Introduction:
The Institute of Medicine published “Unequal Treatment: Confronting Racial and Ethnic Disparities in Healthcare” in 2002 1. This landmark document highlighted the disparities in healthcare between non-Hispanic Whites and other racial and ethnic groups in the United States which contributed to poorer health outcomes. In response to this report, many national oncology societies released their own reports to address this significant gap in care including the National Cancer Institute, the American Cancer Society, and the American Society of Clinical Oncology 2,3.
The Society of Gynecologic Oncology created a Healthcare Disparities Task Force in 2010 to assess the extent of healthcare disparities in gynecologic cancer care and to propose interventions to decrease these disparities going forward 4. The taskforce found that while there had been increasing attention to healthcare disparities research in the ten years since the Institute of Medicine published its report, the majority of research focused on large retrospective clinical series comparing Black and White women with little attention paid to other races/ethnicities or to systemic and societal factors that contribute to unequal care. The report concluded that lack of access to equal care is a major driver of healthcare disparities and that the minimum standard should be equivalent adherence to evidence-based guidelines. They additionally encouraged that future efforts be targeted at broadening the understanding of what contributes to healthcare disparities and developing a comprehensive approach to both identify and mitigate the complex multifactorial reasons for unequal care.
Prior to delving into the literature on disparities within the field of gynecologic oncology, it is important to define some key terms 5.
-
○Disparity:
-
■An uneven rate of a given health outcome or risk between populations. One’s ability to achieve and maintain good health may be influenced by factors that include: race or ethnicity, sexual or gender identity, socioeconomic status, age, and disability, among others.
-
■
-
○Difference:
-
■An element that separates or distinguishes contrasting people, things, or situations. Individual people differ on characteristics such as race and gender; it is when these factors lead to unequal outcomes, risk levels, or access to care that disparities occur.
-
■
-
○Inequity:
-
■An avoidable, systematic difference in the distribution of resources between groups. Examples of inequities that lead to disparity include: health insurance, education, fresh and healthy food, and clean air.
-
■
-
○Social determinants of health:
-
■Social determinants of health (SDOH) are conditions in the environment in which people are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning, and quality-of-life outcomes and risks. Broadly, most SDOH fall under one of five categories: Economic Stability, Education, Social and Community Context, Health and Health Care, Neighborhood and Built Environment.
-
■
By considering the above definitions it becomes possible to begin to develop a framework by which to consider disparities in gynecologic cancers.
Endometrial Cancer
Endometrial cancer is the most common gynecologic malignancy in the United States 6. Incidence rates of endometrial cancer are rising among all racial/ethnic subgroups (Figure 1), with Black and Asian women experiencing the most rapid increase 7. The increasing incidence in Black women is particularly concerning given that Black women are 98% more likely to die of endometrial cancer than their non-Hispanic White counterparts, a mortality disparity that ranks in the top three of all malignancies 8.
Figure 1.
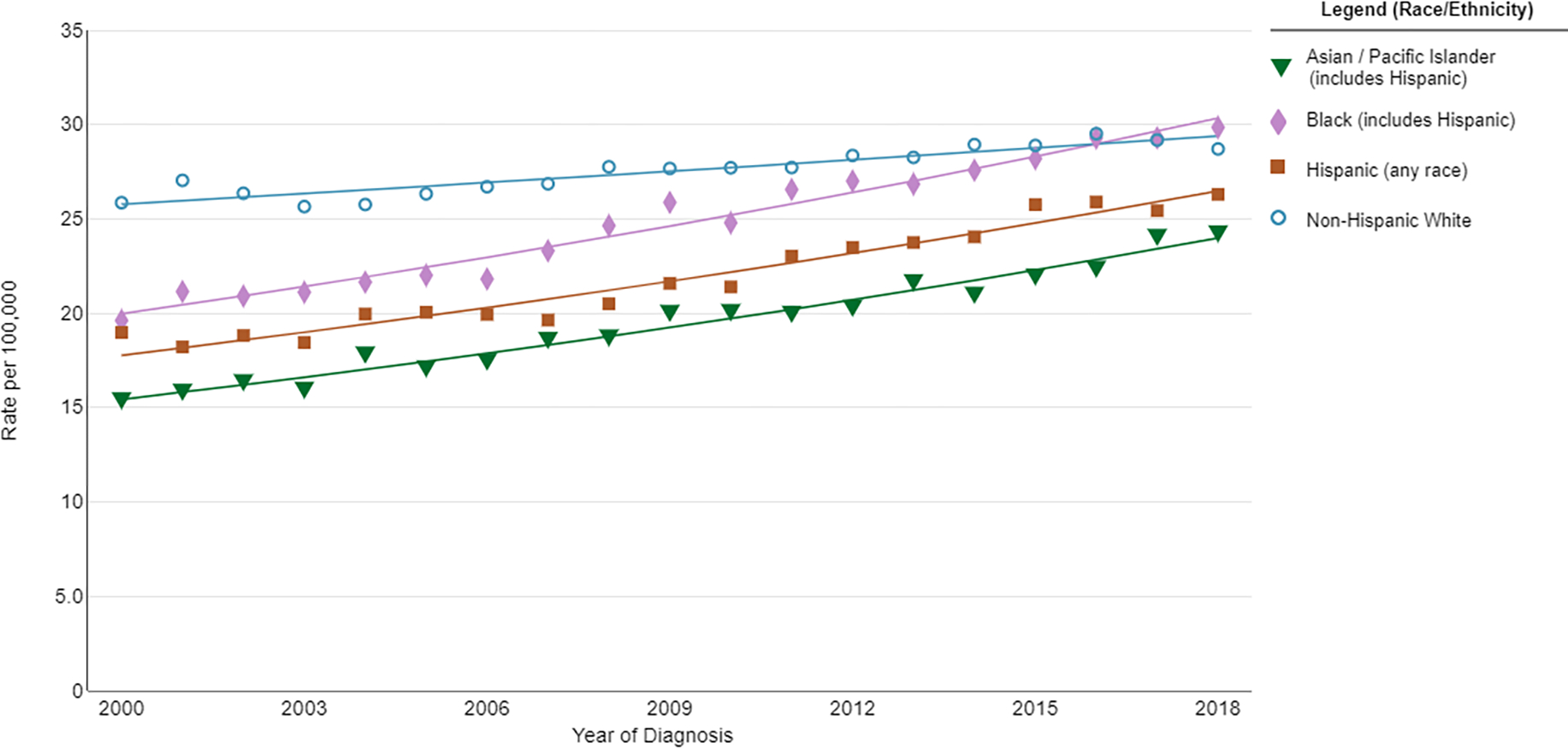
Trends in age-adjusted incidence rates of uterine cancer between 2000–2018. The Annual Percentage Change (APC) for Asian, Black and Hispanic women during this time period was 2.5, 2.3 and 2.2, respectively. The APC for white non-hispanic women was 0.7. Source: https://seer.cancer.gov/explorer
Multiple reports have highlighted the many differences in endometrial cancer characteristics among different racial/ethnic groups at the time of diagnosis. While Black women make up about 7% of all endometrial cancer diagnoses, they comprise 17% of all Type II tumors 9. Type II endometrial tumors consist mostly of serous and clear-cell carcinomas, which are characterized by a more aggressive course and poorer prognosis 10. Black women are 3.4 times more likely than white women to be diagnosed with a serous histology and 1.5 times more likely to be diagnosed with a clear cell histology 11,12. Even within endometrioid histologies, Black women with early stage tumors were noted to have higher grades, higher rates of lymphovascular space invasion, and higher rates of recurrence 13. Black women are also approximately two times more likely to be diagnosed with more advanced disease 11,14 (Figure 2).
Figure 2.
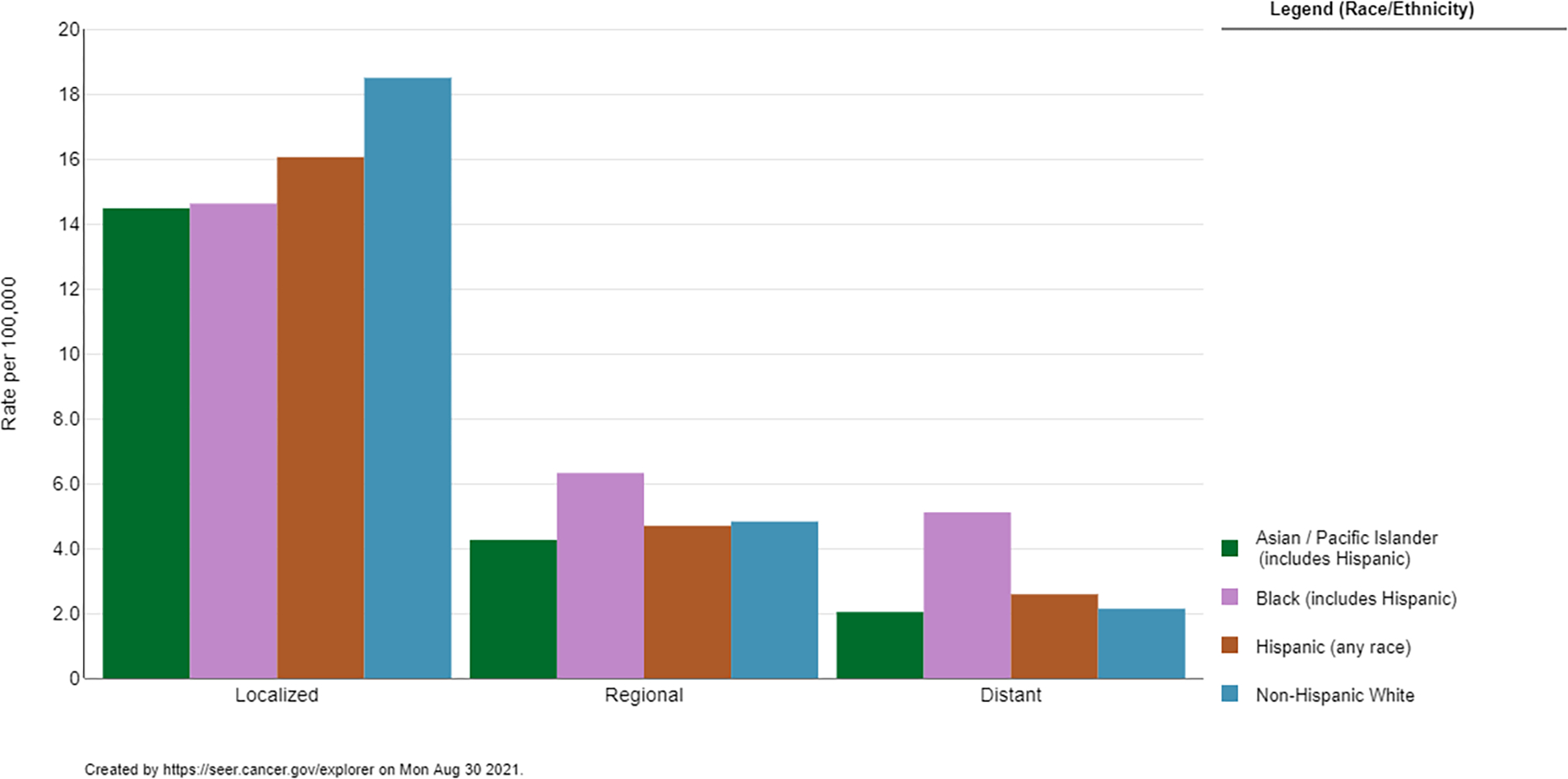
Five-year Age-Adjusted Incidence Rates of Uterine Cancer by Disease Stage and Race/Ethnicity. Source: https://seer.cancer.gov/explorer
Endometrial cancer often presents at an early stage with onset of postmenopausal bleeding. This symptom triggers a guideline-driven workup which ideally leads to rapid diagnosis and definitive treatment with surgery. Doll et al investigated the role of bleeding recognition and subsequent evaluation in Black and White women 15. By evaluating the association of race with diagnostic workup, they demonstrated that Black women were less likely to receive guideline-concordant care with a significant difference in appropriate diagnostic evaluation after presenting with bleeding (Figure 3). Delay in diagnosis could contribute to a more advanced stage at presentation.
Figure 3.
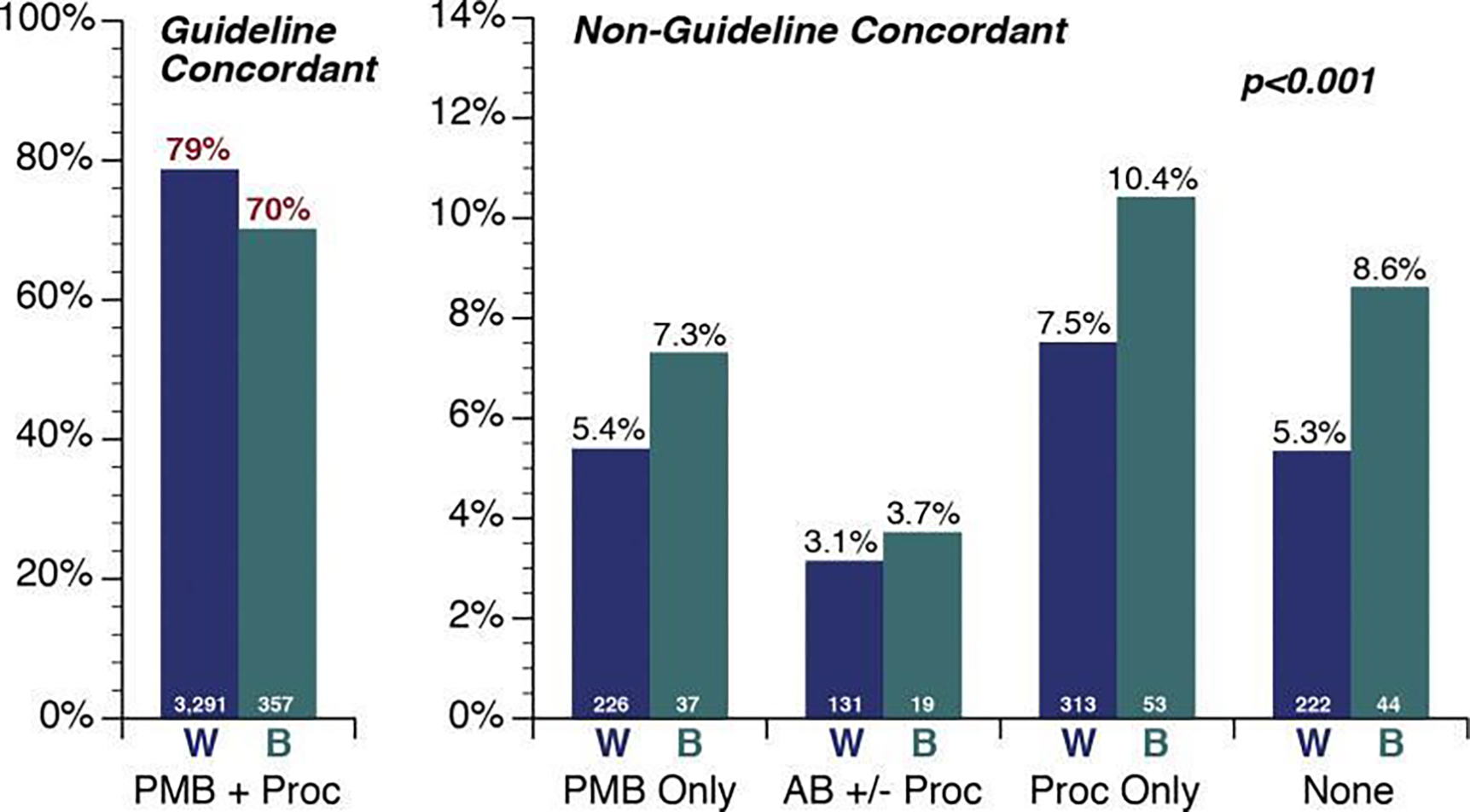
Frequency of recognition and evaluation of post-menopausal bleeding (PMB), by race, prior to diagnosis of endometrial cancer. Women are grouped into 1 guideline-concordant and 4 nonguideline-concordant pathways. AB ± proc, alternative bleeding characterization (eg, menorrhagia) with or without diagnostic procedures; B, black; None, neither PMB documented nor diagnostic procedures performed; PMB + proc, PMB documented and at least 1 diagnostic procedure (endometrial biopsy, transvaginal ultrasound, and/or uterine dilation and curettage) was performed; PMB only, PMB documented with no diagnostic procedures; Proc only, diagnostic procedures with no bleeding documented; W, white.
From Doll KM, Khor S, Odem-Davis K, et al. Role of bleeding recognition and evaluation in Black-White disparities in endometrial cancer. Am J Obstet Gynecol. 2018;219(6):593.e1-e593.e14; with permission. (Figure 2 in original)
Surgery is the cornerstone of endometrial cancer treatment as the majority of women with early stage disease will be cured with surgery alone 16. However, Black women receive incomplete or no surgical therapy at much higher rates than their white counterparts. Reported rates of no surgical management for Black women range from 2.4–11% 12,14,17. Additionally, Black women are more likely to experience a longer time to surgical evaluation, with only 65% of women meeting the standard of surgical staging within six weeks of diagnosis 18.
Two landmark papers are frequently cited to support the hypothesis that there are inherent differences in the molecular characteristics of endometrial cancers in Black women that affect response to chemotherapy. A retrospective pooled analysis of four GOG trials using an adriamycin/cisplatin doublet found an absolute survival difference of 11.2% between Black and White women (adjusted HR 1.26), despite similar completion rates of therapy 19. A similar pooled analysis of GOG trials noted that Black participants experienced a 35% increased rate of death and were 8.2% less likely to have either a complete or partial response to therapy 20. While the authors initially concluded that biological differences in tumor characteristics must have contributed to these survival differences, a subgroup analysis including 44% of the original study population who received full dose chemotherapy was performed to assess survival after completion of chemotherapy. This analysis found that the characteristic most associated with survival was relative dose of chemotherapy received, a finding not modified by race. Every 10% increase in relative dose was associated with a 9% reduction in risk of death, for both Black and White women 19.
With the advent of The Cancer Genome Atlas (TCGA) classification system for endometrial cancer, there has been increasing interest in exploring if differences exist in molecular classifications between the races. Specific to TCGA molecular classification system, two manuscripts demonstrated increased rates of aggressive subtypes within Black patients, but also found that progression free survival remained similar among all patients within a designated classification 21,22. Additional work will need to be completed to explore the role of epigenetic remodeling, especially as it relates to how environmental or social factors may mediate these molecular findings.
While significant attention has been paid to the contribution of comorbidities such as obesity and diabetes, few studies have found that these characteristics significantly contribute to outcome differences in endometrial cancer 23–25. Correction of inequalities in receiving standard of care treatment and socioeconomic factors such as disparities in insurance access and ability to obtain care at high volume centers, are more likely to reduce treatment and outcome disparities 17,26–28.
Ovarian Cancer
Ovarian cancer is the most lethal gynecologic malignancy in the United States, with an estimated 21,750 new cases diagnosed and 13,940 deaths in 2020 29. Rates of ovarian cancer are higher in White women with an incidence per 100,000 of 13.5 in White women, 10.0 in Black women, and 11.6 in Hispanic women 30. Nevertheless, in Black women all-cause mortality from ovarian cancer is 1.3 times higher than White women and it is increasing 4,31. In an analysis of the SEER database from 1992 to 2008, the five-year survival for White women increased from 40.7% to 45.0%, while that for Black women decreased from 47.9% to 40.3% 32.
The majority of ovarian cancer cases are diagnosed at an advanced stage, which confers a poor prognosis, even with aggressive multimodal therapy 16. Bristow et al defined the three tenets of high quality ovarian cancer care as the quality of the initial surgical effort, receipt of recommended chemotherapy, and the volume of the treating center/surgeon 33. When specifically looking at adherence to the surgery/chemotherapy sequence, multiple studies have demonstrated lower rates of guideline-concordant care in Black women 34–36. Chen et al found that only 60.8% of Black women received appropriate care in contrast to 70.4% of White women and that non-adherence to guidelines contributed 36.4% to the excess risk of death for Black patients 34.
When investigating responses to chemotherapy in a GOG trial setting, race was not associated with either incomplete treatment or survival 37. However outside of a clinical trial controlled environment, Black women were less likely to receive chemotherapy as part of their initial treatment 35,36.
With the advent of molecular testing and the incorporation of targeted therapy with PARP inhibitors in ovarian cancer, it has become increasingly important to identify women who harbor genetic alterations, both to reduce risk to their families and to guide treatment decisions. Black women are less likely than their White counterparts to undergo BRCA testing 38. A positive BRCA mutation allows women the opportunity to undergo risk reducing procedures to prevent the development of breast or ovarian cancer. However, Black women are less likely than their White and Hispanic counterparts to undergo these procedures 39. Lastly, as targeted therapy with PARP inhibitors becomes a mainstay of the treatment paradigm in patients with ovarian cancer, careful attention must be paid to inequities in receipt of these therapies and resulting effects on survival.
Although the receipt of high quality medical and surgical care are the cornerstone of ovarian cancer treatment, the patient-physician relationship is critically important given the grave prognosis of this condition. In a subset of patients in the African American Cancer Epidemiology Study, 486 Black women were stratified into two groups by the duration of their symptoms prior to seeking evaluation 40. After surveying the participants about discrimination in their daily lives as well as trust in the medical system, the authors found that perceived everyday discrimination was associated with prolonged symptom duration and thus, late presentation to care.
In conclusion, for women with ovarian cancer, disparities in cancer care have been identified along the continuum including prevention, surgery, chemotherapy and end of life. As new and more effective treatments, such as PARP inhibitors, become part of how we treat ovarian cancer, disparities may become more pronounced and efforts to combat this are crucial.
Cervical Cancer
Along with uterine cancer, cervical cancer is in the top five of malignancies experiencing significant mortality disparities between Black and White patients 8. Screening with Pap smears allow for the rapid identification of women at risk of progression to cervical cancer. While prior work has demonstrated that women of lower socio-economic status are less likely to undergo health maintenance screening, Black women have high rates of undergoing pap testing 41. Compliance in testing was similar between Black and White women through age 29. From ages 30–69, Black women had increased screening relative to White women, though after age 70, White women had dramatically higher screening rates than Black women. Hispanic women, particularly those who did not speak English, had particularly poor rates of cervical cancer screening. Swan et al. found that 84% of American women reported having a pap smear within the last three years. The women most at risk for missing appropriate preventative screening were women without a usual source of healthcare, without health insurance, who had immigrated to the United States within the past ten years, and who were of post-menopausal age 42. The other breakthrough in cervical cancer prevention was the development of the HPV vaccine. In research assessing HPV knowledge, researchers found that Black women had lower rates of HPV awareness, knowledge that HPV causes cervical cancer, and that an HPV vaccine has been approved and can prevent cervical cancer 43.
While high rates of adherence to cervical cancer screening in Black women should reduce the rate of invasive cervical cancers, Black women continue to experience a higher incidence of disease relative to their White counterparts 44. Some of this disparity is likely driven by Black women being older at diagnosis (Figure 4), particularly due to decreased rates of cervical cancer screening after menopause 45. When correcting for lower socioeconomic status, lack of treatment, rural location or low SES neighborhood, and increasing age, the impact of race on survival nearly vanished 46,47.
Figure 4.
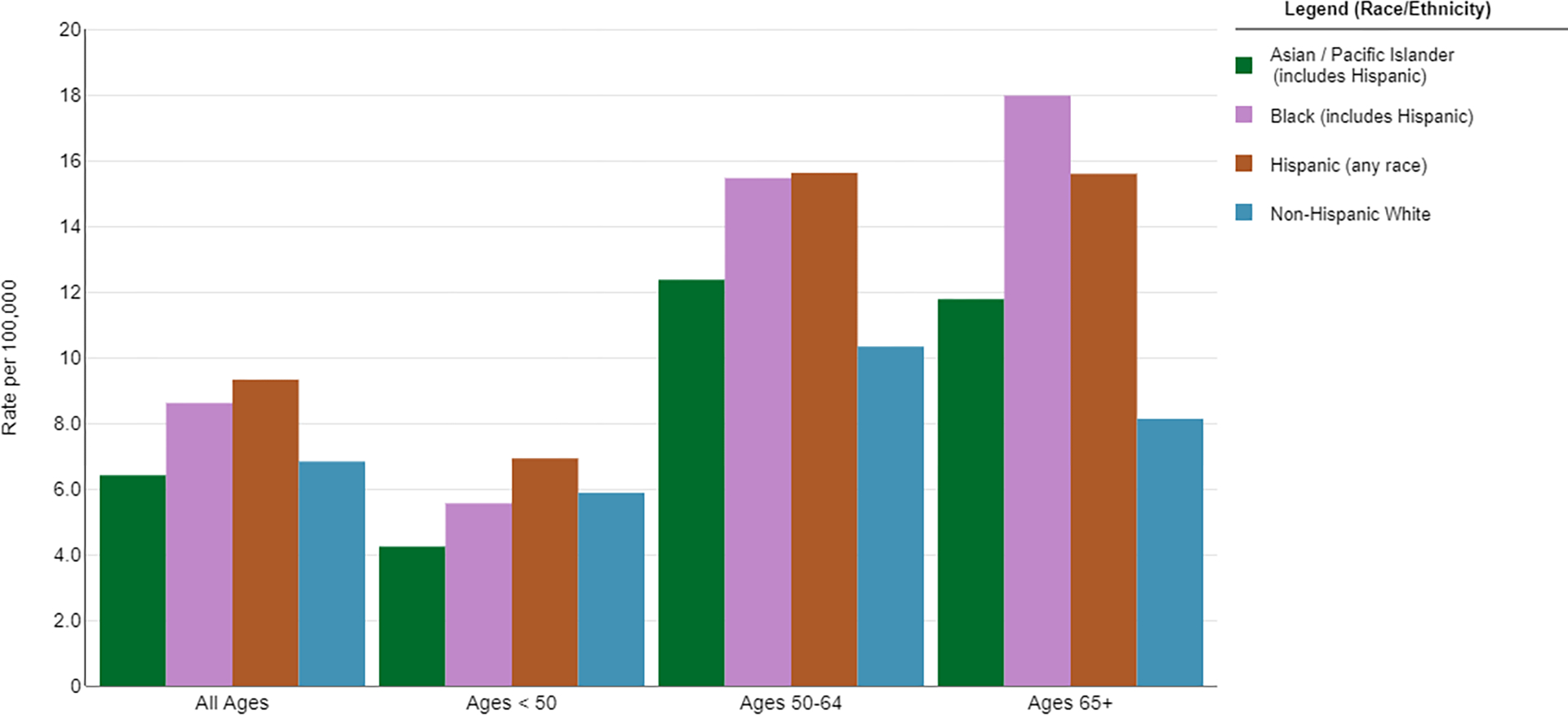
Five-year Incidence Rates of Cervical Cancer by Age and Race/Ethnicity. Source: https://seer.cancer.gov/explorer
Treatment for cervical cancer is dependent on the stage at diagnosis. Radical surgery is the treatment of choice for early stage disease, though Black women are more likely to be diagnosed at more advanced stages 48–50 (Figure 5). Even among patients with early stage disease, Black women were less likely to receive surgery at all due to it not being recommended, contraindicated due to comorbid conditions, or declined by the patient 48,51. Prior research has illustrated the importance of undergoing radical hysterectomy with a high volume surgeon due to the decreased rate of complications, though Black women are less likely than white women to have a high volume surgeon 52.
Figure 5.
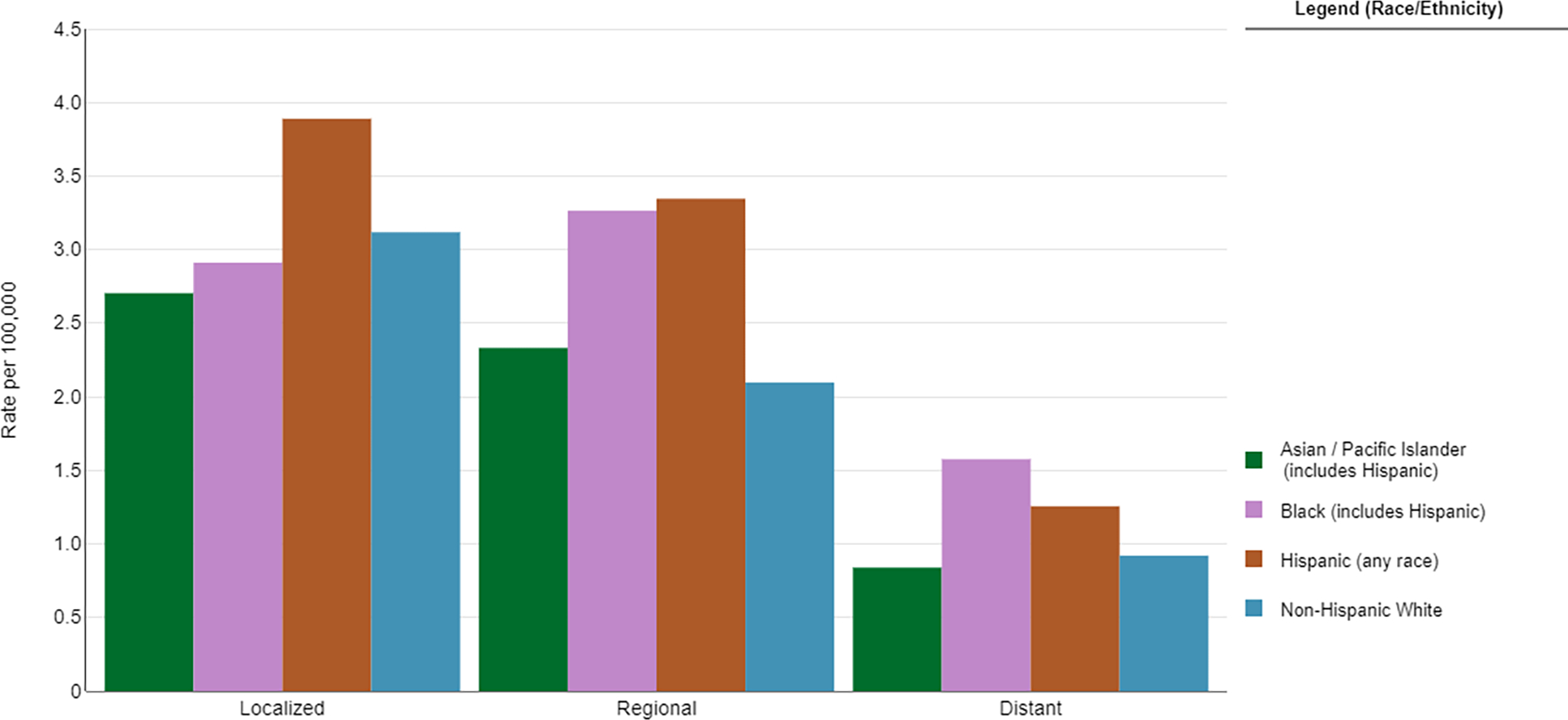
Five-year Age-Adjusted Incidence Rates of Cervical Cancer by Disease Stage and Race/Ethnicity. Source: https://seer.cancer.gov/explorer
Radiation is the cornerstone of therapy in advanced disease, typically provided as a combination of external beam therapy (EBRT) and brachytherapy. While there have not been significant disparities reported in the receipt of EBRT, there have been several reports of differences in receipt of brachytherapy in Black women 49,53,54. A 15-year retrospective cohort out of the University of Chicago found that Black women were less likely to receive brachytherapy and this disparity was more pronounced with more advanced stages of disease 49. Two large NCDB retrospective cohorts found that Black women had a 0.9 adjusted odds ratio of receiving brachytherapy 53,54. Insurance status, non-squamous histology, care at a non-academic center, and care in the southern United States were all associated with decreased rates of brachytherapy treatment.
Other factors in treatment delivery have been implicated in care outcomes between the races, likely due to difficulty in minority patients obtaining treatment at high quality centers. Multiple studies have demonstrated a relationship between hospital volume and survival among all of the key components of cervical cancer treatment. Uppal et al found that guideline adherent care had the largest impact on survival with an adjusted hazard ratio for death of 0.65 55. When investigating survival impact by quartiles of volume, the top and bottom quartiles for chemotherapy administration demonstrated a nearly 30% difference in survival, while the top and bottom quartiles of brachytherapy demonstrated a 81% difference in survival 56. Similarly, rates of guideline concordant therapy were strongly associated with insurance status; and insurance status was strongly associated with race, income, and education 57.
Clinical Trial Participation and Enrollment
The field of gynecologic oncology has experienced tremendous progress over the past several decades, partly due to its ability to enroll patients in clinical trials. The history of minority participation in clinical trials highlights the urgent need to improve participation and to design trials focused on the needs of minority populations. A 2015 analysis of clinical trial participation found that while Black and Hispanic Americans made up approximately 30% of the United States population, they only accounted for 6% of clinical trial participants 58. Additionally, only 2% of all federally funded trials specifically looked at the needs of minority populations 59.
A retrospective analysis of 445 GOG publications aimed to assess racial/ethnic disparities within trial participation found that only 38% of publications had racial/ethnic breakdowns available and only 17% of the study populations were non-White 60. No GOG trial published a racial breakdown of participants prior to 1994. More concerningly, Black participant enrollment decreased from 16% in 1994–2002 to only 5.8% in 2009–2013 (Figure 6). Relative to CDC population incidence, the authors found that Black participants were less likely than White women to participate in clinical trials across all disease sites (Table 1). In a similar analysis of gynecology oncology specific NCI trials, the authors found that ovarian cancer trials were the least diverse, but that minorities were largely underrepresented in all disease sites 61.
Figure 6.
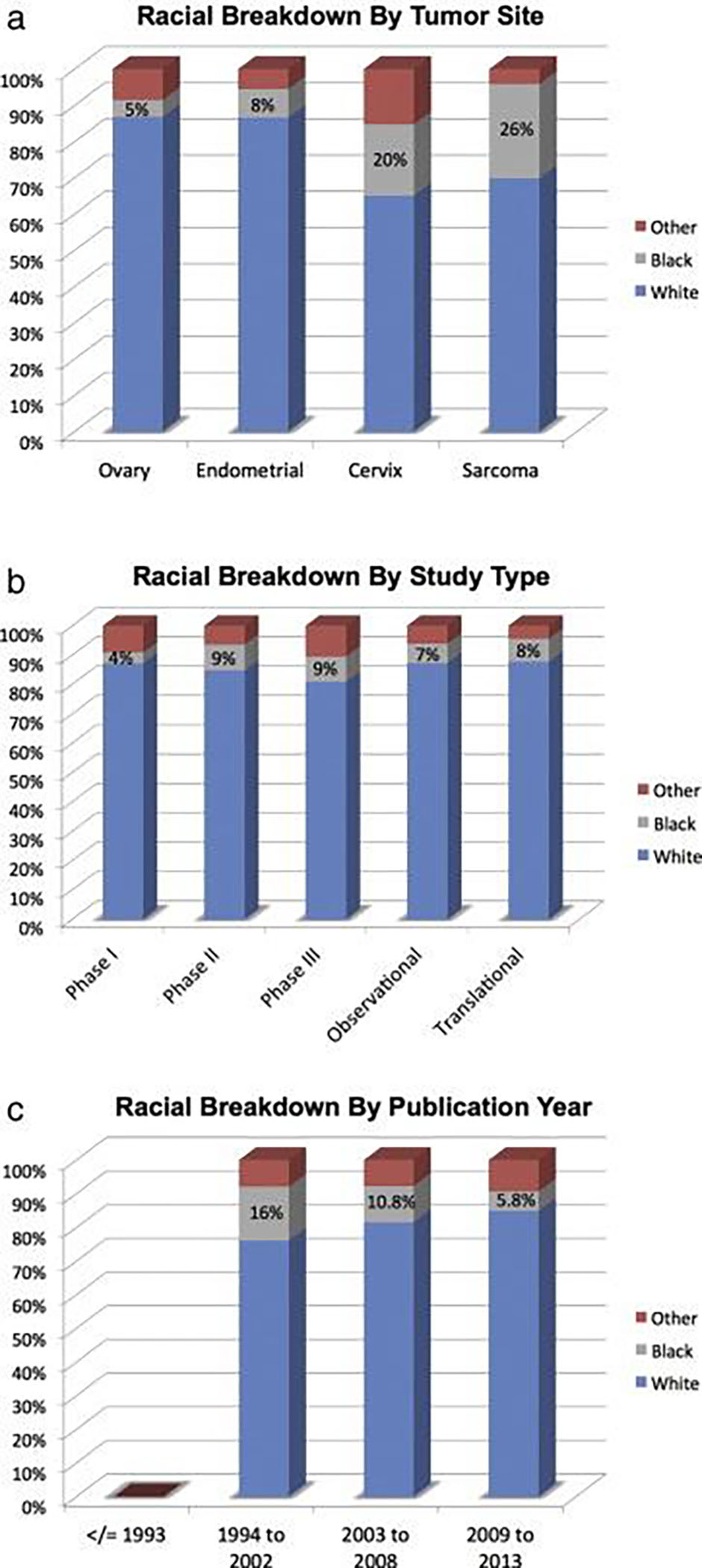
Minority participation in Gynecologic Oncology Group (GOG) Studies from 1985–2013. (a) Racial breakdown by tumor site; (b) racial breakdown by study type; (c) racial breakdown by publication year.
From Scalici J, Finan MA, Black J, et al. Minority participation in Gynecologic Oncology Group (GOG) Studies. Gynecol Oncol. 2015;138(2):441–444.; with permission. (Figure 1 in original).
Table 1.
Expected vs. Observed Enrollment of Black and White women in GOG Studies from 1985–2013.
| White Incidence | Black Incidence | Expected W:B ratio | |
| Expected Incidence per 100,000 | |||
| Ovary | 12.9 | 9.5 | 1 to 0.74 |
| Endometrial | 24.8 | 22.4 | 1 to 0.90 |
| Cervical | 7.7 | 10.3 | 1 to 1.34 |
| Sarcoma | 3.6 | 7 | 1 to 1.94 |
| Observed White | Observed Black | Observed W:B ratio | |
| Observed Incidence | |||
| Ovary | 23,269 | 1,258 | 1 to 0.05 |
| Endometrial | 9,289 | 850 | 1 to 0.09 |
| Cervical | 4,421 | 1,340 | 1 to 0.30 |
| Sarcoma | 638 | 238 | 1 to 0.37 |
| Expected W:B ratio | Observed W:B ratio | Fold Difference in Ratios | |
| Ovary | 1 to 0.74 | 1 to 0.05 | 14.8-fold |
| Endometrial | 1 to 0.90 | 1 to 0.09 | 9.8-fold |
| Cervical | 1 to 1.34 | 1 to 0.30 | 4.5-fold |
| Sarcoma | 1 to 1.94 | 1 to 0.37 | 5.2-fold |
From Scalici J, Finan MA, Black J, et al. Minority participation in Gynecologic Oncology Group (GOG) Studies. Gynecol Oncol. 2015;138(2):441–444.; with permission. (Table 3 in original).
Therefore, it is important to assess barriers to clinical trial participation given the significant correlation between social/environmental factors and race. In addition to the significant differences in minority participation in federally funded trials, the elderly, the publicly insured or uninsured, the medically ill, transportation poor, and non-English speaking patients are all less likely to participate in trials 61. These findings can help direct future interventions to make research within the field of gynecologic oncology more diverse. Trial design could be more inclusive of a range of ages and pre-existing conditions to better reflect the population. Academic medical centers could improve their outreach efforts to target those patients who may have poorer access to high volume centers, a particularly important intervention given that almost 15% of American women live greater than 15 miles from a gynecologic oncologist 61. Perhaps more critical than these specific interventions, is changing the culture on how trial participation is discussed with patients, to normalize the process of participating as a component of evidence-based, rather than purely experimental care.
Addressing Health Disparities in Gynecologic Cancer Care
A significant amount of the literature on health disparities in gynecologic cancer care has focused on descriptively identifying disparities with a particular focus on the dichotomous care received by Black and White patients. While there are actionable improvements that can be made to improve research within this field as it is currently conceived, it is critical to broaden the academic approach to these difficult issues in order to develop frameworks in which both the medical factors and social and environmental factors are treated as an integrated system.
The vast majority of prior research within this topic has relied on large retrospective databases with incompletely classified race and ethnicity information. Additionally, there is no mechanism to adequately capture systems factors such as details about the location of care, volume of center, and provider type, volume, and experience. It also does not capture more nuanced patient factors including individual socioeconomic status, insurance details, language, educational attainment, and types and severity of medical comorbidities.
Aside from optimizing the quality of retrospective cohort studies, there is a substantial gap in prospective work aimed at addressing the lived experience of minority women with gynecologic malignancies. It is only by directly addressing how minority women navigate their health and healthcare experience that researchers can begin to develop appropriate interventions to improve outcomes. By developing initiatives targeted at the aspects of gynecologic cancer that are most amenable to intervention, our field can see the greatest improvement in outcomes.
Doll et al offers a provocative framework by which to begin to allocate research efforts 62. She highlights the concept of amenability, which is a means by which to describe how amenable a specific disease state is to an intervention. Prior research has demonstrated that Black-White differences in mortality were largest in malignancies that were the most amenable to intervention or treatment 63. Therefore, more studies focused on interventions that not only describe, but also improve these well documented disparities are needed.
Keypoints:
Disparities in the treatment, incidence and outcomes of women with gynecologic malignancies are multifactorial.
Identifying systemic and societal factors contributing to unequal care should be part of the academic approach to address disparities in gynecologic oncology.
Studies focused on interventions that improve the well-documented disparities are needed.
Funding Support:
NCI P20 CA233304 (DRR, ELB), the Bristol Myers Squibb Foundation Diversity in Clinical Trials Career Development Program (DRR).
References
- 1.Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. (Smedley BD, Stith AY, Nelson AR, eds.). National Academies Press; (US: ); 2014. [PubMed] [Google Scholar]
- 2.Cancer Disparities. Published August 4, 2016. Accessed July 25, 2021. https://www.cancer.gov/about-cancer/understanding/disparities
- 3.Goss E, Lopez AM, Brown CL, Wollins DS, Brawley OW, Raghavan D. American society of clinical oncology policy statement: disparities in cancer care. J Clin Oncol. 2009;27(17):2881–2885. [DOI] [PubMed] [Google Scholar]
- 4.Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: a report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecol Oncol. 2014;133(2):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Search Healthy People - Healthy People 2030. Accessed August 9, 2021. https://health.gov/healthypeople/search?query=foundation+health+measures
- 6.American Cancer Society. Accessed July 25, 2021. https://cancerstatisticscenter.cancer.org/?_ga=2.38100131.1072989990.1627242312-1724352611.1627242312#!/cancer-site/Uterine%20corpus
- 7.Cote ML, Ruterbusch JJ, Olson SH, Lu K, Ali-Fehmi R. The Growing Burden of Endometrial Cancer: A Major Racial Disparity Affecting Black Women. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1407–1415. [DOI] [PubMed] [Google Scholar]
- 8.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211–233. [DOI] [PubMed] [Google Scholar]
- 9.Long B, Liu FW, Bristow RE. Disparities in uterine cancer epidemiology, treatment, and survival among African Americans in the United States. Gynecol Oncol. 2013;130(3):652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu F-S. Molecular carcinogenesis of endometrial cancer. Taiwan J Obstet Gynecol. 2007;46(1):26–32. [DOI] [PubMed] [Google Scholar]
- 11.Wright JD, Fiorelli J, Schiff PB, et al. Racial disparities for uterine corpus tumors: changes in clinical characteristics and treatment over time. Cancer. 2009;115(6):1276–1285. [DOI] [PubMed] [Google Scholar]
- 12.Hicks ML, Phillips JL, Parham G, et al. The National Cancer Data Base report on endometrial carcinoma in African-American women. Cancer. 1998;83(12):2629–2637. [DOI] [PubMed] [Google Scholar]
- 13.Elshaikh MA, Munkarah AR, Robbins JR, et al. The impact of race on outcomes of patients with early stage uterine endometrioid carcinoma. Gynecol Oncol. 2013;128(2):171–174. [DOI] [PubMed] [Google Scholar]
- 14.Fader AN, Habermann EB, Hanson KT, Lin JF, Grendys EC, Dowdy SC. Disparities in treatment and survival for women with endometrial cancer: A contemporary national cancer database registry analysis. Gynecol Oncol. 2016;143(1):98–104. [DOI] [PubMed] [Google Scholar]
- 15.Doll KM, Khor S, Odem-Davis K, et al. Role of bleeding recognition and evaluation in Black-White disparities in endometrial cancer. Am J Obstet Gynecol. 2018;219(6):593.e1–e593.e14. [DOI] [PubMed] [Google Scholar]
- 16.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2017, National Cancer Institute. Bethesda, MD. Published online 2020. [Google Scholar]
- 17.Madison T, Schottenfeld D, James SA, Schwartz AG, Gruber SB. Endometrial cancer: socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival. Am J Public Health. 2004;94(12):2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang AB, Huang Y, Hur C, et al. Impact of quality of care on racial disparities in survival for endometrial cancer. Am J Obstet Gynecol. 2020;223(3):396.e1–e396.e13. [DOI] [PubMed] [Google Scholar]
- 19.Farley JH, Tian C, Rose GS, et al. Chemotherapy intensity and toxicity among black and white women with advanced and recurrent endometrial cancer: a Gynecologic Oncology Group Study. Cancer. 2010;116(2):355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maxwell GL, Tian C, Risinger J, et al. Racial disparity in survival among patients with advanced/recurrent endometrial adenocarcinoma: a Gynecologic Oncology Group study. Cancer. 2006;107(9):2197–2205. [DOI] [PubMed] [Google Scholar]
- 21.Guttery DS, Blighe K, Polymeros K, Symonds RP, Macip S, Moss EL. Racial differences in endometrial cancer molecular portraits in The Cancer Genome Atlas. Oncotarget. 2018;9(24):17093–17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubil EA, Tian C, Wang G, et al. Racial disparities in molecular subtypes of endometrial cancer. Gynecol Oncol. 2018;149(1):106–116. [DOI] [PubMed] [Google Scholar]
- 23.Olson SH, Atoria CL, Cote ML, et al. The impact of race and comorbidity on survival in endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2012;21(5):753–760. [DOI] [PubMed] [Google Scholar]
- 24.Felix AS, Cook LS, Gaudet MM, et al. The etiology of uterine sarcomas: a pooled analysis of the epidemiology of endometrial cancer consortium. Br J Cancer. 2013;108(3):727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruterbusch JJ, Ali-Fehmi R, Olson SH, et al. The influence of comorbid conditions on racial disparities in endometrial cancer survival. Am J Obstet Gynecol. 2014;211(6):627.e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliver KE, Enewold LR, Zhu K, et al. Racial disparities in histopathologic characteristics of uterine cancer are present in older, not younger blacks in an equal-access environment. Gynecol Oncol. 2011;123(1):76–81. [DOI] [PubMed] [Google Scholar]
- 27.Fedewa SA, Lerro C, Chase D, Ward EM. Insurance status and racial differences in uterine cancer survival: a study of patients in the National Cancer Database. Gynecol Oncol. 2011;122(1):63–68. [DOI] [PubMed] [Google Scholar]
- 28.Al-Wahab Z, Ali-Fehmi R, Cote ML, et al. The impact of race on survival in uterine serous carcinoma: a hospital-based study. Gynecol Oncol. 2011;121(3):577–580. [DOI] [PubMed] [Google Scholar]
- 29.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. [DOI] [PubMed] [Google Scholar]
- 30.Lowe KA, Chia VM, Taylor A, et al. An international assessment of ovarian cancer incidence and mortality. Gynecol Oncol. 2013;130(1):107–114. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava SK, Ahmad A, Miree O, et al. Racial health disparities in ovarian cancer: not just black and white. J Ovarian Res. 2017;10(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terplan M, Schluterman N, McNamara EJ, Tracy JK, Temkin SM. Have racial disparities in ovarian cancer increased over time? An analysis of SEER data. Gynecol Oncol. 2012;125(1):19–24. [DOI] [PubMed] [Google Scholar]
- 33.Bristow RE, Zahurak ML, Ibeanu OA. Racial disparities in ovarian cancer surgical care: a population-based analysis. Gynecol Oncol. 2011;121(2):364–368. [DOI] [PubMed] [Google Scholar]
- 34.Chen F, Bailey CE, Alvarez RD, Shu X-O, Zheng W. Adherence to treatment guidelines as a major determinant of survival disparities between black and white patients with ovarian cancer. Gynecol Oncol. 2021;160(1):10–15. [DOI] [PubMed] [Google Scholar]
- 35.Howell EA, Egorova N, Hayes MP, Wisnivesky J, Franco R, Bickell N. Racial disparities in the treatment of advanced epithelial ovarian cancer. Obstet Gynecol. 2013;122(5):1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hildebrand JS, Wallace K, Graybill WS, Kelemen LE. Racial disparities in treatment and survival from ovarian cancer. Cancer Epidemiol. 2019;58:77–82. [DOI] [PubMed] [Google Scholar]
- 37.Farley JH, Tian C, Rose GS, Brown CL, Birrer M, Maxwell GL. Race does not impact outcome for advanced ovarian cancer patients treated with cisplatin/paclitaxel: an analysis of Gynecologic Oncology Group trials. Cancer. 2009;115(18):4210–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarthy AM, Bristol M, Domchek SM, et al. Health Care Segregation, Physician Recommendation, and Racial Disparities in BRCA1/2 Testing Among Women With Breast Cancer. J Clin Oncol. 2016;34(22):2610–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cragun D, Weidner A, Lewis C, et al. Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer. 2017;123(13):2497–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mullins MA, Peres LC, Alberg AJ, et al. Perceived discrimination, trust in physicians, and prolonged symptom duration before ovarian cancer diagnosis in the African American Cancer Epidemiology Study. Cancer. 2019;125(24):4442–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harlan LC, Bernstein AB, Kessler LG. Cervical cancer screening: who is not screened and why? Am J Public Health. 1991;81(7):885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97(6):1528–1540. [DOI] [PubMed] [Google Scholar]
- 43.Ojeaga A, Alema-Mensah E, Rivers D, Azonobi I, Rivers B. Racial Disparities in HPV-related Knowledge, Attitudes, and Beliefs Among African American and White Women in the USA. J Cancer Educ. 2019;34(1):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo W, Kim S, Huh WK, et al. Recent trends in racial and regional disparities in cervical cancer incidence and mortality in United States. PLoS One. 2017;12(2):e0172548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rauh-Hain JA, Melamed A, Schaps D, et al. Racial and ethnic disparities over time in the treatment and mortality of women with gynecological malignancies. Gynecol Oncol. 2018;149(1):4–11. [DOI] [PubMed] [Google Scholar]
- 46.Coker AL, Desimone CP, Eggleston KS, White AL, Williams M. Ethnic disparities in cervical cancer survival among Texas women. J Womens Health. 2009;18(10):1577–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Movva S, Noone A-M, Banerjee M, et al. Racial differences in cervical cancer survival in the Detroit metropolitan area. Cancer. 2008;112(6):1264–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleming S, Schluterman NH, Tracy JK, Temkin SM. Black and white women in Maryland receive different treatment for cervical cancer. PLoS One. 2014;9(8):e104344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mundt AJ, Connell PP, Campbell T, Hwang JH, Rotmensch J, Waggoner S. Race and clinical outcome in patients with carcinoma of the uterine cervix treated with radiation therapy. Gynecol Oncol. 1998;71(2):151–158. [DOI] [PubMed] [Google Scholar]
- 50.Sheppard CS, El-Zein M, Ramanakumar AV, Ferenczy A, Franco EL. Assessment of mediators of racial disparities in cervical cancer survival in the United States. Int J Cancer. 2016;138(11):2622–2630. [DOI] [PubMed] [Google Scholar]
- 51.Merrill RM, Merrill AV, Mayer LS. Factors associated with no surgery or radiation therapy for invasive cervical cancer in Black and White women. Ethn Dis. 2000;10(2):248–256. [PubMed] [Google Scholar]
- 52.Wright JD, Lewin SN, Deutsch I, Burke WM, Sun X, Herzog TJ. The influence of surgical volume on morbidity and mortality of radical hysterectomy for cervical cancer. Am J Obstet Gynecol. 2011;205(3):225.e1–e7. [DOI] [PubMed] [Google Scholar]
- 53.Alimena S, Yang DD, Melamed A, et al. Racial disparities in brachytherapy administration and survival in women with locally advanced cervical cancer. Gynecol Oncol. 2019;154(3):595–601. [DOI] [PubMed] [Google Scholar]
- 54.Bruce SF, Joshi TV, Chervoneva I, et al. Disparities Among Cervical Cancer Patients Receiving Brachytherapy. Obstet Gynecol. 2019;134(3):559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uppal S, Chapman C, Spencer RJ, et al. Association of Hospital Volume With Racial and Ethnic Disparities in Locally Advanced Cervical Cancer Treatment. Obstet Gynecol. 2017;129(2):295–304. [DOI] [PubMed] [Google Scholar]
- 56.Lin JF, Berger JL, Krivak TC, et al. Impact of facility volume on therapy and survival for locally advanced cervical cancer. Gynecol Oncol. 2014;132(2):416–422. [DOI] [PubMed] [Google Scholar]
- 57.Churilla T, Egleston B, Dong Y, et al. Disparities in the management and outcome of cervical cancer in the United States according to health insurance status. Gynecol Oncol. 2016;141(3):516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oh SS, Galanter J, Thakur N, et al. Diversity in Clinical and Biomedical Research: A Promise Yet to Be Fulfilled. PLoS Med. 2015;12(12):e1001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen MS Jr, Lara PN, Dang JHT, Paterniti DA, Kelly K. Twenty years post-NIH Revitalization Act: enhancing minority participation in clinical trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual: renewing the case for enhancing minority participation in cancer clinical trials. Cancer. 2014;120 Suppl 7:1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scalici J, Finan MA, Black J, et al. Minority participation in Gynecologic Oncology Group (GOG) Studies. Gynecol Oncol. 2015;138(2):441–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mishkin G, Minasian LM, Kohn EC, Noone A-M, Temkin SM. The generalizability of NCI-sponsored clinical trials accrual among women with gynecologic malignancies. Gynecol Oncol. 2016;143(3):611–616. [DOI] [PubMed] [Google Scholar]
- 62.Doll KM. Investigating Black-White disparities in gynecologic oncology: Theories, conceptual models, and applications. Gynecol Oncol. 2018;149(1):78–83. [DOI] [PubMed] [Google Scholar]
- 63.Tehranifar P, Neugut AI, Phelan JC, et al. Medical advances and racial/ethnic disparities in cancer survival. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2701–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]


