Abstract
The effects of Endocrine Disrupting Chemicals (EDCs) on the current obesity epidemic is a growing field of interest. Numerous EDCs have shown the potential to alter energy metabolism, which may increase the risk of obesity, in part, through direct actions on adipose tissue. While white adipose tissue has historically been the primary focus of this work, evidence of the EDC-induced disruption of brown and beige adipose tissues continues to build. Both brown and beige fat are thermogenic adipose depots rich in mitochondria that dispense heat when activated. Due to these properties, brown and beige fat are implicated in metabolic diseases such as obesity, diabetes, and cachexia. This review delves into the current literature of different EDCs, including bisphenols, dioxins, air pollutants, phthalates, and phytochemicals. The possible implications that these EDCs have on thermogenic adipose tissues are covered. This review also introduces the possibility of using brown and beige fat as a therapeutic target organ by taking advantage of some of the properties of EDCs. Collectively, we provide a comprehensive discussion of the evidence of EDC disruption in white, brown, and beige fat and highlight gaps worthy of further exploration.
Keywords: Brown adipose tissue, Beige Adipose Tissue, Endocrine Disrupting Chemicals, Phytochemicals, Obesity
1.1. Introduction
Despite growing acknowledgement and therapeutic advancements, the obesity epidemic continues worldwide and is estimated to reach a global prevalence of one billion adults by 2030 (Kelly et al. 2008). This increase in weight cannot solely be explained by one outstanding factor, as social, economic, and cultural components all contribute to the spread (Wang and Beydoun 2007). Lifestyle factors, such as diet and physical activity, in addition to an individual’s genetics, have been long considered to be the leading contributors to the development of obesity (Zhang et al. 2014). However, it has become increasingly recognized that various chemicals in our built environment can interact with the endocrine system, altering the homeostatic mechanisms that control energy imbalance that can increase susceptibility to obesity and metabolic disease (Darbre 2017). These classes of environmental chemicals that are known to disrupt the regulatory actions of hormones, interfering in health and disease, have been termed ‘endocrine-disrupting chemicals’ (EDCs).
EDCs are inclusive of a wide range of synthetic compounds that are used in either the manufacturing of or as a component of the final product in various consumer goods. As of 2020, over 86,000 unique manufactured chemicals have been registered with the U.S. Environmental Protection Agency (U.S. 2021); however, it is not well known how many of these may disrupt endocrine function in mammals. EDCs can be found in a wide range of commercial products such as pesticides, plastics, clothing, personal care products, and in both building materials and food packaging, among others. Hence, humans, pets, and wildlife are exposed to numerous EDCs throughout the lifespan, some of which can have long half-lives in the body and can take years to be fully metabolized and excreted (Diamanti-Kandarakis et al. 2009). As compounds that can either directly bind to hormone receptors or interfere with hormone signaling, EDCs can have a wide range of impacts including reduced fertility, cancer, cardiovascular disease, and neurological disorders. A comprehensive summary of systemic effects of EDCs can be found in the recent review by Kahn et al. (2020). However, for the purpose of the current review, there is growing evidence that EDCs may have obesogenic properties thereby contributing to the increasing rates of metabolic disease in both adults and children.
1.2. Obesity and EDCs
The endocrine system plays a pivotal role in regulating the metabolism of fats, carbohydrates, and proteins. Thus, interference in the hormonal control of metabolism can lead to deposition of fat, and hence, obesity (Darbre 2017). Interestingly, EDCs have the potential to mimic hormonal actions, allowing them to exhibit a wide range of effects. Most examples of EDCs studied act through the nuclear hormone receptor superfamily, which includes steroid hormone receptors, thyroid hormone receptors, retinoid X receptors (RXR), peroxisome proliferator-activated receptors (PPAR), liver X receptors, and farnesoid X receptors (Grun and Blumberg 2007). A common feature of nuclear receptor ligands is their small size and lipophilicity. Not surprisingly, many EDCs share these same characteristics. For example, activation of the nuclear hormone receptor PPARγ is known to promote fat cell differentiation (Rosen et al. 1999). PPARs dimerize with RXR and bind to PPAR-responsive DNA regulatory elements controlling the expression of genes involved in adipogenesis, glucose, lipid, and cholesterol metabolism (Maradonna and Carnevali 2018).
Considering the physiological role of PPARγ in adipose tissue development and maintenance, it has been proposed that disruption of the regulatory pathways under the control of PPARγ may be involved in the onset of obesity and other metabolic syndromes (Delfosse et al. 2015). Poly- and mono-unsaturated fatty acids, eicosanoids, and lipophilic hormones are known to be PPAR natural ligands which induce the expression of genes and enzymes involved in lipid metabolism (Maradonna and Carnevali 2018). In addition to these natural ligands, PPARγ also binds to and is activated by molecules such as derivatives of bisphenol A (BPA), perfluorinated compounds (PFCs), and certain phthalates. Activation of this receptor by various xenobiotic compounds, as listed above, has been shown to stimulate adipogenesis in vitro and in vivo by inducing the differentiation of preadipocytes into mature adipocytes (Delfosse et al. 2015). This has led to the “obesogen hypothesis”, which suggests that the rapidly growing obesity epidemic may likely be attributed to increased exposure to chemicals that interfere with any aspects of energy metabolism (Grun and Blumberg 2007). Furthermore, while the direct effect of EDCs (e.g., PFCs and BPA) on white adipocytes have long been studied, the impacts of exogenous EDCs on modulating the function of other types of adipocytes that play critical roles in energy balance such as brown and beige adipocytes are an emerging area of focus.
1.3. Adipose Tissue Populations
While white adipose tissue (WAT) is best known for its role in fat storage, specific sub-types of adipose tissue can also serve to suppress weight gain and promote a healthy metabolic state (Symonds et al. 2018). These additional populations are known as brown adipose tissue (BAT) and beige fat. BAT, which is embryologically derived from the Myf5 progenitor cell population, has thermogenic properties that is primarily mediated through the expression of uncoupling protein 1 (UCP1) (Harms and Seale 2013). Uncoupling proteins have a wide variety of roles. UCP1, specifically, helps to regulate body temperature through non-shivering thermogenesis. To do this, UCP1 inserts itself into the inner mitochondrial membrane, permitting leakage of protons back into the mitochondrial matrix and allows for brown fat to produce heat at the expense of ATP production (Bertholet and Kirichok 2019). As a secondary effect of thermogenesis, UCP1 also controls fat metabolism and hence, energy balance.
BAT is activated rapidly at the time of birth in both humans and rodents to maintain core body temperature. However in humans the contribution of BAT for thermogenesis is thought to decrease with age (Harms and Seale 2013). In contrast, beige fat, also known as inducible “brown-like” or ‘brite’ adipocytes, is thought to differentiate postnatally from white adipocytes in response to environmental cues (e.g., cold temperatures) and endocrine metabolic regulators including thyroid hormone, adipokines and myokines, and even immune cells and cytokines. As such, adipocyte beiging plays a crucial adaptive role, allowing the body to maintain core body temperature and respond to changing energetic demands of the system. While the two distinct types of thermogenic adipose tissue have different developmental origins, brown and beige adipocytes have important shared properties that include dense mitochondria and the expression of UCP1 (Lidell et al. 2014). The energy expenditure that comes as a result of thermogenesis in brown and beige cells serves to both prevent hypothermia and to metabolize excess lipid through the uncoupling of oxidative phosphorylation (Himms-Hagen 1990). Therefore, due to their ability to contribute to basal metabolic rate, both BAT and beige fat appear to have anti-obesity properties. In fact, the loss of BAT (“whitening”) and the reduced capacity to induce beiging are associated with aging, obesity, and overall poor metabolic responses (Harms and Seale 2013).
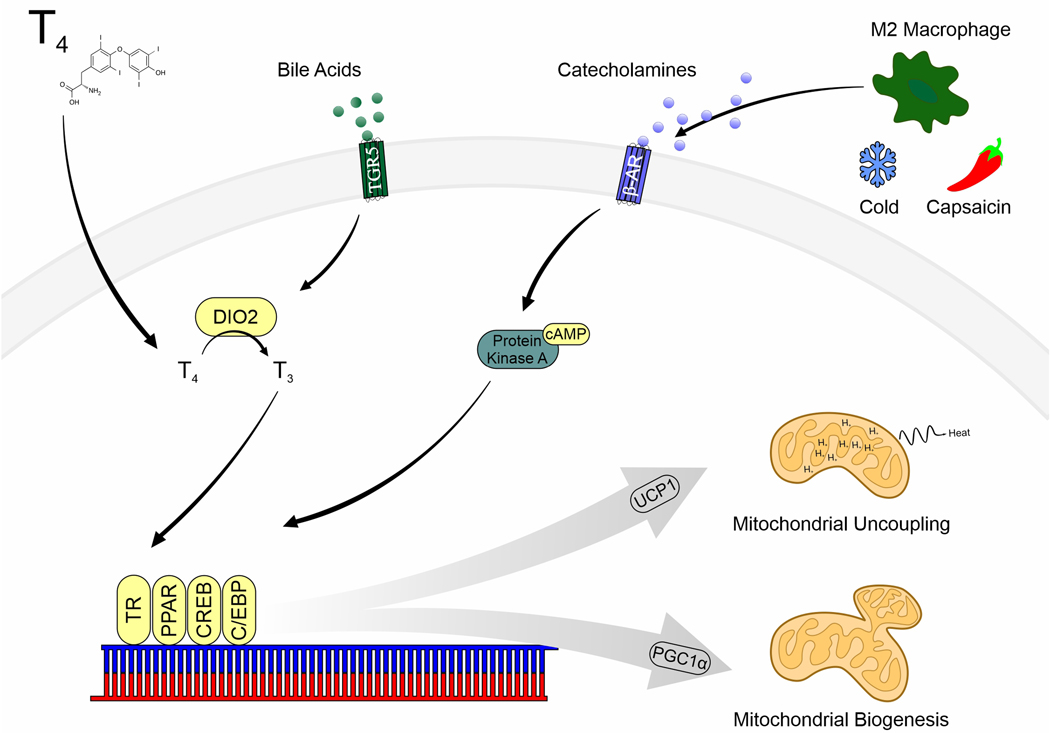
Because BAT assists in the thermoregulation, brown and beige adipocytes are highly responsive to the environment. The most widely studied pathway that upregulates mitochondrial biogenesis and uncoupling via PGC1α and UCP1 expression is through the activation of adrenergic receptors on adipocytes, which is primarily how cold exposure activates BAT for non-shivering thermogenesis (Harms and Seale 2013). Thyroid hormone is also a potent regulator of thermogenic adipose tissues, with its’ addition being crucial for the differentiation of brown adipocytes in vitro and in the development of the fetal BAT depot (Bianco and McAninch 2013). While some of the major pathways involved in the regulation of brown and beige fat are shown in Figure 1, the transcriptional regulation of peroxisome proliferator-activated receptor gamma coactivator-1 α (PGC1α) and UCP1 includes a varied set of hormone receptors such as the PPARs and thyroid receptor. Thus, as various EDCs interfere with thyroid or PPAR signaling, there is a strong likelihood that such compounds can also induce dysfunction in both brown and beige fat depots.
Figure 1:
Major signaling pathways involved in the regulation of brown and beige adipose tissue activity. Abbreviations: β-Adrenergic Receptor (β-AR), CCAAT-enhancer-binding proteins (C/EBP), cyclic adenosine monophosphate (cAMP), cAMP-response element binding protein (CREB), Type II iodothyronine deiodinase (DIO2), peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC1α), peroxisome proliferator-activated receptor (PPAR), triiodothyronine (T3), thyroxine (T4), G-protein-coupled bile acid receptor (TGR5), thyroid receptor (TR).
Our understanding of the possibility for environmental pollutants to disrupt energy metabolism and promote weight gain has grown significantly over time. With the recent advancements in the field of brown and beige fat, we are beginning to realize the unique interplay between the endocrine system, metabolism, and the environment. This review aims to delve into the aspects of common environmental chemicals and their impact on metabolism in brown and beige fat. In addition, this review introduces the potential use of phytochemicals, some of which are considered EDCs themselves, as therapeutic strategies to counteract the adverse effects of environmental pollutants in the thermogenic fat depots.
2.1. Bisphenols
As one of the most widely studied environmental obesogens, the impact of BPA on metabolic disease has received increasing attention. Its high volume use in the manufacture of polycarbonate plastics and epoxy resin contributes significantly to its ubiquitous presence in food storage containers such as water bottles and the protective internal lining of cans used for food and beverages (Le et al. 2008). Despite recent voluntary removal of BPA from many of these products, human exposure to BPA and it’s replacement compounds are widespread (Pelch et al. 2019).
The effects of bisphenols on adipogenesis have been well studied with exposure upregulating markers of adipocyte differentiation such as PPARγ and CCAT/enhancer-binding protein (C/EBPα) in 3T3-L1s, uncommitted NIH3T3 cells, and in adipose-derived stem cells (Ariemma et al. 2016; Dong et al. 2018; Longo et al. 2020). Further, analogs of BPA have shown similar effects across the various adipocyte model systems (Martinez et al. 2020; Reina-Perez et al. 2021). Hence, through stimulation of adipogenesis signaling, bisphenol exposure induces mature adipocyte differentiation and lipid accumulation. Perhaps unsurprisingly, the most convincing evidence of bisphenol’s obesogenic effects are following early-life exposure in vivo. Such studies suggest that BPA exposure may drive the commitment of stem cells to the preadipocyte program during development, laying the foundation for the expansion of white adipose tissue later in life (Alonso-Magdalena et al. 2016).
In accordance with its obesogenic properties, bisphenols have also shown the potential to alter BAT activity (Heindel et al. 2017). While work in this area has only scratched the surface, BPA appears to preferentially accumulate in the BAT at levels far exceeding those found in the blood, brain, or WAT of female rats exposed to BPA between 1 – 5 mg/day via an osmotic minipump (Nunez et al. 2001). Interestingly, the accumulation of BPA in brown fat was accompanied by a reduction in body weight gain without a commensurate reduction in caloric intake, owing to the likelihood that BPA may transiently increase energy expenditure. Additionally, gestational exposure to BPA appears to not only alter WAT, but BAT as well. Female offspring from dams exposed to dietary BPA (< 3,000 μg/kg body weight) had increases in both the weight of the interscapular BAT depot and in the expression of the thermogenic protein, Ucp1 (van Esterik et al. 2014). Male offspring, on the other hand, showed signs of reduced brown adipogenesis and BAT activity. The sexual dimorphism observed with BPA may be attributed to its agonistic effects on the estrogen receptor, which has shown to be indispensable for mitochondrial biogenesis and thermogenesis in BAT (Zhou et al. 2020). In fact, the potential for sex to mediate the effects of BPA in beige and brown fat has also been proposed by Taylor et al. (2018).
At least in the male sex, some of the negative effects of BPA on brown fat has been attributed to impaired thyroid signaling. BAT is a thyroid sensitive tissue, expressing relatively high levels of the type II deiodinase (DIO2) that leads to the T3-mediated upregulation of Ucp1 expression (de Jesus et al. 2001). Furthermore, thyroid hormone also appears to be critical for the “beiging” of WAT (Martinez-Sanchez et al. 2017), an effect that is both centrally and peripherally mediated. In ex vivo cultures of male BAT, exposure to BPA (< 5 mM) resulted in concentration-dependent reductions in DIO2 activity (da Silva et al. 2019). Importantly, however, these effects could not be reproduced in male rats exposed to BPA by gavage (40 mg/kg body weight). Hence, it is likely that additional factors may be responsible for BPA-induced BAT dysfunction beyond just localized alterations in estrogen or thyroid signaling. Furthermore, the potential for differing effects between the bisphenols is likely critical as bisphenol F (BPF) has shown some opposing effects of BPA in both in vitro and in vivo model systems (Drobna et al. 2019).
One potential modulator of BPA-induced WAT and BAT dysfunction is the distribution of the resident macrophage sub-populations (Keuper 2019). Macrophages comprise ~5% of the total cell population in WAT, with the fraction increasing to 50% during obesity (Weisberg et al. 2003). Specifically, activated macrophages secrete factors that contribute to the regulation of adipose tissue and consequently the regulation of energy metabolism. Classically activated macrophages, referred to as M1 macrophages, contribute to WAT inflammation through the secretion of proinflammatory cytokines including interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNFα) (Chawla et al. 2011). In contrast, alternatively activated macrophages (M2) secrete anti-inflammatory cytokines such as interleukin-4 (IL-4) and interleukin-10 (IL-10) (Nguyen et al. 2011). Importantly, M2 macrophages also secrete norepinephrine, which has been hypothesized to contribute to the beiging of white adipocytes (Y Lv et al. 2016; Miller et al. 2019). Unlike in WAT depots, the macrophage population in BAT is generally thought to be low (Fitzgibbons et al. 2011). However, it has been frequently noted that macrophage infiltrations accompany the ‘whitening’ of brown fat, suggesting the role of inflammation in mediating impairments in BAT function (Kotzbeck et al. 2018).
The well-studied immunomodulatory effects of BPA and its analogues gains a new perspective when one considers the importance of macrophage polarization in adipose tissue. Both BPA and its replacements appear to induce M1 polarization of macrophages, which can serve to maintain the white adipocyte population. Such findings have been reported using in vitro model systems (Chen et al. 2018; Hong et al. 2004; Y Liu et al. 2014; Lu et al. 2019; Zhao et al. 2019). BPA treatment (10 μM) in macrophage-like Kupffer cells obtained from BPA-exposed mice induced a classically activated M1 phenotype with elevated production of IL-6, TNFα, IL-1β. Interestingly, conditioned media from the BPA-exposed Kupffer cells increased lipogenesis and lipid droplet formation in HepG2 hepatocytes (Lv et al. 2017). Furthermore, elevated M1 macrophages have also been observed in the gonadal WAT from male mice from dams orally exposed to BPA throughout gestation and up to weaning (50 μg/kg body weight daily) (Malaise et al. 2017). Interestingly, the BPF-induced elevation in M1 polarization was preventable through chemical inhibition of estrogen receptor-α (Shi et al. 2020). Hence, it is likely that the propensity of the bisphenols to induce M1 activation, thereby affecting adipocyte beiging, is likely attributed to estrogen-driven signaling. Nonetheless, further research is needed to better understand the role of bisphenol-induced inflammation in the whitening of brown and beige fat.
2.2. Dioxins
Another group of EDCs that have been shown to modulate metabolism are the persistent organic pollutants (POPs). Included in this class of environmental contaminants are dioxins, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibensofurans (PCDFs), and dioxin-like compounds like the polychlorinated biphenyls (PCBs) (Regnier and Sargis 2014). They are introduced to our environment as by-products of manufacturing processes that include waste incineration, smelting, bleaching of paper pulp and burning of fossil fuels. Most exposure occurs through dietary consumption of meat, fish, shellfish, and dairy products, where concentrations accumulate up the food chain. As POPs primarily reside in the adipose tissue and are released slowly into the bloodstream, their half-lives can be upwards of a decade or more and lends to their potential to impact health throughout the lifespan (Milbrath et al. 2009).
Although many dioxins and dioxin-like compounds have been recognized, TCDD is often regarded as the most toxic of the class (Bruner-Tran et al. 2017). It has been long thought that some of the overt effects of TCDD are mediated by its interaction with the aryl hydrocarbon receptor (Ahr), a ligand activated transcription factor that plays a major role in regulating xenobiotic metabolism, cellular proliferation/differentiation, and the immune response (Larigot et al. 2018). More recent evidence suggests that TCDD may also regulate energy metabolism. Exposure to TCDD is associated with the development of a “wasting syndrome,” likely akin to cachexia, which is a progressive loss of adipose tissue and reduction in body weight (Girer et al. 2021; Linden et al. 2014). Similar findings have also been noted in numerous species environmentally exposed to dioxins including birds and cattle (Debacker et al. 2003; Sani 2015). In contrast, others have demonstrated that chronic exposure of TCDD may be pro-obesogenic, especially within the diet-induced obesity rodent model (Brulport et al. 2017; Hoyeck et al. 2020).
Considering the evidence that high dioxin exposure may lead to a cachexia-like, wasting syndrome and that a hallmark of cachexia is rampant activation of brown and beige fat, it is not surprising that there are numerous reports which demonstrate that TCDD preferentially acts upon BAT (Sun et al. 2020). Notably, however, whether brown fat contributes to dioxin-induced cachexia has only been hypothesized and not rigorously investigated (Rozman 1984). Nonetheless, BAT has long been identified as a target tissue of TCDD (Rozman et al. 1986; Viluksela et al. 1995). Following a single intraperitoneal exposure to TCDD (15 μg/kg body weight), brown adipocytes increased in size and contained fewer, but larger lipid droplets (Rozman et al. 1987). These morphological changes were also accompanied by mitochondrial swelling observable up to 14 days following exposure. Cumulatively, these alterations in cell structure, including mitochondrial biogenesis and the presence of multilocular lipid droplets, are indicative of activated BAT. In addition to rapid changes in BAT lipids, glucose uptake in the tissue occurs swiftly following intravenous injection of [14C] glucose in TCDD exposed rats (125 μg/kg body weight), again a similar phenomenon during cold-induced brown fat activation (Nedergaard et al. 2007; Weber et al. 1987a). Despite the likelihood that TCDD alters BAT activity, reports have not found that exposure is associated with subsequent increases in body temperature (Weber et al. 1987b). Specifically, in rats housed well below thermoneutrality (4°C vs. 25°C), TCDD-induced wasting (60 μg/kg body weight, i.p.) occurs at a faster clip, alongside a failure to increase core body temperature (Rozman and Greim 1986). Further, a single oral exposure to TCDD on gestation day (GD) 15 (1.0 μg/kg body weight) was associated with reduced body temperature in the offspring (Gordon et al. 1995).
Despite this work, the understanding of the relationship between BAT and TCDD is incomplete. From what is currently known, TCDD-induced activation of brown fat is likely a complex interaction between both direct and indirect signaling factors. TCDD is a potent disruptor of thyroid signaling. In TCDD-exposed rats (125 μg/kg body weight, i.p.), plasma levels of both triiodothyronine (T3) and thyroxine (T4) were reduced as well as lipogenesis in BAT (Gorski et al. 1988). Thyroidectomy inhibited these outcomes, suggesting that TCDD effects in brown fat is partially mediated through peripheral signaling. In BAT, however, oral TCDD exposure (10 – 40 mg/kg body weight) in rats was associated with dose-dependent increases in DIO2 (Raasmaja et al. 1996). Similar results were also found in TCDD-exposed rats (60 mg/kg body weight, oral gavage), alongside reductions in circulating T4 (Viluksela et al. 2004). Together, this suggests that the effects of dioxins on BAT may be mediated through the modification of thyroid signaling.
Finally, it is important to note the effects of TCDD on white adipocytes. In the 3T3-L1 white adipocyte line, exposure to TCDD (10 nM) during the early induction stage suppressed formation of the large, unilocular lipid droplet reminiscent of mature white adipocytes (Phillips et al. 1995). Accordingly, the impairment of terminal differentiation in adipocytes exposed to TCDD (30 nM) was associated with reduced expression of adipogenic markers including CCAAT/enhancer-binding protein-α (Cebpα), Pparγ, and glucose transporter type 4 (Glut4) (Hsu et al. 2010). Similar effects have also been reported in primary preadipocytes obtained from rats orally exposed to TCDD (175 μg/kg body weight) or in exposed mesenchymal stem cells, with impaired adipogenesis and maturation mirroring closely what is observed in 3T3-L1 cell line (Brodie et al. 1997; Li et al. 2008). Interestingly, such effects are seemingly lost if mature adipocytes are exposed to TCDD, suggesting that the chemical may regulate an early step in the differentiation pathway of white adipocytes. The 3T3-L1 adipocyte line is capable of differentiation into a beige phenotype (Miller et al. 2015b). Hence, the above findings suggest the likelihood that TCDD may induce beige differentiation from the white preadipocyte. However, to the best of our knowledge, the effects of TCDD on mitochondrial biogenesis and UCP1 expression in 3T3-L1s has yet to be investigated.
2.3. Air Pollutants
2.3.1. Particulate Matter
Air pollution is a complex mixture of particulate matter (PM) and gases, derived from both natural and anthropogenic sources. PM refers to a broad class of microscopic particles that vary by size and chemical composition. Particles originate from a variety of sources, including wildfire smoke, industrial emissions and motor exhaust. Over the last century, rapid industrialization and urbanization have greatly contributed to the global burden of diseases attributable to air pollution (Babatola 2018). Among the criteria air pollutants regulated by the National Ambient Air Quality Standards, PM2.5 (aerodynamic diameter ≤ 2.5 μm) has long been recognized as a significant contributor to cardiopulmonary disease and mortality (Kurt et al. 2016). Exposure to air pollutants, however, is now being implicated in the development of metabolic disorders (Kim et al. 2019).
Epidemiological evidence has linked exposure to air pollution to the development of metabolic syndrome, which is a cluster of risk factors characterized by abdominal obesity, insulin resistance, hypertension, and dyslipidemia (Alderete et al. 2017; Auchincloss et al. 2008; Wallwork et al. 2017). The biological mechanism underlying this association remains unclear. However, it has been hypothesized that the pulmonary immune response to inhaled particles triggers systemic inflammation and oxidative stress pathways that can perturb downstream metabolic processes (Haberzettl et al. 2016; Pope et al. 2004; Riggs et al. 2020).
As discussed previously, adipose tissue macrophages are recognized as an important link between cellular responses to injury and metabolic dysregulation. Numerous studies in vivo have shown that inhalation of PM2.5 induces macrophage infiltration in WAT (Mendez et al. 2013; Sun et al. 2009; Zhong et al. 2016). Once more, macrophages undergo polarization to produce distinct functional phenotypes. M2 macrophages are generally associated with an anti-inflammatory profile and release catecholamines, whereas M1 macrophages are associated with a pro-inflammatory profile (Nguyen et al. 2011). In diet-induced obese mice, exposure to PM2.5 (72.7 μg/m3 average concentration) shifted the macrophage population in visceral white adipose tissue from an M2 phenotype to an M1 phenotype (Sun et al. 2009). In similar studies, PM2.5-exposed mice (70 – 97 μg/m3 average concentration) showed reduced mitochondrial density, disrupted mitochondrial biogenesis, and reduced UCP1 expression in WAT (X Xu et al. 2011; Xu et al. 2012; Z Xu et al. 2011). Considering that M2 macrophages function as a local source of norepinephrine, which can stimulate UCP1 expression in thermogenic adipose tissue, exposure to PM appears to result in a less favorable environment to support beiging in WAT depots.
Unlike in WAT, brown fat is more resistant to macrophage infiltration (Fitzgibbons et al. 2011). Nonetheless, C Liu et al. (2014a) demonstrated that exposure to PM2.5 (102.9 μg/m3 average) resulted in elevated levels of pro-inflammatory cytokines in BAT using a genetically susceptible mouse model of type II diabetes mellitus. Prolonged adipose tissue inflammation affects other metabolically active organs, contributing to ectopic lipid storage, insulin resistance and can exacerbate systemic inflammation. Both in vitro and in vivo evidence have shown that inflammation impairs thermogenesis by inhibiting BAT development and suppressing the induction of UCP1 (Porras et al. 1997; Sakamoto et al. 2016). Consistent with inflammatory-induced mitochondrial dysfunction, PM2.5 exposure alters thermogenic gene profiles in BAT. In apolipoprotein E knockout mice, subchronic exposure to PM2.5 (2 months, 96.9 μg/m3 average concentration) downregulated the expression of Ucp1, Dio2, and PPARG coactivator 1-α (Ppargc1α) (Z Xu et al. 2011). Similar decreases in the BAT thermogenic gene program and increases in oxidative stress were also reported with long-term, chronic inhalation to PM2.5 in mice (10 months, 94.4 μg/m3 average concentration) (X Xu et al. 2011). It is hypothesized that during exposure to obesogenic stimuli, the brown to white conversion of adipose tissue may be a physiological adaptation to store excess lipids (Cinti 2018). Notably, in apolipoprotein E knockout mice, the expression of WAT-specific genes were upregulated, suggesting that BAT can shift towards a WAT phenotype following PM2.5 exposure (96.9 μg/m3 average) (Z Xu et al. 2011).
Due to their unique physical characteristics, silicon dioxide (SiO2) nanoparticles (aerodynamic diameter ≤ 0.1 μm) have been extensively used in a variety of applications, including pharmaceutical drug delivery, cosmetics and electrical insulation (Mebert et al. 2017). Due to their relative size, SiO2 and other nanoparticles can translocate from the respiratory interstitium to other tissues. Accordingly, SiO2 nanoparticles have been detected in brown adipocytes of rats following exposure (Zhang et al. 2017). Further, in thermal stress-exposed rats (4°C), pulmonary exposure to SiO2 (3 mg/mL; instillation) prevented cold-induced upregulations in BAT thermogenesis (Lin et al. 2016). This was accompanied by both whitening of the interscapular brown fat depot and prevention of beiging in the epididymal white fat depot. As such, inflammatory signaling has been implicated as a driver of these outcomes (Zhang et al. 2017).
Deficits in brown and beige fat function may be associated with hypothalamic inflammation (Arruda et al. 2011). In fact, exposure to PM2.5 (107 μg/m3 average concentration) was shown to elevate hypothalamic Tnfα and Il-6 expression in rodents (Ying et al. 2014). Further, genetic ablation of the TLR4 receptor in the hypothalamus protected mice from the obesogenic effects of prolonged PM2.5 exposure (600 μg/m3 average) (Campolim et al. 2020; C Liu et al. 2014b; Qiu et al. 2018). Taken together, these findings support that air pollution may promote metabolic and brown/beige adipose tissue dysfunction through both indirect hypothalamic-mediated inflammation or direct effects on the adipocyte.
2.3.2. Heavy Metals
Beyond particle size, the health effects of PM can be further mediated by the unique make up of chemicals that are bound to a particle. Heavy metals are naturally abundant elements, with varied uses in the agricultural, biomedical, and technological fields that have led to their widespread distribution in the environment. Some heavy metals can serve important biological functions, although many are considered to be potent toxicants and carcinogens (Tchounwou et al. 2012). In addition to the consumption of contaminated foods and water, exposure to heavy metals can also occur through inhalation. Several heavy metals have been implicated in the health effects of PM, including cadmium (Cd), nickel (Ni) and zinc (Zn) (Gavett et al. 2003; Srimuruganandam and Shiva Nagendra 2012). Both Cd and Ni are toxic heavy metals produced during the manufacturing of alloys, batteries and tobacco products (Pappas 2011; Tchounwou et al. 2012). Conversely, Zn is an essential heavy metal primarily emitted through the metal mining, fossil fuel and electroplating industries (Wuana and Okieimen 2011).
Heavy metals are hypothesized to contribute to the etiology of PM-induced obesity and its sequelae (Wang et al. 2018). Many of the toxic effects of heavy metals can be attributed to their long half-lives and tendency to accumulate in multiple tissues (Li et al. 2015). Recent evidence suggests that adipose tissue is an important target for heavy metal toxicity, although their effects on fat have not been extensively studied (Egger et al. 2019; Freire et al. 2020). Nonetheless, exposure to metals (e.g., mercury or lead) may increase lipid accumulation in 3T3-L1 adipocytes through C/EBPβ induction and corroborates epidemiological associations between exposure and obesity (Tinkov et al. 2021). As part of the obesogenic properties of air pollution may be attributed to heavy metals, it is likely that such compounds have direct actions on brown and beige fat.
Heavy metals, such as Cd, have the potential to disrupt metabolism through direct toxicity or displacing essential micronutrients (Iavicoli et al. 2009). Accordingly, CdCl2 (> 3 mM) can inhibit mitochondrial glycerol-3-phophate dehydrogenase (GPDH) activity in BAT-derived mitochondria, an enzyme important in redSucing dihydroflavine-adenine dinucleotide (FADH2) during oxidative phosphorylation (Rauchová et al. 1985). Such effects on GPDH function were also observed in the murine white adipocyte 3T3-L1 line treated with 3 μM CdCl2 (Lee et al. 2012). Cd is also suspected to interfere with thyroid signaling; in vitro CdCl2 exposure (5 – 10 mM) inhibits the conversion of T4 to active T3 (Paier et al. 1997). Such effects have been attributed to the strong affinity of Cd for the sulfhydryl groups of DIO2 (Vallee and Ulmer 1972). The thyroid receptor is a transcriptional regulator of several mitochondrial biogenesis genes and its appropriate signaling is indispensable for both brown and beige thermogenesis (Yau and Yen 2020). Unsurprisingly, the known interaction between Cd and thyroid signaling has led some to use BAT as a model tissue system for Cd-induced toxicity (Paier et al. 1997). Due to impaired thyroid signaling, cold stress in rats that have been exposed to CdCl2 (200 μg/100 g body weight, i.p.) showed a reduced capacity to upregulate thermogenesis in brown fat (Noli et al. 1998). These effects were recapitulated in excised BAT exposed to CdCl2 ex vivo (2.5 – 5 mM). Considering the more recent findings that Cd storage in adipose tissue may have been underestimated in the past (Tinkov et al. 2017), a better understanding as to how its exposure may interfere with the specific classes of adipocytes is increasingly relevant.
Zn is another metal that has been shown to inhibit T4 deiodination, lending itself as another potential heavy metal that may perturb brown and beige adipose tissue. In primary rat brown adipocytes, exposure to Zn (1 or 5 mM as ZnSO4) impaired T3 production regardless of the thermal conditions in the exposed animal (Pavia et al. 1999). As expected, ZnSO4 exposure (10 mg/kg, subcutaneous) in cold-stressed rats resulted in reduced serum T3 levels and BAT weight, alongside decreased oxygen consumption and reduced guanosine diphosphate (GDP) binding in isolated BAT mitochondria from healthy rats (Rebagliati et al. 2001). Notably, exogenous T3 supplementation only partially reversed these outcomes, suggesting that the entirety of effects of Zn exposure on BAT may not fully be explained by impaired thyroid signaling. Zn-induced impairments in GDP binding (< 200 μM of ZnSO4), a marker of thermogenic potential, was found in brown adipocytes derived from mice with genetic obesity (ob/ob), an effect not seen in lean mice (Chen et al. 1997). On the other hand, it has also been reported that ZnSO4 (1 – 10 mg/kg body weight) may upregulate UCP1 in BAT of cold exposed rats (Beattie et al. 2000; Luo et al. 2004). As dietary Zn deficiency has also shown adverse effects on BAT through the downregulation of thyroid signaling, adverse effects of toxic exposures to Zn are likely dependent on other factors including dietary sources, the presence and status of other metals that share transporters, and body condition (Lukaski et al. 1992).
Considering that PM may contain various heavy metals, discriminating the individual health effects of exposure to the metal from its exposure as a component of particulate matter is critical. In mice, exposure to either concentrated ambient particles ( 69.6 μg/m3 PM2.5) or NiSO4 (0.44 μg/m3) produced independent adverse metabolic deficits, which in some cases resulted in additive impacts when exposures were combined (Xu et al. 2012). Nonetheless, like what is seen in models of PM exposure, NiSO4 inhalation induced mitochondrial dysfunction, impaired expression of the BAT gene program, and macrophage infiltration of the adipose tissue depots in the study by Xu et al. (2012). Furthermore, these effects were implicated to be related to reduced 5’ AMP-activated protein kinase (AMPK) signaling, which is key regulator of cellular metabolism and mitochondrial biogenesis. Taken together, these findings suggest that the elemental composition of PM plays an important role in driving the metabolic and thermogenic responses to exposure. The mechanisms of action likely occur through multiple, intersecting signaling pathways and targets. Importantly, metals preferentially accumulate in different tissues (Li et al. 2015). How the composition of metals in PM alters BAT and WAT has not been comprehensively described.
2.4. Phthalates
An additional chemical class known to impact BAT function is phthalate esters. As a plasticizer, phthalates are added to plastic packaging to increase their flexibility and strength. They are also a critical ingredient in fragrances, acting as a solvent for the aromatic scents that can improve their strength and longevity. Accordingly, exposure to phthalates occurs through varied sources such as food packaging, vinyl flooring, medical tubing, and personal care products (e.g., shampoo, perfumes, lotions, and soap). While both dietary consumption and skin absorption are the primary routes of phthalate exposure, phthalates are also introduced to the body via inhalation of dust particles (Calafat and McKee 2006). Considering the breadth of potential sources of phthalates, and their overall short half-lives (~5 – 24 hours), levels of the metabolites are widely found throughout the population (Wittassek and Angerer 2008).
Having first been introduced to the market in the 1930s, the potential for health effects of phthalates has long been of concern. As an EDC, some phthalates have shown to have numerous modulating effects on numerous receptors and transcription factors such as estrogen receptor, androgen receptor, and on the PPARs (Desvergne et al. 2009; Engel et al. 2017). Perhaps unsurprising, some of the most noted relationships related to high phthalate exposure is on reproductive and developmental toxicity. Phthalate esters and some of their active metabolites have also demonstrated the potential to impair immune cell functionality (Li et al. 2013; Wang and Dong 2012) and increase the production of reactive oxygen species (Sedha et al. 2015). Hence, their potential effects are thought to be systemic.
Considering phthalates regulate PPAR signaling, phthalate exposure has been implicated in the pathogenesis of obesity and other metabolic disorders (Grun and Blumberg 2007). In vitro exposures to monoethyl-hexyl-phthalate (MEHP; 100 μM), benzyl butyl phthalate (BBP; 100 μM), or dicyclohexyl phthalate (DCHP; 1 μM) have shown the potential to increase lipid accumulation and lipogenic gene expression in both the 3T3-L1 adipocyte cell line and in primary human adipocytes (Ellero-Simatos et al. 2011; Feige et al. 2007; Sargis et al. 2010; Yin et al. 2016). Exposure to either 20 μM MEHP or mono-(2-ethylhexyl) tetrabromophthalate (METBP) shifted the differentiation of bone marrow-derived mesenchymal stromal cells towards the adipocyte lineage and away from osteoblasts (Watt and Schlezinger 2015). Furthermore, gestational exposure to di(2-ethylhexyl) phthalate (DEHP; 300 mg/kg body weight, oral gavage) was associated with increases in adipose tissue size and inflammation in the adult male offspring (Campioli et al. 2014; Hao et al. 2013; Strakovsky et al. 2015). This work demonstrates the potential for phthalate esters to induce a pro-obesogenic phenotype. However, in some models, dietary DEHP (2% w/w) has been associated with reduced WAT size in healthy, adult rodents (Martinelli et al. 2010; Xie et al. 2002). This suggests that the type of ester, route, and developmental window of exposure are likely important in determining the obesogenic potential of phthalates.
In addition to promoting WAT dysfunction, evidence also points to the potential of phthalates to regulate the activity of thermogenic adipose tissues. As an activator of PPARα, which regulates the expression of enzymes involved in lipolysis and β-oxidation, DEHP exposure in vivo has been associated with increased acetate production and short-chain acyl-CoA metabolism (Engberg et al. 1997; Yamashita et al. 2006). This suggests the likelihood that DEHP exposure induces a systemic shift to aerobic metabolism. In fact, literature dating back into the 1980s identified phthalates as a potent mitochondrial toxicant capable of uncoupling the respiratory chain and increasing mitochondrial density in the rat liver (Ganning et al. 1983; Inouye et al. 1978; Melnick and Schiller 1985). Utilizing radiolabeled DEHP, BAT was found to be one of the tissues with the highest DEHP uptake at 4 hours after injection in mice (Lindgren et al. 1982). Further, BAT was the only tissue with persistently high levels of radioactivity at 24 hours post-injection, outside of the bladder, gall bladder, and intestinal contents.
Despite evidence indicating that BAT is a major target organ of DEHP, few studies have considered the effects of phthalates on BAT morphometry or function. Following developmental exposure to DEHP (30 mg/kg body weight pre-pregnancy through lactation, oral gavage), offspring had hyperplastic brown fat, with both increases in scapular brown depot size and adipocyte number (Lee et al. 2016). However, in male mice exposed to DEHP for 5 weeks (0.5 mg/kg body weight, oral gavage), expression of UCP1 in BAT was reduced, which was accompanied by hypothermia, increased food intake, and an increase in body weight (Z Lv et al. 2016). Thus, as previously noted, the effects of phthalates on BAT may also be highly dependent on numerous factors including life stage and sex.
In a recent study by Hsu et al. (2020), treatment of MEHP during differentiation in 3T3-L1s induced beiging when adipocytes reached maturity. Specifically, MEHP treated cells had a higher energy demand, showing increases in both basal respiration and glycolytic capacity compared to vehicle-treated adipocytes (≥ 100 μM), an increased transcriptional phenotype resembling beige adipocytes (≥ 30 μM), and an increased number of small lipid droplets per cell (≥ 30 μM). Similar elevations in thermogenic gene expression in visceral WAT were found in mice exposed to DEHP for 25 weeks (1.0 mg/kg body weight, oral gavage), in addition to a noted elevation in BAT weight (Hsu et al. 2020). Intriguingly, certain phthalates including DEHP may induce macrophage M2 polarization (Hansen et al. 2015; Kim et al. 2014; Lee et al. 2018), which would promote an environment conducive to beiging and may provide some explanation behind the in vivo observations in the report by Hsu et al. (2020). While there appears to be differential effects of the unique phthalate esters on adipose tissue, growing evidence suggests that phthalates target and modulate the activity of brown and beige fat. In some instances, exposure to specific phthalate esters may promote thermogenesis and increase in metabolic rate, however, the circumstances that drives this response renders further study.
2.5. Phytochemicals
Phytochemicals constitute a large class of non-nutritive compounds produced by plants often to protect against stress from temperature, sunlight, and infectious agents (Ramakrishna and Ravishankar 2011). In addition to protecting the host, many of these compounds have bioactive properties in mammals if consumed. Of note, phytochemicals offer potential as a weight loss strategy due to its known anti-obesity properties. For instance, polyphenols have been reported to increase lipolysis and induce fatty acid oxidation via modulation of hormone sensitive lipase, acetyl-coA carboxylase, carnitine acyl transferase, and PGC1α (Rupasinghe et al. 2016). Most of these phytochemicals, in addition to their weight loss properties, can also have added health benefits including anti-inflammatory and antioxidant functions (Zhang et al. 2015). These added benefits may further the anti-obesity effects seen with phytochemicals, as the decrease in inflammation would subsequently cause a further reduction in the risk of metabolic disease development.
Outside of notable therapeutic examples (e.g., salicylic acid and paclitaxel), much of the current interest and attention in phytochemicals are as preventatives. The specific structure of individual phytochemicals predicates the mechanism of their bioactivity. Polyphenols, as an example, are made up of a group of phytochemicals constituted of multiple phenolic units (Meulenberg 2009). Accordingly, polyphenols are most widely known and commercially marketed as antioxidants. However, some also share structural similarities with steroid hormones, resulting in their capacity to modulate hormone signaling and their classification as endocrine-disrupting compounds (Meulenberg 2009).
Numerous polyphenols have been identified and studied as modulators of brown and beige adipose tissue, which is partially attributed to their ability to bind to a wide array of steroid receptors. While an in-depth review of the evidence is beyond the breadth of the current discussion, readers are directed to topical reviews by Mele et al. (2017) and Azhar et al. (2016). In brief, polyphenols appear to regulate numerous pathways involved in the function of thermogenic adipose tissue (Figure 2). Resveratrol, both an estrogen receptor agonist and antagonist, has been shown to decrease adipogenesis and viability in maturing 3T3-L1 white preadipocytes (Rayalam et al. 2008). Additionally, resveratrol treatment in mature adipocytes resulted in reduced lipogenesis and increased lipolysis, which was associated with alterations in mitochondrial function. The mechanism behind such effects were later ascribed to increased intracellular cAMP which resulted in the downstream induction of sirtuin 1 (SIRT1) and PGC1α mediated mitochondrial biogenesis (Li et al. 2020). Through a similar pathway, many of the flavanols, which include compounds from tea and cocoa, upregulate PGC1α-mediated mitochondrial biogenesis via AMPK activity (Mele et al. 2017). On the other hand, capsinoids, menthol, and curcumin are sympathetic nervous system stimulants that increase UCP1 expression through the transient receptor potential channels and eventual beta-adrenergic receptor activation (Saito et al. 2020). Furthermore, some polyphenols such as guggulsterone, allspice, and clove are suspected to activate the G-protein-coupled bile acid receptor, which increases UCP1 expression through the upregulation of deiodinases and thyroid signaling (Azhar et al. 2016; Ladurner et al. 2017; Thomas et al. 2008). While the direct effect of phytochemicals on adipocytes appears to be varied, they are also hypothesized to promote macrophage M2 polarization, increasing norepinephrine production and inducing beiging in WAT (Miller et al. 2019; Nishikawa et al. 2019).
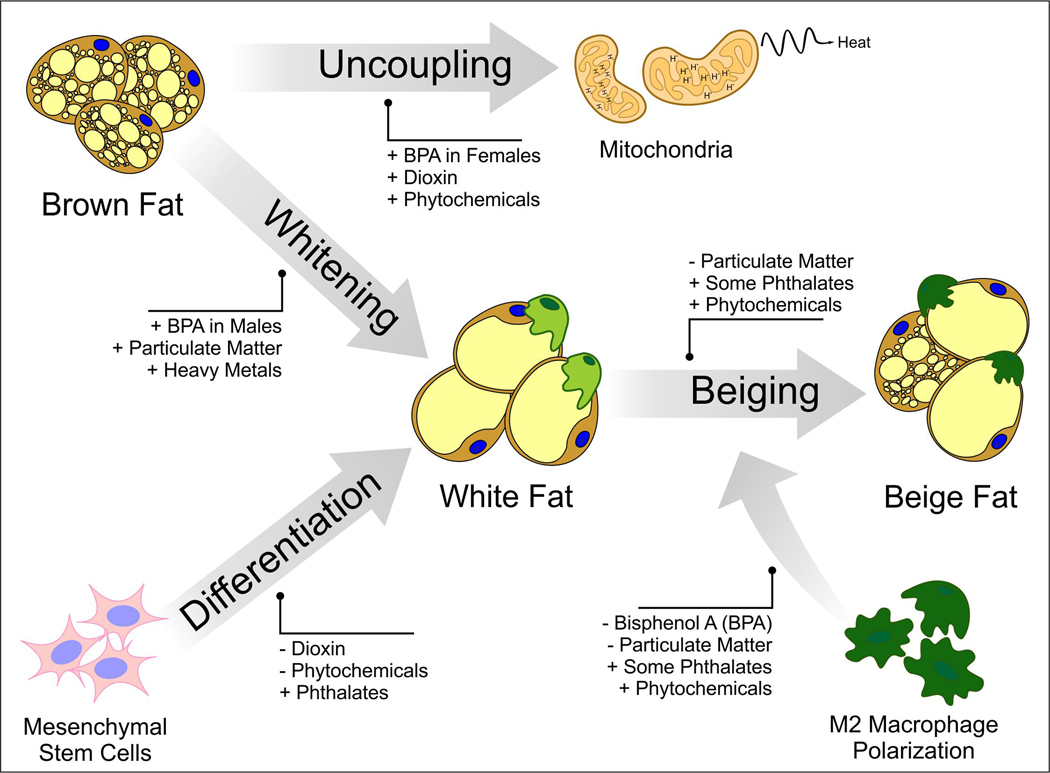
Figure 2:
Summary of potential points where synthetic and natural EDCs may alter brown and beige adipogenesis.
Numerous phytochemicals have shown an improved efficacy when they are dosed in combination compared to when they are dosed independently. Such synergistic and anti-obesogenic effects have been observed both in 3T3-L1 adipocytes (Baile et al. 2011; Park et al. 2008) and in vivo (Miller et al. 2015a; Nishikawa et al. 2019). Similar additive effects have also been observed with EDCs, with noteworthy examples including the reported relationship with phthalate mixtures and alterations in male reproductive tract development (Howdeshell et al. 2017). Importantly, the pathways that phytochemicals interact with often coincide with those perturbed by environmental pollutants. Hence, this implies that interactions between the synthetic and natural endocrine disruptors are likely to exist.
3.1. Brown and Beige Fat as a Therapeutic Target Organ
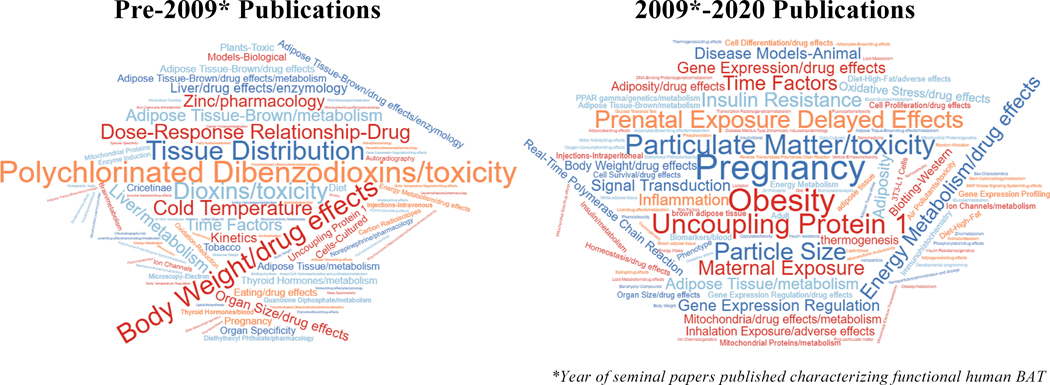
Historically, humans were thought to lose function of thermoregulatory adipose tissue early after infancy. Rodents, however, remain dependent on brown and beige adipose tissues throughout their lifespan to maintain body temperature. Attributable to several seminal publications in the 2000s [see Nedergaard et al. (2007) and Kiefer (2017)], the interest in human brown fat re-emerged following observations of glucose uptake in known BAT regions in FDG-PET/CT imaging. Accordingly, this has led to a refocus of the value of human thermogenic tissues. The discovery of beige adipose tissue has only heightened this interest, leading to a shift in the publications investigating the role that beige and brown fat may have in humans (Figure 3).
Figure 3:
Word cloud of keywords derived from manuscripts that investigated the effects of bisphenols, dioxins, air pollutants, or phthalates on brown or beige adipose tissues. Studies were collated from Web of Science, split by year, and keywords were pulled to generate word clouds.
Importantly, the amount of brown and beige fat is reduced in obesity (Symonds et al. 2018). Hence, the challenge presented is to determine how to either prevent its loss or to reactivate it. Nonetheless, significant methodological hurdles exist in assessing human brown and beige fat activity. Testing is often performed by measuring radiolabeled glucose in the fasting state and is dependent on cold exposure (Wu et al. 2013). This measurement modality frequently gives results that vary between repeated scanning, making accurate functionality measurements difficult to obtain (Wu et al. 2013). Because of this, human studies involving imaging of BAT often rely on the use of medications such as beta agonists for functional analysis, some of which have known cardiovascular contraindications.
In humans, β3-adrenergic receptor (ADRβ3) mRNA has been found in multiple tissues, such as BAT and WAT, bladder, gallbladder, brain, and in the gastrointestinal tract (Mund and Frishman 2013). Agonists of ADRβ3 increase lipolysis, fat oxidation, energy expenditure, and improve insulin sensitivity (de Souza and Burkey 2001). These effects have led many to believe that targeting this receptor might serve as an effective treatment of diabetes and obesity. While there remains continued promise of therapeutic use of β3-adrenergic agonists for obesity, significant limitations persist. Although ADRβ3 is considered the main receptor to stimulate BAT, it is unclear as to whether it is the only adrenergic receptor involved in BAT activation in rodents. For example, ADRβ3 knockout mice can maintain cold-induced BAT function (de Jong et al. 2017). This has been hypothesized to be due to non-sympathetic mechanisms and/or compensatory β1-adrenergic receptor signaling, creating possible interest in the manipulation of the β1- receptor to stimulate BAT. While this receptor may play an important role in BAT activation, administration of β1-agonists for the purpose of treating obesity in humans would create several systemic side effects, most notably in the cardiovascular system (Mund and Frishman 2013).
With the tangible utilization of beta-adrenergic agonists still debated, it would be remiss not to mention the recently approved anti-obesity medications. In addition to their most widely recognized mechanism of action, many of the pharmaceutical options appear to have modulatory capacity in brown adipocytes. For example, the glucagon like peptide 1 (GLP-1) receptor agonist, Liraglutide (Saxenda®), was shown to induce brown adipocyte differentiation in the skeletal muscle of diet induced diabetic mice (Zhou et al. 2019). These findings were associated with the increased expression of UCP1 and PR domain containing 16 (PRDM16), attributed to activation of SIRT1 signaling. Qsymia® is a potent appetite suppressor composed of two medications, phentermine and topiramate, that both have unique CNS effects. The impacts of these two compounds either in separate or in combination on brown/beige fat has not been extensively investigated. Nonetheless, limited findings suggest that topiramate may induce an upregulation of uncoupling proteins 2 and 3 in muscle and white and brown fat in high fat diet-fed rats (York et al. 2000). Adjunctive treatment of Contrave® (naltrexone and bupropion) in gastric banded rats resulted in synergistic improvements in both energy expenditure and BAT activity (Stefanidis et al. 2017). Attributable to their various impacts on the CNS, however, many of the appetite suppressant drugs have extensive side effects. Of note, phentermine is contraindicated in patients where sympathomimetic drugs may pose a significant risk, including those with unstable cardiovascular disease, high blood pressure, hyperthyroidism, and unstable cardiac dysthymias (Bays et al. 2007). Topiramate can result in various vision and eye conditions (Cereza et al. 2005).
Due to the side effect profile associated with many anti-obesity medications, they are often removed from the market. With comparatively less severe potential for adversity, the interest in natural compounds as anti-obesity therapies has surged. Phytochemicals do not work through direct adrenergic signaling, thus creating a pharmacologic advantage that may circumvent many of the systemic side effects associated with beta agonists. As reviewed in the previous section, specific phytochemicals are hypothesized to counteract the loss of BAT function seen in obesity by increasing brown fat activation and white adipocyte beiging (Azhar et al. 2016; Mele et al. 2017). While phytochemicals have demonstrated positive, but mixed, efficaciousness for aspects of metabolic syndrome (e.g. hyperlipidemia, hypertension, hyperinsulinemia), clinical trials on the efficacy of phytochemicals on brown or beige adipose tissue are lacking (Jenkins et al. 2008; Minich et al. 2010).
Lastly, it is important to note that such phytochemicals are in of themselves EDCs (Marcoccia et al. 2017). One example is the soy phytochemical, genistein, which is a well characterized estrogen receptor agonist. The developmental effects of genistein, primarily through soy infant formulas, have long been of concern (Rozman et al. 2006). Nonetheless, it is likely that some of the thermogenic effects of many of the phytochemicals may be attributed to their EDC properties. This raises the question as to whether specific classes of EDCs may have the potential to be exploited for their anti-obesity properties. In addition to phytochemicals, one noteworthy example of a synthetic EDC used for weight loss is 2,4-dintrophenol (2,4-DNP). As an EDC used in the manufacturing of dyes, pesticides, and explosives, 2,4-DNP exposure at high levels induces an hyper-metabolic state accompanied with fever, respiratory failure, and death within a few hours (Siegmueller and Narasimhaiah 2010). While the use of 2,4-DNP as a weight loss drug was banned by the Food and Drug Administration in 1938, it is still illicitly used today (Potts et al. 2020). While the use of EDCs to activate brown and beige fat is likely controversial, both the evidence seen with phytoestrogens and historical use of 2,4-DNP for weight loss provides reasonable precedence for continued study.
4.1. Conclusions
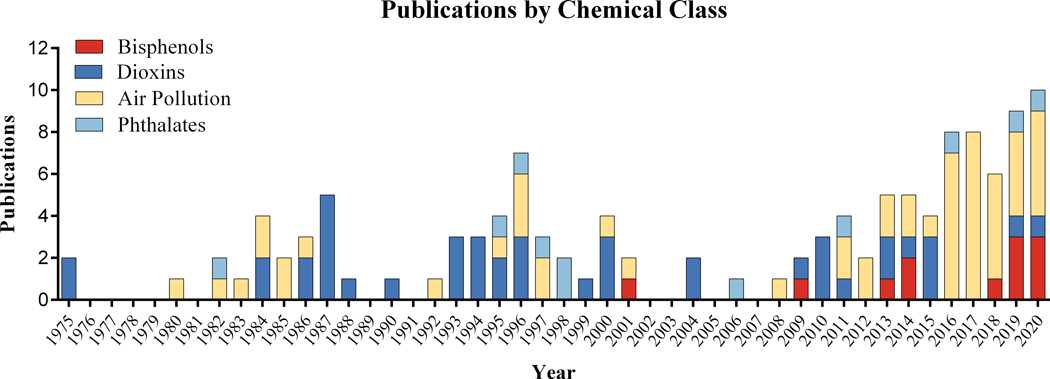
With the evidence of the existence of brown and beige fat in the adult human population, the role of these important depots in contributing to and protecting from metabolic disease has dramatically increased over the past decade (Kiefer 2017). A variety of both synthetic and natural EDCs have shown the potential to induce adipose tissue dysfunction not only in white adipocytes but in brown and beige fat as well (Azhar et al. 2016; Di Gregorio et al. 2018). Interest in this line of research has only recently started to grow, however, the overall number of publications on the topic of environmental pollutants and brown and beige adipose tissue remain scant (Figure 4). Herein, we highlight the likely critical importance these unique fat depots play in modulating the risk and pathogenesis of various metabolic diseases including obesity and cachexia. Considering the public health implications of such conditions, continued efforts should be placed on determining the impacts of EDCs on brown and beige adipogenesis and function.
Figure 4:
Number of publications by year that study the effects of bisphenols, dioxins, air pollutants, or phthalates on brown or beige adipose tissues. Databases searched included PubMed, Web of Science, and ProQuest. Following de-duplication, a total of 126 citations were found. Of note, phytochemicals were not included in this list due to the vast array of chemicals within this class and large number of publications in this area.
Finally, we note the effects of EDCs on thermogenic adipocytes likely exist on a spectrum encompassing both agonistic and antagonistic effects and may be driven by phase I/II metabolism, concentration, and even by life stage or sex. While the chemicals reviewed herein were selected due to the number of reference publications, there is a strong likelihood that many different environmental contaminants have functional effects in brown and beige fat. Furthermore, there is emerging evidence that combinations of EDCs may interact with each other in either additive or inhibitory ways, as others’ have reported in adipocytes (Baile et al. 2011; Choi et al. 2019). Such interactions may provide a unique opportunity to counteract some of the potential adverse effects of certain EDCs on white, brown, and beige fat, necessitating further exploration.
5.1. Acknowledgements
The authors thank the U.S. EPA Library in Research Triangle Park, NC for their assistance in collating references and figure development. Specifically, we appreciate Taylor Johnson for their noted addition and effort in the creation of the word clouds. Authors also thank Drs. Ian Gilmour, Urmila Kodavanti, and Brian Chorley for their suggestions on earlier versions of the manuscript.
6.1. Disclaimer
This project was supported in partS by an appointment to the Research Participation Program at the Center for Public Health and Environmental Assessment at the U.S. EPA administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and U.S. EPA. The research described in this article has been reviewed by the Center Public Health and Environmental Assessment of the U.S. EPA and approved for publication. Approval does not signify that the contents necessarily reflect the views and the policies of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
7.1. References
- Alderete TL, Habre R, Toledo-Corral CM, Berhane K, Chen Z, Lurmann FW, et al. 2017. Longitudinal associations between ambient air pollution with insulin sensitivity, beta-cell function, and adiposity in los angeles latino children. Diabetes 66:1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Rivera FJ, Guerrero-Bosagna C. 2016. Bisphenol-a and metabolic diseases: Epigenetic, developmental and transgenerational basis. Environ Epigenet 2:dvw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariemma F, D’Esposito V, Liguoro D, Oriente F, Cabaro S, Liotti A, et al. 2016. Low-dose bisphenol-a impairs adipogenesis and generates dysfunctional 3t3-l1 adipocytes. PLoS One 11:e0150762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda AP, Milanski M, Velloso LA. 2011. Hypothalamic inflammation and thermogenesis: The brown adipose tissue connection. J Bioenerg Biomembr 43:53–58. [DOI] [PubMed] [Google Scholar]
- Auchincloss AH, Diez Roux AV, Dvonch JT, Brown PL, Barr RG, Daviglus ML, et al. 2008. Associations between recent exposure to ambient fine particulate matter and blood pressure in the multi-ethnic study of atherosclerosis (mesa). Environ Health Perspect 116:486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar Y, Parmar A, Miller CN, Samuels JS, Rayalam S. 2016. Phytochemicals as novel agents for the induction of browning in white adipose tissue. Nutr Metab (Lond) 13:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babatola SS. 2018. Global burden of diseases attributable to air pollution. J Public Health Afr 9:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baile CA, Yang JY, Rayalam S, Hartzell DL, Lai CY, Andersen C, et al. 2011. Effect of resveratrol on fat mobilization. Annals of the New York Academy of Sciences 1215:40–47. [DOI] [PubMed] [Google Scholar]
- Bays H, Rodbard HW, Schorr AB, Gonzalez-Campoy JM. 2007. Adiposopathy: Treating pathogenic adipose tissue to reduce cardiovascular disease risk. Current treatment options in cardiovascular medicine 9:259–271. [DOI] [PubMed] [Google Scholar]
- Beattie JH, Wood AM, Trayhurn P, Jasani B, Vincent A, McCormack G, et al. 2000. Metallothionein is expressed in adipocytes of brown fat and is induced by catecholamines and zinc. Am J Physiol Regul Integr Comp Physiol 278:R1082–1089. [DOI] [PubMed] [Google Scholar]
- Bertholet AM, Kirichok Y. 2019. The mechanism fa-dependent h(+) transport by ucp1. Handbook of experimental pharmacology 251:143–159. [DOI] [PubMed] [Google Scholar]
- Bianco AC, McAninch EA. 2013. The role of thyroid hormone and brown adipose tissue in energy homoeostasis. The lancet Diabetes & endocrinology 1:250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie AE, Azarenko VA, Hu CY. 1997. Inhibition of increases of transcription factor mrnas during differentiation of primary rat adipocytes by in vivo 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd) treatment. Toxicol Lett 90:91–95. [DOI] [PubMed] [Google Scholar]
- Brulport A, Le Corre L, Chagnon MC. 2017. Chronic exposure of 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd) induces an obesogenic effect in c57bl/6j mice fed a high fat diet. Toxicology 390:43–52. [DOI] [PubMed] [Google Scholar]
- Bruner-Tran KL, Gnecco J, Ding T, Glore DR, Pensabene V, Osteen KG. 2017. Exposure to the environmental endocrine disruptor tcdd and human reproductive dysfunction: Translating lessons from murine models. Reprod Toxicol 68:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, McKee RH. 2006. Integrating biomonitoring exposure data into the risk assessment process: Phthalates [diethyl phthalate and di(2-ethylhexyl) phthalate] as a case study. Environ Health Perspect 114:1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campioli E, Martinez-Arguelles DB, Papadopoulos V. 2014. In utero exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate promotes local adipose and systemic inflammation in adult male offspring. Nutrition & diabetes 4:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolim CM, Weissmann L, Ferreira CKO, Zordao OP, Dornellas APS, de Castro G, et al. 2020. Short-term exposure to air pollution (pm2.5) induces hypothalamic inflammation, and long-term leads to leptin resistance and obesity via tlr4/ikbke in mice. Sci Rep 10:10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereza G, Pedros C, Garcia N, Laporte JR. 2005. Topiramate in non-approved indications and acute myopia or angle closure glaucoma. British journal of clinical pharmacology 60:578–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Nguyen KD, Goh YP. 2011. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol 11:738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MD, Lin PY, Chen PS, Cheng V, Lin WH. 1997. Zinc attenuation of gdp binding to brown adipocytes mitochondria in genetically obese (ob/ob) mice. Biol Trace Elem Res 57:139–145. [DOI] [PubMed] [Google Scholar]
- Chen Y, Xu HS, Guo TL. 2018. Modulation of cytokine/chemokine production in human macrophages by bisphenol a: A comparison to analogues and interactions with genistein. J Immunotoxicol 15:96–103. [DOI] [PubMed] [Google Scholar]
- Choi EM, Suh KS, Park SY, Chin SO, Rhee SY, Chon S. 2019. Biochanin a prevents 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced adipocyte dysfunction in cultured 3t3-l1 cells. J Environ Sci Health A Tox Hazard Subst Environ Eng 54:865–873. [DOI] [PubMed] [Google Scholar]
- Cinti S 2018. Adipose organ development and remodeling. Compr Physiol 8:1357–1431. [DOI] [PubMed] [Google Scholar]
- da Silva MM, Gonçalves CFL, Miranda-Alves L, Fortunato RS, Carvalho DP, Ferreira ACF. 2019. Inhibition of type 1 iodothyronine deiodinase by bisphenol a. Horm Metab Res 51:671–677. [DOI] [PubMed] [Google Scholar]
- Darbre PD. 2017. Endocrine disruptors and obesity. Current obesity reports 6:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, et al. 2001. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest 108:1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong JMA, Wouters RTF, Boulet N, Cannon B, Nedergaard J, Petrovic N. 2017. The beta3-adrenergic receptor is dispensable for browning of adipose tissues. Am J Physiol Endocrinol Metab 312:E508–E518. [DOI] [PubMed] [Google Scholar]
- de Souza CJ, Burkey BF. 2001. Beta 3-adrenoceptor agonists as anti-diabetic and anti-obesity drugs in humans. Current pharmaceutical design 7:1433–1449. [DOI] [PubMed] [Google Scholar]
- Debacker V, Eppe G, Massart A-C, Xhrouet C, Jauniaux T, Huart P, et al. 2003. Polychlorinated dibenzo-p-dioxins and dibenzofurans in livers of an atlantic seabird, the common guillemot uria aalge : Influence of the general body condition. Organohalogen Compounds 64:443–446. [Google Scholar]
- Delfosse V, Maire AL, Balaguer P, Bourguet W. 2015. A structural perspective on nuclear receptors as targets of environmental compounds. Acta pharmacologica Sinica 36:88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvergne B, Feige JN, Casals-Casas C. 2009. Ppar-mediated activity of phthalates: A link to the obesity epidemic? Mol Cell Endocrinol 304:43–48. [DOI] [PubMed] [Google Scholar]
- Di Gregorio I, Busiello RA, Burgos Aceves MA, Lepretti M, Paolella G, Lionetti L. 2018. Environmental pollutants effect on brown adipose tissue. Front Physiol 9:1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. 2009. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocrine reviews 30:293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Yao X, Liu S, Yin N, Faiola F. 2018. Non-cytotoxic nanomolar concentrations of bisphenol a induce human mesenchymal stem cell adipogenesis and osteogenesis. Ecotoxicol Environ Saf 164:448–454. [DOI] [PubMed] [Google Scholar]
- Drobna Z, Talarovicova A, Schrader HE, Fennell TR, Snyder RW, Rissman EF. 2019. Bisphenol f has different effects on preadipocytes differentiation and weight gain in adult mice as compared with bisphenol a and s. Toxicology 420:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger AE, Grabmann G, Gollmann-Tepekoylu C, Pechriggl EJ, Artner C, Turkcan A, et al. 2019. Chemical imaging and assessment of cadmium distribution in the human body. Metallomics 11:2010–2019. [DOI] [PubMed] [Google Scholar]
- Ellero-Simatos S, Claus SP, Benelli C, Forest C, Letourneur F, Cagnard N, et al. 2011. Combined transcriptomic-(1)h nmr metabonomic study reveals that monoethylhexyl phthalate stimulates adipogenesis and glyceroneogenesis in human adipocytes. J Proteome Res 10:5493–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg ST, Aoyama T, Alexson SE, Hashimoto T, Svensson LT. 1997. Peroxisome proliferator-induced acyl-coa thioesterase from rat liver cytosol: Molecular cloning and functional expression in chinese hamster ovary cells. The Biochemical journal 323 ( Pt 2):525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A, Buhrke T, Imber F, Jessel S, Seidel A, Volkel W, et al. 2017. Agonistic and antagonistic effects of phthalates and their urinary metabolites on the steroid hormone receptors eralpha, erbeta, and ar. Toxicol Lett 277:54–63. [DOI] [PubMed] [Google Scholar]
- Feige JN, Gelman L, Rossi D, Zoete V, Metivier R, Tudor C, et al. 2007. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J Biol Chem 282:19152–19166. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. 2011. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol 301:H1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire C, Vrhovnik P, Fiket Z, Salcedo-Bellido I, Echeverria R, Martin-Olmedo P, et al. 2020. Adipose tissue concentrations of arsenic, nickel, lead, tin, and titanium in adults from gramo cohort in southern spain: An exploratory study. Sci Total Environ 719:137458. [DOI] [PubMed] [Google Scholar]
- Ganning AE, Brunk U, Dallner G. 1983. Effects of dietary di(2-ethylhexyl)phthalate on the structure and function of rat hepatocytes. Biochimica et biophysica acta 763:72–82. [DOI] [PubMed] [Google Scholar]
- Gavett SH, Haykal-Coates N, Copeland LB, Heinrich J, Gilmour MI. 2003. Metal composition of ambient pm2.5 influences severity of allergic airways disease in mice. Environ Health Perspect 111:1471–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girer NG, Tomlinson CR, Elferink CJ. 2021. The aryl hydrocarbon receptor in energy balance: The road from dioxin-induced wasting syndrome to combating obesity with ahr ligands. Int J Mol Sci 22:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CJ, Gray LE, Jr., Monteiro-Riviere NA, Miller DB 1995. Temperature regulation and metabolism in rats exposed perinatally to dioxin: Permanent change in regulated body temperature? Toxicol Appl Pharmacol 133:172–176. [DOI] [PubMed] [Google Scholar]
- Gorski JR, Weber LW, Rozman K. 1988. Tissue-specific alterations of de novo fatty acid synthesis in 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd)-treated rats. Arch Toxicol 62:146–151. [DOI] [PubMed] [Google Scholar]
- Grun F, Blumberg B. 2007. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Reviews in endocrine & metabolic disorders 8:161–171. [DOI] [PubMed] [Google Scholar]
- Haberzettl P, O’Toole TE, Bhatnagar A, Conklin DJ. 2016. Exposure to fine particulate air pollution causes vascular insulin resistance by inducing pulmonary oxidative stress. Environ Health Perspect 124:1830–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JF, Nielsen CH, Brorson MM, Frederiksen H, Hartoft-Nielsen ML, Rasmussen AK, et al. 2015. Influence of phthalates on in vitro innate and adaptive immune responses. PLoS One 10:e0131168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao C, Cheng X, Guo J, Xia H, Ma X. 2013. Perinatal exposure to diethyl-hexyl-phthalate induces obesity in mice. Frontiers in bioscience 5:725–733. [DOI] [PubMed] [Google Scholar]
- Harms M, Seale P. 2013. Brown and beige fat: Development, function and therapeutic potential. Nature medicine 19:1252–1263. [DOI] [PubMed] [Google Scholar]
- Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, et al. 2017. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol 68:3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J 1990. Brown adipose tissue thermogenesis: Interdisciplinary studies. FASEB J 4:2890–2898. [PubMed] [Google Scholar]
- Hong C-C, Shimomura-Shimizu M, Muroi M, Tanamoto K. 2004. Effect of endocrine disrupting chemicals on lipopolysaccharide-induced tumor necrosis factor-aand nitric oxide production by mouse macrophages. BIol Pharm BUll 27:1136–1139. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Hotchkiss AK, Gray LE Jr, 2017. Cumulative effects of antiandrogenic chemical mixtures and their relevance to human health risk assessment. International journal of hygiene and environmental health 220:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyeck MP, Merhi RC, Blair HL, Spencer CD, Payant MA, Martin Alfonso DI, et al. 2020. Female mice exposed to low doses of dioxin during pregnancy and lactation have increased susceptibility to diet-induced obesity and diabetes. Mol Metab 42:101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HF, Tsou TC, Chao HR, Kuo YT, Tsai FY, Yeh SC. 2010. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on adipogenic differentiation and insulin-induced glucose uptake in 3t3-l1 cells. J Hazard Mater 182:649–655. [DOI] [PubMed] [Google Scholar]
- Hsu JW, Nien CY, Yeh SC, Tsai FY, Chen HW, Lee TS, et al. 2020. Phthalate exposure causes browning-like effects on adipocytes in vitro and in vivo. Food Chem Toxicol 142:111487. [DOI] [PubMed] [Google Scholar]
- Iavicoli I, Fontana L, Bergamaschi A. 2009. The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev 12:206–223. [DOI] [PubMed] [Google Scholar]
- Inouye B, Ogino Y, Ishida T, Ogata M, Utsumi K. 1978. Effects of phthalate esters on mitochondrial oxidative phosphorylation in the rat. Toxicol Appl Pharmacol 43:189–198. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Kendall CW, Faulkner DA, Kemp T, Marchie A, Nguyen TH, et al. 2008. Long-term effects of a plant-based dietary portfolio of cholesterol-lowering foods on blood pressure. European journal of clinical nutrition 62:781–788. [DOI] [PubMed] [Google Scholar]
- Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L. 2020. Endocrine-disrupting chemicals: Implications for human health. The lancet Diabetes & endocrinology 8:703–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T, Yang W, Chen CS, Reynolds K, He J. 2008. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 32:1431–1437. [DOI] [PubMed] [Google Scholar]
- Keuper M 2019. On the role of macrophages in the control of adipocyte energy metabolism. Endocr Connect 8:R105–R121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer FW. 2017. The significance of beige and brown fat in humans. Endocrine connections 6:R70–R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Chen Z, Alderete TL, Toledo-Corral C, Lurmann F, Berhane K, et al. 2019. Associations of air pollution, obesity and cardiometabolic health in young adults: The meta-air study. Environ Int 133:105180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Yeon SM, Kim HG, Choi HS, Kang H, Park HD, et al. 2014. Diverse influences of androgen-disrupting chemicals on immune responses mounted by macrophages. Inflammation 37:649–656. [DOI] [PubMed] [Google Scholar]
- Kotzbeck P, Giordano A, Mondini E, Murano I, Severi I, Venema W, et al. 2018. Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J Lipid Res 59:784–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt OK, Zhang J, Pinkerton KE. 2016. Pulmonary health effects of air pollution. Curr Opin Pulm Med 22:138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladurner A, Zehl M, Grienke U, Hofstadler C, Faur N, Pereira FC, et al. 2017. Allspice and clove as source of triterpene acids activating the g protein-coupled bile acid receptor tgr5. Frontiers in pharmacology 8:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larigot L, Juricek L, Dairou J, Coumoul X. 2018. Ahr signaling pathways and regulatory functions. Biochim Open 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le HH, Carlson EM, Chua JP, Belcher SM. 2008. Bisphenol a is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Letters 176:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Moon JY, Yoo BS. 2012. Cadmium inhibits the differentiation of 3t3-l1 preadipocyte through the c/ebpalpha and ppargamma pathways. Drug Chem Toxicol 35:225–231. [DOI] [PubMed] [Google Scholar]
- Lee JW, Park S, Han HK, Gye MC, Moon EY. 2018. Di-(2-ethylhexyl) phthalate enhances melanoma tumor growth via differential effect on m1-and m2-polarized macrophages in mouse model. Environ Pollut 233:833–843. [DOI] [PubMed] [Google Scholar]
- Lee KI, Chiang CW, Lin HC, Zhao JF, Li CT, Shyue SK, et al. 2016. Maternal exposure to di-(2-ethylhexyl) phthalate exposure deregulates blood pressure, adiposity, cholesterol metabolism and social interaction in mouse offspring. Arch Toxicol 90:1211–1224. [DOI] [PubMed] [Google Scholar]
- Li L, Li HS, Song NN, Chen HM. 2013. The immunotoxicity of dibutyl phthalate on the macrophages in mice. Immunopharmacology and immunotoxicology 35:272–281. [DOI] [PubMed] [Google Scholar]
- Li Q, Liu H, Alattar M, Jiang S, Han J, Ma Y, et al. 2015. The preferential accumulation of heavy metals in different tissues following frequent respiratory exposure to pm2.5 in rats. Sci Rep 5:16936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Vogel CF, Fujiyoshi P, Matsumura F. 2008. Development of a human adipocyte model derived from human mesenchymal stem cells (hmsc) as a tool for toxicological studies on the action of tcdd. Biol Chem 389:169–177. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang Z, Ke L, Sun Y, Li W, Feng X, et al. 2020. Resveratrol promotes white adipocytes browning and improves metabolic disorders in sirt1-dependent manner in mice. FASEB J 34:4527–4539. [DOI] [PubMed] [Google Scholar]
- Lidell ME, Betz MJ, Enerback S. 2014. Brown adipose tissue and its therapeutic potential. Journal of internal medicine 276:364–377. [DOI] [PubMed] [Google Scholar]
- Lin Y, Li X, Zhang L, Zhang Y, Zhu H, Zhang Y, et al. 2016. Inhaled sio2 nanoparticles blunt cold-exposure-induced wat-browning and metabolism activation in white and brown adipose tissue. Toxicology Research 5:1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J, Lensu S, Pohjanvirta R. 2014. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd) on hormones of energy balance in a tcdd-sensitive and a tcdd-resistant rat strain. Int J Mol Sci 15:13938–13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren A, Lindquist NG, Lydén A, Olsson T, Ullberg S. 1982. A whole body autoradiographic study on the distribution of 14c-labelled di-(2-ethylhexyl)phthalate in mice. Toxicology 23:149–158. [DOI] [PubMed] [Google Scholar]
- Liu C, Bai Y, Xu X, Sun L, Wang A, Wang TY, et al. 2014a. Exaggerated effects of particulate matter air pollution in genetic type ii diabetes mellitus. Part Fibre Toxicol 11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Fonken LK, Wang A, Maiseyeu A, Bai Y, Wang TY, et al. 2014b. Central ikkbeta inhibition prevents air pollution mediated peripheral inflammation and exaggeration of type ii diabetes. Part Fibre Toxicol 11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Mei C, Liu H, Wang H, Zeng G, Lin J, et al. 2014. Modulation of cytokine expression in human macrophages by endocrine-disrupting chemical bisphenol-a. Biochem Biophys Res Commun 451:592–598. [DOI] [PubMed] [Google Scholar]
- Longo M, Zatterale F, Naderi J, Nigro C, Oriente F, Formisano P, et al. 2020. Low-dose bisphenol-a promotes epigenetic changes at ppargamma promoter in adipose precursor cells. Nutrients 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Li M, Wu C, Zhou C, Zhang J, Zhu Q, et al. 2019. Bisphenol a promotes macrophage proinflammatory subtype polarization via upregulation of irf5 expression in vitro. Toxicology in vitro : an international journal published in association with BIBRA 60:97–106. [DOI] [PubMed] [Google Scholar]
- Lukaski HC, Hall CB, Marchello MJ. 1992. Impaired thyroid hormone status and thermoregulation during cold exposure of zinc-deficient rats. Horm Metab Res 24:363–366. [DOI] [PubMed] [Google Scholar]
- Luo W, Chen J, Jiang H, Chen Y. 2004. [effects of zinc on content of uncoupling protein in cold stress rats]. Wei Sheng Yan Jiu 33:33–35. [PubMed] [Google Scholar]
- Lv Q, Gao R, Peng C, Yi J, Liu L, Yang S, et al. 2017. Bisphenol a promotes hepatic lipid deposition involving kupffer cells m1 polarization in male mice. J Endocrinol 234:143–154. [DOI] [PubMed] [Google Scholar]
- Lv Y, Zhang SY, Liang X, Zhang H, Xu Z, Liu B, et al. 2016. Adrenomedullin 2 enhances beiging in white adipose tissue directly in an adipocyte-autonomous manner and indirectly through activation of m2 macrophages. J Biol Chem 291:23390–23402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Z, Cheng J, Huang S, Zhang Y, Wu S, Qiu Y, et al. 2016. Dehp induces obesity and hypothyroidism through both central and peripheral pathways in c3h/he mice. Obesity 24:368–378. [DOI] [PubMed] [Google Scholar]
- Malaise Y, Menard S, Cartier C, Gaultier E, Lasserre F, Lencina C, et al. 2017. Gut dysbiosis and impairment of immune system homeostasis in perinatally-exposed mice to bisphenol a precede obese phenotype development. Sci Rep 7:14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maradonna F, Carnevali O. 2018. Lipid metabolism alteration by endocrine disruptors in animal models: An overview. Front Endocrinol (Lausanne) 9:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcoccia D, Pellegrini M, Fiocchetti M, Lorenzetti S, Marino M. 2017. Food components and contaminants as (anti)androgenic molecules. Genes & nutrition 12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli MI, Mocchiutti NO, Bernal CA. 2010. Effect of di(2-ethylhexyl) phthalate (dehp) on lipolysis and lipoprotein lipase activities in adipose tissue of rats. Human & experimental toxicology 29:739–745. [DOI] [PubMed] [Google Scholar]
- Martinez-Sanchez N, Moreno-Navarrete JM, Contreras C, Rial-Pensado E, Ferno J, Nogueiras R, et al. 2017. Thyroid hormones induce browning of white fat. J Endocrinol 232:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez MA, Blanco J, Rovira J, Kumar V, Domingo JL, Schuhmacher M. 2020. Bisphenol a analogues (bps and bpf) present a greater obesogenic capacity in 3t3-l1 cell line. Food Chem Toxicol 140:111298. [DOI] [PubMed] [Google Scholar]
- Mebert AM, Baglole CJ, Desimone MF, Maysinger D. 2017. Nanoengineered silica: Properties, applications and toxicity. Food Chem Toxicol 109:753–770. [DOI] [PubMed] [Google Scholar]
- Mele L, Bidault G, Mena P, Crozier A, Brighenti F, Vidal-Puig A, et al. 2017. Dietary (poly)phenols, brown adipose tissue activation, and energy expenditure: A narrative review. Advances in nutrition 8:694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick RL, Schiller CM. 1985. Effect of phthalate esters on energy coupling and succinate oxidation in rat liver mitochondria. Toxicology 34:13–27. [DOI] [PubMed] [Google Scholar]
- Mendez R, Zheng Z, Fan Z, Rajagopalan S, Sun Q, Zhang K. 2013. Exposure to fine airborne particulate matter induces macrophage infiltration, unfolded protein response, and lipid deposition in white adipose tissue. Am J Transl Res 5:224–234. [PMC free article] [PubMed] [Google Scholar]
- Meulenberg EP. 2009. Phenolics: Occurrence and immunochemical detection in environment and food. Molecules 14:439–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrath MO, Wenger Y, Chang CW, Emond C, Garabrant D, Gillespie BW, et al. 2009. Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast-feeding. Environ Health Perspect 117:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CN, Yang JY, Avra T, Ambati S, Della-Fera MA, Rayalam S, et al. 2015a. A dietary phytochemical blend prevents liver damage associated with adipose tissue mobilization in ovariectomized rats. Obesity 23:112–119. [DOI] [PubMed] [Google Scholar]
- Miller CN, Yang JY, England E, Yin A, Baile CA, Rayalam S. 2015b. Isoproterenol increases uncoupling, glycolysis, and markers of beiging in mature 3t3-l1 adipocytes. PLoS One 10:e0138344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CN, Samuels JS, Azhar Y, Parmar A, Shashidharamurthy R, Rayalam S. 2019. Guggulsterone activates adipocyte beiging through direct effects on 3t3-l1 adipocytes and indirect effects mediated through raw264.7 macrophages. Medicines 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minich DM, Lerman RH, Darland G, Babish JG, Pacioretty LM, Bland JS, et al. 2010. Hop and acacia phytochemicals decreased lipotoxicity in 3t3-l1 adipocytes, db/db mice, and individuals with metabolic syndrome. Journal of nutrition and metabolism 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mund RA, Frishman WH. 2013. Brown adipose tissue thermogenesis: Beta3-adrenoreceptors as a potential target for the treatment of obesity in humans. Cardiology in review 21:265–269. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. 2007. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293:E444–452. [DOI] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, et al. 2011. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S, Kamiya M, Aoyama H, Yoshimura K, Miyata R, Kumazawa S, et al. 2019. Co-administration of curcumin and artepillin c induces development of brown-like adipocytes in association with local norepinephrine production by alternatively activated macrophages in mice. Journal of nutritional science and vitaminology 65:328–334. [DOI] [PubMed] [Google Scholar]
- Noli MI, Pavia MA Jr., Mignone IR, Brignone JA, Hagmuller K, Zaninovich AA. 1998. Cadmium chloride prevents the rise in rat brown adipose tissue mitochondrial respiration in response to acute cold stress. Bull Environ Contam Toxicol 61:31–37. [DOI] [PubMed] [Google Scholar]
- Nunez AA, Kannan K, Giesy JP, Fang J, Clemens LG. 2001. Effects of bisphenol a on energy balance and accumulation in brown adipose tissue in rats. Chemosphere 42:917–922. [DOI] [PubMed] [Google Scholar]
- Paier B, Pavia MA Jr., , Hansi C, Noli MI, Hagmüller K, Zaninovich AA. 1997. Cadmium inhibits the in vitro conversion of thyroxine to triiodothyronine in rat brown adipose tissue. Bull Environ Contam Toxicol 59:164–170. [DOI] [PubMed] [Google Scholar]
- Pappas RS. 2011. Toxic elements in tobacco and in cigarette smoke: Inflammation and sensitization. Metallomics 3:1181–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Yang JY, Ambati S, Della-Fera MA, Hausman DB, Rayalam S, et al. 2008. Combined effects of genistein, quercetin, and resveratrol in human and 3t3-l1 adipocytes. Journal of medicinal food 11:773–783. [DOI] [PubMed] [Google Scholar]
- Pavia M Jr, . Paier B, Hagmuller K, Zaninovich AA 1999. [zinc inhibits the in vitro conversion of thyroxine to triiodothyronine in brown adipose issue]. Medicina (B Aires) 59:265–268. [PubMed] [Google Scholar]
- Pelch K, Wignall JA, Goldstone AE, Ross PK, Blain RB, Shapiro AJ, et al. 2019. A scoping review of the health and toxicological activity of bisphenol a (bpa) structural analogues and functional alternatives. Toxicology 424:152235. [DOI] [PubMed] [Google Scholar]
- Phillips M, Enan E, Liu PCC, Matsumura F. 1995. Inhibition of 3t3-l1 adipose differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Cell Science 108:395–402. [DOI] [PubMed] [Google Scholar]
- Pope CA 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, et al. 2004. Cardiovascular mortality and long-term exposure to particulate air pollution: Epidemiological evidence of general pathophysiological pathways of disease. Circulation 109:71–77. [DOI] [PubMed] [Google Scholar]
- Porras A, Alvarez AM, Valladares A, Benito M. 1997. Tnf-alpha induces apoptosis in rat fetal brown adipocytes in primary culture. FEBS Lett 416:324–328. [DOI] [PubMed] [Google Scholar]
- Potts AJ, Bowman NJ, Seger DL, Thomas SHL. 2020. Toxicoepidemiology and predictors of death in 2,4-dinitrophenol (dnp) toxicity. Clin Toxicol (Phila):1–6. [DOI] [PubMed] [Google Scholar]
- Qiu L, Chen M, Wang X, Qin X, Chen S, Qian Y, et al. 2018. Exposure to concentrated ambient pm2.5 compromises spermatogenesis in a mouse model: Role of suppression of hypothalamus-pituitary-gonads axis. Toxicol Sci 162:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raasmaja A, Viluksela M, Rozman KK. 1996. Decreased liver type i 5’-deiodinase and increased brown adipose tissue type ii 5’-deiodinase activity in 2,3,7,8-tetrachlorobibenzo-p-dioxin (tcdd)-treated long-evans rats. Toxicology 114:199–205. [DOI] [PubMed] [Google Scholar]
- Ramakrishna A, Ravishankar GA. 2011. Influence of abiotic stress signals on secondary metabolites in plants. Plant signaling & behavior 6:1720–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchová H, Kaul PP, Drahota Z. 1985. Activation of mitochondrial glycerol 3-phosphate dehydrogenase by cadmium ions. Gen Physiol Biophys 4:29–33. [PubMed] [Google Scholar]
- Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. 2008. Resveratrol induces apoptosis and inhibits adipogenesis in 3t3-l1 adipocytes. Phytother Res 22:1367–1371. [DOI] [PubMed] [Google Scholar]
- Rebagliati I, Raices M, Ricci C, Weisstaub A, Hagmüller K, Zaninovich AA. 2001. Effects of zinc on brown fat thermal response to cold in normal and triiodothyronine-treated hypothyroid rats. Bull Environ Contam Toxicol 67:641–648. [DOI] [PubMed] [Google Scholar]
- Regnier SM, Sargis RM. 2014. Adipocytes under assault: Environmental disruption of adipose physiology. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1842:520–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina-Perez I, Olivas-Martinez A, Mustieles V, Ruiz-Ojeda FJ, Molina-Molina JM, Olea N, et al. 2021. Bisphenol f and bisphenol s promote lipid accumulation and adipogenesis in human adipose-derived stem cells. Food Chem Toxicol 152:112216. [DOI] [PubMed] [Google Scholar]
- Riggs DW, Zafar N, Krishnasamy S, Yeager R, Rai SN, Bhatnagar A, et al. 2020. Exposure to airborne fine particulate matter is associated with impaired endothelial function and biomarkers of oxidative stress and inflammation. Environ Res 180:108890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al. 1999. Ppar gamma is required for the differentiation of adipose tissue in vivo and in vitro. Molecular cell 4:611–617. [DOI] [PubMed] [Google Scholar]
- Rozman K 1984. Hexadecane increases the toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd): Is brown adipose tissue the primary target in tcdd-induced wasting syndrome? Biochem Biophys Res Commun 125:996–1004. [DOI] [PubMed] [Google Scholar]
- Rozman K, Greim H. 1986. Toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in cold-adapted rats. Arch Toxicol 59:211–215. [DOI] [PubMed] [Google Scholar]
- Rozman K, Strassle B, Iatropoulos MJ. 1986. Brown adipose tissue is a target tissue in 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd) induced toxicity. Arch Toxicol Suppl 9:356–360. [DOI] [PubMed] [Google Scholar]
- Rozman K, Pereira D, Iatropoulos MJ. 1987. Effect of a sublethal dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin on interscapular brown adipose tissue of rats. Toxicol Pathol 15:425–430. [DOI] [PubMed] [Google Scholar]
- Rozman KK, Bhatia J, Calafat AM, Chambers C, Culty M, Etzel RA, et al. 2006. Ntp-cerhr expert panel report on the reproductive and developmental toxicity of genistein. Birth defects research Part B, Developmental and reproductive toxicology 77:485–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupasinghe HP, Sekhon-Loodu S, Mantso T, Panayiotidis MI. 2016. Phytochemicals in regulating fatty acid beta-oxidation: Potential underlying mechanisms and their involvement in obesity and weight loss. Pharmacol Ther 165:153–163. [DOI] [PubMed] [Google Scholar]
- Saito M, Matsushita M, Yoneshiro T, Okamatsu-Ogura Y. 2020. Brown adipose tissue, diet-induced thermogenesis, and thermogenic food ingredients: From mice to men. Front Endocrinol (Lausanne) 11:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Nitta T, Maruno K, Yeh YS, Kuwata H, Tomita K, et al. 2016. Macrophage infiltration into obese adipose tissues suppresses the induction of ucp1 level in mice. Am J Physiol Endocrinol Metab 310:E676–E687. [DOI] [PubMed] [Google Scholar]
- Sani Y 2015. Pathological changes of suspected tetrachloro dibenzo-ρ-dioxins/tetrachloro dibenzofurans toxication in beef cattle. Jurnal Ilmu Ternak dan Veteriner 20:214–223. [Google Scholar]
- Sargis RM, Johnson DN, Choudhury RA, Brady MJ. 2010. Environmental endocrine disruptors promote adipogenesis in the 3t3-l1 cell line through glucocorticoid receptor activation. Obesity 18:1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedha S, Kumar S, Shukla S. 2015. Role of oxidative stress in male reproductive dysfunctions with reference to phthalate compounds. Urology journal 12:2304–2316. [PubMed] [Google Scholar]
- Shi M, Lin Z, Ye L, Chen X, Zhang W, Zhang Z, et al. 2020. Estrogen receptor-regulated socs3 modulation via jak2/stat3 pathway is involved in bpf-induced m1 polarization of macrophages. Toxicology 433–434:152404. [DOI] [PubMed] [Google Scholar]
- Siegmueller C, Narasimhaiah R. 2010. ‘Fatal 2,4-dinitrophenol poisoning... Coming to a hospital near you’. Emergency medicine journal : EMJ 27:639–640. [DOI] [PubMed] [Google Scholar]
- Srimuruganandam B, Shiva Nagendra SM. 2012. Source characterization of pm10 and pm2.5 mass using a chemical mass balance model at urban roadside. Sci Total Environ 433:8–19. [DOI] [PubMed] [Google Scholar]
- Stefanidis A, Man Lee CC, Brown WA, Oldfield BJ. 2017. Improving efficacy of the adjustable gastric band: Studies of the use of adjuvant approaches in a rodent model. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 13:291–304. [DOI] [PubMed] [Google Scholar]
- Strakovsky RS, Lezmi S, Shkoda I, Flaws JA, Helferich WG, Pan YX. 2015. In utero growth restriction and catch-up adipogenesis after developmental di (2-ethylhexyl) phthalate exposure cause glucose intolerance in adult male rats following a high-fat dietary challenge. The Journal of nutritional biochemistry 26:1208–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, et al. 2009. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 119:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Feng X, Wu X, Lu Y, Chen K, Ye Y. 2020. Fat wasting is damaging: Role of adipose tissue in cancer-associated cachexia. Front Cell Dev Biol 8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds ME, Aldiss P, Pope M, Budge H. 2018. Recent advances in our understanding of brown and beige adipose tissue: The good fat that keeps you healthy. F1000Research 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Shioda K, Mitsunaga S, Yawata S, Angle BM, Nagel SC, et al. 2018. Prenatal exposure to bisphenol a disrupts naturally occurring bimodal DNA methylation at proximal promoter of fggy, an obesity-relevant gene encoding a carbohydrate kinase, in gonadal white adipose tissues of cd-1 mice. Endocrinology 159:779–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. 2012. Heavy metal toxicity and the environment. Exp Suppl 101:133–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Auwerx J, Schoonjans K. 2008. Bile acids and the membrane bile acid receptor tgr5--connecting nutrition and metabolism. Thyroid : official journal of the American Thyroid Association 18:167–174. [DOI] [PubMed] [Google Scholar]
- Tinkov AA, Filippini T, Ajsuvakova OP, Aaseth J, Gluhcheva YG, Ivanova JM, et al. 2017. The role of cadmium in obesity and diabetes. Sci Total Environ 601–602:741–755. [DOI] [PubMed] [Google Scholar]
- Tinkov AA, Aschner M, Ke T, Ferrer B, Zhou JC, Chang JS, et al. 2021. Adipotropic effects of heavy metals and their potential role in obesity. Fac Rev 10:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. E. 2021. Tsca chemical substance inventory. Available: https://www.epa.gov/tsca-inventory [accessed February 10 2021].
- Vallee BL, Ulmer DD. 1972. Biochemical effects of mercury, cadmium, and lead. Annu Rev Biochem 41:91–128. [DOI] [PubMed] [Google Scholar]
- van Esterik JC, Dollé ME, Lamoree MH, van Leeuwen SP, Hamers T, Legler J, et al. 2014. Programming of metabolic effects in c57bl/6jxfvb mice by exposure to bisphenol a during gestation and lactation. Toxicology 321:40–52. [DOI] [PubMed] [Google Scholar]
- Viluksela M, Stahl BU, Rozman KK. 1995. Tissue-specific effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd) on the activity of phosphoenolpyruvate carboxykinase (pepck) in rats. Toxicol Appl Pharmacol 135:308–315. [DOI] [PubMed] [Google Scholar]
- Viluksela M, Raasmaja A, Lebofsky M, Stahl BU, Rozman KK. 2004. Tissue-specific effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd) on the activity of 5’-deiodinases i and ii in rats. Toxicol Lett 147:133–142. [DOI] [PubMed] [Google Scholar]
- Wallwork RS, Colicino E, Zhong J, Kloog I, Coull BA, Vokonas P, et al. 2017. Ambient fine particulate matter, outdoor temperature, and risk of metabolic syndrome. Am J Epidemiol 185:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Dong S. 2012. Icam-1 and il-8 are expressed by dehp and suppressed by curcumin through erk and p38 mapk in human umbilical vein endothelial cells. Inflammation 35:859–870. [DOI] [PubMed] [Google Scholar]
- Wang X, Mukherjee B, Park SK. 2018. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among u.S. Adults in nhanes 2003–2014. Environ Int 121:683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Beydoun MA. 2007. The obesity epidemic in the united states--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiologic reviews 29:6–28. [DOI] [PubMed] [Google Scholar]
- Watt J, Schlezinger JJ. 2015. Structurally-diverse, ppargamma-activating environmental toxicants induce adipogenesis and suppress osteogenesis in bone marrow mesenchymal stromal cells. Toxicology 331:66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber LW, Greim H, Rozman KK. 1987a. Metabolism and distribution of [14c]glucose in rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd). J Toxicol Environ Health 22:195–206. [DOI] [PubMed] [Google Scholar]
- Weber LW, Haart TW, Rozman K. 1987b. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (tcdd) on thermogenesis in brown adipose tissue of rats. Toxicol Lett 39:241–248. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr, 2003. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittassek M, Angerer J. 2008. Phthalates: Metabolism and exposure. Int J Androl 31:131–138. [DOI] [PubMed] [Google Scholar]
- Wu J, Cohen P, Spiegelman BM. 2013. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes & development 27:234–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuana RA, Okieimen FE. 2011. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecology 2011:402647. [Google Scholar]
- Xie Y, Yang Q, Nelson BD, DePierre JW. 2002. Characterization of the adipose tissue atrophy induced by peroxisome proliferators in mice. Lipids 37:139–146. [DOI] [PubMed] [Google Scholar]
- Xu X, Liu C, Xu Z, Tzan K, Zhong M, Wang A, et al. 2011. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol Sci 124:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Rao X, Wang TY, Jiang SY, Ying Z, Liu C, et al. 2012. Effect of co-exposure to nickel and particulate matter on insulin resistance and mitochondrial dysfunction in a mouse model. Part Fibre Toxicol 9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Xu X, Zhong M, Hotchkiss IP, Lewandowski RP, Wagner JG, et al. 2011. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part Fibre Toxicol 8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, Itsuki A, Kimoto M, Hiemori M, Tsuji H. 2006. Acetate generation in rat liver mitochondria; acetyl-coa hydrolase activity is demonstrated by 3-ketoacyl-coa thiolase. Biochimica et biophysica acta 1761:17–23. [DOI] [PubMed] [Google Scholar]
- Yau WW, Yen PM. 2020. Thermogenesis in adipose tissue activated by thyroid hormone. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Yu KS, Lu K, Yu X. 2016. Benzyl butyl phthalate promotes adipogenesis in 3t3-l1 preadipocytes: A high content cellomics and metabolomic analysis. Toxicology in vitro : an international journal published in association with BIBRA 32:297–309. [DOI] [PubMed] [Google Scholar]
- Ying Z, Xu X, Bai Y, Zhong J, Chen M, Liang Y, et al. 2014. Long-term exposure to concentrated ambient pm2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: A role for hypothalamic inflammation. Environ Health Perspect 122:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York DA, Singer L, Thomas S, Bray GA. 2000. Effect of topiramate on body weight and body composition of osborne-mendel rats fed a high-fat diet: Alterations in hormones, neuropeptide, and uncoupling-protein mrnas. Nutrition 16:967–975. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu J, Yao J, Ji G, Qian L, Wang J, et al. 2014. Obesity: Pathophysiology and intervention. Nutrients 6:5153–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lin Y, Li X, Zhang L, Pan W, Zhu H, et al. 2017. Silica dioxide nanoparticles combined with cold exposure induce stronger systemic inflammatory response. Environ Sci Pollut Res Int 24:291–298. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Gan RY, Li S, Zhou Y, Li AN, Xu DP, et al. 2015. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 20:21138–21156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Tang Z, Xie P, Lin K, Chung ACK, Cai Z. 2019. Immunotoxic potential of bisphenol f mediated through lipid signaling pathways on macrophages. Environ Sci Technol 53:11420–11428. [DOI] [PubMed] [Google Scholar]
- Zhong J, Allen K, Rao X, Ying Z, Braunstein Z, Kankanala SR, et al. 2016. Repeated ozone exposure exacerbates insulin resistance and activates innate immune response in genetically susceptible mice. Inhal Toxicol 28:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JY, Poudel A, Welchko R, Mekala N, Chandramani-Shivalingappa P, Rosca MG, et al. 2019. Liraglutide improves insulin sensitivity in high fat diet induced diabetic mice through multiple pathways. European journal of pharmacology 861:172594. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Moore TM, Drew BG, Ribas V, Wanagat J, Civelek M, et al. 2020. Estrogen receptor alpha controls metabolism in white and brown adipocytes by regulating polg1 and mitochondrial remodeling. Sci Transl Med 12. [DOI] [PMC free article] [PubMed] [Google Scholar]