Abstract
Background:
Precision medicine and prediction of therapeutic response requires monitoring potential biomarkers before and after treatment. Liquid biopsies provide noninvasive prognostic markers such as circulating tumor DNA and RNA. Circulating tumor RNA (ctRNA) in blood is also used to identify mutations in genes of interest, but additionally, provides information about relative expression levels of important genes. In this study, we analyzed PD-L1 expression in ctRNA isolated from various cancer types. Tumors inhibit antitumor response by modulating the immune checkpoint proteins programmed death ligand 1 (PD-L1) and its cognate receptor PD1. The expression of these genes has been implicated in evasion of immune response and resistance to targeted therapies.
Methods:
Blood samples were collected from gastric (GC), colorectal (CRC), lung (NSCLC), breast (BC), prostate cancer (PC) patients, and a healthy control group. ctRNA was purified from fractionated plasma, and following reverse transcription, levels of PD-L1 expression were analyzed using qPCR.
Results:
PD-L1 expression was detected in the plasma ctRNA of all cancer types at varying frequencies but no PD-L1 mRNA was detected in cancer-free individuals. The frequencies of PD-L1 expression were significantly different among the various cancer types but the median relative PD-L1 expression values were not significantly different. In 12 cases where plasma and tumor tissue were available from the same patients, there was a high degree of concordance between expression of PD-L1 protein in tumor tissues and PD-L1 gene expression in plasma, and both methods were equally predictive of response to nivolumab.
Conclusions:
PD-L1 mRNA can be detected and quantitated in ctRNA of cancer patients. These results pave the way for further studies aimed at determining whether monitoring the levels of PD-L1 mRNA in blood can identify patients who are most likely to benefit from the conventional treatment.
Keywords: PD-L1, Circulating tumor DNA, Liquid biopsy, Cancer
1. Background
Past efforts in improving cancer treatment have largely consisted of screening, development of new anti-cancer agents, multi-drug combinations and advances in the radiation therapy. A more recent approach is precision medicine, which takes individual variability into account in order to design personalized treatment strategies [1]. An important goal of precision medicine is to identify molecular markers indicative of therapy selection by analyzing the factors involved in the therapeutic effects and prognosis [2,3]. So far, such information has been obtained by analysis of genes and proteins in cancer tissue biopsies. However, the use of tissue biopsies has many problems, including possible sampling bias and a limited ability to monitor tumor markers in patients during the course of the therapy. In 1977, Leon et al. discovered that serum ctDNA levels were higher in patients with cancer [4], suggesting that the extra serum DNA in cancer patients originates from the tumor. Subsequent work confirmed this and established that the ctDNA reveals the same information about the patient’s genes as that found in the tumor without an invasive tissue biopsy [5‒7]. The genetic information from liquid biopsies resides in circulating cancer cells (CTC), circulating tumor DNA (ctDNA), circulating tumor RNA (ctRNA) and exosomes [8]. Cell-free or extracellular RNAs have been detected in many human bodily fluids, including serum/ plasma, saliva, cerebrospinal fluid, synovial fluid, tear fluid, amniotic fluid and urine [9]. For those genes that are expressed, ctRNA contains the same mutational information as does ctDNA, but in addition, can also provide information about the quantitative expression levels of genes of interest.
It is now well established that tumors can inhibit autoimmune antitumor activity by modulating the expression of inhibitory factors and immune checkpoint proteins like programmed death ligand 1 (PD-L1) and its cognate receptor, programmed cell death 1 (PD-1) [10‒14]. As an immune suppression mechanism, the expression of PD-L1 is elevated in many types of cancer and is often correlated with poor patient prognosis and predictive of responses to the antibodies against PD-1/PD-L1 [15‒17]. Therapies that block this interaction have demonstrated very promising clinical activity in several cancer types [11,12,17,18]. Excluding currently used antibodies for such therapies, inhibition of PD-L1 expression could also have a therapeutic effect. As PD-L1 expression is regulated by mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)/AKT signaling pathways, inhibition of these pathways should reduce PD-L1. Indeed, receptor tyrosine kinase inhibitors result in better treatment outcome in lung cancers with high-expression of PD-L1 [17,19,20]. The PD-1 inhibitor, pembrolizumab is now under evaluation in 13 trials across more than 30 types of cancer including bladder, colorectal, gastric, head and neck, melanoma, non-small and small cell lung (NSCLC), renal, pancreatic, prostate, triple negative and estrogen-receptor positive human epidermal growth factor receptor 2 (HER2)-negative breast, gynecologic, and hematologic malignancies [21].
There has to date been no report about using ctRNA to detect PD-L1 expression and there are only a few studies across the various cancers about ctDNA [22]. The purpose of this study was to analyze the frequency and level of PD-L1 expression in ctRNA isolated from various cancer types.
2. Methods
2.1. Patient samples and samples transportation
A total of 760 patient samples were blinded and accessioned by Liquid Genomics, Inc.(later acquired by NantHealth, Inc.) The cancer types comprising the samples included; 44 gastric cancers (GC), 212 colorectal cancers (CRC), 320 non-small cell lung cancers (NSCLC), 24 breast cancers (BC), and 88 prostate cancers (PC). Only cancer types (not histologic subtypes) were known of the samples. All cancer patients had distant metastases and had scheduled chemotherapy. Patient samples were from University of Southern California Norris Comprehensive Cancer Center, Memorial Cancer Institute or University Hospital Essen, West German Cancer Center. This study was approved by the Institutional Review Board of each facility. Ten milliliters of blood were collected in each of two tubes containing a proprietary nucleic acid preservation cocktail and transferred to Liquid Genomics, Inc. as soon as possible. After blind accessioning, all samples proceeded to the isolation process within five days after collection. A two-dimensional bar code was placed on all plasma samples for automatic identification.
2.2. Fractionation of plasma and extraction of ctRNA
Whole blood in 10 mL tubes was centrifuged to fractionate plasma at 16,000 rcf for 20 min ctRNA was extracted from 2 mL of plasma with a proprietary in-house developed protocol especially designed to remove potential contaminating blood cells during the extraction. All nucleic acids were kept in bar-coded matrix storage tubes. RNA was stored at −80 °C or reverse-transfected to complementary DNA (cDNA) and cDNA was stored at −4 °C.
2.3. The detection of PD-L1 with quantitative real-time PCR
Quantitative real-time PCR was used to detect the expression of PD-L1 in ctRNA. We designed the primers to investigate the expression of PD-L1. Amplification was performed in 10 μL reactions containing 2 μL cDNA, the primers, the probe, and the reaction mix with qPCR and an in-house developed assay. β-actin was used as an internal control. PD-L1 delta-Cts (dCts) were calculated from the PD-L1 Ct value by using b-actin as the positive control dCts were the Ct value of PD-L1 subtracted by the Ct value of β-actin. Next we calculated K using universal human reference (UHR) as a positive control. With using this K, we can reach to the Relative PDL1 Gene Expression.
Relative PD-L1 Gene Expression= (2^-dCT)*K
Relative PD-L1 Gene Expressions were compared across various cancers.
2.4. Digital droplet PCR
Digital PCR was performed on QX200 digital PCR system (BioRad, Hercules, CA). The 96-well PCR plate was prepared with 2×ddPCR Supermix for probes (no dUTP) (Bio-Rad), PD-L1 primer and β-actin VIC primer, and partitioned into a median of ~14,000 droplets per well in QX200 droplet generator according to the manufacturer’s instructions. After PCR amplfication, the plate was read on QX200 droplet reader with QuantaSoft v1.7.4.0917 software (Bio-Rad). At least two negative control wells with no DNA were included in every run. The amount of PD-L1 was calculated from the concentration provided by the software.
2.5. Statistics analysis
Statistical analysis was carried out using JMP version 12.2 (SAS Institute Inc., Cary NC). The frequency of expression in cancer types were calculated and compared with each other using analysis of variance (ANOVA). The relative expression levels were compared across cancer type using the Median test. The Chi-Square test compared the normal and control groups. We considered p < 0.05 as statistically significant.
3. Result and discussion
An important goal of this study was to demonstrate the feasibility of quantitating PD-L1 gene expressions using ctRNA isolated from blood samples taken from cancer patients as a potential alternative to assaying PD-L1 from solid tumor tissue biopsies. Besides just the detection of PD-L1 expression, which is a prerequisite for the use of PD-L1 directed immunotherapy, we also wanted to set the stage for future studies to determine if the actual quantity of PD-L1 expressed in tumors might provide additional predictive insight into the patient’s outcome from therapy with PD-L1 directed agents.
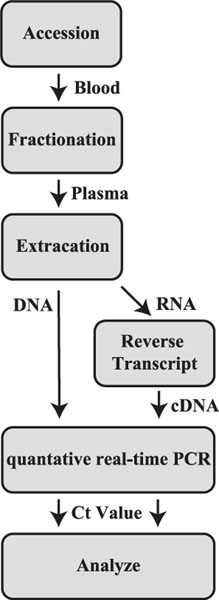
The procedure that was followed in this study to isolate ctRNA is summarized as a flow chart in Fig. 1. Plasma contains both ctRNA as well as ctDNA, but the procedure described here results in isolation of ctRNA that is essentially free of contaminating DNA. In contrast to ctDNA, ctRNA is relatively unstable in biological fluids presumably due to the presence of ribonucleases, and thus extra precautions are needed when working with ctRNA. To deal with this potential problem, blood drawn in the clinics of origin was immediately placed into storage tubes specially designed to stabilize RNA and to prevent lysis of normal blood cells. However, even then the RNA is not stabilized indefinitely and must be received in the analytical laboratory and processing begun within 7 days. Once RNA is isolated from the plasma, it can be stored at 80 C if necessary, but we usually proceeded with the PCR analysis as soon as possible to avoid any potential effects of freeze-thawing the sample. The isolation-analysis process generally could be completed within 2 days.
Fig. 1.
Flow diagram of measuring ctDNA and ctRNA. The blood sample was accessioned, fractionated and extracted. After extraction, ctDNA was analyzed with qPCR. ctRNA was reverse-transcripted to cDNA and analyzed with qPCR.
For validation purposes, the detection accuracy of the conventional qPCR, which was our method of choice, was assessed by comparing the results of qPCR analysis of PD-L1 from 50 clinical blood samples against those of the Digital Droplet PCR methodology. Among the 50 samples tested, 5 contained insufficient cDNA for analysis and 2 samples were discordant between the two platforms. The remainder of the samples was concordant between the two platforms, corresponding to a specificity of 92.7% and a sensitivity of 96.7% for qPCR. We therefore judged the qPCR platform to be of sufficient accuracy for use in this study. The performances of these two systems also had previously been shown to be very comparable in an EGFR mutation assay [23].
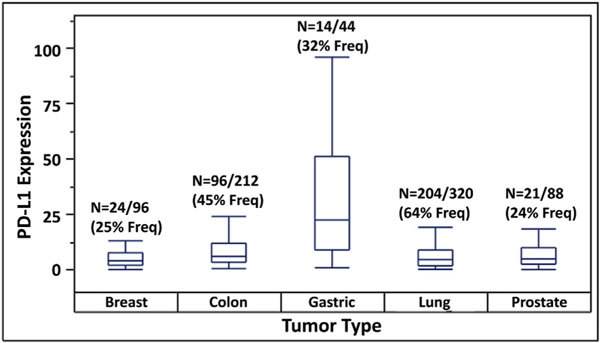
Frequencies, medians and ranges of PD-L1 expression as detected by qPCR in ctRNA isolated from blood samples of patients with the various types of cancer are shown as box plots in Fig. 2. These frequencies were statistically different across the five cancer types (p < 0.0001, Fisher’s Exact Test). For comparison, we also listed PD-L1 protein expression frequencies independently determined in previous studies by immunohistochemistry of tissue biopsies from the same tumor types (Table 1). The frequency values for NSCLC, breast, gastric and colorectal cancers determined by the two approaches are similar, while those for prostate cancer are somewhat discordant, being lower with qPCR than with IHC. This discrepancy may be due to the relatively small sample sizes in our study and/or to methodological variations in the evaluation and reporting of IHC data, which are often seen as somewhat subjective and semi-quantitative. While variations were noted in median relative PD-L1 expressions among the various cancer types, the differences were not statistically significantly different across the five cancer types (p = 0.406, Median Test).
Fig. 2.
Frequencies, medians and ranges of PD-L1 expression determined by analysis of ctRNA isolated from blood samples of cancer patients.
Table 1.
Detection frequencies and relative values of PD-L1 gene expression in plasma from patients with various cancer types and in cancer free individuals.
| Plasma from individuals with | Detection frequency (%) | Detection frequency by IHC (ref) | P values for cancer-healthy difference |
|---|---|---|---|
| No cancer | 0.0 (0/19) | — | — |
| Gastric Ca | 31.8 (14/44) | 29.6 [24] | 0.006 |
| CRC | 44.8 (96/212) | 25.8 [25] | <0.001 |
| NSCLC | 63.8 (204/320) | 25.0 [26] | <0.001 |
| Breast Ca | 25.0 (24/96) | 56.6 [27] | 0.012 |
| Prostate Ca | 23.9 (21/88) | 52.2 [28] | 0.022 |
| All cancer | 47.2 (359/760) | — | <0.001 |
No PD-L1 mRNA was detected in any individuals of the cancer-free control group (0/19). As shown in Table 1, the difference in PD-L1 expression between cancer-free individuals and cancer patients was highly statistically significant by Fischer’s Exact Test, not only with the entire group of cancer patients but with each individual cancer as well. This result suggests a possible use of plasma PD-L1 expression as a cancer detection method.
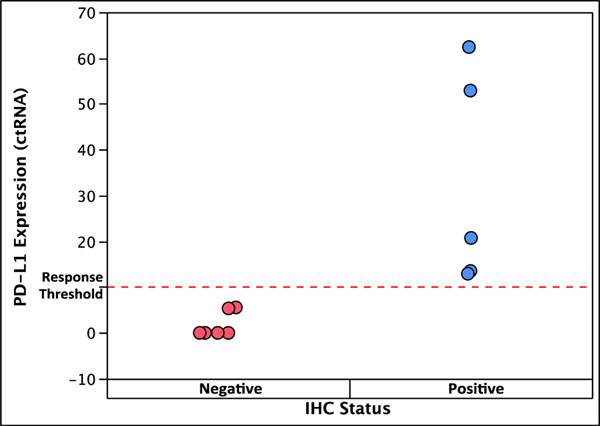
Previous studies of frequency of mutation detection in DNA have reported a high concordance (95%) between ctDNA analysis from plasma and tissue DNA analysis [29,30]. We had available 12 NSCLC tumor tissue samples from patients who had been treated with nivolumab, thus allowing us to perform concordance analysis between PD-L1 protein in tumor tissue and PD-L1 plasma gene expression in the same patients. All 5 of these samples that were positive for PD-L1 by IHC also had various measurable levels of PD-L1 gene expression (Fig. 3). Of the 7 samples that were negative by IHC, 5 also had undetectable gene expression, while the remaining 2 had low but detectable gene expression. This result indicates a high degree of concordance for PD-L1 analysis between the two methodologies and shows that either one can effectively predict response to nivolumab.
Fig. 3.
Concordance between PD-L1 protein analysis by IHC in tumor tissue and gene expression analysis by PCR in plasma in the same patients and association with nivolumab response. Blue color: response; red color: no response.
4. Conclusions
PD-L1 mRNA (gene expression) can be detected and quantitated in the ctRNA of cancer patients with frequencies of detection similar to those obtained by previous IHC analysis of solid tumor tissues. Gene expression in plasma predicts response to Nivolumab as effectively as IHC of tumor tissue. These results pave the way for further studies aimed at determining whether monitoring the quantitative levels of PD-L1 mRNA in blood before and during treatment can provide additional predictive value for anti-PD-L1 therapy. Moreover, the lack of PD-L1 mRNA in any of the cancer-free individuals suggests that the presence of PD-L1 mRNA in blood may be a potential marker for detecting the presence of cancer.
Abbreviations
- QOL
quality of life
- CTC
circulating cancer cells
- CtDNA
circulating tumor DNA
- CtRNA
circulating tumor RNA
- PD-L1
proteins like programmed death ligand 1
- PD-1
programmed cell death 1
- MAPK
mitogen-activated protein kinase
- PI3K
phospho-inositide 3-kinase
- HER2
human epidermal growth factor receptor 2
- GC
gastric cancer
- CRC
colorectal cancer
- NSCLC
nonesmall cell lung cancer
- BC
breast cancer
- PC
prostate cancer
- QPCR
quantitative polymerase chain reaction
- DCT
delta Ct
- ANOVA
analysis of variance
Footnotes
Transparency document
Transparency document related to this article can be found online at https://doi.org/10.1016/j.bbrc.2018.04.120.
References
- [1].Collins FS, Varmus H, A new initiative on precision medicine, N. Engl. J. Med. 372 (2015) 793–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Raphael BJ, Dobson JR, Oesper L, Vandin F, Identifying Driver Mutations in Sequenced Cancer Genomes: Computational Approaches to Enable Precision Medicine, vol. 6, 2014, p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jameson JL, Longo DL, Precision medicine - personalized, problematic, and promising, N. Engl. J. Med. 372 (2015) 2229–2234. [DOI] [PubMed] [Google Scholar]
- [4].Leon SA, Shapiro B, Sklaroff DM, Yaros MJ, Free DNA in the serum of cancer patients and the effect of therapy, Canc. Res. 37 (1977) 646–650. [PubMed] [Google Scholar]
- [5].Ignatiadis M, Dawson S, Circulating Tumor Cells and Circulating Tumor DNA for Precision Medicine: Dream or Reality?, vol. 25, 2014, pp. 2304–23138. [DOI] [PubMed] [Google Scholar]
- [6].Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, Ponzetti A, Cremolini C, Amatu A, Lauricella C, Lamba S, Hobor S, Avallone A, Valtorta E, Rospo G, Medico E, Motta V, Antoniotti C, Tatangelo F, Bellosillo B, Veronese S, Budillon A, Montagut C, Racca P, Marsoni S, Falcone A, Corcoran RB, Di Nicolantonio F, Loupakis F, Siena S, Sartore-Bianchi A, Bardelli A, Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients, Nat. Med. 21 (2015), 827–827b. [DOI] [PubMed] [Google Scholar]
- [7].Karachaliou N, Mayo-de las Casas C, Queralt C, de Aguirre I, Melloni B, Cardenal F, Garcia-Gomez R, Massuti B, Sanchez JM, Porta R, Ponce-Aix S, Moran T, Carcereny E, Felip E, Bover I, Insa A, Reguart N, Isla D, Vergnenegre A, de Marinis F, Gervais R, Corre R, Paz-Ares L, Morales-Espinosa D, Viteri S, Drozdowskyj A, Jordana-Ariza N, Ramirez-Serrano JL, Molina-Vila MA, Rosell R, Spanish Lung Cancer Group, Association of EGFR L858R mutation in circulating free DNA with survival in the EURTAC trial, JAMA Oncol 1 (2015) 149–157. [DOI] [PubMed] [Google Scholar]
- [8].Isobe K, Hata Y, Kobayashi K, Hirota N, Sato K, Sano G, Sugino K, Sakamoto S, Takai Y, Shibuya K, Takagi K, Homma S, Clinical significance of circulating tumor cells and free DNA in non-small cell lung cancer, Anticancer Res. 32 (2012) 3339–3344. [PubMed] [Google Scholar]
- [9].Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K, The microRNA spectrum in 12 body fluids, Clin. Chem. 56 (2010) 1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hirokawa K, Utsuyama M, Ishikawa T, Kikuchi Y, Kitagawa M, Fujii Y, Nariuchi H, Uetake H, Sugihara K, Decline of T cell-related immune functions in cancer patients and an attempt to restore them through infusion of activated autologous T cells, Mech. Ageing Dev. 130 (2009) 86–91. [DOI] [PubMed] [Google Scholar]
- [11].Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M, Safety, activity, and immune correlates of anti-PD-1 antibody in cancer, N. Engl. J. Med. 366 (2012) 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM, Safety and activity of anti-PD-L1 antibody in patients with advanced cancer, N. Engl. J. Med. 366 (2012) 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Herbst RS, Soria J, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HEK, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS, Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients, Nature 515 (2014), 563-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kleffel S, Posch C, Barthel SR, Mueller H, Schlapbach C, Guenova E, Elco CP, Lee N, Juneja VR, Zhan Q, Lian CG, Thomi R, Hoetzenecker W, Cozzio A, Dummer R, Mihm MC Jr., Flaherty KT, Frank MH, Murphy GF, Sharpe AH, Kupper TS, Schatton T, Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth, Cell 162 (2015) 1242–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS, Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients, Nature 515 (2014) 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S, Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer, Proc. Natl. Acad. Sci. U. S. A. 104 (2007) 3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen J, Jiang CC, Jin L, Zhang XD, Regulation of PD-L1: a novel role of prosurvival signalling in cancer, Ann. Oncol. 27 (2016) 409–416. [DOI] [PubMed] [Google Scholar]
- [18].Sundar R, Cho BC, Brahmer JR, Soo RA, Nivolumab in NSCLC: latest evidence and clinical potential, Ther Adv Med Oncol 7 (2015) 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L, Rimm DL, Programmed death ligand-1 expression in non-small cell lung cancer, Lab. Invest. 94 (2014) 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lin C, Chen X, Li M, Liu J, Qi X, Yang W, Zhang H, Cai Z, Dai Y, Ouyang X, Programmed death-ligand 1 expression predicts tyrosine kinase inhibitor response and better prognosis in a cohort of patients with epidermal growth factor receptor mutation-positive lung adenocarcinoma, Clin. Lung Canc. 16 (2015) e25–e35. [DOI] [PubMed] [Google Scholar]
- [21].Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, KEYNOTE-001 Investigators, Pembrolizumab for the treatment of non-small-cell lung cancer, N. Engl. J. Med. 372 (2015) 2018–2028. [DOI] [PubMed] [Google Scholar]
- [22].Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, Antonarakis ES, Azad NS, Bardelli A, Brem H, Cameron JL, Lee CC, Fecher LA, Gallia GL, Gibbs P, Le D, Giuntoli RL, Goggins M, Hogarty MD, Holdhoff M, Hong S, Jiao Y, Juhl HH, Kim JJ, Siravegna G, Laheru DA, Lauricella C, Lim M, Lipson EJ, Marie SKN, Netto GJ, Oliner KS, Olivi A, Olsson L, Riggins GJ, Sartore-Bianchi A, Schmidt K, Shih I, Oba-Shinjo SM, Siena S, Theodorescu D, Tie J, Harkins TT, Veronese S, Wang T, Weingart JD, Wolfgang CL, Wood LD, Xing D, Hruban RH, Wu J, Allen PJ, Schmidt CM, Choti MA, Velculescu VE, Kinzler KW, Vogelstein B, Papadopoulos N, Diaz LA Jr., Detection of Circulating Tumor DNA in Early- and Late-stage Human Malignancies, vol. 6, 2014, 224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang BO, Xu CW, Shao Y, Wang HT, Wu YF, Song YY, Li XB, Zhang Z, Wang WJ, Li LQ, Cai CL, Comparison of droplet digital PCR and conventional quantitative PCR for measuring gene mutation, Exp Ther Med 9 (2015) 1383–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tamura T, Ohira M, Tanaka H, Muguruma K, Toyokawa T, Kubo N, Sakurai K, Amano R, Kimura K, Shibutani M, Maeda K, Hirakawa K, Programmed Death-1 Ligand-1 (PDL1) expression is associated with the prognosis of patients with stage II/III gastric cancer, Anticancer Res. 35 (2015) 5369–5376. [PubMed] [Google Scholar]
- [25].Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, Zlobec I, Eppenberger-Castori S, Tzankov A, Rosso R, Zuber M, Muraro MG, Amicarella F, Cremonesi E, Heberer M, Iezzi G, Lugli A, Terracciano L, Sconocchia G, Oertli D, Spagnoli GC, Tornillo L, Clinical impact of programmed cell death ligand 1 expression in colorectal cancer, Eur. J. Canc. 49 (2013) 2233–2242. [DOI] [PubMed] [Google Scholar]
- [26].Herbst RS, Bass P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr., Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB, Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial, Lancet 387 (10027) (2016) 1540–1550. [DOI] [PubMed] [Google Scholar]
- [27].Sabatier R, Finetti P, Mamessier E, Adelaide J, Chaffanet M, Ali HR, Viens P, Caldas C, Birnbaum D, Bertucci F, Prognostic and predictive value of PDL1 expression in breast cancer, Oncotarget 6 (2015) 5449–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gevensleben H, Dietrich D, Golletz C, Steiner S, Jung M, Thiesler T, Majores M, Stein J, Uhl B, Muller S, Ellinger J, Stephan C, Jung K, Brossart P, Kristiansen G, The immune checkpoint regulator PD-l1 is highly expressed in aggressive primary prostate cancer, Clin. Canc. Res. (2015). [DOI] [PubMed] [Google Scholar]
- [29].Spindler KL, Pallisgaard N, Appelt AL, Andersen RF, Schou JV, Nielsen D, Pfeiffer P, Yilmaz M, Johansen JS, Hoegdall EV, Jakobsen A, Jensen BV, Clinical utility of KRAS status in circulating plasma DNA compared to archival tumour tissue from patients with metastatic colorectal cancer treated with anti-epidermal growth factor receptor therapy, Eur. J. Canc. 51 (2015) 2678–2685. [DOI] [PubMed] [Google Scholar]
- [30].Douillard JY, Ostoros G, Cobo M, Ciuleanu T, Cole R, McWalter G, Walker J, Dearden S, Webster A, Milenkova T, McCormack R, Gefitinib treatment in EGFR mutated caucasian NSCLC: circulating-free tumor DNA as a surrogate for determination of EGFR status, J. Thorac. Oncol. 9 (2014) 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]