Dear Editor,
Bedaquiline (BDQ) is a diarylquinoline antimycobacterial that specifically inhibits mycobacterial ATP synthase. BDQ has been positively associated with treatment success and reduced mortality in multi-drug-resistant TB (MDR-TB),1–4 and is now approved in >60 countries. However, non-target-based mechanisms can result in decreased susceptibility to BDQ.5–7 Mutations in Rv0678, a transcriptional repressor of genes encoding the MmpS5-MmpL5 efflux pump, with concomitant upregulation of the efflux pump, MmpL5, account for cross-resistance between clofazimine (CFZ) and BDQ.5,6,8,9 The presence of Rv0678 resistance-associated variants (RAVs) led to increased BDQ and CFZ minimal inhibitory concentrations (MICs) of 2- to 8-fold and 2- to 4-fold, respectively, in murine isolates, and increased BDQ MICs of 2- to 16-fold in clinical isolates.6,7 In murine isolates, a moderate BDQ MIC increase (3-fold) could be overcome by increasing the BDQ dose by 8-fold (from 6.25 to 50 mg/kg), but resistance of mutations yielding an 8-fold MIC increase could not be completely overcome.6 The efflux pump inhibitors verapamil (40 μg/mL) or reserpine (3 μg/mL) decreased the MICs of BDQ and CFZ in both drug-susceptible and drug-resistant isolates, but verapamil did not improve the bactericidal effect of BDQ in mice, and was unable to reverse efflux-based resistance in vivo.6 In many cases, Rv0678 RAVs in clinical isolates are not associated with prior use of BDQ or CFZ, and do not result in MICs above the BDQ susceptible breakpoint (≤0.12 μg/mL).7,10 Mutations in clinically relevant Rv0678 RAVs include single-nucleotide insertions, deletions and substitutions, large deletions, and random insertions of sequence elements.6 Previous studies have shown treatment failure on a BDQ-containing MDR-TB regimen, with emergence of Rv0678 RAVs.8,11–13 However, there is little evidence from controlled trials on whether acquisition of Rv0678 RAVs results in treatment failure, or if treatment failure results in acquisition of Rv0678 RAVs, or if patients with Rv0678 RAVs at baseline are more likely to fail BDQ treatment.
In an ad-hoc analysis of two Phase 2b BDQ clinical trial data, we investigated the impact on culture conversion rates of 1) presence of Rv0678 RAVs in Mycobacterium tuberculosis isolates prior to treatment initiation; 2) Rv0678 RAVs acquired during treatment; and 3) baseline BDQ MIC values of wild-type and Rv0678 isolates. In the 120-week TMC207-C208 Stage 2 (NCT00449644) and TMC207-C209 (NCT00910871) studies, BDQ was given for 24 weeks (400 mg once-daily for 2 weeks, then 200 mg three times a week for 22 weeks) with a background regimen of anti-TB drugs given for 18–24 months in TMC207-C2081 and up to 30 months in TMC207-C209.2 TMC207-C208 Stage 2 was a randomized trial involving 160 MDR-TB patients, including pre-extensively drug-resistant-TB (pre-XDR-TB), comparing the efficacy and safety of BDQ vs. placebo.1 TMC207-C209 was an open-label, single-arm trial involving 233 newly diagnosed or previously treated patients with MDR-TB (including pre-XDR-TB or XDR-TB) confirming the safety and efficacy of BDQ.2 Patients recruited to both studies had a broad range of characteristics, including many with known risk factors for delayed sputum conversion (e.g., HIV, diabetes and/or cavitary disease).1,2 Patients’ M. tuberculosis isolates were target-sequenced for previously described Rv0678 RAVs using the Sanger method.7 Corresponding BDQ MICs were determined by the 7H11 agar-dilution method.7,14 Microbiological outcomes (sputum culture conversion rates, no overruling for discontinuation) were assessed at Week 24 and endpoint (Week 120). Protocols were approved by an independent ethics committee/institutional review board, and all patients provided written informed consent.1,2
In the pooled TMC207-C208 and -C209 analysis, patients with baseline isolates without and with Rv0678 RAVs (n = 226) had comparable conversion rates at the end of BDQ treatment (Week 24) (148/188, 78.7% and 28/38, 73.7%, respectively; Fisher’s exact test P = 0.52) (Figure). In analyses performed for the subset of patients (n = 165) with ≥1 positive sputum culture post-baseline, patients with wild-type isolates who acquired Rv0678 RAVs post-baseline were more likely to fail treatment than those who did not acquire Rv0678 RAVs (Figure). In the subset of patients with ≤2 active drugs in their treatment regimen, the culture conversion rate among patients who remained wild-type post-baseline was significantly higher than those whose post-baseline isolates acquired Rv0678 RAVs (39/54, 72.2% vs. 2/9, 22.2%; P = 0.0065), and similarly, in the partially overlapping subset of patients with ≤3 active drugs (130/155, 83.9% vs. 4/10, 40.0%, respectively; P = 0.0034). Similar findings were seen at endpoint (Week 120: ≤2 active drugs: 37/50, 74.0% vs. 5/10, 50.0%; P = 0.1491; ≤3 active drugs: 86/109, 78.9% vs. 5/11, 45.5%; P = 0.0231).
Figure.
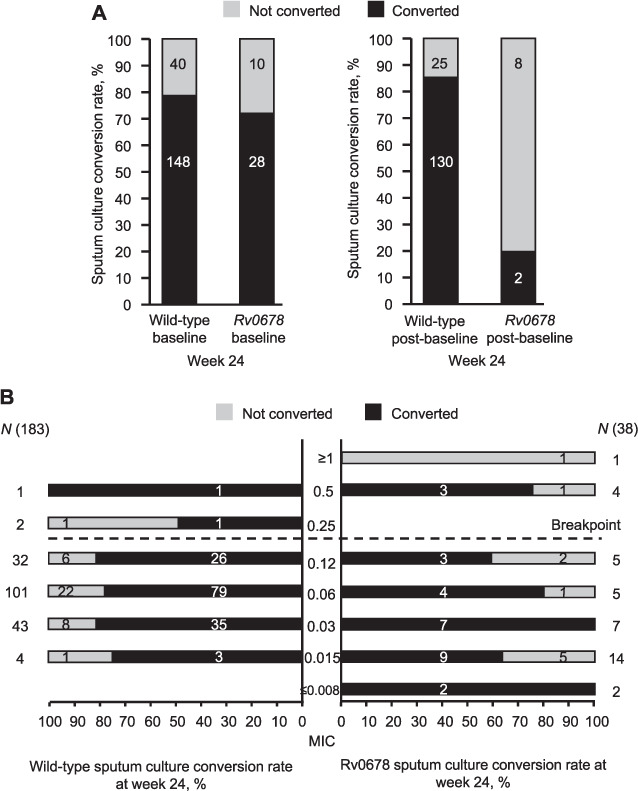
Effects of A) Rv0678 RAVs in baseline isolates and Rv0678 RAVs acquired during treatment, and B) baseline BDQ MIC for wild-type and Rv0678 baseline isolates on sputum culture conversion rates at Week 24 (no overruling for discontinuation) for BDQ-treated patients in the Phase 2b studies, TMC207-C208 Stage 2 and TMC207-C209. Sputum culture conversion was defined as two consecutive negative cultures from sputum samples collected at least 25 days apart. All intermediate cultures had to be negative as well. This condition was overruled when followed by a confirmed positive result, defined as two consecutive visits with positive sputum results, not taking into account intermittent missing/contaminated results. In the no-overrulingfor-discontinuation analysis, discontinuation information was not taken into account (patients who converted, then discontinued afterwards were considered as converted). In A), the numbers in the bars represent the actual number of isolates in each category (Week 24, P = 0.52 for culture conversion rate with baseline wild-type isolates vs. baseline Rv0678 RAVs; Week 24, P < 0.0001 for culture conversion rate with post-baseline wild type isolates vs. post-baseline Rv0678 RAVs). In B), the numbers in the bars represent the actual number of isolates in each category. RAV = resistance-associated variant; BDQ = bedaquiline; MIC = minimal inhibitory concentration.
For patients with BDQ MICs below the breakpoint, there was no correlation with culture conversion at Week 24 (Figure). For patients with BDQ MICs above the breakpoint, there were insufficient data to draw conclusions. Similar findings were seen at endpoint (Week 120). Based on this ad-hoc analysis in MDR-TB patients receiving BDQ in the Phase 2b TMC207-C208 Stage 2 and TMC207-C209 studies,1,2 the presence of Rv0678 RAVs at baseline was not associated with poor treatment outcome.
We and others have described treatment failure on a BDQ-containing MDR-TB regimen coinciding with the emergence of Rv0678 RAVs.8,11–13 However, given the apparent lack of effect of baseline Rv0678 RAVs on treatment outcome, this is not a straightforward process to explain. We show that culture conversion can be achieved even in the presence of high BDQ MICs, provided the background regimen remains strong (it should be noted that the concurrent emergence of resistance to background drugs may also occur). This observation is made with caution because of the low incidence of baseline isolates with high MICs, which is to be expected for a drug with a new mechanism of action and limited clinical exposure at the time of conducting the Phase 2b studies. We did not evaluate any correlation between increased baseline BDQ MICs and treatment outcome in patients with wild-type vs. Rv0678 RAVs at baseline. However, there are a variety of RAVs in Rv0678 with variable effects on the BDQ MIC,7 and it is not possible to develop an algorithm to predict BDQ MICs based on specific Rv0678 RAVs. Consequently, based upon the limited available information, sequencing for Rv0678 RAVs is not useful to rule in BDQ susceptibility – it could only be used to exclude the likelihood of resistance due to Rv0678 RAVs. This makes development of a rapid genotypic drug susceptibility test (DST) challenging. Thus, a standardized phenotypic DST method should be considered to determine susceptibility of M. tuberculosis to BDQ, especially among pretreated MDR-TB patients.11
In conclusion, no clear relationship was observed between the presence of isolates with Rv0678 RAVs at baseline and poor treatment outcome. However, patients with wild-type isolates who acquired Rv0678 RAVs post-baseline were more likely to fail treatment than those who did not acquire Rv0678 RAVs.
Acknowledgements
The authors thank the participants, study center staff and Janssen (Beerse, Belgium) study personnel. Medical writing and editorial support were provided by I Woolveridge and M Urbacz of Ashfield MedComms (Macclesfield, UK). Janssen supported the work and BDQ Phase 2b studies. At the time of writing, all authors were in full-time Janssen employment and are potential stockholders of Johnson and Johnson (Titusville, NJ, USA).
References
- 1.Diacon AH, et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med. 2014;371:723–732. doi: 10.1056/NEJMoa1313865. [DOI] [PubMed] [Google Scholar]
- 2.Pym AS, et al. Bedaquiline in the treatment of multidrug- and extensively drug-resistant tuberculosis. Eur Respir J. 2016;47:564–574. doi: 10.1183/13993003.00724-2015. [DOI] [PubMed] [Google Scholar]
- 3.Schnippel K, et al. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir Med. 2018;6:699–706. doi: 10.1016/S2213-2600(18)30235-2. [DOI] [PubMed] [Google Scholar]
- 4.Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment–2017 et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018;392:821–834. doi: 10.1016/S0140-6736(18)31644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartkoorn RC, Uplekar S, Cole ST. Cross-resistance between clofazimine and bedaquiline through upregulation of MmpL5 in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2014;58:2979–2981. doi: 10.1128/AAC.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andries K, et al. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One. 2014;9:e102135. doi: 10.1371/journal.pone.0102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villellas C, et al. Unexpected high prevalence of resistance-associated Rv0678 variants in MDR-TB patients without documented prior use of clofazimine or bedaquiline. J Antimicrob Chemother. 2017;72:684–690. doi: 10.1093/jac/dkw502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghodousi A, et al. Acquisition of cross-resistance to bedaquiline and clofazimine following treatment for tuberculosis in Pakistan. Antimicrob Agents Chemother. 2019;63:e00915–19. doi: 10.1128/AAC.00915-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ismail N, et al. Clofazimine exposure in vitro selects efflux pump mutants and bedaquiline resistance. Antimicrob Agents Chemother. 2019;26:63. doi: 10.1128/AAC.02141-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaniga K, et al. Validation of bedaquiline phenotypic drug susceptibility testing methods and breakpoints: a multilaboratory, multicountry study. J Clin Microbiol. 2020;58:e01677–19. doi: 10.1128/JCM.01677-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vos M et al. Bedaquiline microheteroresistance after cessation of tuberculosis treatment. N Engl J Med. 2019;380:2178–2180. doi: 10.1056/NEJMc1815121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peretokina IV, et al. Reduced susceptibility and resistance to bedaquiline in clinical M. tuberculosis isolates. J Infect. 2020;80:527–535. doi: 10.1016/j.jinf.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, et al. Reduced susceptibility of Mycobacterium tuberculosis to bedaquiline during antituberculosis treatment and its correlation with clinical outcomes in China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1002. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Kaniga K, et al. A multilaboratory, multicountry study to determine bedaquiline MIC quality control ranges for phenotypic drug susceptibility testing. J Clin Microbiol. 2016;54:2956–2962. doi: 10.1128/JCM.01123-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


