Abstract
Imaging of dopaminergic transmission in neurodegenerative disorders such as Parkinson disease (PD) or dementia with Lewy bodies plays a major role in clinical practice and in clinical research. We here review the role of imaging of the nigrostriatal pathway, as well as of striatal receptors and dopamine release, in common neurodegenerative disorders in clinical practice and research. Imaging of the nigrostriatal pathway has a high diagnostic accuracy to detect nigrostriatal degeneration in disorders characterized by nigrostriatal degeneration, such as PD and dementia with Lewy bodies, and disorders of more clinical importance, namely in patients with clinically uncertain parkinsonism. Imaging of striatal dopamine D2/3 receptors is not recommended for the differential diagnosis of parkinsonian disorders in clinical practice anymore. Regarding research, recently the European Medicines Agency has qualified dopamine transporter imaging as an enrichment biomarker for clinical trials in early PD, which underlines the high diagnostic accuracy of this imaging tool and will be implemented in future trials. Also, imaging of the presynaptic dopaminergic system plays a major role in, for example, examining the extent of nigrostriatal degeneration in preclinical and premotor phases of neurodegenerative disorders and to examine subtypes of PD. Also, imaging of postsynaptic dopamine D2/3 receptors plays a role in studying, for example, the neuronal substrate of impulse control disorders in PD, as well as in measuring endogenous dopamine release to examine, for example, motor complications in the treatment of PD. Finally, novel MRI sequences as neuromelanin-sensitive MRI are promising new tools to study nigrostriatal degeneration in vivo.
Keywords: dopamine, neurodegeneration, PET, SPECT, Parkinson
Imaging of dopaminergic transmission in the brain is an important tool in neurodegenerative disorders such as Parkinson disease (PD) and dementia with Lewy bodies (DLB), not only as a research topic but also, frequently, for use in routine practice. In the first part of this review, we describe the role of dopaminergic imaging in routine practice. In the second part, we discuss its role in research.
IMAGING BRAIN DOPAMINERGIC NEUROTRANSMISSION IN NEURODEGENERATIVE DISORDERS IN ROUTINE PRACTICE
Imaging of Presynaptic Nigrostriatal Dopaminergic Pathway
In routine practice, imaging of the presynaptic nigrostriatal dopaminergic pathway is used to determine whether this is degenerated and, therefore, to differentiate patients with nigrostriatal degeneration from those without degeneration. The most common, and the relatively common, diseases characterized by nigrostriatal degeneration are PD, DLB, multiple-system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal degeneration (1–3). PD, including PD dementia, and DLB are increasingly considered a disease continuum in view of their similar pathology (4). We will here focus on imaging of the dopaminergic system in these disorders.
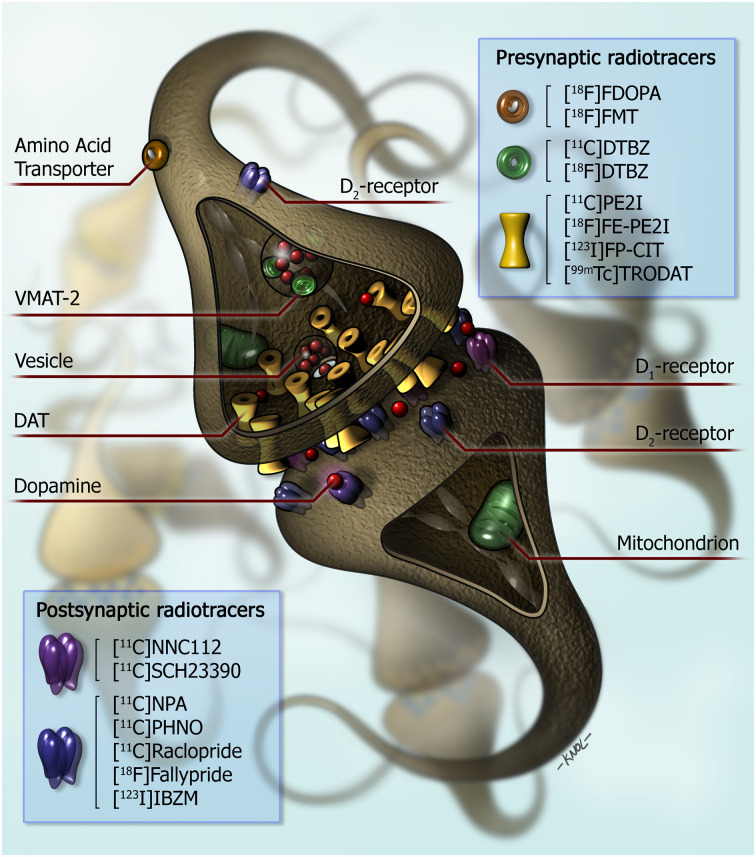
The nigrostriatal dopaminergic pathway can be imaged using radiopharmaceuticals for the dopamine transporter (DAT), for the vesicular monoamine transporter-2 (VMAT-2), or for aromatic L-amino-acid decarboxylase (AADC) activity (mainly using 18F-6-fluoro-l-dopa [18F-FDOPA]) (Fig. 1) (5). Regarding DAT imaging, many SPECT and PET tracers have been developed successfully (6). For VMAT-2 imaging, only PET tracers have been developed successfully (Fig. 1). Since the radiopharmaceutical 123I-labeled 2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl) nortropane (123I-FP-CIT, or 123I-ioflupane, commercialized as DaTscan [in the United States; GE Healthcare], DaTSCAN [in Europe; GE Healthcare], or Striascan [Curium]) is the only licensed radiotracer to image the nigrostriatal dopaminergic pathway by the Food and Drug Administration and European Medicines Agency, most hospitals and institutes use this radiopharmaceutical to assess the integrity of the nigrostriatal pathway in routine clinical studies.
FIGURE 1.
Simplified diagram of striatal dopaminergic synapse. On presynaptic side, markers for imaging of integrity of dopaminergic neurons in humans are shown. 18F-FDOPA and 18F-FMT PET provide measures of structural and biochemical integrity of dopaminergic neurons. 11C-DTBZ and 18F-DTBZ are radiopharmaceuticals for vesicular monoaminergic transporter. Substituted (nor)phenyltropanes (11C-PE2I, 18F-FE-PE2I, 123I-FP-CIT, and 99mTc-TRODAT) are frequently used PET and SPECT radioligands for imaging of DAT. On postsynaptic side, 11C-NNC112 and 11C-SCH23390 radiopharmaceuticals for dopamine D1 receptor are shown. Dopamine D2 receptors are expressed predominantly on postsynaptic side as compared with presynaptic side of dopaminergic synapse. 11C-NPA and 11C-PHNO are agonist radioligands for dopamine D2/3 receptors. Commonly used antagonist radioligands for D2/3 receptors are substituted benzamides (11C-raclopride, 11C-FLB 457, 18F-fallypride, and 123I-IBZM). (Reprinted from (6).)
DAT Imaging
In PD, the loss of striatal DAT binding is typically more pronounced in the putamen than in the caudate nucleus (6). Characteristically, the loss of DAT binding starts in the posterior part of the putamen and is more pronounced in the dorsal than in the ventral part of the putamen (Fig. 2). Also, commonly, binding of the DAT tracer is lower at the contralateral than the ipsilateral striatum (i.e., contralateral to the clinically most affected body side) (Fig. 2). The loss of striatal DAT binding in PD can already be detected in the early motor phases of the disease and even at the premotor and preclinical stage (7–10). In line with this fact, systematic reviews and metaanalyses showed that DAT imaging is a sensitive and specific imaging tool to detect nigrostriatal degeneration in PD (5,11). The diagnostic accuracy of DAT imaging is also high in patients with clinically uncertain parkinsonism (CUPS) and impacts clinical decision making, emphasizing its usefulness in clinical practice (11–14). This is of relevance since it can be challenging to diagnose PD clinically, especially in the early motor stage of disease (15,16). In addition to visual inspection, a quantitative or semiquantitative approach may increase reader confidence and create more reproducible reporting (17,18).
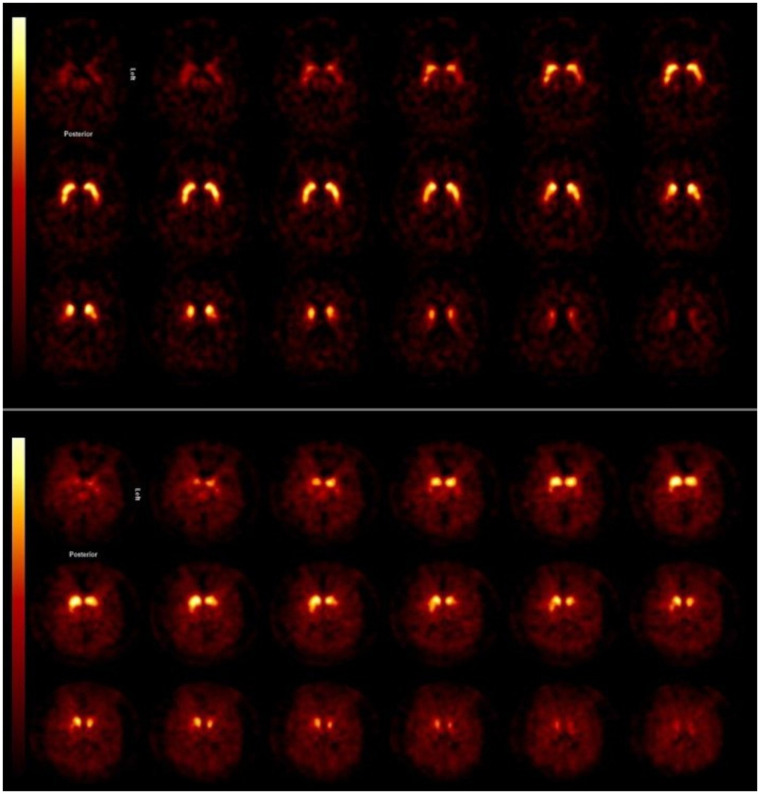
FIGURE 2.
Transversal 123I-FP-CIT SPECT images obtained in patient with CUPS without striatal DAT loss (top) and in CUPS patient with striatal DAT loss (bottom). Asymmetric striatal binding can be seen, as well as severe loss of DAT binding, especially in putamen, in subject with dopaminergic deficit. This study was acquired on brain-dedicated SPECT system (InSPira; NeuroLogica).
Clinically and neuropathologically, MSA can be divided into MSA with predominantly parkinsonian signs (MSA-P) or MSA with cerebellar features (MSA-C). As in PD, DAT binding is lower in the putamen than in the caudate nucleus (19). Some studies have demonstrated lower and more symmetric striatal DAT binding in MSA-P patients than in PD patients. However, the differences are relatively small, and findings consequently are inconsistent and cannot differentiate between these diagnoses at an individual level (20,21). Nevertheless, at the individual level there is a clear overlap in binding ratios between MSA-P and PD patients (20,21), thus precluding a role for DAT SPECT imaging in differentiating between degenerative parkinsonian diseases in daily clinical practice. DAT PET imaging offers the advantage of a better spatial resolution than DAT SPECT and, subsequently, a subregional analysis of striatal DAT binding. In this regard, Oh et al. showed that DAT PET imaging may indeed be able to differentiate PD from MSA-P at a group level (22). However, also in that study, MSA-P patients could not be differentiated completely from PD patients at an individual level.
In MSA-C, on average, striatal DAT binding is higher than in MSA-P and PD (19) and can even sometimes be normal (21,23). Fortunately, in routine practice, MSA-C patients can frequently be differentiated clinically from PD patients rather easily.
As in MSA-P and PD, DAT imaging is a sensitive means to detect loss of striatal DAT binding in PSP (21). Interestingly, a recent systematic review showed that striatal DAT binding in PSP is clearly lower than in PD and MSA-P (5). More specifically, DAT binding in PSP was on average approximately 34% and 18% lower than in PD in the caudate nucleus and putamen, respectively. Although studies on PSP, PD, and MSA-P do show an overlap in striatal DAT binding at an individual level, a very low DAT binding, particularly of the caudate nucleus, in an individual parkinsonian patient with a short disease duration (e.g., <2 y) might indicate the development of atypical parkinsonism. In corticobasal degeneration, the loss of striatal DAT binding can be very asymmetric, although it can also mimic the typical pattern of PD and can sometimes even be normal (24).
In Europe, 123I-FP-CIT SPECT is also frequently used to differentiate DLB from Alzheimer disease, since it is also approved by the European Medicines Agency for this indication. In Alzheimer disease, striatal DAT is typically not reduced, whereas DLB is characterized by loss of striatal DAT binding (25,26). Characteristically, in DLB, DAT binding is lower in the putamen than in the caudate nucleus; however, this posterior–anterior gradient may be more pronounced in PD than in DLB (27). Recent metaanalyses on the value of DAT imaging in DLB concluded that DAT imaging has a high diagnostic value for detecting DLB versus Alzheimer disease (sensitivity of 86.5% and specificity of 93%) and is more accurate than the clinical diagnosis (28,29). However, some recent studies suggested that DAT imaging may initially be normal in a relatively rare DLB subtype (∼10% of cases), with possibly a different severity or spread of α-synuclein pathology (neocortical predominant subtype) (30–32).
Among patients with clinically diagnosed PD who were enrolled in PD trials, around 10%–15% have been found to have normal DAT SPECT findings, also referred to as scans without evidence of dopaminergic deficit (33). Interestingly, in most patient with such scans, abnormal DAT SPECT scans do not develop on long-term follow up (11). In line with this observation, it is now well accepted that normal DAT SPECT findings exclude PD (34).
Although most institutes use 123I-FP-CIT SPECT to assess striatal DAT binding in routine practice, some institutes also use DAT PET tracers such as 18F-FE-PE2I (6).
AADC Imaging
Many studies have assessed striatal AADC activity in neurodegenerative disorders, particularly PD, using 18F-FDOPA PET (5). The pattern loss of striatal AADC mimics the loss of striatal DAT binding in diseases such as PD, MSA-P and PSP (Fig. 3) (5,19). Although 123I-FP-CIT SPECT is used in most hospitals as a diagnostic tool to support or exclude dopaminergic degeneration in routine practice, some institutes do use 18F-FDOPA PET for this purpose (35). Like DAT imaging, 18F-FDOPA PET is a sensitive technique, but a recent metaanalysis showed that the loss of striatal AADC activity is consistently smaller than that of striatal DAT activity in PD (5), possibly because of upregulation of AADC activity in surviving monoaminergic neurons. Consequently, especially in early stages of PD, 18F-FDOPA PET might be less sensitive to detect the dopaminergic deficit, but this postulate has not been proven yet (36).
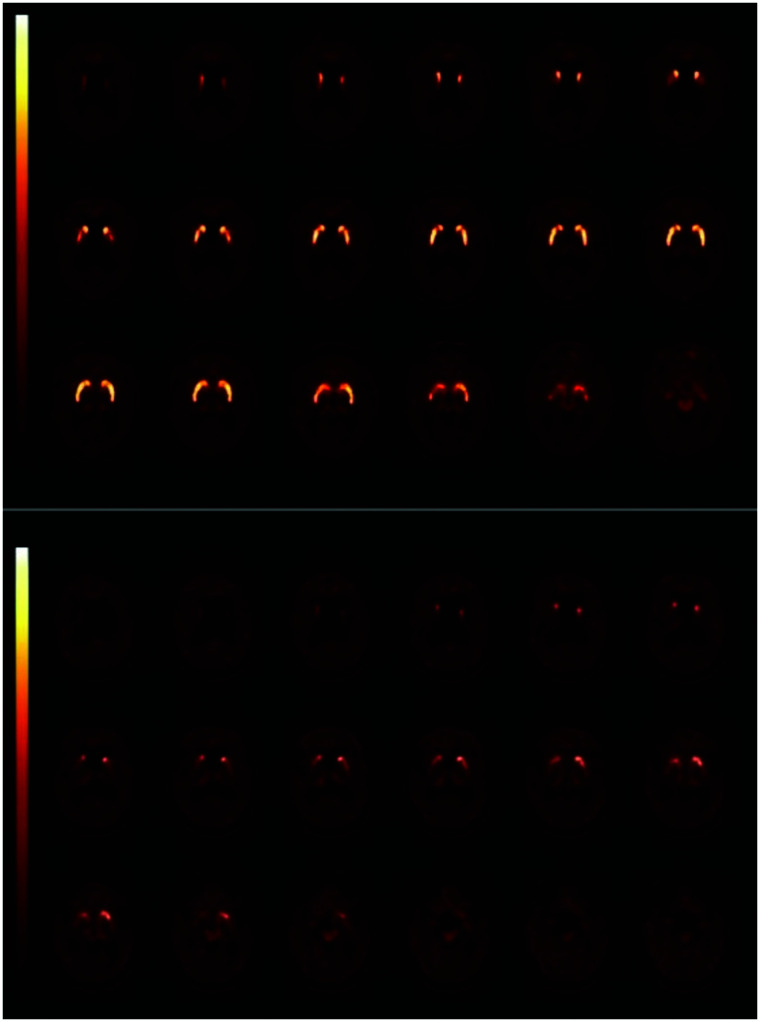
FIGURE 3.
Transversal planes of 18F-FDOPA PET images obtained in patient with CUPS without nigrostriatal cell loss (top) and in CUPS patient with nigrostriatal degeneration (bottom). Asymmetric striatal uptake can be seen, as well as severe loss of 18F-FDOPA uptake, especially in putamen, of subject with nigrostriatal degeneration.
VMAT-2 Imaging
Although the number of studies on VMAT-2 PET imaging in neurodegenerative disorders such as PD is much smaller than the number of studies on DAT imaging and 18F-FDOPA, the above-mentioned patterns of loss of striatal binding do—generally speaking—match the findings of DAT studies (37–40). Also, a 2018 study showed that the diagnostic accuracy of VMAT-2 imaging is high in patients with CUPS (41).
Imaging of Postsynaptic Striatal Dopaminergic D2/3 Receptors
Dopamine receptors can be differentiated in dopamine D1- and D2-like receptors. D1 and D5 receptors belong to the group of D1-like receptors, whereas D2, D3, and D4 receptors belong to the group of D2-like receptors. Importantly, most radiopharmaceuticals used in PET/SPECT studies are nonselective tracers and bind to D1/5 or D2/3 receptors (Fig. 1) (6,35).
Dopamine D2 receptors are expressed presynaptically in dopaminergic neurons. These receptors are called autoreceptors and play a role in the regulation of dopamine release (Fig. 1). Dopamine D2/3 receptors, located in the striatum, are expressed predominantly postsynaptically (6).
MSA-P and PSP are characterized not only neuropathologically by degeneration of nigrostriatal dopaminergic neurons but also by loss of striatal D2-like receptors (42,43). PET and SPECT studies showed loss of postsynaptic dopamine D2/3 receptors in PSP and MSA-P (44). For many years, radiotracers such as 123I-IBZM or 11C-raclopride (Fig. 1) have been used in clinical practice to differentiate PSP/MSA-P from PD. Importantly, a recent metaanalysis of dopamine D2/3 receptor studies in PD showed increased D2/3 receptor binding (particularly on the contralateral side) in an early state of PD compared with control values (probably reflecting upregulation), but after a disease duration of approximately 4 y, PD patients had lower striatal D2/3 receptor binding values than did controls (probably due to downregulation). This metaanalysis also showed that PSP and MSA-P patients indeed had lower striatal dopamine D2/3 receptor binding than in PD, but this loss was only around 14% and 22%, respectively (44). This result indicates that imaging findings are in line with autopsy findings but also that the intraindividual values for striatal dopamine D2/3 receptor binding do show a clear overlap between PD and MSA-P or PSP. Although striatal D2/3 receptor binding has been used for many years in routine practice, in light of these diagnostic uncertainties, the diagnostic use of dopamine D2/3 receptor imaging is not recommended anymore. Interestingly, the use of 18F-FDG PET is probably superior to dopamine D2/3 receptor binding in differentiating PD from MSA-P or PSP and may be used in clinical practice for diagnostic support (44–47).
IMAGING DOPAMINERGIC NEUROTRANSMISSION IN NEURODEGENERATIVE DISORDERS IN A RESEARCH SETTING
In the past 2 decades, an important research topic, related to imaging of the presynaptic pathway, has been the detection of nigrostriatal dopaminergic degeneration in the preclinical phase of neurodegenerative disorders. This topic is relevant, not only from a scientific point of view (e.g., to examine how many years before the motor signs of PD the nigrostriatal degeneration starts) but also to examine whether molecular imaging is able to detect subjects in the preclinical phase of neurodegeneration and, if so, to determine the extent of degeneration. This ability is relevant, because when the motor signs of PD start, approximately half of DAT expression in the putamen is already lost (5), potentially hampering a potentially successful intervention aimed to slow disease progression. Fortunately, molecular imaging studies showed the ability to detect nigrostriatal degeneration in subjects with rapid-eye-movement sleep behavior disorder (RBD), hyposmia, and late-onset depression, all of which are related to an increased risk for developing a movement disorder characterized by a dopaminergic deficit (7–10).
There is consensus among PD researchers that disease-modifying drugs and neuroprotective drugs are likely to be most effective at an early stage of PD, when delaying disease progression will be most effective (16). At this stage, a large number of the nigrostriatal dopaminergic neurons are already lost (5). Also, at this stage it can be especially difficult to diagnose PD clinically (15,16). Since DAT imaging is a sensitive imaging tool to detect PD in an early disease stage (11) and there is consensus that subjects having scans without evidence of dopaminergic deficit show a much slower motor deterioration than subjects whose scans do show dopaminergic degeneration (33,48), the European Medicines Agency has qualified DAT imaging as an enrichment biomarker for clinical trials targeting early stages of PD (i.e., within 1–2 y of clinical diagnosis) (16). Data for the large Parkinson Research Examination of CEP-1347 study and the Parkinson Progression Markers Initiative study were essential to reach this important milestone (33,49,50). The first application of DAT imaging as an enrichment biomarker has been published recently (51). It is likely that DAT imaging will be increasingly used in clinical trials that evaluate the efficacy of potential drug-modifying drugs in early PD.
The Parkinson Progression Markers Initiative data are publicly available, offering the unique opportunity for all interested research groups to perform analyses on this large dataset. For example, studies using this dataset have been performed to test the relationship between striatal DAT binding and cognitive executive impairment in PD, as well as on the relationship between DAT binding and α-synuclein in the cerebral spinal fluid (52,53).
It is now well accepted that neurodegenerative diseases such as PD, MSA, and PSP are not single disease entities (54,55). In this regard, it is of interest that molecular imaging studies showed the loss of striatal DAT binding to be more pronounced in the akinetic-rigid subtype than in the tremor-dominant subtype (56). Also, striatal DAT binding may be higher in women than men with PD, at symptom onset and throughout the course of PD, as is in line with the observation that women more often present with tremor than do men (57). These findings suggest a more benign phenotype in women with PD (57). Interestingly, Horsager et al. recently proposed that PD may comprise 2 subtypes: brain-first versus body-first (58). They postulated that in the brain-first subtype, degeneration starts in a single hemisphere, leading to asymmetric nigrostriatal degeneration, whereas in the body-first form, the initial enteric pathology will spread through vagal innervation, leading to a more symmetric degeneration. Indeed, in line with their postulation, a recent combined study of 18F-FDOPA PET and 123I-FP-CIT SPECT on isolated RBD (which is suggested to be the prototype of the body-first subtype) and on de novo PD patients with and without RBD showed a more symmetric degeneration in isolated RBD subjects than in PD patients without RBD (59). Finally, DAT binding is lower in MSA-P than in MSA-C (60).
The SPECT tracer 123I-FP-CIT is not a selective DAT tracer, as this radiotracer also shows a modest affinity for the serotonin transporter. Studies on healthy controls showed that it is actually extrastriatal, but not striatal, 123I-FP-CIT binding that can be blocked by a selective serotonin reuptake inhibitor (61,62). Previous work has shown that analyses of extrastriatal 123I-FP-CIT binding may contribute to the differential diagnosis of parkinsonian syndromes, in that not only striatal DAT binding is lower in PSP and MSA-P than in PD but also extrastriatal binding may be lower in some brain areas such as the diencephalon (21,63). Also, DLB patients may show lower 123I-FP-CIT binding in the thalamus than do PD patients (64).
Selective DAT tracers have been developed successfully (62,65); one example is 18F-FE-PE2I (Fig. 1). As expected, 18F-FE-PE2I PET studies showed the ability to detect the loss of striatal DAT binding in PD (66), and small head-to-head studies with 123I-FP-CIT SPECT showed that 18F-FE-PE2I is not inferior to 123I-FP-CIT in detecting striatal DAT loss (65,67). However, it is still unclear whether this PET tracer will replace 123I-FP-CIT as a diagnostic tool in the future.
SPECT and PET tracers for dopamine D2/3 receptors (Fig. 1) can be used not only to assess the baseline in vivo availability of these receptors but also to assess endogenous dopamine release (displacement experiments). Many studies have been performed to assess the baseline availability of these receptors in vivo in PD (44,68). Generally speaking, these studies showed an upregulation of striatal dopamine D2/3 receptors in early PD, likely a compensating effect on the presynaptic dopaminergic deficit, that faded when the disease duration increased (44). Using the dopamine release paradigm, Piccini et al. showed that a methamphetamine challenge was able to induce a detectable dopamine release in the putamen of advanced PD cases, although this release was much lower than in healthy controls (69). Also, impulse control disorders are common in PD, and impulse control disorders in PD are associated with relatively increased dopamine in the ventral striatum (70). Interestingly, a 11C-raclopride PET study showed that although striatal dopamine D2/3 binding is similar at baseline between PD patients with and those without impulse control disorders, dopamine release after presentation of reward-related visual cues (and after a levodopa challenge) was higher in PD patients with impulse control disorders (71). Finally, dopamine release is also assessed to better understand motor fluctuation in PD. Using 11C-raclopride PET, de la Fuente-Fernández et al. showed that 1 h after a levodopa challenge, the dopamine levels were increased in the putamen of PD patients with motor fluctuations as compared with those without such fluctuations (72). All in all, these findings highlight that disturbance of the dopaminergic system in neurodegenerative disorders is sometimes detectable only when the dopaminergic system is challenged.
The number of studies on dopamine D1/5 receptors, using tracers such as 11C-SCH 23390 (Fig. 1), is much lower than the number on D2/3 receptors. In general, these studies have not shown a significant difference in striatal D1 receptor binding between PD and controls (68).
Different aspect of the dopaminergic system can be assessed directly only by imaging techniques such as PET or SPECT. However, novel MRI sequences are capable to indirectly assess the dopaminergic system in vivo. The so-called neuromelanin-sensitive MRI is capable of visualizing the loss of neuromelanin-containing dopaminergic cells in the substantia nigra (73,74). Recently, the correlation between signal intensity on neuromelanin-sensitive MRI and neuromelanin concentration in the substantia nigra was elegantly proven by an autopsy study by Cassidy et al. (75). Indeed, many studies have shown that the neuromelanin signal in the substantia nigra is lower in PD, MSA, and PSP than in controls (Fig. 4) (76,77). Although this new technique is promising, with potential advantages over DAT imaging (e.g., lower costs, faster acquisitions), large prospective clinical studies on patients with CUPS, and studies on CUPS patients with autopsy conformation, are needed to assess its diagnostic power in clinical practice.
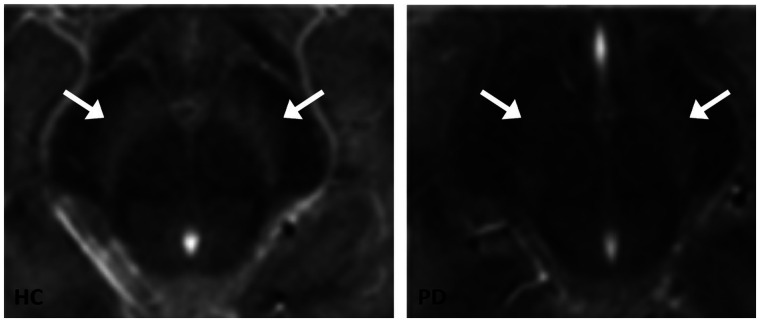
FIGURE 4.
Transversal images of neuromelanin-sensitive MRI scans of mesencephalon. Substantia nigra is visible as hyperintense area next to cerebral peduncles. Left panel shows example of neuromelanin-sensitive MRI in healthy control, and right panel shows example of patient with PD. Loss of signal can be seen in substantia nigra in PD patient. Arrows point to substantia nigra. (Reprinted from (74).)
CONCLUSION
Imaging of the nigrostriatal pathway has high diagnostic accuracy in detecting nigrostriatal degeneration in common movement disorders characterized by a presynaptic dopaminergic deficit and in patients with CUPS. In clinical practice, imaging of striatal dopamine D2/3 receptors no longer plays a major diagnostic role in the differential diagnosis of parkinsonian disorders. Regarding research, imaging of the dopaminergic system plays a major role in, for example, examining nigrostriatal degeneration in preclinical and premotor stages of neurodegenerative disorders or motor complications in the treatment of PD. Finally, neuromelanin-sensitive MRI is a promising new tool to study nigrostriatal degeneration in vivo.
DISCLOSURE
Jan Booij is consultant at GE Healthcare and received research grants from GE Healthcare (all payments to the institution). Rob de Bie received research grants from GE Healthcare (paid to the institution). No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1. Piggott MA, Marshall EF, Thomas N, et al. Striatal dopaminergic markers in dementia with Lewy bodies, Alzheimer’s and Parkinson’s diseases: rostrocaudal distribution. Brain. 1999;122:1449–1468. [DOI] [PubMed] [Google Scholar]
- 2. Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease: pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–880. [DOI] [PubMed] [Google Scholar]
- 3. Kraemmer J, Kovacs GG, Perju-Dumbrava L, Pirker S, Traub-Weidinger T, Pirker W. Correlation of striatal dopamine transporter imaging with post mortem substantia nigra cell counts. Mov Disord. 2014;29:1767–1773. [DOI] [PubMed] [Google Scholar]
- 4. Walker L, Stefanis L, Attems J. Clinical and neuropathological differences between Parkinson’s disease, Parkinson’s disease dementia and dementia with Lewy bodies: current issues and future directions. J Neurochem. 2019;150:467–474. [DOI] [PubMed] [Google Scholar]
- 5. Kaasinen V, Vahlberg T. Striatal dopamine in Parkinson disease: a meta-analysis of imaging studies. Ann Neurol. 2017;82:873–882. [DOI] [PubMed] [Google Scholar]
- 6. Booij J, van Wieringen J-P, van de Giessen E, Knol RJJ, Finnema SJ. PET and SPECT imaging of the central dopamine system in humans. In: Dierckx RAJO, Otte A, de Vries EFJ, van Waarde A, Lammertsma AA, eds. PET and SPECT of Neurobiological Systems . Springer; 2021:295–318. [Google Scholar]
- 7. Ponsen MM, Stoffers D, Booij J, van Eck-Smit BL, Wolters E, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann Neurol. 2004;56:173–181. [DOI] [PubMed] [Google Scholar]
- 8. Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12:443–453. [DOI] [PubMed] [Google Scholar]
- 9. Kazmi H, Walker Z, Booij J, et al. Late onset depression: dopaminergic deficit and clinical features of prodromal Parkinson’s disease: a cross-sectional study. J Neurol Neurosurg Psychiatry. 2021;92:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doppler K, Jentschke HM, Schulmeyer L, et al. Dermal phospho-alpha-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson’s disease. Acta Neuropathol (Berl). 2017;133:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suwijn SR, van Boheemen CJ, de Haan RJ, Tissingh G, Booij J, de Bie RM. The diagnostic accuracy of dopamine transporter SPECT imaging to detect nigrostriatal cell loss in patients with Parkinson’s disease or clinically uncertain parkinsonism: a systematic review. EJNMMI Res. 2015;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marshall VL, Reininger CB, Marquardt M, et al. Parkinson’s disease is overdiagnosed clinically at baseline in diagnostically uncertain cases: a 3-year European multicenter study with repeat [123I]FP-CIT SPECT. Mov Disord. 2009;24:500–508. [DOI] [PubMed] [Google Scholar]
- 13. Isaacson JR, Brillman S, Chhabria N, Isaacson SH. Impact of DaTscan imaging on clinical decision making in clinically uncertain Parkinson’s disease. J Parkinsons Dis. 2021;11:885–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bega D, Kuo PH, Chalkidou A, et al. Clinical utility of DaTscan in patients with suspected Parkinsonian syndrome: a systematic review and meta-analysis. NPJ Parkinsons Dis. 2021;7:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adler CH, Beach TG, Hentz JG, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology. 2014;83:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stephenson D, Hill D, Cedarbaum JM, et al. The qualification of an enrichment biomarker for clinical trials targeting early stages of Parkinson’s disease. J Parkinsons Dis. 2019;9:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Booij J, Dubroff J, Pryma D, et al. Diagnostic performance of the visual reading of 123I-ioflupane SPECT images with or without quantification in patients with movement disorders or dementia. J Nucl Med. 2017;58:1821–1826. [DOI] [PubMed] [Google Scholar]
- 18. Söderlund TA, Dickson JC, Prvulovich E, et al. Value of semiquantitative analysis for clinical reporting of 123I-2-β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl)nortropane SPECT studies. J Nucl Med. 2013;54:714–722. [DOI] [PubMed] [Google Scholar]
- 19. Kaasinen V, Kankare T, Joutsa J, Vahlberg T. Presynaptic striatal dopaminergic function in atypical parkinsonism: a metaanalysis of imaging studies. J Nucl Med. 2019;60:1757–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Varrone A, Marek KL, Jennings D, Innis RB, Seibyl JP. [123I]beta-CIT SPECT imaging demonstrates reduced density of striatal dopamine transporters in Parkinson’s disease and multiple system atrophy. Mov Disord. 2001;16:1023–1032. [DOI] [PubMed] [Google Scholar]
- 21. Joling M, Vriend C, van den Heuvel OA, et al. Analysis of extrastriatal 123I-FP-CIT binding contributes to the differential diagnosis of parkinsonian diseases. J Nucl Med. 2017;58:1117–1123. [DOI] [PubMed] [Google Scholar]
- 22. Oh M, Kim JS, Kim JY, et al. Subregional patterns of preferential striatal dopamine transporter loss differ in Parkinson disease, progressive supranuclear palsy, and multiple-system atrophy. J Nucl Med. 2012;53:399–406. [DOI] [PubMed] [Google Scholar]
- 23. McKinley J, O’Connell M, Farrell M, Lynch T. Normal dopamine transporter imaging does not exclude multiple system atrophy. Parkinsonism Relat Disord. 2014;20:933–934. [DOI] [PubMed] [Google Scholar]
- 24. Cilia R, Rossi C, Frosini D, et al. Dopamine transporter SPECT imaging in corticobasal syndrome. PLoS One. 2011;6:e18301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McKeith I, O’Brien J, Walker Z, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6:305–313. [DOI] [PubMed] [Google Scholar]
- 26. Shirvan J, Clement N, Ye R, et al. Neuropathologic correlates of amyloid and dopamine transporter imaging in Lewy body disease. Neurology. 2019;93:e476–e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O’Brien JT, Colloby S, Fenwick J, et al. Dopamine transporter loss visualized with FP-CIT SPECT in the differential diagnosis of dementia with Lewy bodies. Arch Neurol. 2004;61:919–925. [DOI] [PubMed] [Google Scholar]
- 28. McCleery J, Morgan S, Bradley KM, Noel-Storr AH, Ansorge O, Hyde C. Dopamine transporter imaging for the diagnosis of dementia with Lewy bodies. Cochrane Database Syst Rev. 2015;1:CD010633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Papathanasiou ND, Boutsiadis A, Dickson J, Bomanji JB. Diagnostic accuracy of 123I-FP-CIT (DaTSCAN) in dementia with Lewy bodies: a meta-analysis of published studies. Parkinsonism Relat Disord. 2012;18:225–229. [DOI] [PubMed] [Google Scholar]
- 30. van der Zande JJ, Booij J, Scheltens P, Raijmakers PG, Lemstra AW. [123]FP-CIT SPECT scans initially rated as normal became abnormal over time in patients with probable dementia with Lewy bodies. Eur J Nucl Med Mol Imaging. 2016;43:1060–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Colloby SJ, McParland S, O’Brien JT, Attems J. Neuropathological correlates of dopaminergic imaging in Alzheimer’s disease and Lewy body dementias. Brain. 2012;135:2798–2808. [DOI] [PubMed] [Google Scholar]
- 32. Thomas AJ, Attems J, Colloby SJ, et al. Autopsy validation of 123I-FP-CIT dopaminergic neuroimaging for the diagnosis of DLB. Neurology. 2017;88:276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marek K, Seibyl J, Eberly S, et al. Longitudinal follow-up of SWEDD subjects in the PRECEPT Study. Neurology. 2014;82:1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–1601. [DOI] [PubMed] [Google Scholar]
- 35. Morbelli S, Esposito G, Arbizu J, et al. EANM practice guideline/SNMMI procedure standard for dopaminergic imaging in Parkinsonian syndromes 1.0. Eur J Nucl Med Mol Imaging. 2020;47:1885–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eshuis SA, Jager PL, Maguire RP, Jonkman S, Dierckx RA, Leenders KL. Direct comparison of FP-CIT SPECT and F-DOPA PET in patients with Parkinson’s disease and healthy controls. Eur J Nucl Med Mol Imaging. 2009;36:454–462. [DOI] [PubMed] [Google Scholar]
- 37. Hsiao IT, Weng YH, Hsieh CJ, et al. Correlation of Parkinson disease severity and 18F-DTBZ positron emission tomography. JAMA Neurol. 2014;71:758–766. [DOI] [PubMed] [Google Scholar]
- 38. Okamura N, Villemagne VL, Drago J, et al. In vivo measurement of vesicular monoamine transporter type 2 density in Parkinson disease with 18F-AV-133. J Nucl Med. 2010;51:223–228. [DOI] [PubMed] [Google Scholar]
- 39. Bohnen NI, Albin RL, Koeppe RA, et al. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab. 2006;26:1198–1212. [DOI] [PubMed] [Google Scholar]
- 40. Villemagne VL, Okamura N, Pejoska S, et al. Differential diagnosis in Alzheimer’s disease and dementia with Lewy bodies via VMAT2 and amyloid imaging. Neurodegener Dis. 2012;10:161–165. [DOI] [PubMed] [Google Scholar]
- 41. Xu SS, Alexander PK, Lie Y, et al. Diagnostic accuracy of imaging brain vesicular monoamine transporter type 2 (VMAT2) in clinically uncertain parkinsonian syndrome (CUPS): a 3-year follow-up study in community patients. BMJ Open. 2018;8:e025533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. González AM, Berciano J, Figols J, Pazos A, Pascual J. Loss of dopamine uptake sites and dopamine D2 receptors in striatonigral degeneration. Brain Res. 2000;852:228–232. [DOI] [PubMed] [Google Scholar]
- 43. Pascual J, Berciano J, Grijalba B, et al. Dopamine D1 and D2 receptors in progressive supranuclear palsy: an autoradiographic study. Ann Neurol. 1992;32:703–707. [DOI] [PubMed] [Google Scholar]
- 44. Kaasinen V, Vahlberg T, Stoessl AJ, Strafella AP, Antonini A. Dopamine receptors in Parkinson’s disease: a meta-analysis of imaging studies. Mov Disord. 2021;36:1781–1791. [DOI] [PubMed] [Google Scholar]
- 45. Hellwig S, Amtage F, Kreft A, et al. [18F]FDG-PET is superior to [123I]IBZM-SPECT for the differential diagnosis of parkinsonism. Neurology. 2012;79:1314–1322. [DOI] [PubMed] [Google Scholar]
- 46. Tang CC, Poston KL, Eckert T, et al. Differential diagnosis of parkinsonism: a metabolic imaging study using pattern analysis. Lancet Neurol. 2010;9:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schindlbeck KA, Gupta DK, Tang CC, et al. Neuropathological correlation supports automated image-based differential diagnosis in parkinsonism. Eur J Nucl Med Mol Imaging. 2021;48:3522–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marek K, Jennings D, Seibyl J. Single-photon emission tomography and dopamine transporter imaging in Parkinson’s disease. Adv Neurol. 2003;91:183–191. [PubMed] [Google Scholar]
- 49. The Parkinson Progression Marker Initiative. The Parkinson progression marker initiative (PPMI). Prog Neurobiol. 2011;95:629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Parkinson Study Group. Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA. 2002;287:1653–1661. [DOI] [PubMed] [Google Scholar]
- 51. Hutchison RM, Evans KC, Fox T, et al. Evaluating dopamine transporter imaging as an enrichment biomarker in a phase 2 Parkinson’s disease trial. BMC Neurol. 2021;21:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Siepel FJ, Brønnick KS, Booij J, et al. Cognitive executive impairment and dopaminergic deficits in de novo Parkinson’s disease. Mov Disord. 2014;29:1802–1808. [DOI] [PubMed] [Google Scholar]
- 53. Mollenhauer B, Zimmermann J, Sixel-Döring F, et al. Baseline predictors for progression 4 years after Parkinson’s disease diagnosis in the De Novo Parkinson Cohort (DeNoPa). Mov Disord. 2019;34:67–77. [DOI] [PubMed] [Google Scholar]
- 54. Thenganatt MA, Jankovic J. Parkinson disease subtypes. JAMA Neurol. 2014;71:499–504. [DOI] [PubMed] [Google Scholar]
- 55. Koga S, Dickson DW. Recent advances in neuropathology, biomarkers and therapeutic approach of multiple system atrophy. J Neurol Neurosurg Psychiatry. 2018;89:175–184. [DOI] [PubMed] [Google Scholar]
- 56. Kaasinen V, Kinos M, Joutsa J, Seppänen M, Noponen T. Differences in striatal dopamine transporter density between tremor dominant and non-tremor Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2014;41:1931–1937. [DOI] [PubMed] [Google Scholar]
- 57. Haaxma CA, Bloem BR, Borm GF, et al. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Horsager J, Andersen KB, Knudsen K, et al. Brain-first versus body-first Parkinson’s disease: a multimodal imaging case-control study. Brain. 2020;143:3077–3088. [DOI] [PubMed] [Google Scholar]
- 59. Knudsen K, Fedorova TD, Horsager J, et al. Asymmetric dopaminergic dysfunction in brain-first versus body-first Parkinson’s disease subtypes. J Parkinsons Dis. 2021;11:1677–1687. [DOI] [PubMed] [Google Scholar]
- 60. Bu LL, Liu FT, Jiang CF, et al. Patterns of dopamine transporter imaging in subtypes of multiple system atrophy. Acta Neurol Scand. 2018;138:170–176. [DOI] [PubMed] [Google Scholar]
- 61. Booij J, de Jong J, de Bruin K, Knol R, de Win MM, van Eck-Smit BL. Quantification of striatal dopamine transporters with 123I-FP-CIT SPECT is influenced by the selective serotonin reuptake inhibitor paroxetine: a double-blind, placebo-controlled, crossover study in healthy control subjects. J Nucl Med. 2007;48:359–366. [PubMed] [Google Scholar]
- 62. Ziebell M, Holm-Hansen S, Thomsen G, et al. Serotonin transporters in dopamine transporter imaging: a head-to-head comparison of dopamine transporter SPECT radioligands 123I-FP-CIT and 123I-PE2I. J Nucl Med. 2010;51:1885–1891. [DOI] [PubMed] [Google Scholar]
- 63. Nicastro N, Fleury V, Broc N, Burkhard PR, Garibotto V. Extrastriatal 123I-FP-CIT SPECT impairment in degenerative parkinsonisms. Parkinsonism Relat Disord. 2020;78:38–43. [DOI] [PubMed] [Google Scholar]
- 64. Pilotto A, Schiano di Cola F, Premi E, et al. Extrastriatal dopaminergic and serotonergic pathways in Parkinson’s disease and in dementia with Lewy bodies: a 123I-FP-CIT SPECT study. Eur J Nucl Med Mol Imaging. 2019;46:1642–1651. [DOI] [PubMed] [Google Scholar]
- 65. Delva A, Van Weehaeghe D, van Aalst J, et al. Quantification and discriminative power of 18F-FE-PE2I PET in patients with Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2020;47:1913–1926. [DOI] [PubMed] [Google Scholar]
- 66. Fazio P, Svenningsson P, Forsberg A, et al. Quantitative analysis of 18F-(E)-N-(3-iodoprop-2-enyl)-2β-carbofluoroethoxy-3β-(4′-methyl-phenyl) nortropane binding to the dopamine transporter in Parkinson disease. J Nucl Med. 2015;56:714–720. [DOI] [PubMed] [Google Scholar]
- 67. Jakobson Mo S, Axelsson J, Jonasson L, et al. Dopamine transporter imaging with [18F]FE-PE2I PET and [123I]FP-CIT SPECT: a clinical comparison. EJNMMI Res. 2018;8:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Niccolini F, Su P, Politis M. Dopamine receptor mapping with PET imaging in Parkinson’s disease. J Neurol. 2014;261:2251–2263. [DOI] [PubMed] [Google Scholar]
- 69. Piccini P, Pavese N, Brooks DJ. Endogenous dopamine release after pharmacological challenges in Parkinson’s disease. Ann Neurol. 2003;53:647–653. [DOI] [PubMed] [Google Scholar]
- 70. Vriend C, Pattij T, van der Werf YD, et al. Depression and impulse control disorders in Parkinson’s disease: two sides of the same coin? Neurosci Biobehav Rev. 2014;38:60–71. [DOI] [PubMed] [Google Scholar]
- 71. O’Sullivan SS, Wu K, Politis M, et al. Cue-induced striatal dopamine release in Parkinson’s disease-associated impulsive-compulsive behaviours. Brain. 2011;134:969–978. [DOI] [PubMed] [Google Scholar]
- 72. de la Fuente-Fernández R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293:1164–1166. [DOI] [PubMed] [Google Scholar]
- 73. Sasaki M, Shibata E, Tohyama K, et al. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson’s disease. Neuroreport. 2006;17:1215–1218. [DOI] [PubMed] [Google Scholar]
- 74. Reneman L, van der Pluijm M, Schrantee A, van de Giessen E. Imaging of the dopamine system with focus on pharmacological MRI and neuromelanin imaging. Eur J Radiol. 2021;140:109752. [DOI] [PubMed] [Google Scholar]
- 75. Cassidy CM, Zucca FA, Girgis RR, et al. Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proc Natl Acad Sci USA. 2019;116:5108–5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cho SJ, Bae YJ, Kim JM, et al. Diagnostic performance of neuromelanin-sensitive magnetic resonance imaging for patients with Parkinson’s disease and factor analysis for its heterogeneity: a systematic review and meta-analysis. Eur Radiol. 2021;31:1268–1280. [DOI] [PubMed] [Google Scholar]
- 77. Matsuura K, Ii Y, Maeda M, et al. Neuromelanin-sensitive magnetic resonance imaging in disease differentiation for parkinsonism or neurodegenerative disease affecting the basal ganglia. Parkinsonism Relat Disord. 2021;87:75–81. [DOI] [PubMed] [Google Scholar]