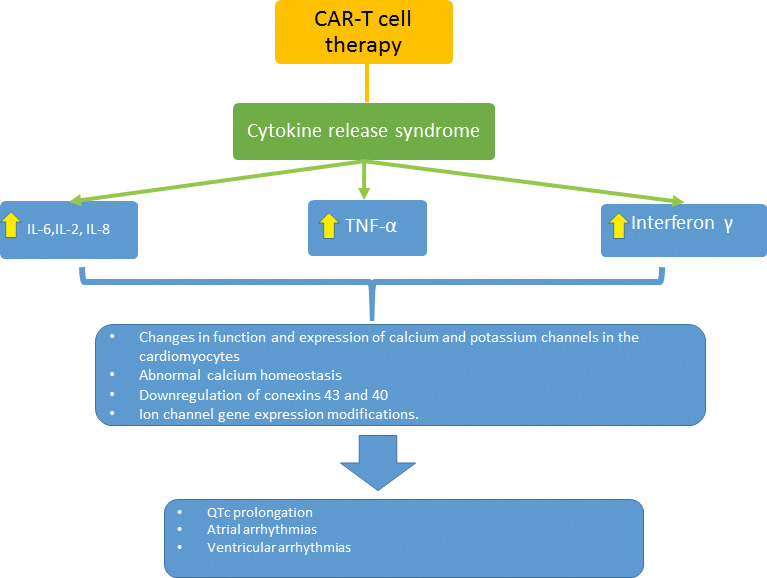
Possible mechanisms of arrhythmias in chimeric antigen receptor (CAR) T-cell therapy. IL, interleukin; TNF, tumor necrosis factor.
The association between severe inflammatory states and arrhythmias has been suspected, with higher levels of inflammatory biomarkers like CRP,[40,41] IL-6,[42] leukocyte count,[43,44] and complement activation[40] associated with a higher incidence of atrial tachyarrhythmias.
Increases in circulating cytokines may play a role in the development of acquired long QTc, by a direct effect on cardiomyocytes in addition to cardiac stimulation from an activated sympathetic nervous system. The direct effects on cardiomyocytes are related to changes in function and expression of calcium and potassium channels, which lead to a prolongation of the action potential duration.[45–47] Lazzerini et al[48] found that systemic inflammation and IL-6 may be an important contributing risk factor for QT prolongation and the development of Torsade de pointes while presenting concomitantly with other well-known risk factors.
Similarly, inflammation has been associated with the initiation and maintenance of atrial fibrillation.[49] The proposed mechanisms for the development of atrial fibrillation include abnormal calcium homeostasis caused by ILs and tumor necrosis factor α[39] as well as downregulation of connexins 43 and 40 by IL-6.[50] There are multiple studies demonstrating the association with elevated inflammatory biomarkers, especially CRP with persistent atrial fibrillation.[51]
Even in hearts without structural abnormalities, supraventricular ectopic beats can be seen with fever as well as recurrent ventricular tachycardia or idiopathic ventricular fibrillation.[52] However, the incidence of sudden cardiac death and major arrhythmias is low in patients without major cardiac structural abnormalities.[53,54] On the other hand, in patients with channelopathies, fever has been associated with ion channel gene expression modifications.[55]
TREATMENT OF CRS
The treatment of CRS depends on the severity of the clinical presentation. The ASBMT grading system is helpful to guide management[22] (Table).
Grade 1 (fever without hypotension or hypoxia) is managed with supportive care with intravenous (IV) hydration and antipyretics. Evaluation and management are necessary to exclude a concomitant infection. Growth factors and antibiotics can be used if neutropenic.[7]
Grade 2 (fever with hypotension but not needing vasopressors and/or hypoxia needing oxygen by nasal cannula) is managed with antipyretics, IV fluids, and/or oxygen.[7] According to Maude et al,[18] providers may use tocilizumab, a monoclonal IL-6 receptor antibody that has been found efficacious in reversing CRS.[18] The tocilizumab dose for patients weighing less than 30 kg is 12 mg/kg IV and for patients weighing greater than or equal to 30 kg is 8 mg/kg IV; the total tocilizumab dose should not exceed 800 mg. Doses may be repeated every 8 hours up to three times if needed. If no meaningful clinical response occurs, steroid may be added.
Grade 3 (fever with hypotension needing one vasopressor with or without vasopressin and/or hypoxia needing a higher fraction of inspired oxygen [FiO2] by high-flow nasal cannula, facemask, non-rebreather, or venturi mask)[7] requires management of the fever, IV hydration, vasopressor, supplemental oxygen, tocilizumab (dosing as for grade 2), and dexamethasone 10 to 20 mg IV every 6 hours or equal methylprednisolone dose. Monitoring in the medical intensive care unit should be considered.[7]
Grade 4 (fever with hypotension needing several vasopressors but not vasopressin and/or hypoxia needing continuous positive airway pressure [CPAP], bilevel positive airway pressure [BIPAP], and/or mechanical ventilation). The management includes antipyretics, IV hydration, vasopressors, oxygen by positive pressure ventilation as before and monitoring in the intensive care unit with administration of tocilizumab and methylprednisolone 1 mg/kg/day.[7]
MANAGEMENT OF CRS-ASSOCIATED CARDIAC ARRHYTHMIAS
Cardiac arrhythmias should be treated depending on the type of arrhythmia, ideally by the cardiology team. Cardiac monitoring during and following treatment with CAR is essential for early identification of arrhythmias. These patients benefit from multidisciplinary care and close collaboration between cardiologists and oncologists. An important part of the management of these arrhythmias related to CRS includes the use of specific antiarrhythmic medications, assessment of risk versus benefits of anticoagulation on a case-by-case basis, and cardioversion or defibrillation if necessary. Well-supported ancillary staff trained in advanced cardiovascular life support and intensive care triaging should be an essential part of the care team of these patients.
Supportive care with IV fluids and/or vasopressors to ensure adequate perfusion of the heart and other vital organs is important, as well as the correction of electrolyte abnormalities.
In patients with unstable arrhythmias, tocilizumab may be used. However, consideration regarding severity of illness and CRS comes into play given the possibility that it may decrease the efficacy of the CAR T-cell therapy.[56]
In severe cases with poor response to the management, high-dose steroids may be used; however, there is similar concern about the potential decreased efficacy of the CAR T-cell therapy, and individual risk versus benefits should be assessed.
Interleukin-1 is a proinflammatory cytokine that has been associated with cardiovascular disease. Interleukin-1 blockage may have a beneficial effect in inflammatory cardiovascular conditions refractory to standard treatment[57] like CRS and potentially the associated arrhythmias. Medications like anakinra, a recombinant human receptor antagonist that blocks IL-1α and IL-1β, has been used in clinical trials for ST segment elevation myocardial infarction, non-ST segment elevation myocardial infarction, heart failure with reduced ejection fraction, and heart failure with preserved ejection fraction[57]; it could be helpful in the treatment of CRS-related cardiac arrhythmias.
CONCLUSION
Chimeric antigen receptor T-cell immune therapy has been proven to provide remission and increase survival in patients with refractory cancer with otherwise very poor outcomes and prognosis.
As a novel therapy there is still much to be learned about this treatment, involving cardiac risk stratification, prediction and prognosis regarding complications like CRS and cardiac toxicities, animal models of cytokine-mediated left ventricular dysfunction, development of cardiac screening algorithms,[58] preclinical screening for cross-reactivity with non-tumor antigens,[31] and assessing outcomes with the use of immunosuppressive therapies.[59]
While in a majority of patients the side effects of treatment are well tolerated, there are potential severe complications, some life-threatening, that warrant close monitoring of these patients. Early recognition of the main and most common adverse reaction, the CRS and potential life-threatening arrhythmias, is important, as most resolve if adequately treated. Future studies are needed to better understand risk factors, prevention, pathophysiology, and adequate management of CRS and its cardiovascular complications.
Funding Statement
Source of Support: None.
Footnotes
Conflict of Interest: None.
References
- 1.American Cancer Society. Cancer facts & figures 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf Accessed June 6, 2020.
- 2.Kroschinsky F, Stolzel F, von Bonin S, et al. New drugs, new toxicities: severe side effects of modern targeted and immunotherapy of cancer and their management. Crit Care . 2017;21:89. doi: 10.1186/s13054-017-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration. FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma. 2017. https://www.fda.gov/news-events/press-announcements/fda-approves-car-t-cell-therapy-treat-adults-certain-types-large-b-cell-lymphoma Accessed June 6, 2020.
- 4.Ye B, Stary CM, Li X, Gao Q, Kang C, Xiong X. Engineering chimeric antigen receptor-T cells for cancer treatment. Mol Cancer . 2018;17:32. doi: 10.1186/s12943-018-0814-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganatra S, Parikh R, Neilan TG. Cardiotoxicity of immune therapy. Cardiol Clin . 2019;37:385–397. doi: 10.1016/j.ccl.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Strati P, Neelapu SS. Chimeric antigen receptor-engineered T cell therapy in lymphoma. Curr Oncol Rep . 2019;21:38. doi: 10.1007/s11912-019-0789-z. [DOI] [PubMed] [Google Scholar]
- 7.Neelapu SS. Managing the toxicities of CAR T-cell therapy. Hematol Oncol . 2019;37(suppl 1):48–52. doi: 10.1002/hon.2595. [DOI] [PubMed] [Google Scholar]
- 8.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood . 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochenderfer JN, Somerville RPT, Lu T, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol . 2017;35:1803–1813. doi: 10.1200/JCO.2016.71.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brudno JN, Somerville RP, Shi V, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol . 2016;34:1112–1121. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med . 2019;380:1726–1737. doi: 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang ML, Munoz J, Goy A, et al. KTE-X19, an anti-CD19 chimeric antigen receptor (CAR) T cell therapy, in patients (Pts) with relapsed/refractory (R/R) mantle cell lymphoma (MCL): results of the phase 2 ZUMA-2 study. Blood . 2019;134(suppl 1):754. [Google Scholar]
- 13.Yan W, Hu H, Tang B. Advances of chimeric antigen receptor T cell therapy in ovarian cancer. Onco Targets Ther . 2019;12:8015–8022. doi: 10.2147/OTT.S203550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang RY, Wei D, Liu ZK, et al. Doxycycline inducible chimeric antigen receptor T cells targeting CD147 for hepatocellular carcinoma therapy. Front Cell Dev Biol . 2019;7:233. doi: 10.3389/fcell.2019.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen AD, Raje N, Fowler JA, Mezzi K, Scott EC, Dhodapkar MV. How to train your T cells: overcoming immune dysfunction in multiple myeloma. Clin Cancer Res . 2019 Oct 31 [epub ahead of print] [DOI] [PMC free article] [PubMed]
- 16.Jiang C, Zhao W, Qin M, Jin M, Chang L, Ma X. CD56-chimeric antigen receptor T-cell therapy for refractory/recurrent rhabdomyosarcoma: a 3.5-year follow-up case report. Medicine . 2019;98:e17572. doi: 10.1097/MD.0000000000017572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood . 2017;130:2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med . 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet . 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol . 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood . 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant . 2019;25:625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 23.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med . 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldoss I, Khaled SK, Budde E, Stein AS. Cytokine release syndrome with the novel treatments of acute lymphoblastic leukemia: pathophysiology, prevention, and treatment. Curr Oncol Rep . 2019;21:4. doi: 10.1007/s11912-019-0753-y. [DOI] [PubMed] [Google Scholar]
- 25.Burstein DS, Maude S, Grupp S, Griffis H, Rossano J, Lin K. Cardiac profile of chimeric antigen receptor T cell therapy in children: a single-institution experience. Biol Blood Marrow Transplant . 2018;24:1590–1595. doi: 10.1016/j.bbmt.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Ganatra S, Carver JR, Hayek SS, et al. Chimeric antigen receptor T-cell therapy for cancer and heart: JACC Council perspectives. J Am Coll Cardiol . 2019;74:3153–3163. doi: 10.1016/j.jacc.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvi RM, Frigault MJ, Fradley MG, et al. Cardiovascular events among adults treated with chimeric antigen receptor T-cells (CAR-T) J Am Coll Cardiol . 2019;74:3099–3108. doi: 10.1016/j.jacc.2019.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sano D, Nastoupil LJ, Fowler NH, et al. Safety of axicabtagene ciloleucel CD19 CAR T-cell therapy in elderly patients with relapsed or refractory large B-cell lymphoma. Blood . 2018;132(suppl 1):96. [Google Scholar]
- 29.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med . 2014;6 doi: 10.1126/scitranslmed.3008226. 224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol . 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cameron BJ, Gerry AB, Dukes J, et al. Identification of a titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med . 2013;5 doi: 10.1126/scitranslmed.3006034. 197ra03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linette GP, Stadtmauer EA, Maus MV, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood . 2013;122:863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato R, Nasu M. A review of sepsis-induced cardiomyopathy. J Intens Care . 2015;3:48. doi: 10.1186/s40560-015-0112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kochenderfer JN, Dudley ME, Carpenter RO, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood . 2013;122:4129–4139. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med . 2018;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 36.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med . 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med . 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kouvas N, Kontogiannis C, Georgiopoulos G, et al. The complex crosstalk between inflammatory cytokines and ventricular arrhythmias. Cytokine . 2018;111:171–177. doi: 10.1016/j.cyto.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Vonderlin N, Siebermair J, Kaya E, Kohler M, Rassaf T, Wakili R. Critical inflammatory mechanisms underlying arrhythmias. Herz . 2019;44:121–129. doi: 10.1007/s00059-019-4788-5. [DOI] [PubMed] [Google Scholar]
- 40.Bruins P, te Velthuis H, Yazdanbakhsh AP, et al. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation . 1997;96:3542–3548. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 41.Ishii Y, Schuessler RB, Gaynor SL, et al. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation . 2005;111:2881–2888. doi: 10.1161/CIRCULATIONAHA.104.475194. [DOI] [PubMed] [Google Scholar]
- 42.Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA . 2004;291:1720–1729. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 43.Lamm G, Auer J, Weber T, Berent R, Ng C, Eber B. Postoperative white blood cell count predicts atrial fibrillation after cardiac surgery. J Cardiothorac Vasc Anesth . 2006;20:51–56. doi: 10.1053/j.jvca.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 44.Amar D, Goenka A, Zhang H, Park B, Thaler HT. Leukocytosis and increased risk of atrial fibrillation after general thoracic surgery. Ann Thorac Surg . 2006;82:1057–1061. doi: 10.1016/j.athoracsur.2006.03.103. [DOI] [PubMed] [Google Scholar]
- 45.Lazzerini PE, Capecchi PL, Laghi-Pasini F. Long QT syndrome: an emerging role for inflammation and immunity. Front Cardiovasc Med . 2015;2:26. doi: 10.3389/fcvm.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petkova-Kirova PS, Gursoy E, Mehdi H, McTiernan CF, London B, Salama G. Electrical remodeling of cardiac myocytes from mice with heart failure due to the overexpression of tumor necrosis factor-alpha. Am J Physiol Heart Circ Physiol . 2006;290:H2098–H2107. doi: 10.1152/ajpheart.00097.2005. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Wang H, Zhang Y, Gao H, Nattel S, Wang Z. Impairment of HERG K(+) channel function by tumor necrosis factor-alpha: role of reactive oxygen species as a mediator. J Biol Chem . 2004;279:13289–13292. doi: 10.1074/jbc.C400025200. [DOI] [PubMed] [Google Scholar]
- 48.Lazzerini PE, Laghi-Pasini F, Bertolozzi I, et al. Systemic inflammation as a novel QT-prolonging risk factor in patients with torsades de pointes. Heart (British Cardiac Society) . 2017;103:1821–1829. doi: 10.1136/heartjnl-2016-311079. [DOI] [PubMed] [Google Scholar]
- 49.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol . 2015;12:230–243. doi: 10.1038/nrcardio.2015.2. [DOI] [PubMed] [Google Scholar]
- 50.Lazzerini PE, Laghi-Pasini F, Acampa M, et al. Systemic inflammation rapidly induces reversible atrial electrical remodeling: the role of interleukin-6-mediated changes in connexin expression. J Am Heart Assoc . 2019;8:e011006. doi: 10.1161/JAHA.118.011006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dernellis J, Panaretou M. Relationship between C-reactive protein concentrations during glucocorticoid therapy and recurrent atrial fibrillation. Eur Heart J . 2004;25:1100–1107. doi: 10.1016/j.ehj.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 52.Pasquie JL, Sanders P, Hocini M, et al. Fever as a precipitant of idiopathic ventricular fibrillation in patients with normal hearts. J Cardiovasc Electrophysiol . 2004;15:1271–1276. doi: 10.1046/j.1540-8167.2004.04388.x. [DOI] [PubMed] [Google Scholar]
- 53.Muller D, Agrawal R, Arntz HR. How sudden is sudden cardiac death? Circulation . 2006;114:1146–1150. doi: 10.1161/CIRCULATIONAHA.106.616318. [DOI] [PubMed] [Google Scholar]
- 54.Fabre A, Sheppard MN. Sudden adult death syndrome and other non-ischaemic causes of sudden cardiac death. Heart (British Cardiac Society) . 2006;92:316–320. doi: 10.1136/hrt.2004.045518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patruno N, Pontillo D, Achilli A, Ruggeri G, Critelli G. Electrocardiographic pattern of Brugada syndrome disclosed by a febrile illness: clinical and therapeutic implications. Europace . 2003;5:251–255. doi: 10.1016/s1099-5129(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 56.Le RQ, Li L, Yuan W, Shord SS, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist . 2018;23:943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buckley LF, Abbate A. Interleukin-1 blockade in cardiovascular diseases: a clinical update. Eur Heart J . 2018;39:2063–2069. doi: 10.1093/eurheartj/ehy128. [DOI] [PubMed] [Google Scholar]
- 58.Asnani A. Cardiotoxicity of immunotherapy: incidence, diagnosis, and management. Curr Oncol Rep . 2018;20:44. doi: 10.1007/s11912-018-0690-1. [DOI] [PubMed] [Google Scholar]
- 59.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics . 2016;3:16011. doi: 10.1038/mto.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]


