Abstract
The first α-boryl diazo compound that is capable of engaging in classic synthetic organic diazo reaction chemistry is described. The diazomethyl-1,2-azaborine 1, which is a BN isostere of phenyldiazomethane, is significantly more stable than phenyldiazomethane; its reaction chemistry ranges from C–H activation, O–H activation, [3+2] cycloaddition, and halogenation, to Ru-catalyzed carbonyl olefination. The demonstrated broad range of reactivity of diazomethyl-1,2-azaborine 1 makes it an exceptionally versatile synthetic building block for the 1,2-azaborine heterocyclic motif.
Main group element substituted diazo compounds have been well studied and utilized as versatile reagents in organic synthesis. Illustrative examples include trimethylsilyldiazomethane (Si)1 and Ohira–Bestmann reagent2 (P). In contrast, α-boryl diazo compounds are rare, with only about a handful of examples reported to date (Scheme 1, top).3 Lewis acidic boranes have been demonstrated to decompose diazo compounds;4 thus all the reported α-boryl diazo compounds either contain quaternized boron or involve trigonal planar boron atoms attached to two π-donating heteroatoms (O, N) to minimize boron’s Lewis acidity (see Scheme 1, top). Despite the broad applicability of diazo compounds as versatile reagents in synthetic organic applications,5 no instances of organic reaction chemistry have been described for α-boryl diazo compounds, presumably due to their perceived highly sensitive nature toward reaction conditions for organic synthesis. We envisioned that when the boron atom of an α-boryl diazo species is embedded in an aromatic scaffold such as in diazomethyl-1,2-azaborine 16 (Scheme 1, bottom), sufficient stability could still be achieved to enable diverse reaction chemistry of the diazo functional group. In this communication, we demonstrate that diazomethyl-1,2-azaborine 1 is an exceptional example of the α-boryl diazo family of compounds that is remarkably stable and capable of engaging in a wide range of diazo activation modes, in some cases with distinct reaction selectivities, thus rendering 1 a powerful synthetic building block for 1,2-azaborine chemistry.7 In view of emerging applications of BN heterocycles in materials chemistry,8 biomedical research,9 and organic synthesis,10 the development of new versatile 1,2-azaborine building blocks is a significant objective.
Scheme 1.

Novel α-Boryl Diazo Compound with Diverse Reaction Chemistry
Diazomethyl-1,2-azaborine 1 can be prepared in a two-step procedure from commercially available starting materials. Treatment of N-TBS-B-Cl-1,2-azaborine with AgOTf furnishes the B-OTf adduct,11 which is more electrophilic toward nucleophilic attack by TMSCHN2 to generate the target compound 1 with concomitant release of TMSOTf (Scheme 2, top). It is worth noting that the outcome of this process contrasts with the reaction observed between TMSCHN2 and other B–X-containing compounds, where insertion chemistry with elimination of N2 and retention of the TMS group is typically observed.12,13 Diazomethyl-1,2-azaborine 1 is the BN isosteric analogue of phenyldiazomethane; thus comparisons are warranted: (1) Stability: We determined that diazomethyl-1,2-azaborine 1 is a stable oil. When stored at room temperature as a neat compound, no decomposition of 1 has been observed for over a month. In stark contrast, phenyldiazomethane is an unstable and potentially explosive liquid that decomposes readily at room temperature.14 (2) IR: The presence of the diazo functional group in 1 is indicated by an intense band at ν = 2062 cm−1 for the asymmetric CN2 stretching vibration. In comparison, the CN2 vibration for phenyldiazomethane15 is at 2053 cm−1.16 The higher CN2 vibrational frequency observed for 1 in comparison to phenyldiazomethane is consistent with some additional stabilization of the negative charge at the CN2 carbon position.17
Scheme 2.
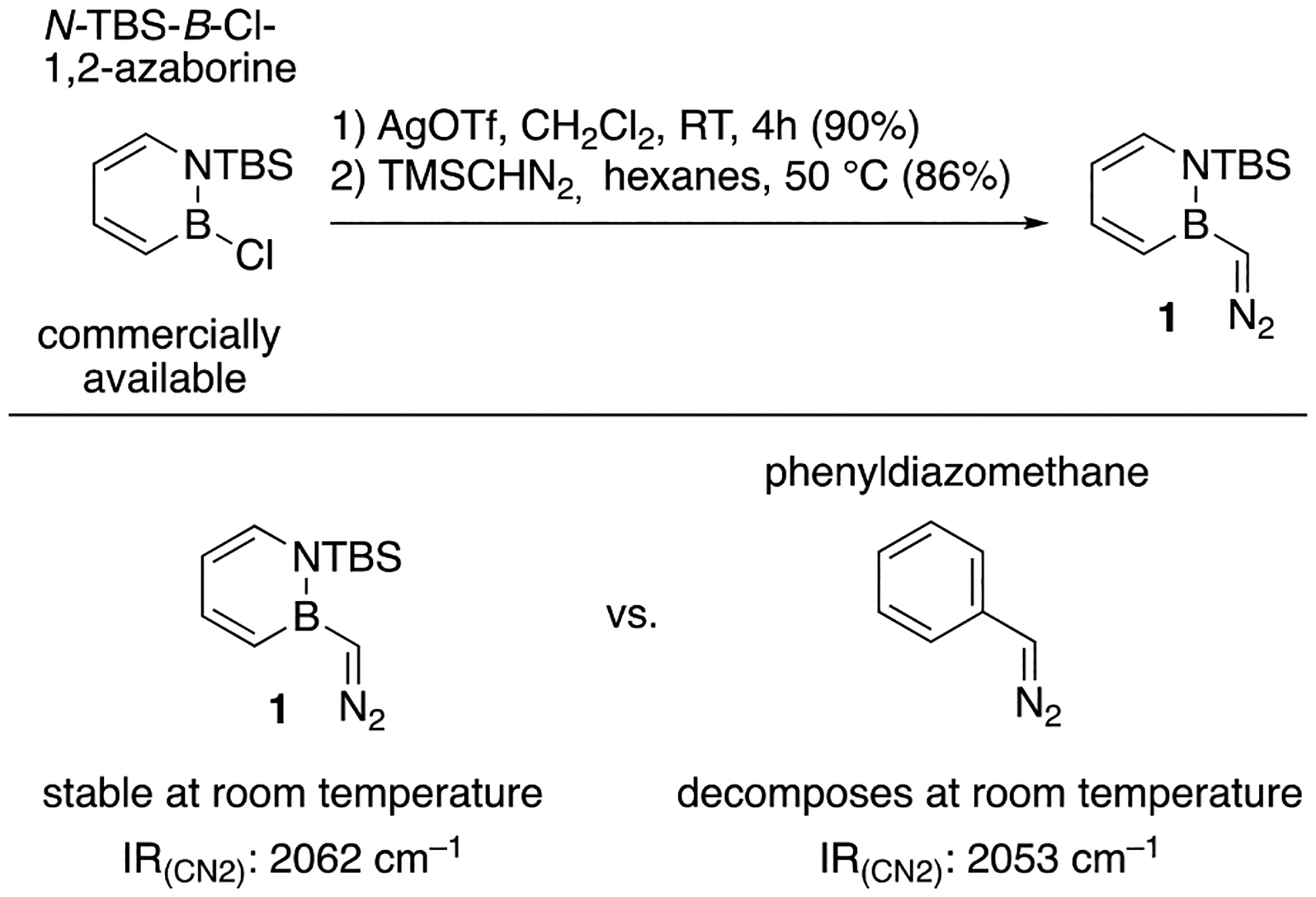
Synthesis and Comparative Characterization of Diazomethyl-1,2-azaborine 1a
aReported isolated yields are the average of two runs. TBS: tert-butyldimethylsilyl.
Undaunted by the lack of precedent of synthetic applications of α-boryl diazo compounds, we investigated the reaction chemistry of diazomethyl-1,2-azaborine 1 in the context of diversity-oriented synthesis18,19 using 1 as a universal building block. The first reaction we examined is the catalytic cross-metathesis of a diazo compound and a carbonyl to form an alkene.20 In the presence of Cl2Ru(PPh3)3 as the catalyst, diazo compound 1 undergoes metathesis with benzaldehyde to furnish BN stilbene derivative 2 with PPh3 as the stoichiometric reductant (Scheme 3, eq 1).21 We were able to isolate the corresponding Ru-α-borylalkylidine complex 3 (Scheme 3, eq 2) and demonstrate its chemical competency as a catalyst for the transformation (Scheme 3, eq 3). Gratifyingly, we are able to grow single crystals of complex 3 from a pentane/dichloromethane solution that are suitable for X-ray diffraction analysis. To the best of our knowledge, complex 3 is the first isolated Ru-α-borylalkylidine complex.22,23 Not many trans-bistriphenylphosphine dichlororuthenium alkylidene complexes have been crystallographically characterized, and we were unfortunately unable to obtain the structure of the corresponding carbonaceous Ru-benzylidine complex for direct comparison due to its facile decomposition in solution.24,25 Instead, we used the reported Cl2(PPh3)2Ru-vinylcarbene 4 as the reference compound.26 While most structural parameters are quite similar between 3 and 4, we note a few features for the Ru-α-borylalkylidine 3 that are distinct from the reference complex 4 (Scheme 3, table): (1) The two Ru–P bonds in 3 (2.484 Å, 2.326 Å; Δ = 0.158 Å) are significantly more nonsymmetrical than the ones observed in 4 (2.400 Å, 2.372 Å; Δ = 0.028 Å). (2) The ∠Ru–C1–B angle (124.1°) in 3 is smaller than the corresponding angle in 4 (131.2°). (3) The Ru–C1–B plane in 3 is somewhat perpendicular to the plane of the BN-heterocyclic ring (∠Ru–C1–B–N = 135.8(9)°). This is in contrast to compound 4, where the alkenyl group is more aligned to conjugate with the Ru alkylidine (∠Ru–C1–C–C = −158.6(4)°).
Scheme 3.

Ru-Catalyzed Carbonyl Olefination with Diazomethyl-1,2-azaborine 1 via Ru-α-borylalkylidine Intermediate 3
Next, we investigated the Cu-catalyzed C–H activation of terminal alkynes in the presence of the α-boryl diazo compound 1. Fu et al. reported a simple CuI-catalyzed procedure that couples terminal alkynes with diazoesters and diazoamides to furnish 3-alkynoates (Scheme 4, eq 4).27 In a number of cases, the corresponding allene isomer was observed as a minor byproduct. Fox and co-workers subsequently optimized the conditions for allene formation for α-alkyl-α-diazoesters with terminal alkynes and determined that the initially formed alkynoate intermediates isomerize to the allenoate products in the presence of the stoichiometrically employed K2CO3 base (Scheme 4, eq 5).28 Thus, it appears that the alkynoate species is the kinetically preferred product under Cu catalysis that subsequently can isomerize to the allenoates in the presence of a base. Surprisingly, when we adapted the protocol developed by Fu and treated diazomethyl-1,2-azaborine 1 with terminal alkynes in the presence of catalytic amounts of CuI in MeCN, and in the absence of any base, the corresponding allene 5 is formed exclusively instead of the expected internal alkyne (Scheme 4, eq 6).
Scheme 4.

Cu-Catalyzed C–H Activation of Terminal Alkynes with Diazomethyl-1,2-azaborinea
aYields are the average of two runs.
The formation of allene 5 is indicated by the two diastereotopic Si–Me signals of the N-tert-butyldimethylsilyl (TBS) group in the 1H NMR, which is consistent with the existence of an axially chiral stereogenic unit. The distinct product selectivity of CuI-catalyzed alkyne–diazo coupling for α-boryl diazo compound 1 again highlights its unique electronic structure that is likely underlying the observed selectivity.29
We also note that in the absence of a viable external C–H source and in the presence of the Rh2OAc4 catalyst an intramolecular C–H insertion occurs at the methyl or t-Bu group (on the N-TBS moiety), respectively, yielding the corresponding bicyclic 1,2-azaborine compounds 6a and 6b with a strong preference for the formation of the fivemembered 6a (eq 7).30

In addition to C–H activation chemistry, diazo compounds are known to engage in uncatalyzed O–H insertion chemistry.31 This reactivity feature has been utilized in chemical biology applications, where stabilized diazo compounds have been developed to label proteins and nucleic acids via O-alkylation.32 For an α-boryl diazo compound the high oxophilicity of boron may present an additional challenge to successfully O-alkylate suitable oxygen-based nucleophiles such as carboxylic acids. Gratifyingly, we determined that diazomethyl-1,2-azaborine 1 reacts quite rapidly at room temperature with carboxylic acids to form esters in acetonitrile in the absence of a catalyst. As can be seen from Scheme 5, aryl and alkyl carboxylic acids are suitable substrates. An electrophilic alkyl bromide functional group is also tolerated (Scheme 5, entry 7c).
Scheme 5.

O-Alkylation of Carboxylic Acids with Diazomethyl-1,2-azaborinea
aReported isolated yields are the average of two runs.
To explore additional reactivity types that diazo compounds can engage in, we investigated the [3+2] cycloaddition of diazomethyl-1,2-azaborine 1 with alkenes. A diazo compound is typically considered electron-rich and thus reacts as a nucleophile in normal-electron-demand cycloadditions with electron-deficient dipolarophiles.33 We are pleased to find that diazomethyl-1,2-azaborine 1 undergoes regioselective [3+2] cycloaddition reactions with α,β-unsaturated esters, ketones, and nitriles in the presence of a Pd catalyst to presumably initially form intermediates 8′, which then subsequently undergoes a formal 1,3-boryl shift to furnish adducts 8 (Scheme 6). In contrast, phenyldiazomethane preferentially forms cyclopropane compounds with elimination of N2 (see Supporting Information for experimental details). As an additional comparison, while phenyldiazomethane reacts relatively cleanly with styrene to furnish 1,2-diphenylcyclopropane in the presence of cobalt(II) tetraphenylporphyrin,34 diazomethyl-1,2-azaborine 1 remains mostly unreactive, producing minor amounts of the intramolecular C–H activation product 6a (see Supporting Information for experimental details).
Scheme 6.

[3+2] Cycloaddition Reaction with Diazomethyl-1,2-azaborinea
aReported isolated yields are the average of two runs.
Finally, we envisioned that diazomethyl-1,2-azaborine 1 can serve as a precursor to the halomethyl building blocks 9 and 10 (Scheme 7). Benzyl halides are versatile intermediates in diversity-oriented synthesis in medicinal chemistry,35 and compounds 9 and 10 represent the direct BN isosteres of benzylic halides. While examples of halomethyl BN naphthalenes have been reported,36 to the best of our knowledge, such a building block for the monocyclic 1,2-azaborine heterocycle has not been prepared. Importantly, the annulation method involving potassium chloromethyltrifluoroborate and 2-aminostyrene to yield the chloromethyl BN naphthalenes36 is not applicable to the monocyclic benzene-type BN heterocycle. We envisioned that similar to how O-alkylation proceeds with protonation of the α-boryl carbon followed by nucleophilic attack of the oxygen nucleophile,31 treatment of 1 with a suitably matched proton/halide source should yield the corresponding halomethyl 1,2-azaborines 9 and 10.37 After screening a number of conditions, we determined that 1-ethynyl-4-nitrobenzene as the proton source in combination with CuI as the iodide nucleophile works well for the iodination of diazomethyl-1,2-azaborine 1 (Scheme 7, eq 8). Similarly, the combination of bromoacetic acid as the proton source and cetrimonium (hexadecyltrimethylammonium) bromide is suitable for converting 1 to the bromomethyl 1,2-azaborine 10 (Scheme 7, eq 9).38
Scheme 7.

Synthesis of Halomethyl 1,2-Azaborine Building Blocks from 1a
aReported isolated yields are the average of two runs.
We chose bromomethyl 1,2-azaborine 10 to demonstrate the ability of halomethyl 1,2-azaborines to serve as an electrophilic building block in a variety of coupling and substitution reactions.36,39 As can be seen from Scheme 8, compound 10 can engage in Suzuki–Miyaura cross-coupling reaction to produce BN diarylmethanes 11. Compound 10 also reacts with a number of nucleophiles such as thiocyanate, azide, amine, and phthalimide anion to furnish the corresponding adducts 12–15 in moderate to good yields, further demonstrating the diversity of compounds that can be accessed with diazomethyl-1,2-azaborine 1 as the universal precursor.
Scheme 8.

Bromomethyl 1,2-Azaborine 10 as a Versatile Electrophilic Building Blocka
aReported isolated yields are the average of two runs.
In summary, we synthesized the first α-boryl diazo compound that is capable of engaging in classic synthetic organic diazo reaction chemistry, including C–H activation, O–H activation, [3+2] cycloaddition, halogenation, and Rucatalyzed carbonyl olefination. Furthermore, we showed that diazomethyl-1,2-azaborine 1, a BN isostere of phenyldiazomethane, is a stable compound and that its corresponding Ru carbene complex 3 exhibits bonding features that are distinct from an analogous Ru-alkylidine complex. Overall, we believe that diazomethyl-1,2-azaborine 1 as a new member of the α-boryldiazo compound family and as a remarkably versatile 1,2-azaborine building block will advance both the basic science of diazo chemistry and the multifaceted chemistry of 1,2-azaborine heterocycles.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM136920 and by Boston College start-up funds. R.P. and A.B.C. thank the Universitat Ramon Llull and “la Caixa” Foundation (2017-URL-Internac-010 grant), the MICINN (PID2020-113661GB-I00) grant, and the AGAUR SGR-00294 grant for support.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c06112.
Experimental procedures, compound characterization data, crystallographic information, and NMR spectra for all new compounds (PDF)
Accession Codes
CCDC 2082462 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Complete contact information is available at: https://pubs.acs.org/10.1021/jacs.1c06112
The authors declare no competing financial interest.
Contributor Information
Yao Liu, Department of Chemistry, Boston College, Chestnut Hill, Massachusetts 02467-3860, United States.
Raimon Puig de la Bellacasa, Department of Organic and Pharmaceutical Chemistry, Institut Químic de Sarrià, Universitat Ramon Llull, E-08017 Barcelona, Spain.
Bo Li, Department of Chemistry, Boston College, Chestnut Hill, Massachusetts 02467-3860, United States.
Ana Belén Cuenca, Department of Organic and Pharmaceutical Chemistry, Institut Químic de Sarrià, Universitat Ramon Llull, E-08017 Barcelona, Spain.
Shih-Yuan Liu, Department of Chemistry, Boston College, Chestnut Hill, Massachusetts 02467-3860, United States.
REFERENCES
- (1).(a) Seyferth D; Dow AW; Menzel H; Flood TC Trimethylsilyldiazomethane and trimethylsilylcarbene. J. Am. Chem. Soc 1968, 90, 1080–1082. [Google Scholar]; (b) Shioiri T; Aoyama T; Snowden T; Lee D; Gupta In Encyclopedia of Reagents for Organic Synthesis; Wiley, 2001; DOI: 10.1002/047084289X.rt298.pub3. [DOI] [Google Scholar]
- (2).Dhameja M; Pandey J Bestmann-Ohira Reagent: A Convenient and Promising Reagent in the Chemical World. Asian J. Org. Chem 2018, 7, 1502–1523. [Google Scholar]
- (3).(a) Schollkopf U; Banhidai B; Frasnelli H; Meyer R; Beckhaus H Metal-Substituted Carbenes and C-Metalated Diazoalkanes.6. Alpha-Diazo-Beta-Hydroxy-Carboxylates and Alpha-Diazo-Beta-Hydroxy-Ketones from Carbonyl and Diazo Lithio Compounds and Their Rearrangement into Beta-Ketocarboxylates and Beta-Diketones. Liebigs Ann. Chem 1974, 11, 1767–1783. [Google Scholar]; (b) Arthur MP; Baceiredo A; Bertrand G Synthesis of the 1st Distillable Alpha-Boranyldiazomethane - Direct Evidence of Lithioboranyldiazo-methane-Lithioboranylisodiazo-Methane [(BCNN−,Li+)-(BNNC−,Li +)] Rearrangement. J. Am. Chem. Soc 1991, 113, 5856–5857. [Google Scholar]; (c) Weber L; Wartig HB; Stammler H-G; Neumann B Synthesis and Structure of Stable α-Boranyldiazomethanes: 1,3-Di-tert-butyl-2,3-dihydro-1H-1,3,2-diazaborolyl-(trimethylsilyl)-diazomethane and 1,3-diethyl-2,3-dihydro-1H-1,3,2-benzodiazaborolyl-(trimethylsilyl)diazomethane. Organometallics 2001, 20, 5248–5250. [Google Scholar]; (d) Sotiropoulos JM; Baceiredo A; Vonlocquenghien KH; Dahan F; Bertrand G Reactivity of a (Diazomethylene)-Phosphorane with Alkylating-Agents and Lewis-Acids - Synthesis of the First Alpha-Diazoalkylborates. Angew. Chem., Int. Ed. Engl 1991, 30, 1154–1156. [Google Scholar]; (e) Ansorge A; Brauer DJ; Burger H; Hagen T; Pawelke G BNC Isosteres of Cyclopropane and a Borylated Diazoester from Diazo-Compounds and (Dimethylamino)Bis-(Trifluoromethyl)Borane. Angew. Chem., Int. Ed. Engl 1993, 32, 384–385. [Google Scholar]
- (4).Hooz J; Gunn DM Reaction of Organoboranes with Diazo Esters and Diazo Ketones in Presence of Deuterium Oxide. A New Synthesis of Alpha-Deuterio- and Alpha,Alpha-Dideuterio Esters and Ketones. J. Am. Chem. Soc 1969, 91, 6195–6198. [Google Scholar]
- (5).Maas G New syntheses of diazo compounds. Angew. Chem., Int. Ed 2009, 48 (44), 8186–95. [DOI] [PubMed] [Google Scholar]
- (6).Campbell PG; Abbey ER; Neiner D; Grant DJ; Dixon DA; Liu S-Y Resonance stabilization energy of 1,2-azaborines: a quantitative experimental study by reaction calorimetry. J. Am. Chem. Soc 2010, 132, 18048–50. [DOI] [PubMed] [Google Scholar]
- (7).For an overview of 1,2-azaborine chemistry, see:; (a) Liu Z; Marder TB B-N versus C-C: how similar are they? Angew. Chem., Int. Ed 2008, 47, 242–244. [DOI] [PubMed] [Google Scholar]; (b) Bosdet MJD; Piers WE BN as a CC substitute in aromatic systems. Can. J. Chem 2009, 87, 8–29. [Google Scholar]; (c) Campbell PG; Marwitz AJV; Liu S-Y Recent advances in azaborine chemistry. Angew. Chem., Int. Ed 2012, 51, 6074–6092. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Belanger-Chabot G; Braunschweig H; Roy DK Recent Developments in Azaborinine Chemistry. Eur. J. Inorg. Chem 2017, 38–39, 4353–4368. [Google Scholar]; (e) Giustra ZX; Liu SY The State of the Art in Azaborine Chemistry: New Synthetic Methods and Applications. J. Am. Chem. Soc 2018, 140, 1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).For an overview, see:; (a) Wang X-Y; Wang J-Y; Pei J BN Heterosuperbenzenes: Synthesis and Properties. Chem. - Eur. J 2015, 21 (9), 3528–3539. [DOI] [PubMed] [Google Scholar]; (b) Morgan MM; Piers WE Efficient synthetic methods for the installation of boron-nitrogen bonds in conjugated organic molecules. Dalton Trans 2016, 45, 5920–5924. [DOI] [PubMed] [Google Scholar]; (c) Huang J; Li Y BN Embedded Polycyclic pi-Conjugated Systems: Synthesis, Optoelectronic Properties, and Photovoltaic Applications. Front. Chem 2018, 6, 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).For select examples, see:; (a) Vlasceanu A; Jessing M; Kilburn JP BN/CC isosterism in borazaronaphthalenes towards phosphodiesterase 10A (PDE10A) inhibitors. Bioorg. Med. Chem 2015, 23, 4453–4461. [DOI] [PubMed] [Google Scholar]; (b) Rombouts FJ; Tovar F; Austin N; Tresadern G; Trabanco AA Benzazaborinines as Novel Bioisosteric Replacements of Naphthalene: Propranolol as an Example. J. Med. Chem 2015, 58, 9287–9295. [DOI] [PubMed] [Google Scholar]; (c) Lee H; Fischer M; Shoichet BK; Liu SY Hydrogen Bonding of 1,2-Azaborines in the Binding Cavity of T4 Lysozyme Mutants: Structures and Thermodynamics. J. Am. Chem. Soc 2016, 138, 12021–12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) Burford RJ; Li B; Vasiliu M; Dixon DA; Liu S-Y Diels-Alder Reactions of 1,2-Azaborines. Angew. Chem., Int. Ed 2015, 54 (27), 7823–7827. [DOI] [PubMed] [Google Scholar]; (b) Edel K; Yang X; Ishibashi JSA; Lamm AN; Maichle-Mossmer C; Giustra ZX; Liu S-Y; Bettinger HF The Dewar Isomer of 1,2-Dihydro-1,2-azaborinines: Isolation, Fragmentation, and Energy Storage. Angew. Chem., Int. Ed 2018, 57, 5296–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Giustra ZX; Yang X; Chen M; Bettinger HF; Liu S-Y Accessing 1,2-Substituted Cyclobutanes through 1,2-Azaborine Photoisomerization. Angew. Chem., Int. Ed 2019, 58, 18918–18922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).(a) Marwitz AJV; Jenkins JT; Zakharov LN; Liu S-Y 1,2-Azaborine cations. Angew. Chem., Int. Ed 2010, 49, 7444–7447. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Marwitz AJV; Jenkins JT; Zakharov LN; Liu S-Y Cationic 1,2-Azaborine Adducts of Trimethylphosphine, Triphenylphosphine oxide, and Pyridine-N-oxide. Organometallics 2011, 30, 52–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).(a) Li H; Zhang Y; Wang JB Reaction of Diazo Compounds with Organoboron Compounds. Synthesis 2013, 45, 3090–3098. [Google Scholar]; (b) Buynak JD; Geng B Synthesis and reactivity of Silylboranes. Organometallics 1995, 14, 3112–3115. [Google Scholar]; (c) Burgos CH; Canales E; Matos K; Soderquist JA Asymmetric Allyl- and Crotylboration with the Robust, Versatile, and Recyclable 10-TMS-9-borabicyclo[3.3.2]decanes. J. Am. Chem. Soc 2005, 127, 8044–8049. [DOI] [PubMed] [Google Scholar]; (d) Wommacka AJ; Kingsbury JS On the scope of the Ptcatalyzed Srebnikdiborylation of diazoalkanes. An efficient approach to chiral tertiary boronic esters and alcohols via B-stabilized carbanions. Tetrahedron Lett 2014, 55, 3163–3166. [Google Scholar]; (e) Cuenca AB; Cid J; García-López D; Carbó JJ; Fernández E Unsym-metrical 1,1-diborated multisubstituted sp3-carbons formed via a metal-free concerted-asynchronous mechanism. Org. Biomol. Chem 2015, 13, 9659–9664. [DOI] [PubMed] [Google Scholar]; (f) La Cascia E; Cuenca AB; Fernandez E Opportune gem-Silylborylation of Carbonyl Compounds: A Modular and Stereocontrolled Entry to Tetrasubstituted Olefins. Chem. - Eur. J 2016, 22, 18737–18741. [DOI] [PubMed] [Google Scholar]; (g) Civit MG; Royes J; Vogels CM; Westcott SA; Cuenca AB; Fernandez E Strategic Trimethylsilyldiazomethane Insertion into pinB-SR Followed by Selective Alkylations. Org. Lett 2016, 18, 3830–3833. [DOI] [PubMed] [Google Scholar]; (h) Trofimova A; LaFortune JHW; Qu Z-W; Westcott SA; Stephan DW 1,1-Phosphinoboration of diazomethanes. Chem. Commun 2019, 55, 12100–12103. [DOI] [PubMed] [Google Scholar]; (i) Bartholome TA; Bluer KR; Martin CD Successive Carbene Insertion into 9-Phenyl-9-Borafluorene. Dalton Trans 2019, 48, 6319–6322. [DOI] [PubMed] [Google Scholar]
- (13).No reaction was observed between N-TBS-B-Cl-1,2-azaborine and TMSCHN2.
- (14).(a) Creary X Tosylhydrazone Salt Pyrolyses: Phenyldiazomethanes. Org. Synth 1986, 64, 207. [Google Scholar]; (b) Sammakia T In Encyclopedia of Reagents for Organic Synthesis; Wiley, 2001; DOI: 10.1002/047084289X.rp060. [DOI] [Google Scholar]; (c) Green SP; Wheelhouse KM; Payne AD; Hallett JP; Miller PW; Bull JA Thermal Stability and Explosive Hazard Assessment of Diazo Compounds and Diazo Transfer Reagents. Org. Process Res. Dev 2020, 24, 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Yates P; Shapiro BL Aliphatic Diazo Compounds.3. Infrared Spectra. J. Am. Chem. Soc 1957, 79, 5756–5760. [Google Scholar]
- (16).The IR spectrum of phenyldiazomethane was independently measured for a direct comparison; see Supporting Information for details.
- (17).Three-coordinate boron has been demonstrated to stabilize anions at adjacent carbon centers; see:; Hong K; Liu X; Morken JP Simple access to elusive alpha-boryl carbanions and their alkylation: an umpolung construction for organic synthesis. J. Am. Chem. Soc 2014, 136, 10581–15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).For an overview, see:; McConnell CR; Liu SY Late-stage functionalization of BN-heterocycles. Liu. Chem. Soc. Rev 2019, 48, 3436–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).For examples, see:; (a) Baggett AW; Vasiliu M; Li B; Dixon DA; Liu S-Y Late-Stage Functionalization of 1,2-Dihydro-1,2-azaborines via Regioselective Iridium-Catalyzed C-H Borylation: The Development of a New N,N-Bidentate Ligand Scaffold. J. Am. Chem. Soc 2015, 137, 5536–5541. [DOI] [PubMed] [Google Scholar]; (b) Brown AN; Li B; Liu SY Negishi Cross-Coupling Is Compatible with a Reactive B-Cl Bond: Development of a Versatile Late-Stage Functionalization of 1,2-Azaborines and Its Application to the Synthesis of New BN Isosteres of Naphthalene and Indenyl. J. Am. Chem. Soc 2015, 137, 8932–8935. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) McConnell CR; Haeffner F; Baggett AW; Liu SY 1,2-Azaborine’s Distinct Electronic Structure Unlocks Two New Regioisomeric Building Blocks via Resolution Chemistry. J. Am. Chem. Soc 2019, 141, 9072–9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Fujimura O; Honma T Olefination of aldehydes by ethyl diazoacetate catalyzed by a ruthenium(II)complex. Tetrahedron Lett 1998, 39, 625–626. [Google Scholar]
- (21).Two plausible mechanistic pathways have been proposed for metal-catalyzed olefination of carbonyls with PPh3; see:; (a) Lu X; Fang H; Ni Z An organometallic analogue of the Wittig reaction. A one-pot reaction for C = C bond formation catalyzed by a molybdenum complex. J. Organomet. Chem 1989, 373, 77–84. [Google Scholar]; (b) Cheng GL; Mirafzal GA; Woo LK Iron porphyrincatalyzed olefination of carbonyl compounds with ethyl diazoacetate. Organometallics 2003, 22, 1468–1474. [Google Scholar]; (c) Lebel H; Paquet V Rhodium-catalyzed methylenation of aldehydes. J. Am. Chem. Soc 2004, 126, 320–328. [DOI] [PubMed] [Google Scholar]
- (22).For an example of a Ru-methylidyne complex, see:; Batsanov AS; Cabeza JA; Crestani MG; Fructos MR; Garcia-Alvarez P; Gille M; Lin Z; Marder TB Fully Borylated Methane and Ethane by Ruthenium-Mediated Cleavage and Coupling of CO. Angew. Chem., Int. Ed 2016, 55, 4707–4710. [DOI] [PubMed] [Google Scholar]
- (23).For select examples of metal-α-borylalkylidine complexes, see:; (a) Townsend EM; Kilyanek SM; Schrock RR; Muller P; Smith SJ; Hoveyda AH Synthesis of High Oxidation State Molybdenum Imido Heteroatom-Substituted Alkylidene Complexes. Organometallics 2013, 32, 4612–4617. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bailey BC; Fout AR; Fan H; Tomaszewski J; Huffman JC; Mindiola DJ An alkylidyne analogue of Tebbe’s reagent: trapping reactions of a titanium neopentylidyne by incomplete and complete 1,2-additions. Angew. Chem., Int. Ed 2007, 46, 8246–8249. [DOI] [PubMed] [Google Scholar]; (c) Braunschweig H; Celik MA; Dewhurst RD; Kachel S; Wennemann B Mild and Complete Carbonyl Ligand Scission on a Mononuclear Transition Metal Complex. Angew. Chem., Int. Ed 2016, 55, 5076–5080. [DOI] [PubMed] [Google Scholar]
- (24).Schwab P; France MB; Ziller JW; Grubbs RH A Series of Well-Defined Metathesis Catalysts- Synthesis of [RuCl2(=CHR’)-(PR3)2] and Its Reactions. Angew. Chem., Int. Ed. Engl 1995, 34, 2039–2041. [Google Scholar]
- (25).Both complex 3 and the corresponding Ru benzylidene complex are inactive catalysts for ring-closing metathesis (RCM) of diethyl diallylmalonate as the substrate; see Supporting Information for experimental details. For a discussion of the RCM reactivity of (Ph3P)2Cl2Ru=CH-CH=CPh2, see:; Nguyen ST; Grubbs RH; Ziller JW Syntheses and Activities of New Single-Component, Ruthenium-Based Olefin Metathesis Catalysts. J. Am. Chem. Soc 1993, 115, 9858–9859. [Google Scholar]
- (26).Volland MAO; Rominger F; Eisentrager F; Hofmann P An ‘Old Hydride’ in a new synthesis: a convenient approach to Grubbs-type carbene complexes (PPh3)2Cl2Ru = CH-CH = CR2 and their hexacoordinate acetonitrile adducts. J. Organomet. Chem 2002, 641, 220–226. [Google Scholar]
- (27).Suarez A; Fu GC A straightforward and mild synthesis of functionalized 3-alkynoates. Angew. Chem., Int. Ed 2004, 43, 3580–3582. [DOI] [PubMed] [Google Scholar]
- (28).Hassink M; Liu X; Fox JM Copper-catalyzed synthesis of 2,4-disubstituted allenoates from alpha-diazoesters. Org. Lett 2011, 13, 2388–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).The detailed reaction mechanism of the formation of allene 5 is presently unknown. We speculate that the anion-stabilizing effect of boron might increase the acidity of the propargylic C–H protons to potentially allow for a spontaneous isomerization to the allene structure.
- (30).Doyle MP; Westrum LJ; Wolthuis WNE; See MM; Boone WP; Bagheri V; Pearson MM Electronic and Steric Control in Carbon Hydrogen Insertion Reactions of Diazoacetoacetates Catalyzed by Dirhodium(II) Carboxylates and Carboxamides. J. Am. Chem. Soc 1993, 115, 958–964. [Google Scholar]
- (31).Kuhnel E; Laffan DD; Lloyd-Jones GC; Martinez Del Campo T; Shepperson IR; Slaughter JL Mechanism of methyl esterification of carboxylic acids by trimethylsilyldiazomethane. Angew. Chem., Int. Ed 2007, 46, 7075–7078. [DOI] [PubMed] [Google Scholar]
- (32).(a) Mix KA; Aronoff MR; Raines RT Diazo Compounds: Versatile Tools for Chemical Biology. ACS Chem. Biol 2016, 11, 3233–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mix KA; Raines RT Optimized diazo scaffold for protein esterification. Org. Lett 2015, 17, 2359–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).(a) Jangra H; Chen Q; Fuks E; Zenz I; Mayer P; Ofial AR; Zipse H; Mayr H Nucleophilicity and Electrophilicity Parameters for Predicting Absolute Rate Constants of Highly Asynchronous 1,3-Dipolar Cycloadditions of Aryldiazomethanes. J. Am. Chem. Soc 2018, 140, 16758–16772. [DOI] [PubMed] [Google Scholar]; (b) Gold B; Aronoff MR; Raines RT 1,3-Dipolar Cycloaddition with Diazo Groups: Noncovalent Interactions Overwhelm Strain. Org. Lett 2016, 18, 4466–4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Wang Y; Wen X; Cui X; Wojtas L; Zhang XP Asymmetric Radical Cyclopropanation of Alkenes with In Situ-Generated Donor-Substituted Diazo Reagents via Co(II)-Based Metalloradical Catalysis. J. Am. Chem. Soc 2017, 139, 1049–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).For leading references, see:; (a) Ref 30 .; (b) Davies KA; Abel RC; Wulff JE Operationally Simple Copper-Promoted Coupling of Terminal Alkynes with Benzyl Halides. J. Org. Chem 2009, 74, 3997–4000. [DOI] [PubMed] [Google Scholar]; (c) Chen X; Zhou L; Li Y; Xie T; Zhou S Synthesis of Heteroaryl Compounds through Cross-Coupling Reaction of Aryl Bromides or Benzyl Halides with Thienyl and Pyridyl Aluminum Reagents. J. Org. Chem 2014, 79, 230–239. [DOI] [PubMed] [Google Scholar]
- (36).Molander GA; Wisniewski SR; Amani J Accessing an Azaborine Building Block: Synthesis and Substitution Reactions of 2-Chloromethyl-2,1-borazaronaphthalene. Org. Lett 2014, 16, 5636– 5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).For an overview of halo-functionalization of diazo compounds, see:; Khanal HD; Thombal RS; Maezono SMB; Lee YR Designs and Strategies for the Halo-Functionalization of Diazo Compounds. Adv. Synth. Catal 2018, 360, 3185–3212. [Google Scholar]
- (38).Treatment of diazomethyl-1,2-azaborine 1 with anhydrous HCl (as an HX model) in CHCl3 did not produce the halogenated products in substantial amounts; see Supporting Information for experimental details.
- (39).Molander GA; Amani J; Wisniewski SR Accessing 2-(hetero)arylmethyl-,-allyl-, and -propargyl-2,1-borazaronaphthalenes: palladium-catalyzed cross-couplings of 2-(chloromethyl)-2,1-borazaronaphthalenes. Org. Lett 2014, 16, 6024–6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


