SUMMARY
BACKGROUND:
Optimal drug dosing is important to ensure adequate response to treatment, prevent development of drug resistance and reduce drug toxicity. The aim of these clinical standards is to provide guidance on ‘best practice’ for dosing and management of TB drugs.
METHODS:
A panel of 57 global experts in the fields of microbiology, pharmacology and TB care were identified; 51 participated in a Delphi process. A 5-point Likert scale was used to score draft standards. The final document represents the broad consensus and was approved by all participants.
RESULTS:
Six clinical standards were defined: Standard 1, defining the most appropriate initial dose for TB treatment; Standard 2, identifying patients who may be at risk of sub-optimal drug exposure; Standard 3, identifying patients at risk of developing drug-related toxicity and how best to manage this risk; Standard 4, identifying patients who can benefit from therapeutic drug monitoring (TDM); Standard 5, highlighting education and counselling that should be provided to people initiating TB treatment; and Standard 6, providing essential education for healthcare professionals. In addition, consensus research priorities were identified.
CONCLUSION:
This is the first consensus-based Clinical Standards for the dosing and management of TB drugs to guide clinicians and programme managers in planning and implementation of locally appropriate measures for optimal person-centred treatment to improve patient care.
Keywords: tuberculosis, pharmacokinetics, pharmacodynamics, adverse drug reaction, management, dosing
Abstract
CONTEXTE :
Une posologie optimale est importante afin de garantir une réponse adéquate au traitement, de prévenir le développement de résistances aux médicaments et de réduire la toxicité liée aux médicaments. L’objectif de ces normes cliniques est de donner des indications de « bonne pratique » en matière de posologie et de gestion des agents antituberculeux.
MÉTHODES :
Un panel de 57 experts internationaux spécialisés en microbiologie, pharmacologie et soins antituberculeux a été identifié ; 51 ont participé à un processus Delphi. Une échelle de Likert à 5 points a été utilisée pour noter les premières ébauches des normes. Le document final est fondé sur un large consensus puisqu’il a été approuvé par tous les participants.
RÉSULTATS :
Six normes cliniques ont été définies : Norme 1, définir la dose initiale la mieux adaptée au traitement de la TB ; Norme 2, identifier les patients potentiellement à risque d’exposition sous-optimale aux médicaments ; Norme 3, identifier les patients à risque de développer une toxicité liée aux médicaments et déterminer comment diminuer au mieux ce risque ; Norme 4, identifier les patients pouvant bénéficier d’un suivi thérapeutique pharmacologique (TDM) ; Norme 5, définir les informations et conseils à fournir aux patients placés sous traitement antituberculeux et Norme 6, enseigner les fondamentaux aux professionnels de santé. Les priorités de recherche ont également été définies, sur la base d’un consensus.
CONCLUSION :
Il s’agit des premières normes cliniques, fondées sur un consensus, en matière de posologie et de gestion des antituberculeux. Elles ont pour objectif d’orienter les cliniciens et les responsables de programme en matière de planification et de mise en place de mesures locales adéquates pour un traitement optimal centré sur le patient, afin d’améliorer la prise en charge.
Treatment of TB is aimed at more than simply curing a patient. Possible drug-related adverse effects (AEs) must be balanced against effective treatment to reduce ongoing transmission, prevent future disease, development of drug resistance and chronic post-TB disease. Effective treatment for TB is highly dependent on early diagnosis, and rapid and adequate treatment initiation. Sub-optimal drug exposure often facilitates the emergence of drug-resistant TB, and it is now well-known that drug-related AEs are also common. By studying the absorption, distribution, metabolism and excretion (ADME) of individual TB drugs, as well as the effect of drug transporters,1 important differences in pharmacokinetics (PK) have been observed between patients. These inter-individual differences help to explain why some patients show poor treatment response, or have a higher risk of suffering from significant AEs.2 The introduction of hollow-fibre infection models has contributed significantly to our understanding of the relation between drug exposure and antibacterial effect.3 Detailed dose fractionation studies have identified optimal drug dosing strategies that maximise the treatment efficacy and reduce the risk of acquired drug resistance.4 An example of the value of these critical evaluations is our reconsideration of how best to dose rifampicin (RIF),5 a drug that has been in clinical use since the 1960s. Initial dosing recommendations were mainly influenced by price considerations and not optimal efficacy. It is now widely known that its maximum therapeutic effect is not achieved at the standard recommended dose,6,7 a recognised limitation of current treatment regimens. Newer TB drugs such as bedaquiline (BDQ), delamanid (DLM) and pretomanid (Pa),8–10 as well as repurposed antibiotics (such as moxifloxacin [MFX] and linezolid [LZD]) are widely used in the treatment of drug-resistant TB, but optimal dosing strategies are still being investigated.11–13
AIM OF THE CLINICAL STANDARDS
Our aim is to provide guidance on ‘best practice’ for dosing and management of TB drugs, identifying important clinical considerations to inform dosing decisions for both adults and children. For some TB drugs, selecting the most appropriate dose is challenging, as there is limited evidence to inform dose adjustments in specific circumstances. In these situations, the pharmacological principles described should guide dosing decisions.14 Fortunately, there is a rapidly growing body of literature informing better TB drug dosing, including in children.15
This consensus-based document describes the following activities:
Defining the most appropriate initial dose for TB treatment (Standard 1).
Identify patients who may be at risk of sub-optimal drug exposure (Standard 2)
Identify patients at risk of developing drug-related toxicity and how best to manage this risk (Standard 3).
To identify patients who can benefit from TDM (Standard 4).
Highlighting education and counselling that should be provided to people initiating TB treatment (Standard 5).
Provide essential education for healthcare professionals (Standard 6).
In addition, consensus research priorities were identified.
METHODS
A panel of global experts was identified to represent the main scientific societies, associations and groups active in global TB management and TB treatment research. Of the 57 experts initially invited, six did not respond after one invitation reminder. All respondents (n = 51) were asked to comment via a Delphi process on six draft standards developed by a core team (n = 8) of researchers; everyone provided valid answers and constructive input. The final panel included TB clinicians (n = 24), TB public health specialists (n = 5), TB paediatricians (n = 5), pharmacologists (n = 12), microbiologists/biologists (n = 4) and a TB trials methodologist (n = 1). A 5-point Likert scale (5: high agreement; 1: low agreement) was used to indicate agreement with the standards. At the first Delphi round, agreement was high, with a median value of >4.6 (for all standards). Due to the high agreement (defined as quartile deviation [Q3-Q1/2] ≤0.6),16 no major changes to the draft standards were made. Based on substantial initial agreement, a draft document was developed by the expert panel. The document underwent two rounds of revisions, and the final version was approved by consensus (100% agreement).
STANDARD 1
Every patient should receive the most appropriate drug dose when starting TB treatment to avoid too low or too high drug exposure, which could result in treatment failure or adverse drug effects
In the current WHO guideline, advice is provided regarding dose adjustment for bodyweight and renal function.17 However, other factors contribute to variability in the pharmacokinetics of TB drugs, such as age, malnutrition, hepatic function, diabetes (DM) and HIV status, pregnancy, disease severity, genetic factors predisposing for rapid drug metabolism, drug–drug interactions with concomitant treatment, drug absorption and food-drug interactions. Selecting the most appropriate doses for children is hindered even more by insufficient data on PK, bioavailability and treatment efficacy, especially among the most vulnerable paediatric populations (including those living with HIV, those who are malnourished and those who fall in understudied age groups such as <2 years). These aspects therefore require careful consideration in conjunction with drug susceptibility testing (DST) results (phenotypic and/or genotypic) collected from the patient (or likely source in the context of children) when selecting the optimal treatment regimen and drug dosages to avoid treatment failure and/or AEs.18 Details on individual drugs and factors that require consideration are presented in Figure 1.
Figure.
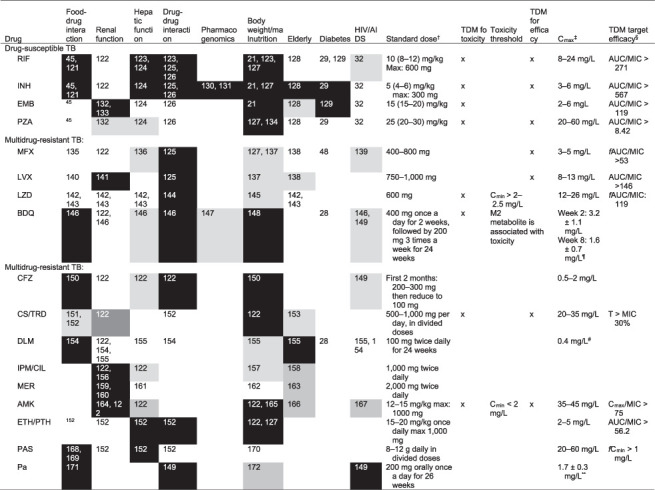
Factors contributing to variability in pharmacokinetics, as well as the efficacy and toxicity of drugs used to treat TB.* *Factors which are likely (black), might (light grey) or are unlikely (white) to contribute to variability in drug efficacy or toxicity and which should be considered when making drug selections or dose adjustments. †WHO-recommended doses for adults.173 ‡Reference values for Cmax after a standard dose.64 §The PK/PD targets were previously reported and are dependent on the precise MIC methodology used in the respective studies.104 Because of the systematic differences between some MIC methods, these targets cannot be used directly with some MIC methods.114 The PK/PD targets should be used in a multiprofessional team experienced in TDM. ¶Reference values for Cmax after standard dose.174 **Reference value for Cmax after standard dose.175 TDM=therapeutic drug monitoring; Cmax=maximum concentration (of a drug); RIF = rifampicin; AUC = area under the curve; INH = isoniazid; EMB= ethambutol; PZA = pyrazinamide; MFX ¼moxifloxacin; LVX = levofloxacin; LZD = linezolid; BDQ = bedaquiline; CFZ = clofazimine; CS = cycloserine; TRD = terizidone; DLM = delamanid; IPM/CIL = imipenem/cilastatin; MER = meropenem; AMK = amikacin; ETH = ethionamide; PTH = prothionamide; PAS = para-aminosalicylic acid; Pa = pretomanid; MIC = minimum inhibitory concentration; fAUC = free area under the concentration time curve; Cmin = minimum concentration (of a drug); PK = pharmacokinetics; PD = pharmacodynamics.
Weight-based dose selection of TB drugs should be carefully considered in cases with extremely high or low body weights, and in children, especially when no maximal dose limit is provided. It is important to appreciate that severe states of malnutrition are associated with changes in body composition, hypoproteinaemia, gastrointestinal disturbances (such as diarrhoea and malabsorption) and decreased renal function.19,20 This can impact the PK of some TB drugs, thereby necessitating dose adjustments.21 In children, age-related maturation of drug metabolism pathways may be more important than weight, although the two are usually closely correlated. Age-related effects on renal clearance and metabolism of TB drugs are also present in very young children (<2 years old), often resulting in reduced absorption, metabolism and elimination. A full understanding of the age, weight and potential hormonal impacts on drug metabolism in adolescents is understudied. Increasing age is associated with a decline in renal function, requiring dose adjustment of renally excreted drugs (e.g., amikacin [AMK]). The risk of hepatotoxicity (e.g., for isoniazid [INH], pyrazinamide [PZA]) also increases with age.22 Therefore, age and weight must be taken into consideration when deciding on the dose.23–25
For renally cleared drugs, dose adjustments are required in individuals with renal impairment, including those receiving renal dialysis, to avoid supra-therapeutic drug exposure and the potential for increased risk of AEs.26 Similarly, for TB drugs that are predominantly metabolised by the liver or may cause hepatotoxicity, dose adjustments or alternative drugs require consideration in patients with severe hepatic impairment.26 In patients with chronic liver disease (e.g., cirrhosis), potentially hepatotoxic drugs should be avoided, particularly if there are alternatives available, given that the risk of severe hepatoxicity and even liver failure is markedly increased.
A risk factor for developing TB disease in case of TB infection,27 DM is also associated with delayed treatment response and lower cure rates. The increased risk of relapse and the emergence of drug resistance, particularly in case uncontrolled glucose, are potentially related to altered drug exposure due to delayed gastric emptying and/or drug–drug interactions with hypoglycaemic agents.28,29 Metformin can be considered a preferred agent as its hypoglycaemic effect was not affected by TB drugs, especially RIF.30,31 Patients who develop nausea and vomiting while on metformin require alternative treatment.
As to the effect of HIV infection, more than half of the studies that included both HIV-positive and HIV-negative TB groups showed statistically significant alterations in total exposure and/or peak plasma concentrations for at least one first-line TB drug, but studies were too heterogeneous to derive consistent conclusions.32 Special attention should be paid to drug–drug interactions.
Pharmacogenomics is known to play an important role in the metabolism of INH, influencing efficacy and risk of AEs.33 A meta-analysis demonstrated a 2-fold higher likelihood of bacteriological failure in rapid acetylators compared to slow acetylators.34 A randomised controlled trial demonstrated that INH drug-induced liver injury could be prevented in slow acetylators by a dose reduction to 2.5 mg/kg/day and without early treatment failure.35 However, determination of NAT-2 genotype (acetylator status) is not routinely available in most settings. Repurposing the widely available GeneXpert (Cepheid, Sunnyvale, CA, USA) platform to perform such tests holds promise for implementation in programmatic care.36 The acetylator status can also be assessed by therapeutic drug monitoring (TDM) if this incorporates the measurement of both INH and its metabolite acetyl INH. One single sample of plasma or saliva is enough for the assessment of the concentration ratio (or metabolic ratio) of acetyl INH to INH, which can be translated to fast or slow acetylator status of the patient.37–39
Rifamycins (RIF, rifapentine [RPT], rifabutin) induce several cytochrome P450 (CYP450) enzymes and drug transporters, which can significantly decrease concentrations of drugs eliminated via these routes.40,41 Conversely, INH inhibits a range of CYP450 enzymes, including CYP3A4 and therefore can increase concentrations of drugs metabolised by this enzyme.42 As the inductive effect of RIF generally outweighs the inhibitory effect of INH, the overall effect is a net decrease in the concentrations of many drugs. Caution should be exercised in the co-administration of nephrotoxic TB drugs (e.g., AMK) with other nephrotoxic drugs (e.g., tenofovir), with regular monitoring of renal function. Older patients, people living with HIV and other immunocompromised patients with TB requiring polypharmacy are at particular risk of drug–drug interactions.
The presence of food can either enhance or reduce drug absorption. As described in the product information, co-administration with food is required for drugs such as BDQ, DLM, Pa, RPT and clofazimine (CFZ), whereas RIF and INH should preferably be taken on an empty stomach,43–45 but they can be taken with a light meal or snack to prevent or alleviate gastrointestinal AEs and subsequent non-adherence.
When DST demonstrates low-level resistance (LLR), clinicians should consider these findings and, ideally, select a different drug to which the isolate is fully susceptible or increase the dose and use TDM when available. For some drugs, a higher dose can be tolerated and achieve the target exposure (Table 1). Although such a decision can be justified based on pharmacological principles, supportive clinical data are scarce and higher doses to overcome LLR should be avoided where possible. Lower-than-standard doses should be avoided and may only be considered with an otherwise strong core regimen when severe AEs to some drugs, such as LZD, cannot be otherwise avoided or managed.46
Table 1.
Overview of low-level drug resistance mechanisms for key first and second-line TB drugs and their corresponding PK/PD targets for TDM and increased dosing strategies
| Drug | Range* | Mode of susceptible MIC distribution* | CC* | LLR mutation(s)† | Typical LLR MIC-range* | Standard dose?‡ | High dose‡ | Maximum dose§ | TDM? | Target AUC/MIC¶ |
|---|---|---|---|---|---|---|---|---|---|---|
| RIF | 0.016–0.25 | 0.06 | 0.5 | Borderline resistance mutations# | 0.125–4 | No | 20–35 mg/kg | 2,100 mg | Yes | >271 |
| INH | 0.016–0.125 | 0.06 | 0.1 | inhA (c-15t) | 0.25–1 | No | 10 mg/kg | 900 mg | Yes | >567 |
| LVX | 0.125–1 | 0.5 | 1 | gyrA A90V, S91P and D94A | 2–4 | No | 15–20 mg/kg | 1,500 mg | Yes | >146 |
| MFX | 0.064–0.25 | 0.125 | 0.25 | gyrA A90V, S91P and D94A | 0.125–2 | No | 10–15 mg/kg | 800 mg | Yes | >53 |
* All figures in mg/L tested using non-standardised protocols as reported in the literature.109,110 These values apply to MGIT and cannot necessarily be used for other growth media because systematic differences may exist compared with MGIT.114
† A higher dose should only be considered, if no additional mutations are present that may raise the MIC even further, thereby conferring high-level resistance (e.g., katG S315T in addition to inhA c-15t or gyrA D94G in addition to gyrA A90V).109,110,176,177 Therefore, the detection of high-level resistance mutations or MICs of >1 mg/L for MFX (i.e., the WHO clinical breakpoint) and, >1 mg/L for INH (CLSI currently recommends >0.4 mg/L) are exclusion criteria for the use of these agents, irrespective of the dose used.109,110,176
‡ When one of these low-level resistance mutations is present, the standard dose is insufficient and should not be used. The level of evidence for whether, and to what extent, low-level resistance can be overcome with a high dose is very low and largely based on expert opinion.178–180 The use of high-dose MFX has been endorsed by the WHO to overcome low-level resistance as part of the long individualised regimen by extrapolating data to high-dose GFX, which is being extrapolated further to high-dose LVX in this publication.110 Given these uncertainties, increased dosing for low-level resistant isolates should be avoided but may be critical where only less effective or more toxic drugs are available. If higher doses are used in this context, the cautious approach would be to use TDM to verify the drug exposure and not to consider the agent in question as a core drug of the regimen.
§ Drug safety at higher dosages is important, active monitoring and use of TDM can help to increase safety.
¶ The PK/PD targets were previously reported and are dependent on the precise MIC methodology used in the respective studies.104 Because of the systematic differences between some MIC methods, these targets cannot be used directly with other MIC methods.114 The PK/PD targets should be used in a multi-professional team experienced in TDM.
# L430P, D435Y, H445L/N/S, L452P, and I491F.109
PK = pharmacokinetics; PD = pharmacodynamics; TDM = therapeutic drug monitoring; CC = critical concentration; LLR = low-level resistance; MIC = minimum inhibitory concentration; AUC = area-under the concentration time curve; RIF = rifampicin; INH = isoniazid; LVX = levofloxacin; MFX = moxifloxacin; MGIT = Mycobacteria Growth Indicator Tube; CLSI = Clinical and Laboratory Standards Institute.
STANDARD 2
Patients should be re-evaluated when demonstrating slower response to TB treatment than expected
Further investigation is warranted when patients are showing signs of sub-optimal treatment response, such as lack of clinical improvement or persistent sputum smear or culture positivity (>2 months for drug-susceptible TB), despite phenotypically proven drug susceptibility. Patients should be evaluated for the following risk factors associated with sub-optimal drug exposure: sub-optimal drug dosing, drug–drug interactions, food–drug interactions, gastrointestinal conditions (e.g., malabsorption and diarrhoea), diabetes, HIV/AIDS, non-adherence, host genetic factors predisposing for rapid drug metabolism (e.g., NAT2 genotype), pathogen factors strains with borderline or LLR as determined using phenotypic and/or genotypic DST. As drug penetration in TB cavities can be sub-optimal, verification of drug concentrations and/or higher dosages may be required for adequate treatment response.47
Although it is critically important to assess for medication adherence and underlying reasons contributing to potential non-adherence, it is equally important to assess for other factors that could contribute to sub-optimal drug exposure (e.g., the right dose has been prescribed and no drug– or food–drug interactions compromising drug exposure are present). Patients with other medical conditions such as gastrointestinal conditions, DM48,49 and/or HIV/AIDS50 may benefit from TDM, which allows for dose individualisation based on measurement of drug concentrations (Figure 1).51,52 If these issues have been ruled out or considered unlikely, repeating DST is recommended to exclude any possibility of drug-resistant disease, especially in situations including presence of cavitary disease, and/or prior history of TB treatment, or pregnancy. When DST results are discrepant, appropriate adjustments should be made. If drug susceptibility of the Mycobacterium tuberculosis isolate is reconfirmed for a patient who is not responding adequately to treatment, it is important to consider the potential for rapid metabolism of INH.34,35 Ideally, a pharmacogenetic test or TDM should be performed to determine the acetylator status of the patient.
As drug exposure reflects all these underlying factors, TDM may be recommended in patients not responding to treatment when available (Figure 1).51–54
STANDARD 3
The risk of TB drug toxicity should be minimised by initial screening and ongoing clinical monitoring. Toxicity specific to TB drugs should be prevented and appropriately managed to prevent harm and limit its contribution to poor treatment-adherence
As a result of receiving a multidrug combination, TB patients may experience various AEs.55 Although the global prevalence of AEs is generally underreported, AEs are more commonly experienced (range: 8–96%) than previously appreciated.56 AEs include gastrointestinal disturbances, hepatotoxicity, ototoxicity, nephrotoxicity and peripheral neuropathy (Table 2).56 Cutaneous reactions (not dose-/concentration-dependent) to first- and second-line TB drugs have also been reported.57 Certain AEs can be life-threatening if not identified early and promptly managed.58,59 Several risk factors, which can be classified as patient, drug and social, contribute to AE risk and require consideration of dose adjustments (Table 2).60
Table 2.
Toxicity associated with TB drugs, potential risk factors for developing drug toxicity and recommended monitoring practices *
| Drug | Toxicity | Patient populations at potentially greater risk of toxicity) | Suggested monitoring (frequency) | Comments |
|---|---|---|---|---|
| Drug-susceptible TB | ||||
| INH |
|
Older patients (>65 years), sex (male),181 diabetes mellitus,182 host genetics (NAT2 variants for isoniazid slow or intermediate acetylators,131,183,184 HIV,185 liver disease | Liver function tests, peripheral sensibility (monthly) | To minimise risk of peripheral neuropathy, co-administrated of vitamin B6 is recommended, particularly in patients at risk (e.g., children, during pregnancy or if alcohol abuse or other predisposing condition is present). In patients with liver disease (without cirrhosis) and with AST and ALT >5x ULN, consider an alternative regimen like RIF, EMB and LVX. In patients with cirrhosis, consider use of an alternative regimen (with an injectable, EMB and LVX) |
| RIF |
|
Sex (male),181 HIV,185 liver disease, polypharmacy (drug-drug interactions) | Liver function (monthly) | Check for drug-drug interactions with all accompanying drugs, online data bases available. Educate patients about discoloration of body fluids. In patients with cirrhosis, consider use of an alternative regimen (with CPM, EMB, and LVX) |
| RPT |
|
Sex (male),181 HIV,185 liver disease, polypharmacy (drug-drug interactions) | Liver function (monthly) | Check for drug-drug interactions with all accompanying drugs, online data bases available |
| PZA |
|
Older patients (>65 years), sex (male),181 HIV,185 liver disease, renal disease | Liver function (monthly) | Watch out for rash, drug-induced liver injury and arthralgia. Omit in older patients (>65 years). In patients with liver disease (without cirrhosis) and with AST and ALT > 5x ULN, consider an alternative regimen with RIF, EMB and LVX. In patients with cirrhosis, consider use of an alternative regimen (with CPM, EMB and LVX). In patients with creatinine clearance <30 mL/min, consider using EMB and PZA only 3 times a week (at the usual dose) |
| EMB |
|
Children (<2 years),186,187 diabetes mellitus,188 older patients (>65 years), HIV,185 renal disease189 | Colour/visual acuity (monthly) | Reduce or stop with renal insufficiency. Use commonly avoided in young children and patients that cannot reliably report colour/visual acuity |
| • Multidrug-resistant TB | ||||
| FQs (LVX/MFX) |
|
HIV (185), children (<2 years), children and older patients (>65 years) (increased risk of tendon damage) | (Electrolytes), QTcF interval,1 painful tendons/joints (monthly) | LVX is less likely to cause QTcF prolongation than MFX.190 MFX can cause liver toxicity. LVX needs dose reduction with renal insufficiency Risk of Achilles tendinitis or rupture (especially when combined with corticosteroids), arthralgia and and aortic aneurysm/dissection (rare events). Reduced seizure threshold. Clostridium difficile associated diarrhoea |
| BDQ |
|
Children (<2 years),186,187 HIV185 | Electrolytes, liver function, QTcF interval (2 weeks, 12 weeks and 24 weeks) | An increased monitoring of baseline, 2 weeks and monthly ECGs during the treatment is recommended if BDQ is used in combination with other QT-prolonging drugs such as FQs and CFZ. Check for drug-drug interactions191 |
| LZD |
|
Older patients (>65 years), HIV,185 host genetics | Colour/visual acuity, full blood count, peripheral neuropathy (monthly) | Beware of lactic acidosis and serotonin syndrome due to drug interactions |
| CS/TRD |
|
Epilepsy, depression, psychosis, severe anxiety | Mental health evaluation (monthly) | Beware of suicide ideations and peripheral neuropathy. Avoid or monitor combination with INH and thionamides (increased risk of neurotoxicity). Administer concomitantly pyridoxine (vitamin B6) |
| CFZ |
|
Severe hepatic impairment | Electrolytes, liver function, QTcF interval | Skin discoloration or hyperpigmentation is common and can be disturbing to patients. Take with food to improve bioavailability and gastrointestinal tolerance. Protect skin from the sun |
| DLM |
|
Children (<2 years),186,187 HIV185 | Electrolytes, liver function, QTcF interval (2 weeks, 12 weeks and 24 weeks) | |
| Imipenem-cilastatin/meropenem3 |
|
History of seizures, renal impairment | Used in combination with amoxicillin-clavulanic acid. Beware of LFT rise with meropenem and reduced seizure threshold with imipenem/cilastatin. Clostridium difficile associated diarrhoea | |
| AMK |
|
Older patients (>65 years), HIV (185), renal, vestibular, auditory or severe hepatic impairment | Renal and electrolyte function, audiometry (monthly) | TDM for AMK involves trough levels to avoid toxic concentrations and is highly recommended if available.76,92 Formal hearing testing must be done for children and adults at baseline and at 2 weeks and regularly thereafter at fortnightly or monthly intervals. Avoid in patients that cannot perform a hearing test |
| ETH/PTH |
|
HIV185 | Liver function, TSH/T4 | Monitor combination with CS or TRD (increased risk of seizures) and PAS (increased risk of gastrointestinal disturbances and hypothyroidism). Administer concomitantly pyridoxine (vitamin B6) |
| PAS |
|
Electrolytes, liver function, TSH/T4 | Monitor combination with ETH/PTH (increased risk of hypothyroidism and gastrointestinal disturbances) | |
| Pa |
|
Liver function |
* ECG monitoring is recommended using the Fridericia method of QT correction. Check concomitant QTc prolonging drugs if QTcF > 450 ms; STOP all QTc prolonging drugs if QTcF> 500 ms. Sometimes other drugs can be spared to avoid stopping TB treatment.
INH = isoniazid; AST = aspartate transaminase; ALT = alanine aminotransferase; ULN = upper limit of normal; RIF = rifampicin; EMB = ethambutol; LVX = levofloxacin; CPM = capreomycin; RPT = rifapentine; PZA = pyrazinamide; FQ = fluoroquinolone; MFX = moxifloxacin; BDQ = bedaquiline; ECG = electrocardiogram; CFZ = clofazimine; LZD = linezolid; CS = cycloserine; TRD = terizidone; DLM = delamanid; LFT = liver function test; TDM = therapeutic drug monitoring; AMK = amikacin; ETH = ethionamide; PTH = prothionamide; TSH = thyroid stimulating hormone; PAS = para-aminosalicylic acid; Pa = pretomanid.
Patient-related risk factors for AEs include age, sex and whether pregnant or nursing, which contribute to individual variability in PK and associated exposure-dependent AEs. Drug exposure can be affected by ADME, which naturally varies with the extremes of age. For example, the NAT2 enzyme does not seem to fully mature until later childhood.61 Older patients exhibit changes in body composition and reduction of renal and liver function, predisposing them to AEs.62 Furthermore, comorbidities such as HIV/AIDS, DM, and liver and renal diseases are frequently associated with PK variability and AEs, either through organ-specific changes or drug–drug interactions.63,64 First-line TB drugs can be used during pregnancy, but safety data to support the use of TB drugs during pregnancy are scarce for second-line drugs.65 FQs and BDQ should be used with caution, while ETH and aminoglycosides should be avoided.66 Human data on DLM and Pa are lacking. The characteristic AE profiles of individual TB drugs require careful consideration in specific patient populations and dose adjustments, and avoidance of the drug may be required/indicated (Table 2).
The joint effects of excessive alcohol consumption and smoking, especially cigarettes, can increase the frequency of severe AEs, most notably in patients with prior hepatic steatosis or cirrhosis.67 Alcohol use during TB treatment has also been associated with peripheral neuropathy, hyperuricaemia and optic neuritis.68 In addition to monitoring drug exposure and potential dose adjustment, assessment of potential substance use and the need for specific support for lifestyle improvements should be integral to TB care.69 States of malnutrition also increase the risk of toxicity.70
Routine monitoring and patient counselling/health education is important during TB treatment to avoid and identify AEs early.71,72 Careful medical history taking is crucial as AEs are not always volunteered by the patient.73 Patients should be reassured that TB treatment is generally safe and AEs can be managed if they occur. Although children tend to experience fewer AEs than adults, monitoring recommendations follow the same principles as in adults (Table 2).74
Although nausea is the most common, the most important AE to first-line TB drugs is hepatotoxicity, as it can be life-threatening. Liver function test derangements are often mild and transient but can be severe. Drug-induced liver toxicity has been described for all first-line drugs (i.e., RIF, INH, PZA) apart from EMB.75 In addition, all patients/parents/care givers should be informed about the red/orange discoloration of urine and other body fluids by RIF, which is universal and not an AE.
Patient awareness and routine monitoring of visual acuity and colour vision (Ishihara chart) to detect optic neuritis is important if treatment involves EMB or LZD (baseline and monthly checks recommended).76 Monitoring for visual changes can be challenging in young children, or critically ill patients, and may not be feasible in many settings or where patients have pre-existing eye disease, especially cataract. Despite known challenges in testing visual changes in young children or critically ill patients, this should still be an objective of routine follow-up, or an alternative agent should be considered.
In currently recommended regimens for multidrug-resistant TB (MDR-TB) treatment, one of the Group A drugs most commonly associated with serious AEs is LZD.72 The risk of bone marrow suppression with LZD requires regular full blood count monitoring (baseline, 2 weeks, then monthly). Peripheral neuropathy is commonly associated with long-term use of LZD and less frequently with INH, CS and FQs.72,77 These possible and serious AEs require patient and care provider awareness, as well as monthly peripheral neuropathy and vision assessment. Peripheral neuropathy in young children can be difficult to diagnose and signs may include refusal to bear weight or complaints of pain or irritability.
Several drugs increase the QTc interval, which when significantly elevated (>500 ms), can increase the risk of heart arrhythmias and even cardiac arrest.78 Adults and children alike require regular ECG monitoring (baseline, 2 weeks, monthly) when receiving BDQ, DLM, or when two drugs that prolong the QT interval are being used in combination (such as FQs and CFZ).71 Fortunately, BDQ-related QTc prolongation is uncommonly associated with clinically significant outcomes such as torsades de pointes. Drug interactions, electrolyte levels and thyroid function should be checked and if necessary, corrected.
The risk of Achilles tendinitis (mostly presenting with pain along the tendon or back of the heel) and rupture, as well as aortic aneurysm/dissection (although uncommon) is important and should be considered with long-term FQ use.79 Despite fear of bone and joint abnormalities in children treated with FQs, a growing body of literature indicate no major safety concerns in this population.80
Depression with suicidal thoughts and other psychiatric illnesses might be associated with TB disease itself, but it is a particular concern with CS/terizidone use, and has also been reported with INH treatment. Regular review, support and counselling is recommended, especially in at-risk groups, not only at the time of diagnosis, but also throughout treatment.81
STANDARD 4
Patients can benefit from TDM in specific situations for specific drugs using resource-and setting-appropriate assays
TDM is intended to detect patients with sub- or supra-therapeutic (potentially toxic) concentrations (Figure 1). TDM should be considered for people at highest risk of PK variability, with clinical conditions in which PK variability carries serious consequences, and for drugs which make up the backbone of multidrug regimens or for which the therapeutic window is narrow.53,82 The implementation of TDM can be tailored for specific TB services making use of various types of assays (e.g., high-performance liquid chromatography-ultraviolet, liquid chromatography–mass spectrometry or nanophotometer).54,83 Dose changes guided by TDM should take other clinical parameters into consideration (e.g., severe cavitary disease).47 High priority populations to consider for TDM include those with HIV co-infection, DM, malnutrition, or children, because these factors increase the probability of pharmacokinetic variability and are independently associated with poor TB treatment outcomes (Figure 1). In many TB-endemic settings, these conditions frequently overlap.84 In malnourished children with TB, sub-therapeutic exposure has been demonstrated despite patients receiving WHO-recommended doses.85 Furthermore, PK variability and sub-therapeutic exposures are likely exacerbated by a concurrent enteropathogen burden, which can present additional challenges for TB eradication.86
Both DM and/or HIV co-infection conditions predispose patients to malabsorption or delayed drug absorption, depending on the stage of treatment or disease severity, but also represent a priority situation for TDM given the potential for drug–drug interactions.50,87,88 Certain programmatic settings have adopted routine TDM for people with HIV47 and DM who initiate TB treatment, and have found that frequent dose adjustments are required to achieve timely microbiological cure.89
In patients with central nervous system TB, cerebrospinal fluid concentrations of RIF are only a small fraction of exposure in the serum, and TDM should be routinely performed. High-dose intravenous and oral RIF combined with TDM can be used to target high exposure in serum,90 and thereby increase concentrations in the cerebrospinal fluid.
Specific drugs to prioritise for TDM are show in Figure 1. For certain drugs, such as LZD, measurement of the trough concentration is important to mitigate AEs such a mitochondrial toxicity associated with myelosuppression and neuropathy.11,46,91 In the uncommon scenario where AMK is used, TDM should be used to avoid ototoxicity and nephrotoxicity.92
Although TDM is currently not readily available in several settings, new developments will facilitate broader implementation.83 Modifications to adjust to different settings include investment in high-throughput equipment, such as mass spectrometry, and human expertise at a central level, bypassing cold chain requirements with microsampling techniques such as dried blood spots (DBS)93 and volumetric absorptive microsampling, but also the utilisation of currently experimental matrices such as saliva- and urine-based point-of-care testing.94–97 As TDM technology becomes more readily available, costs are becoming more affordable for resource-limited settings, and with the rise of digital health technologies, access to experts can be more readily facilitated.
STANDARD 5
Each patient should undergo counselling/health education regarding their TB treatment and potential adverse effects to improve treatment results, organised according to feasibility and cost-effectiveness criteria, based on the local organisation of health services and tailored to the individual patient’s needs
A patient-centred approach is an important pillar of the WHO’s End TB Strategy. A key aspect of this approach is to provide counselling and education to all TB patients as described in the Clinical Standards.71,98 AEs are a frequent cause of poor adherence, treatment interruption and loss to follow-up.99 Patients who are aware of potential AEs may be more likely to notify their healthcare team, facilitating more timely management of AEs, potentially reducing the severity of AEs and preventing unfavourable outcomes.100 Early consultation with the TB healthcare team may also allow symptomatic treatment of some AEs, such as nausea or skin rash, and reassurance that such effects often improve as treatment progresses.
The initial education of patients (or parents of children with TB) should include information focussed on the prescribed TB medications and the most common AEs, as well as less common, but more severe or important AEs. Anticipatory guidance about the natural history of these AEs could be shared, such as that many AEs will resolve or substantially improve after the first 1–2 weeks of treatment. Patients should also be provided with specific guidance about when to contact the healthcare team and who to contact. As AEs pose a significant challenge to uninformed patients and care givers, which could lead a to drop-off in treatment, education about AEs should occur on a regular basis, within a trusting relationship with the patient and as a component of comprehensive psychosocial support.101
STANDARD 6
Education for healthcare professionals is important when applying tailored dosing to better understand the link between clinical condition and drug exposure. Additional technical education is required when TDM is used to ensure the quality of the procedure; this includes sampling requirements, drug exposure targets and how to adjust the dose based on drug concentrations
As TB management is delivered by multidisciplinary teams that include physicians, nurses, counsellors, clinical pharmacologists, laboratory staff, and clinical microbiologists, professional education is required for personalised dosing based on clinical pharmacological principles. Education should include 1) an understanding of how clinical conditions can influence drug exposure, more specifically, how ADME is impacted and how this translates to drug exposure, which should also consider factors such as comorbidities, drug–drug interactions and disease severity;26 2) how the dose-exposure and pathogen susceptibility relate to treatment outcomes (PK and pharmacodynamics [PD] targets) and AEs for balancing efficacy against potential toxicity for prioritised drugs (RIF, INH, PZA, FQs and LZD), including when a “personalised dose” should be considered;53,102,103 and finally, 3) adjustment of dosing based on drug concentrations (TDM) needs to be understood in relation to sampling requirements, including limited sampling schedules.53,103,104 Such training is currently mainly available in specialised TDM centres in high-resource areas and should be expanded for all settings considering or already using TDM.105,106
Specialised TB nurses are essential for TDM and training is required to ensure appropriate timing of blood samples in relation to drug intake and transport logistics, as well as monitoring of AEs. For clinical pharmacologists and pharmacists there is a need for training in TB pathogenesis in relation to PK/PD targets, as the lengthy treatment duration and characteristics of the disease differs from other pathogens where TDM is applied. A close collaboration within laboratory units is critical, as trained laboratory staff are essential to establish drug concentrations assays. Furthermore, there is a need for education in the application of validation and quality control programmes,107 sample stability and transportation requirements, as well as point-of-care testing and the use of alternative sample matrices, such as DBS, other microsampling techniques, saliva or urine.103 Clinical microbiologists at TB laboratories should be trained to provide genotypic and/or phenotypic DST for use in TDM,108 including guidance of standard vs. high dose assisted by quality-assured MIC determination.109–111
The resources and healthcare level affect how educational efforts should be structured.103 At the community level, a dedicated team is needed to understand how to apply personalised dosing based on screening assays for key drugs to determine low, normal and high drug exposure. The regional level should be trained to support local teams on difficult cases and provide basic training, as well as quantitative assays for individualised dosing. Finally, the central level should be able to provide training modules for other levels, a quality assurance programme107 and advanced quantitative assays,112 including multiple sample matrices (blood, DBS, saliva, urine) to facilitate the analysis of samples by mail from rural areas or other outpatient settings and also provide dosing software to calculate the drug dose for optimal exposure.54
PRIORITIES FOR FUTURE RESEARCH
Efficacious and safe TB medication will always be a priority for future research. This is especially important for effective but toxic drugs such as LZD, which would benefit from being replaced by a less toxic derivative.113 High-quality MIC (using a method that is calibrated against the European Committee on Antimicrobial Susceptibility Testing reference method) and PK/PD data need to be collected and correlated with clinical outcome data during clinical trials to set appropriate breakpoints for phenotypic DST and define PK/PD targets that can be subsequently used during TDM.114 This has not been done sufficiently to date, resulting in an incomplete understanding of the mode of action of TB agents. Moreover, operational research can contribute essential evidence on drug dosing in special patient populations including adolescents, pregnant women, malnourished patients and the elderly, as these patients tend to be excluded from Phase 3 clinical trials. Easy-to-use assays facilitating TDM in regional TB clinics and health centres that allow a rapid turnaround time will contribute to better patient management. Cost-effectiveness studies are particularly important in the context of high-burden settings with limited resources, to convince programme managers to offer TDM as part of programmatic care for selected patients without additional costs.
Physicians, pharmacists and other healthcare professionals are encouraged to adequately document personalised dosing practices and impact on treatment.53 Evaluation of clinical programmes results in better management of TB treatment.115–117 When redesigning a TB register, it is recommended to capture data on personalised dosing and TDM, to include a set of core variables which are essential to describe, measure and evaluate the cascade of care in adults, as well as children.98 Individual data are preferred over aggregated data, but this depends on the local arrangements. As systematic evaluation of drug dosing, as well as of AEs, is relatively easy with the implementation of computerised, individual registers, this should be considered. This analysis should be included in the annual TB report compiled by TB programmes in many countries.
New drugs (BDQ, DLM, Pa)115,116 should be included in active drug safety monitoring data to identify AEs of concern, which should be reported to regulatory authorities and the WHO.
Specific attention is needed for children, as child-friendly formulations are not always available for all drugs and in all countries.98,118 Formulation can have substantial effects on PK and thus safety and efficacy, as well as on palatability.119,120 Appropriate use of the available child-friendly formulations, and recording the formulation used where feasible, would contribute useful information.
Data protection laws and other restrictions at the country or regional level may limit the type of data that can be collected and may necessitate amending modalities of data collection and storage. Although the long-term follow-up of patients is not considered feasible,98 if, for any reason patients, are followed up after treatment completion for rehabilitation or research purposes, any change of status (e.g., TB recurrence) or long-term AEs need to be notified to health authorities to update the TB register.98
CONCLUSION
Programmatic TB treatment has saved many lives and is suitable for most patients, but careful risk stratification is warranted, and certain patients would benefit from a more person-centred approach, for example, by tailoring the optimal drug dose needs to relevant patient features, DST results, the drugs required for effective treatment, as well as the local environment and available resources. The Clinical Standards articulated here are intended to ensure that TB treatment is safe and effective in every single patient.
Acknowledgements
This article is sponsored by the Oskar-Helene-Heim Foundation (OHH; Berlin, Germany) and the Günther Labes Foundation (Berlin, Germany) and available as an Open Access article (subject to CC-BY 4.0 licensing rules).
Footnotes
Conflicts of interest: CUK is a consultant for FIND and the TB Alliance (Geneva, Switzerland). CUK’s consulting work for Becton Dickinson (BD; Franklin Lakes, NJ, USA) involves a collaboration with Janssen (Beerse, Belgium) and Thermo Fisher Scientific (Waltham, MA, USA). CUK is collaborating with PZA Innovation (Baltimore, MD, USA). CUK worked as a consultant for QuantuMDx (Newcastle upon Tyne, UK), the Stop TB Partnership, the WHO Global TB Programme, and the WHO Regional Office for Europe. CUK gave a paid educational talk for Oxford Immunotec (Abingdon, UK). Hain Lifescience (Nehren, Germany) covered CUK’s travel and accommodation to present at a meeting. CUK is an unpaid advisor to BioVersys (Basel, Switzerland) and GenoScreen (Lille, France). GJF has received in-kind support from Sanofi Pharmaceuticals (Paris, France) to provide rifapentine for a clinical trial. DMC has participated to studies for the evaluation of epidemiologic cut-off values and critical concentrations for bedaquiline (Janssen), pretomanid (TBA Pharma; Ouagadougou, Burkina Faso), delamanid (Otsuka; Tokyo, Japan) from 2016-2022. CWMO has received speaking fees from Qiagen (Hilden, Germany) outside this work.
References
- 1.Brake LHMT, et al. The role of efflux pumps in tuberculosis treatment and their promise as a target in drug development: unraveling the black box. Annu Rev Pharmacol Toxicol. 2018;58(1):271–291. doi: 10.1146/annurev-pharmtox-010617-052438. [DOI] [PubMed] [Google Scholar]
- 2.Dollery CT. Clinical pharmacology: the first 75 years and a view of the future. Br J Clin Pharmacol. 2006;61(6):650–665. [Google Scholar]
- 3.Romero K, Clay R, Hanna D. Strategic regulatory evaluation and endorsement of the hollow fiber tuberculosis system as a novel drug development tool. Clin Infect Dis. 2015;61(suppl_1):S5–S9. doi: 10.1093/cid/civ424. [DOI] [PubMed] [Google Scholar]
- 4.Gumbo T, Angulo-Barturen I, Ferrer-Bazaga S. Pharmacokinetic-pharmacodynamic and dose-response relationships of antituberculosis drugs: recommendations and standards for industry and academia. J Infect Dis. 2015;211(suppl_3):S96–S106. doi: 10.1093/infdis/jiu610. [DOI] [PubMed] [Google Scholar]
- 5.Van Ingen J et al. Why do we use 600 mg of rifampicin in tuberculosis treatment? Clin Infect Dis. 2011;52(9):e194–e199. doi: 10.1093/cid/cir184. [DOI] [PubMed] [Google Scholar]
- 6.Svensson RJ, et al. Greater early bactericidal activity at higher rifampicin doses revealed by modeling and clinical trial simulations. J Infect Dis. 2018;218(6):991–999. doi: 10.1093/infdis/jiy242. [DOI] [PubMed] [Google Scholar]
- 7.Svensson EM, et al. The potential for treatment shortening with higher rifampicin doses: relating drug exposure to treatment response in patients with pulmonary tuberculosis. Clin Infect Dis. 2018;67(1):34–41. doi: 10.1093/cid/ciy026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasiri MJ, et al. Int J Infect Dis. 2022. Mar 2, Delamanid-containing regimens and multidrug-resistant tuberculosis: A systematic review and meta-analysis. S1201-9712(22)00125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen TVA, et al. Bedaquiline resistance: its emergence, mechanism, and prevention. Clin Infect Dis. 2018;66(10):1625–1630. doi: 10.1093/cid/cix992. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen TVA, et al. Delamanid resistance: update and clinical management. Clin Infect Dis. 2020;71(12):3252–3259. doi: 10.1093/cid/ciaa755. [DOI] [PubMed] [Google Scholar]
- 11.Bolhuis MS, et al. Linezolid-based regimens for multidrug-resistant tuberculosis (TB): a systematic review to establish or revise the current recommended dose for TB treatment. Clin Infect Dis. 2018;67(suppl_3):S327–S335. doi: 10.1093/cid/ciy625. [DOI] [PubMed] [Google Scholar]
- 12.Forsman LD, et al. Suboptimal moxifloxacin and levofloxacin drug exposure during treatment of patients with multidrug-resistant tuberculosis: results from a prospective study in China. Eur Respir J. 2021;57(3):2003463. doi: 10.1183/13993003.03463-2020. [DOI] [PubMed] [Google Scholar]
- 13.Litjens CH, et al. Prediction of moxifloxacin concentrations in tuberculosis patient populations by physiologically based pharmacokinetic modeling. J Clin Pharmacol. 2021;62(3):385–396. doi: 10.1002/jcph.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouton JW, et al. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: an update. J Antimicrob Chemother. 2005;55(5):601–607. doi: 10.1093/jac/dki079. [DOI] [PubMed] [Google Scholar]
- 15.Seddon JA, et al. The evolving research agenda for paediatric tuberculosis infection. Lancet Infect Dis. 2019;19(9):e322–e329. doi: 10.1016/S1473-3099(18)30787-4. [DOI] [PubMed] [Google Scholar]
- 16.Niederberger M, Köberich S. Coming to consensus: the Delphi technique. Eur J Cardiovasc Nurs. 2021;20(7):692–695. doi: 10.1093/eurjcn/zvab059. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization Geneva, Switzerland: WHO; 2017. Guidelines for treatment of drug-susceptible tuberculosis and patient care. [Google Scholar]
- 18.Devaleenal DB, Ramachandran G, Swaminathan S. The challenges of pharmacokinetic variability of first-line anti-TB drugs. Expert Rev Clin Pharmacol. 2017;10(1):47–58. doi: 10.1080/17512433.2017.1246179. [DOI] [PubMed] [Google Scholar]
- 19.Dipasquale V, Cucinotta U, Romano C. Acute malnutrition in children: pathophysiology, clinical effects and treatment. Nutrients. 2020;12(8):2413. doi: 10.3390/nu12082413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Bandt JP. [Understanding the pathophysiology of malnutrition for better treatment] Ann Pharm Fr. 2015;73(5):332–335. doi: 10.1016/j.pharma.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Seneadza NAH, et al. Effect of malnutrition on the pharmacokinetics of anti-TB drugs in Ghanaian children. Int J Tuberc Lung Dis. 2021;25(1):36–42. doi: 10.5588/ijtld.20.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosford JD, et al. Hepatotoxicity from antituberculous therapy in the elderly: a systematic review. Tuberculosis (Edinb) 2015;95(2):112–122. doi: 10.1016/j.tube.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramachandran G, Kumar AK, Swaminathan S. Pharmacokinetics of anti-tuberculosis drugs in children. Indian J Pediatr. 2011;78(4):435–442. doi: 10.1007/s12098-010-0304-x. [DOI] [PubMed] [Google Scholar]
- 24.Kearns GL, et al. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 25.Anderson BJ, Allegaert K, Holford NH. Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr. 2006;165(12):819–829. doi: 10.1007/s00431-006-0189-x. [DOI] [PubMed] [Google Scholar]
- 26.Märtson AG, et al. Therapeutic drug monitoring in patients with tuberculosis and concurrent medical problems. Expert Opin Drug Metab Toxicol. 2021;17(1):23–39. doi: 10.1080/17425255.2021.1836158. [DOI] [PubMed] [Google Scholar]
- 27.Huangfu P, et al. The effects of diabetes on tuberculosis treatment outcomes: an updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2019;23(7):783–796. doi: 10.5588/ijtld.18.0433. [DOI] [PubMed] [Google Scholar]
- 28.Hu M, Zheng C, Gao F. Use of bedaquiline and delamanid in diabetes patients: clinical and pharmacological considerations. Drug Des Devel Ther. 2016;10:3983–3994. doi: 10.2147/DDDT.S121630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alfarisi O, et al. Effect of diabetes mellitus on the pharmacokinetics and pharmacodynamics of tuberculosis treatment. Antimicrob Agents Chemother. 2018;62:e01383–18. doi: 10.1128/AAC.01383-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Crevel R et al. Clinical management of combined tuberculosis and diabetes. Int J Tuberc Lung Dis. 2018;22(12):1404–1410. doi: 10.5588/ijtld.18.0340. [DOI] [PubMed] [Google Scholar]
- 31.Te Brake LHM et al. Rifampicin alters metformin plasma exposure but not blood glucose levels in diabetic tuberculosis patients. Clin Pharmacol Ther. 2019;105(3):730–737. doi: 10.1002/cpt.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daskapan A, et al. A systematic review on the effect of HIV infection on the pharmacokinetics of first-line tuberculosis drugs. Clin Pharmacokinet. 2019;58(6):747–766. doi: 10.1007/s40262-018-0716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erwin ER, et al. Pharmacokinetics of isoniazid: the good, the bad, and the alternatives. Tuberculosis (Edinb) 2019;116(Suppl):S66–S70. doi: 10.1016/j.tube.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Pasipanodya JG, Srivastava S, Gumbo T. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin Infect Dis. 2012;55(2):169–177. doi: 10.1093/cid/cis353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azuma J, et al. NAT2 genotype guided regimen reduces isoniazid-induced liver injury and early treatment failure in the 6-month four-drug standard treatment of tuberculosis: a randomized controlled trial for pharmacogenetics-based therapy. Eur J Clin Pharmacol. 2013;69(5):1091–1101. doi: 10.1007/s00228-012-1429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma R, et al. A rapid pharmacogenomic assay to detect NAT2 polymorphisms and guide isoniazid dosing for tuberculosis treatment. Am J Respir Crit Care Med. 2021;204(11):1317–1326. doi: 10.1164/rccm.202103-0564OC. [DOI] [PubMed] [Google Scholar]
- 37.Hutchings A, Routledge PA. A simple method for determining acetylator phenotype using isoniazid. Br J Clin Pharmacol. 1986;22(3):343–345. doi: 10.1111/j.1365-2125.1986.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seifart HI, et al. Population screening for isoniazid acetylator phenotype. Pharmacoepidemiol Drug Saf. 2001;10(2):127–134. doi: 10.1002/pds.570. [DOI] [PubMed] [Google Scholar]
- 39.Hutchings AD, Routledge PA. A single sample saliva test to determine acetylator phenotype. Br J Clin Pharmacol. 1996;42(5):635–637. doi: 10.1111/j.1365-2125.1996.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 40.Gupta A, et al. Adverse drug reactions & drug interactions in MDR-TB patients. Indian J Tuberc. 2020;67(4s):S69–S78. doi: 10.1016/j.ijtb.2020.09.027. [DOI] [PubMed] [Google Scholar]
- 41.Riccardi N, et al. Tuberculosis and pharmacological interactions: a narrative review. Curr Res Pharmacol Drug Discov. 2021;2:100007. doi: 10.1016/j.crphar.2020.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen X, et al. Isoniazid is a mechanism-based inhibitor of cytochrome P450 1A2, 2A6, 2C19 and 3A4 isoforms in human liver microsomes. Eur J Clin Pharmacol. 2002;57(11):799–804. doi: 10.1007/s00228-001-0396-3. [DOI] [PubMed] [Google Scholar]
- 43.Lin MY, et al. Impact of food and antacids on the pharmacokinetics of anti-tuberculosis drugs: systematic review and meta-analysis. Int J Tuberc Lung Dis. 2010;14(7):806–818. [PubMed] [Google Scholar]
- 44.Bahuguna A, Rawat DS. An overview of new antitubercular drugs, drug candidates, and their targets. Med Res Rev. 2020;40(1):263–292. doi: 10.1002/med.21602. [DOI] [PubMed] [Google Scholar]
- 45.Saktiawati AM, et al. Impact of food on the pharmacokinetics of first-line anti-TB drugs in treatment-naive TB patients: a randomized cross-over trial. J Antimicrob Chemother. 2016;71(3):703–710. doi: 10.1093/jac/dkv394. [DOI] [PubMed] [Google Scholar]
- 46.Bolhuis MS, et al. Treatment of multidrug-resistant tuberculosis using therapeutic drug monitoring: first experiences with sub-300 mg linezolid dosages using in-house made capsules. Eur Respir J. 2019;54(6):1900580. doi: 10.1183/13993003.00580-2019. [DOI] [PubMed] [Google Scholar]
- 47.Dheda K, et al. Drug-penetration gradients associated with acquired drug resistance in patients with tuberculosis. Am J Respir Crit Care Med. 2018;198(9):1208–1219. doi: 10.1164/rccm.201711-2333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dekkers BG, et al. Reduced moxifloxacin exposure in patients with tuberculosis and diabetes. Eur Respir J. 2019;54(3):1900373. doi: 10.1183/13993003.00373-2019. [DOI] [PubMed] [Google Scholar]
- 49.Heysell SK, et al. Early therapeutic drug monitoring for isoniazid and rifampin among diabetics with newly diagnosed tuberculosis in Virginia, USA. Tuberc Res Treat. 2013;2013:129723. doi: 10.1155/2013/129723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daskapan A, et al. A systematic review on the effect of HIV infection on the pharmacokinetics of first-line tuberculosis drugs. Clin Pharmacokinet. 2019;58(6):747–766. doi: 10.1007/s40262-018-0716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nahid P, et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med. 2019;200(10):e93–e142. doi: 10.1164/rccm.201909-1874ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nahid P, et al. Official American thoracic society/centers for disease control and prevention/infectious diseases society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63(7):e147–e195. doi: 10.1093/cid/ciw376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alffenaar J-WC, et al. Integrating pharmacokinetics and pharmacodynamics in operational research to end tuberculosis. Clin Infect Dis. 2020;70(8):1774–1780. doi: 10.1093/cid/ciz942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim HY, et al. Therapeutic drug monitoring of anti-infective drugs: implementation strategies for 3 different scenarios. Ther Drug Monit. 2022;44(1):3–10. doi: 10.1097/FTD.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dobbs TE, Webb RM. Chemotherapy of tuberculosis. Microbiol Spectr. 2017;5(2) doi: 10.1128/microbiolspec.TNMI7-0040-2017. TNMI7-0040-2017. [DOI] [PubMed] [Google Scholar]
- 56.Prasad R, Singh A, Gupta N. Adverse drug reactions in tuberculosis and management. Indian J Tuberc. 2019;66(4):520–532. doi: 10.1016/j.ijtb.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 57.Lehloenya RJ, Dheda K. Cutaneous adverse drug reactions to anti-tuberculosis drugs: state of the art and into the future. Expert Rev Anti Infect Ther. 2012;10(4):475–486. doi: 10.1586/eri.12.13. [DOI] [PubMed] [Google Scholar]
- 58.Soeroto AY, et al. Comparison of serum potassium, magnesium, and calcium levels between kanamycin and capreomycin-based regimen-treated multidrug-resistant tuberculosis patients in Bandung (CEASE MDR-TB): a retrospective cohort study. Int J Microbiol. 2019;2019:5065847. doi: 10.1155/2019/5065847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Avong YK, et al. Doing no harm? Adverse events in a nationwide cohort of patients with multidrug-resistant tuberculosis in Nigeria. PLoS One. 2015;10(3):e0120161. doi: 10.1371/journal.pone.0120161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alomar MJ. Factors affecting the development of adverse drug reactions. Saudi Pharm J. 2014;22(2):83–94. doi: 10.1016/j.jsps.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rogers Z, et al. The non-linear child: ontogeny, isoniazid concentration, and NAT2 genotype modulate enzyme reaction kinetics and metabolism. EBioMedicine. 2016;11:118–126. doi: 10.1016/j.ebiom.2016.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McLachlan AJ, Pont LG. Drug metabolism in older people–a key consideration in achieving optimal outcomes with medicines. J Gerontol A Biol Sci Med Sci. 2012;67(2):175–180. doi: 10.1093/gerona/glr118. [DOI] [PubMed] [Google Scholar]
- 63.Kang YA. Tuberculosis treatment in patients with comorbidities. Tuberc Respir Dis (Seoul) 2014;76(6):257–260. doi: 10.4046/trd.2014.76.6.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alsultan A, Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs. 2014;74(8):839–854. doi: 10.1007/s40265-014-0222-8. [DOI] [PubMed] [Google Scholar]
- 65.Bates M et al. Perspectives on tuberculosis in pregnancy. Int J Infect Dis. 2015;32:124–127. doi: 10.1016/j.ijid.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 66.Loveday M, et al. Maternal and infant outcomes among pregnant women treated for multidrug/rifampicin-resistant tuberculosis in South Africa. Clin Infect Dis. 2021;72(7):1158–1168. doi: 10.1093/cid/ciaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma Y, et al. The joint impact of smoking plus alcohol drinking on treatment of pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis. 2019;38(4):651–657. doi: 10.1007/s10096-019-03489-z. [DOI] [PubMed] [Google Scholar]
- 68.Vilariça AS, et al. Reacçôes adversas aos antibacilares em doentes internados: gravidade e factores de risco. Rev Port Pneumol. 2010;16(3):431–451. doi: 10.1016/s0873-2159(15)30040-4. [Portuguese] [DOI] [PubMed] [Google Scholar]
- 69.Zaverucha-do-Valle C, et al. The role of cigarette smoking and liver enzymes polymorphisms in anti-tuberculosis drug-induced hepatotoxicity in Brazilian patients. Tuberculosis (Edinb) 2014;94(3):299–305. doi: 10.1016/j.tube.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Podewils LJ, et al. Impact of malnutrition on clinical presentation, clinical course, and mortality in MDR-TB patients. Epidemiol Infect. 2011;139(1):113–120. doi: 10.1017/S0950268810000907. [DOI] [PubMed] [Google Scholar]
- 71.Migliori GB, et al. Clinical standards for the diagnosis, treatment and prevention of TB infection. Int J Tuberc Lung Dis. 2022;26(3):190–205. doi: 10.5588/ijtld.21.0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.World Health Organization Geneva, Switzerland: WHO; 2020. WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-resistant tuberculosis treatment. [PubMed] [Google Scholar]
- 73.Zimri K, et al. A novel approach for eliciting adolescent MDR-TB treatment tolerability: qualitative data from South Africa. Int J Tuberc Lung Dis. 2020;24(1):43–47. doi: 10.5588/ijtld.19.0207. [DOI] [PubMed] [Google Scholar]
- 74.4th ed. Boston, MA, USA: The Sentinel Project for Pediatric Drug-Resistant Tuberculosis; 2018. Management of drug-resistant tuberculosis in children: a field guide. [Google Scholar]
- 75.Tostmann A, et al. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol. 2008;23(2):192–202. doi: 10.1111/j.1440-1746.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- 76.Alffenaar J-WC, Akkerman OW, Bothamley G. Monitoring during and after tuberculosis treatment. Eur Respir Monogr. 2018;82:308–325. [Google Scholar]
- 77.Court R, et al. Neuropsychiatric toxicity and cycloserine concentrations during treatment for multidrug-resistant tuberculosis. Int J Infect Dis. 2021;105:688–694. doi: 10.1016/j.ijid.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Monedero-Recuero I, et al. QTc and anti-tuberculosis drugs: a perfect storm or a tempest in a teacup? Review of evidence and a risk assessment. Int J Tuberc Lung Dis. 2018;22:1411–1421. doi: 10.5588/ijtld.18.0423. [DOI] [PubMed] [Google Scholar]
- 79.Pasternak B, Inghammar M, Svanström H. Fluoroquinolone use and risk of aortic aneurysm and dissection: nationwide cohort study. BMJ. 2018;360:k678. doi: 10.1136/bmj.k678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garcia-Prats AJ, et al. Clinical and cardiac safety of long-term levofloxacin in children treated for multidrug-resistant tuberculosis. Clin Infect Dis. 2018;67(11):1777–1780. doi: 10.1093/cid/ciy416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hayward SE, et al. The relationship between mental health and risk of active tuberculosis: a systematic review. BMJ Open. 2022;12(1):e048945. doi: 10.1136/bmjopen-2021-048945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Deun A et al. Principles for constructing a tuberculosis treatment regimen: the role and definition of core and companion drugs. Int J Tuberc Lung Dis. 2018;22(3):239–245. doi: 10.5588/ijtld.17.0660. [DOI] [PubMed] [Google Scholar]
- 83.Alffenaar JC, Heysell SK, Mpagama SG. Therapeutic drug monitoring: the need for practical guidance. Clin Infect Dis. 2019;68(6):1065–1066. doi: 10.1093/cid/ciy787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sinha P, et al. Food for thought: addressing undernutrition to end tuberculosis. Lancet Infect Dis. 2021;21(10):e318–e325. doi: 10.1016/S1473-3099(20)30792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Justine M, et al. Pharmacokinetics of first-line drugs among children with tuberculosis in rural Tanzania. J Pediatric Infect Dis Soc. 2020;9(1):14–20. doi: 10.1093/jpids/piy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Aartsen D Lancet Microbe. 2022. Apr 7, Enteropathogen spectrum and effect on antimycobacterial pharmacokinetics among children with tuberculosis in rural Tanzania: a prospective cohort study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heysell SK, et al. Therapeutic drug monitoring for slow response to tuberculosis treatment in a state control program, Virginia, USA. Emerg Infect Dis. 2010;16(10):1546–1553. doi: 10.3201/eid1610.100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alkabab Y, et al. Early interventions for diabetes related tuberculosis associate with hastened sputum microbiological clearance in Virginia, USA. BMC Infect Dis. 2017;17(1):125. doi: 10.1186/s12879-017-2226-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cresswell FV, et al. High-dose oral and intravenous rifampicin for the treatment of tuberculous meningitis in predominantly human immunodeficiency virus (HIV)-positive Ugandan adults: a Phase II open-label randomized controlled trial. Clin Infect Dis. 2021;73(5):876–884. doi: 10.1093/cid/ciab162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Conradie F, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382(10):893–902. doi: 10.1056/NEJMoa1901814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Altena R et al. Reduced chance of hearing loss associated with therapeutic drug monitoring of aminoglycosides in the treatment of multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2017;61(3):e01400–16. doi: 10.1128/AAC.01400-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Capiau S, et al. Official international association for therapeutic drug monitoring and clinical toxicology guideline: development and validation of dried blood spot-based methods for therapeutic drug monitoring. Ther Drug Monit. 2019;41(4):409–430. doi: 10.1097/FTD.0000000000000643. [DOI] [PubMed] [Google Scholar]
- 93.Mohamed S, et al. Levofloxacin pharmacokinetics in saliva as measured by a mobile microvolume UV spectrophotometer among people treated for rifampicin-resistant TB in Tanzania. J Antimicrob Chemother. 2021;76(6):1547–1552. doi: 10.1093/jac/dkab057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Szipszky C, et al. Determination of rifampin concentrations by urine colorimetry and mobile phone readout for personalized dosing in tuberculosis treatment. J Pediatric Infect Dis Soc. 2021;10(2):104–111. doi: 10.1093/jpids/piaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alffenaar JC, et al. A mobile microvolume UV/visible light spectrophotometer for the measurement of levofloxacin in saliva. J Antimicrob Chemother. 2021;76(2):423–429. doi: 10.1093/jac/dkaa420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim HY, et al. Saliva-based linezolid monitoring on a mobile UV spectrophotometer. J Antimicrob Chemother. 2021;76(7):1786–1792. doi: 10.1093/jac/dkab075. [DOI] [PubMed] [Google Scholar]
- 97.Migliori GB, et al. Clinical standards for the assessment, management and rehabilitation of post-TB lung disease. Int J Tuberc Lung Dis. 2021;25(10):797–813. doi: 10.5588/ijtld.21.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim HW, et al. Reasons why patients with tuberculosis in South Korea stop anti-TB treatment: a cross-sectional study. Int J Tuberc Lung Dis. 2020;24(10):1016–1023. doi: 10.5588/ijtld.19.0684. [DOI] [PubMed] [Google Scholar]
- 99.Alipanah N, et al. Adherence interventions and outcomes of tuberculosis treatment: a systematic review and meta-analysis of trials and observational studies. PLoS Med. 2018;15(7):e1002595. doi: 10.1371/journal.pmed.1002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moodley N, et al. ’They are inconveniencing us’ - exploring how gaps in patient education and patient centred approaches interfere with TB treatment adherence: perspectives from patients and clinicians in the Free State Province, South Africa. BMC Public Health. 2020;20(1):454. doi: 10.1186/s12889-020-08562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lemaitre F. Has the time come for systematic therapeutic drug monitoring of first-line and WHO Group A antituberculosis drugs? Ther Drug Monit. 2022;44(1):133–137. doi: 10.1097/FTD.0000000000000948. [DOI] [PubMed] [Google Scholar]
- 102.Alffenaar JC, et al. Precision and personalized medicine and anti-TB treatment: is TDM feasible for programmatic use? Int J Infect Dis. 2020;92s:S5–S9. doi: 10.1016/j.ijid.2020.01.041. [DOI] [PubMed] [Google Scholar]
- 103.Sturkenboom MGG, et al. Population pharmacokinetics and Bayesian dose adjustment to advance TDM of anti-TB drugs. Clin Pharmacokinet. 2021;60(6):685–710. doi: 10.1007/s40262-021-00997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Margineanu I, et al. Practices of therapeutic drug monitoring in tuberculosis - an international survey. Eur Respir J. 2022 Jan 27;:2102787. doi: 10.1183/13993003.02787-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bolhuis MS, et al. Individualized treatment of multidrugresistant tuberculosis using therapeutic drug monitoring. Int J Mycobacteriol. 2016;5 Suppl 1:S44–S45. doi: 10.1016/j.ijmyco.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 106.Aarnoutse RE, et al. An interlaboratory quality control programme for the measurement of tuberculosis drugs. Eur Respir J. 2015;46(1):268–271. doi: 10.1183/09031936.00177014. [DOI] [PubMed] [Google Scholar]
- 107.Lange C, et al. Management of patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2019;23(6):645–662. doi: 10.5588/ijtld.18.0622. [DOI] [PubMed] [Google Scholar]
- 108.World Health Organization Geneva, Switzerland: WHO; 2021. Technical report on critical concentrations for drug susceptibility testing of isoniazid and the rifamycins (rifampicin, rifabutin and rifapentine) [Google Scholar]
- 109.World Health Organization Geneva, Switzerland: WHO; 2018. Technical report on critical concentrations for drug susceptibility testing of medicines used in the treatment of drug-resistant tuberculosis. [Google Scholar]
- 110.Schön T, et al. Standards for MIC testing that apply to the majority of bacterial pathogens should also be enforced for Mycobacterium tuberculosis complex. Clin Microbiol Infect. 2019;25(4):403–405. doi: 10.1016/j.cmi.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuhlin J, et al. Mass spectrometry for therapeutic drug monitoring of anti-tuberculosis drugs. Clin Mass Spectrom. 2019;14 Pt A:34–45. doi: 10.1016/j.clinms.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bruinenberg P, et al. Single ascending-dose study to evaluate the safety, tolerability, and pharmacokinetics of sutezolid in healthy adult subjects. Antimicrob Agents Chemother. 2022:e0210821. doi: 10.1128/aac.02108-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schön T, et al. What is the role of the EUCAST reference method for MIC testing of the Mycobacterium tuberculosis complex? Clin Microbiol Infect. 2020;26(11):1453–1455. doi: 10.1016/j.cmi.2020.07.037. [DOI] [PubMed] [Google Scholar]
- 114.Koirala S, et al. Outcome of treatment of MDR-TB or drug-resistant patients treated with bedaquiline and delamanid: results from a large global cohort. Pulmonology. 2021;27(5):403–412. doi: 10.1016/j.pulmoe.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 115.Borisov S, et al. Surveillance of adverse events in the treatment of drug-resistant tuberculosis: first global report. Eur Respir J. 2019;54(6):1901522. doi: 10.1183/13993003.01522-2019. [DOI] [PubMed] [Google Scholar]
- 116.World Health Organization Geneva, Switzerland: WHO; 2018. Guidance for country-level TB modelling. [Google Scholar]
- 117.Graham SM, Amanullah F. Updated guidelines for child and adolescent TB. Int J Tuberc Lung Dis. 2022;26(2):81–84. doi: 10.5588/ijtld.21.0747. [DOI] [PubMed] [Google Scholar]
- 118.Garcia-Prats AJ, et al. Pharmacokinetics, safety, and dosing of novel pediatric levofloxacin dispersible tablets in children with multidrug-resistant tuberculosis exposure. Antimicrob Agents Chemother. 2019;63(4):e01865–18. doi: 10.1128/AAC.01865-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Purchase SE, et al. Acceptability of a novel levofloxacin dispersible tablet formulation in young children exposed to multidrug-resistant tuberculosis. Pediatr Infect Dis J. 2019;38(6):608–610. doi: 10.1097/INF.0000000000002268. [DOI] [PubMed] [Google Scholar]
- 120.Requena-Méndez A, et al. Intra-individual effects of food upon the pharmacokinetics of rifampicin and isoniazid. J Antimicrob Chemother. 2019;74(2):416–424. doi: 10.1093/jac/dky444. [DOI] [PubMed] [Google Scholar]
- 121.World Health Organization Geneva, Switzerland: WHO; 2014. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. [PubMed] [Google Scholar]
- 122.Sanofi-Aventis Paris, France: Sanofi-Aventis; 2017. RIFADIN® (rifampin capsules USP) and RIFADIN® IV (rifampin for injection USP), prescribing information. [Google Scholar]
- 123.Dhiman RK, et al. A guide to the management of tuberculosis in patients with chronic liver disease. J Clin Exp Hepatol. 2012;2(3):260–270. doi: 10.1016/j.jceh.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yew WW. Clinically significant interactions with drugs used in the treatment of tuberculosis. Drug Saf. 2002;25(2):111–133. doi: 10.2165/00002018-200225020-00005. [DOI] [PubMed] [Google Scholar]
- 125.Grange JM, Winstanley PA, Davies PD. Clinically significant drug interactions with antituberculosis agents. Drug Saf. 1994;11(4):242–251. doi: 10.2165/00002018-199411040-00003. [DOI] [PubMed] [Google Scholar]
- 126.Verrest L, et al. Influence of malnutrition on the pharmacokinetics of drugs used in the treatment of poverty-related diseases: a systematic review. Clin Pharmacokinet. 2021;60(9):1149–1169. doi: 10.1007/s40262-021-01031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Walubo A, et al. The disposition of antituberculous drugs in plasma of elderly patients. II. Isoniazid, rifampicin and pyrazinamide. Methods Find Exp Clin Pharmacol. 1991;13(8):551–556. [PubMed] [Google Scholar]
- 128.Mtabho CM, et al. Effect of diabetes mellitus on TB drug concentrations in Tanzanian patients. J Antimicrob Chemother. 2019;74(12):3537–3545. doi: 10.1093/jac/dkz368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ramachandran G, Swaminathan S. Role of pharmacogenomics in the treatment of tuberculosis: a review. Pharmgenomics Pers Med. 2012;5:89–98. doi: 10.2147/PGPM.S15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hong BL, et al. A systematic review and meta-analysis of isoniazid pharmacokinetics in healthy volunteers and patients with tuberculosis. Clin Ther. 2020;42(11):e220–e241. doi: 10.1016/j.clinthera.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 131.Saito N, et al. Impact of renal function-based anti-tuberculosis drug dosage adjustment on efficacy and safety outcomes in pulmonary tuberculosis complicated with chronic kidney disease. BMC Infect Dis. 2019;19(1):374. doi: 10.1186/s12879-019-4010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Varughese A, et al. Ethambutol kinetics in patients with impaired renal function. Am Rev Respir Dis. 1986;134(1):34–38. doi: 10.1164/arrd.1986.134.1.34. [DOI] [PubMed] [Google Scholar]
- 133.Graham SM, et al. Low levels of pyrazinamide and ethambutol in children with tuberculosis and impact of age, nutritional status, and human immunodeficiency virus infection. Antimicrob Agents Chemother. 2006;50(2):407–413. doi: 10.1128/AAC.50.2.407-413.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lettieri J, et al. Effect of food on the pharmacokinetics of a single oral dose of moxifloxacin 400mg in healthy male volunteers. Clin Pharmacokinet. 2001;40(Suppl 1):19–25. doi: 10.2165/00003088-200140001-00003. [DOI] [PubMed] [Google Scholar]
- 135.Barth J, et al. Single- and multiple-dose pharmacokinetics of intravenous moxifloxacin in patients with severe hepatic impairment. J Antimicrob Chemother. 2008;62(3):575–578. doi: 10.1093/jac/dkn212. [DOI] [PubMed] [Google Scholar]
- 136.Peloquin CA, et al. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob Agents Chemother. 2008;52(3):852–857. doi: 10.1128/AAC.01036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Falcone M, et al. Considerations for the optimal management of antibiotic therapy in elderly patients. J Glob Antimicrob Resist. 2020;22:325–333. doi: 10.1016/j.jgar.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 138.Naidoo A, et al. Effect of rifampicin and efavirenz on moxifloxacin concentrations when co-administered in patients with drug-susceptible TB. J Antimicrob Chemother. 2017;72(5):1441–1449. doi: 10.1093/jac/dkx004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee LJ, et al. Effects of food and sucralfate on a single oral dose of 500 milligrams of levofloxacin in healthy subjects. Antimicrob Agents Chemother. 1997;41(10):2196–2200. doi: 10.1128/aac.41.10.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pea F, et al. Pharmacokinetics and pharmacodynamics of intravenous levofloxacin in patients with early-onset ventilator-associated pneumonia. Clin Pharmacokinet. 2003;42(6):589–598. doi: 10.2165/00003088-200342060-00008. [DOI] [PubMed] [Google Scholar]
- 141.Dryden MS. Linezolid pharmacokinetics and pharmacodynamics in clinical treatment. J Antimicrob Chemother. 2011;66(Suppl 4):iv7–iv15. doi: 10.1093/jac/dkr072. [DOI] [PubMed] [Google Scholar]
- 142.Stalker DJ, Jungbluth GL. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin Pharmacokinet. 2003;42(13):1129–1140. doi: 10.2165/00003088-200342130-00004. [DOI] [PubMed] [Google Scholar]
- 143.Douros A, Grabowski K, Stahlmann R. Drug-drug interactions and safety of linezolid, tedizolid, and other oxazolidinones. Expert Opin Drug Metab Toxicol. 2015;11(12):1849–1859. doi: 10.1517/17425255.2015.1098617. [DOI] [PubMed] [Google Scholar]
- 144.Tietjen AK, et al. Population pharmacokinetics and target attainment analysis of linezolid in multidrug-resistant tuberculosis patients. Br J Clin Pharmacol. 2022;88(4):1835–1844. doi: 10.1111/bcp.15102. [DOI] [PubMed] [Google Scholar]
- 145.van Heeswijk RP, Dannemann B, Hoetelmans RM. Bedaquiline: a review of human pharmacokinetics and drug-drug interactions. J Antimicrob Chemother. 2014;69(9):2310–2318. doi: 10.1093/jac/dku171. [DOI] [PubMed] [Google Scholar]
- 146.Haas DW, et al. J Infect Dis. 2022. Jan 29, Pharmacogenetics of between-individual variability in plasma clearance of bedaquiline and clofazimine in South Africa; p. jiac024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Svensson EM, Dosne AG, Karlsson MO. Population pharmacokinetics of bedaquiline and metabolite M2 in patients with drug-resistant tuberculosis: the effect of time-varying weight and albumin. CPT Pharmacometrics Syst Pharmacol. 2016;5(12):682–691. doi: 10.1002/psp4.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mukonzo J, et al. Potential drug-drug interactions between antiretroviral therapy and treatment regimens for multi-drug resistant tuberculosis: implications for HIV care of MDR-TB co-infected individuals. Int J Infect Dis. 2019;83:98–101. doi: 10.1016/j.ijid.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Abdelwahab MT, et al. Clofazimine pharmacokinetics in patients with TB: dosing implications. J Antimicrob Chemother. 2020;75(11):3269–3277. doi: 10.1093/jac/dkaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhu M, et al. Pharmacokinetics of cycloserine under fasting conditions and with high-fat meal, orange juice, and antacids. Pharmacotherapy. 2001;21(8):891–897. doi: 10.1592/phco.21.11.891.34524. [DOI] [PubMed] [Google Scholar]
- 151.Arbex MA, et al. Antituberculosis drugs: drug interactions, adverse effects, and use in special situations. Part 2: second line drugs. J Bras Pneumol. 2010;36(5):641–656. doi: 10.1590/s1806-37132010000500017. [DOI] [PubMed] [Google Scholar]
- 152.Zítková L, Tousek J. Pharmacokinetics of cycloserine and terizidone. A comparative study. Chemotherapy. 1974;20(1):18–28. doi: 10.1159/000221787. [DOI] [PubMed] [Google Scholar]
- 153.Lewis JM, Sloan DJ. The role of delamanid in the treatment of drug-resistant tuberculosis. Ther Clin Risk Manag. 2015;11:779–791. doi: 10.2147/TCRM.S71076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang X, Mallikaarjun S, Gibiansky E. Population pharmacokinetic analysis of delamanid in patients with pulmonary multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2020;6565(1)(1):2020. e01202–20. doi: 10.1128/AAC.01202-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gibson TP, Demetriades JL, Bland JA. Imipenem/cilastatin: pharmacokinetic profile in renal insufficiency. Am J Med. 1985;78(6a):54–61. doi: 10.1016/0002-9343(85)90102-0. [DOI] [PubMed] [Google Scholar]
- 156.Anderson BJ, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24(1):25–36. doi: 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- 157.Ritchie DJ, et al. Evaluation and financial impact of imipenem/cilastatin dosing in elderly patients based on renal function and body weight. J Pharm Technol. 1993;9(4):160–163. [PubMed] [Google Scholar]
- 158.Thalhammer F, Hörl WH. Pharmacokinetics of meropenem in patients with renal failure and patients receiving renal replacement therapy. Clin Pharmacokinet. 2000;39(4):271–279. doi: 10.2165/00003088-200039040-00003. [DOI] [PubMed] [Google Scholar]
- 159.Christensson BA, et al. Pharmacokinetics of meropenem in subjects with various degrees of renal impairment. Antimicrob Agents Chemother. 1992;36(7):1532–1537. doi: 10.1128/aac.36.7.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.AstraZeneca Pharmaceuticals Wilmington, DE, USA: AstraZeneca Pharmaceuticals LP; 2013. Merrem® IV (meropenem for injection) package insert. [Google Scholar]
- 161.Lan J, et al. Population pharmacokinetics analysis and dosing simulations of meropenem in critically ill patients with pulmonary infection. J Pharm Sci. 2022 Jan 26;2022 doi: 10.1016/j.xphs.2022.01.015. S0022-3549(22)00025-9. [DOI] [PubMed] [Google Scholar]
- 162.Usman M, Frey OR, Hempel G. Population pharmacokinetics of meropenem in elderly patients: dosing simulations based on renal function. Eur J Clin Pharmacol. 2017;73(3):333–342. doi: 10.1007/s00228-016-2172-4. [DOI] [PubMed] [Google Scholar]
- 163.McHenry MC, et al. Pharmacokinetics of amikacin in patients with impaired renal function. J Infect Dis. 1976;134(suppl):S343–S348. doi: 10.1093/infdis/135.supplement_2.s343. [DOI] [PubMed] [Google Scholar]
- 164.Oshikoya KA, Sammons HM, Choonara I. A systematic review of pharmacokinetics studies in children with protein-energy malnutrition. Eur J Clin Pharmacol. 2010;66(10):1025–1035. doi: 10.1007/s00228-010-0851-0. [DOI] [PubMed] [Google Scholar]
- 165.Fraisse T, et al. Aminoglycosides use in patients over 75 years old. Age Ageing. 2014;43(5):676–681. doi: 10.1093/ageing/afu023. [DOI] [PubMed] [Google Scholar]
- 166.Vivithanaporn P, et al. Potential drug-drug interactions of antiretrovirals and antimicrobials detected by three databases. Sci Rep. 2021;11(1):6089. doi: 10.1038/s41598-021-85586-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Peloquin CA, et al. Pharmacokinetics of para-aminosalicylic acid granules under four dosing conditions. Ann Pharmacother. 2001;35(11):1332–1338. doi: 10.1345/aph.1A088. [DOI] [PubMed] [Google Scholar]
- 168.Abulfathi AA, et al. The pharmacokinetics of para-aminosalicylic acid and its relationship to efficacy and intolerance. Br J Clin Pharmacol. 2020;86(11):2123–2132. doi: 10.1111/bcp.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kock L, et al. Pharmacokinetics of para-aminosalicylic acid in HIV-uninfected and HIV-coinfected tuberculosis patients receiving antiretroviral therapy, managed for multidrug-resistant and extensively drug-resistant tuberculosis. Antimicrob Agents Chemother. 2014;58(10):6242–6250. doi: 10.1128/AAC.03073-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ignatius EH, et al. Pretomanid pharmacokinetics in the presence of rifamycins: interim results from a randomized trial among patients with tuberculosis. Antimicrob Agents Chemother. 2021 Jan 20;65(2):e01196–20. doi: 10.1128/AAC.01196-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Salinger DH, et al. Population pharmacokinetics of the antituberculosis agent pretomanid. Antimicrob Agents Chemother. 2019;63(10):e00907–19. doi: 10.1128/AAC.00907-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.World Health Organization Geneva, Switzerland: WHO; 2010. Treatment of tuberculosis: guidelines. [PubMed] [Google Scholar]
- 173.Diacon AH, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med. 2009;360(23):2397–2405. doi: 10.1056/NEJMoa0808427. [DOI] [PubMed] [Google Scholar]
- 174.Pretomanid Product Information TB Alliance. 2019. https://www.tballiance.org/sites/default/files/assets/Pretomanid_Full-Prescribing-Information.pdf .
- 175.Ghodousi A, et al. Isoniazid resistance in Mycobacterium tuberculosis is a heterogeneous phenotype composed of overlapping MIC distributions with different underlying resistance mechanisms. Antimicrob Agents Chemother. 2019;63(7):e00092–19. doi: 10.1128/AAC.00092-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dean AS, et al. Prevalence and genetic profiles of isoniazid resistance in tuberculosis patients: a multicountry analysis of cross-sectional data. PLoS Med. 2020;17(1):e1003008. doi: 10.1371/journal.pmed.1003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rigouts L, et al. Specific gyrA gene mutations predict poor treatment outcome in MDR-TB. J Antimicrob Chemother. 2016;71(2):314–323. doi: 10.1093/jac/dkv360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dooley KE, et al. Early bactericidal activity of different isoniazid doses for drug resistant TB (INHindsight): a randomized open-label clinical trial. Am J Respir Crit Care Med. 2020;201(11):1416–1424. doi: 10.1164/rccm.201910-1960OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gopie FA, et al. Should treatment of low-level rifampicin mono-resistant tuberculosis be different? J Clin Tuberc Other Mycobact Dis. 2021;23:100222. doi: 10.1016/j.jctube.2021.100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhong T, et al. Predicting antituberculosis drug-induced liver injury using an interpretable machine learning method: model development and validation study. JMIR Med Inform. 2021;9(7):e29226. doi: 10.2196/29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shi L, et al. Interim effectiveness and safety comparison of bedaquiline-containing regimens for treatment of diabetic versus non-diabetic MDR/XDR-TB patients in China: a multicenter retrospective cohort study. Infect Dis Ther. 2021;10(1):457–470. doi: 10.1007/s40121-021-00396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Metaxas EI, Falagas ME. Update on the safety of linezolid. Expert Opin Drug Saf. 2009;8(4):485–491. doi: 10.1517/14740330903049706. [DOI] [PubMed] [Google Scholar]
- 183.Richardson M, et al. NAT2 variants and toxicity related to anti-tuberculosis agents: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2019;23(3):293–305. doi: 10.5588/ijtld.18.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cerrone M, et al. Safety implications of combined antiretroviral and anti-tuberculosis drugs. Expert Opin Drug Saf. 2020;19(1):23–41. doi: 10.1080/14740338.2020.1694901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nahid P, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63(7):e147–e195. doi: 10.1093/cid/ciw376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pecora F, et al. Treatment of multidrug-resistant and extensively drug-resistant tuberculosis in children: the role of bedaquiline and delamanid. Microorganisms. 2021;9(5):1074. doi: 10.3390/microorganisms9051074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Riza AL, et al. Clinical management of concurrent diabetes and tuberculosis and the implications for patient services. Lancet Diab Endocrinol. 2014;2(9):740–753. doi: 10.1016/S2213-8587(14)70110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Citron KM, Thomas GO. Ocular toxicity from ethambutol. Thorax. 1986;41(10):737–739. doi: 10.1136/thx.41.10.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.World Health Organization Geneva, Switzerland: WHO; 2014. Companion Handbook to the WHO Guidelines for the programmatic management of drug-resistant tuberculosis. ANNEX 4 H-tgotuo. [PubMed] [Google Scholar]
- 190.Alffenaar JC, et al. Should we worry about bedaquiline exposure in the treatment of multidrug-resistant and extensively drug-resistant tuberculosis? Eur Respir J. 2020;55(2):1901908. doi: 10.1183/13993003.01908-2019. [DOI] [PubMed] [Google Scholar]


