Abstract
Activation of antigen presenting cells (APCs) is necessary for immune recognition and elimination of cancer. Our lab has developed a liposome nanoparticle that binds to complement C3 proteins present in serum. These C3-liposomes are specifically internalised by APCs and other myeloid cells, which express complement C3-binding receptors. Known immune stimulating compounds, toll-like receptor (TLR) agonists, were encapsulated within the C3-liposomes, including monophosphoryl lipid A (MPLA), R848, and CpG 1826, specific for TLR4, TLR7/8, and TLR9 respectively. When recognised by their respective TLRs within the myeloid cells, these compounds trigger signal cascades that ultimately lead to increased expression of inflammatory cytokines and activation markers (CD80, CD83, CD86 and CD40). RT-PCR analysis of murine bone marrow cells treated with C3-liposomes revealed a significant increase in gene expression of pro-inflammatory cytokines and factors (IL-1β, IL-6, IL-12, TNF-α, IRF7, and IP-10). Furthermore, treatment of 4T1 tumour-bearing mice with C3-liposomes containing TLR agonists resulted in reduced tumour growth, compared to PBS treated mice. Collectively, these results demonstrate that C3-liposome delivery of TLR agonists activates APCs and induces tumour-specific adaptive immune responses, leading to reduced tumour growth in a breast cancer model.
Keywords: Antigen presenting cell, cancer immunotherapy, complement C3, liposome, nanoparticle, targeted delivery, toll-like receptor, breast cancer
Introduction
The innate immune system is the first line of defense against pathogens and results in an immediate but non-specific immune response. Antigen presenting cells (APCs) are critical for the establishment of an innate immune response against bacteria, viruses, and cancer, and play a crucial role in the initiation, regulation and the subsequent direction of an adaptive immune response. APCs include cell types of myeloid lineage (monocytes, macrophages, and dendritic cells), as well as lymphoid B cells [1–3]. Due to the gatekeeping role of APCs in regulating how the immune system responds to an infection or to cancer, there are several therapies that aim to exploit their function, such as ex vivo antigen-loading, expansion of immunostimulatory dendritic cells (DCs), nanoparticle vaccines, and artificial APCs [4–6].
APCs respond to pathogen-associated and danger-associated molecular patterns (PAMPs and DAMPs, respectively) through pattern recognition receptors (PRRs), which include toll-like receptors (TLRs). TLRs are endogenous intracellular and extracellular molecules that bind to ligands with conserved pathogen-associated traits. The binding of ligands to specific TLRs triggers signalling pathways that result in enhanced production of inflammatory cytokines and upregulation of costimulatory molecules on APCs, required for activation of T cells [7,8]. There are numerous TLR agonists in clinical trials as monotherapies or as components of vaccines and drug formulations, but there are only three that are currently approved for use in humans. Monophosphoryl lipid A (MPLA), a TLR4 agonist, is a component of the human papillomavirus (HPV) vaccine, Cervarix®, and several other vaccines [9,10]. Imiquimod, a TLR7 agonist, is approved for use against basal cell carcinoma, several skin conditions, and viral diseases [9–11]. The Bacillus Calmette-Guérin (BCG) vaccine, developed from Mycobacterium bovis, is composed of a mixture of TLR agonists that stimulate TLR2, 3, 4, and 9, and is approved for use against bladder cancers [11].
Our lab has developed a liposome nanoparticle targeted to myeloid cells, specifically APCs [12]. These liposomes are bound to complement component 3 (C3), a central member of the complement system, which works alongside the innate immune system in the initial defense against pathogens. C3 actively binds to and coats foreign particles, priming them for uptake into myeloid cells through the complement receptors. Previous publications from our lab have described the formulation, targeting specificity, and usage of these C3-liposomes to deliver antigens directly to APCs in order to increase antigen presentation to effector cells [13–15]. While having a specific immunogenic tumour antigen is ideal for cancer treatment, there may not always be one readily available, depending on the cancer type, mutational burden, variability between cancers/patients, and availability/cost of sequencing patient samples. Therefore, this study aims to evaluate the use of C3-liposomes to deliver TLR agonist compounds, in the absence of specific tumour antigens, to activate APCs. We selected three TLR agonists, MPLA (recognised by TLR4), R848 (recognised by TLR7/8), and CpG 1826 (recognised by TLR9), based on their use in other cancer vaccine studies, their ability to activate innate immunity, and their ability to break immunosuppression and tolerance [16,17]. Furthermore, we utilised the 4T1 breast cancer mouse model, which is known to have tumours infiltrated with myeloid cells that are polarised towards tumour-associated macrophages and myeloid derived suppressor cells (TAMs and MDSCs, respectively) [18,19]. Using a combination of in vitro and in vivo experiments we tested the ability of C3-liposomes containing TLR agonists to activate APCs and induce tumour-specific adaptive immune responses.
Materials and methods
Reagents
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[poly(ethylene glycol)-2000] (DSPE-PEG(2000)), and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[PDP-poly(ethylene glycol)-2000] (DSPE-PEG(2000)-PDP) for liposome preparation were purchased from Avanti Polar Lipids (Alabaster, AL). Consistent with our previous publications, we use the term OPSS to refer to the PDP group [13]. Fluorescently-tagged lipid, Lissamine rhodamine B 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (RhodaminePE), was purchased from Life Technologies (Grand Island, NY, USA). CL-4B sepharose gel was used for size exclusion chromatography (Sigma-Aldrich, St. Louis, MO, USA). BALB/c mouse serum with complement C3 was purchased from Innovative Research (Novi, MI, USA). MPLA was purchased from Innaxon (United Kingdom), R848 from InvivoGen (San Diego, CA), and CpG 1826 from TriLink Biotechnologies (San Diego, CA). Fluorescent anti-mouse antibodies, FITC CD45 (#103107), PerCP Ly-6G (#127616), PerCP CD40 (#124623), PE/Cy7 CD80 (#104729), PE/Cy7 CD11c (#117317), APC CD3 (#100236), APC CD83 (#121509), Alexa Flour 700 CD11b (#101222), BV421 CD86 (#105031), BV421 CD127 (#135023), BV510 Ly-6C (#128033), BV605 I-A/I-E (#107639), BV650 F4/80 (#123149), BV785 CD4 (#100551), BV785 Ly-6G (#127645), APC/Cy7 CD8b (#126619), and APC/Cy7 CD103 (#121431) were purchased from BioLegend (San Diego, CA, USA). TRIZOL reagent was purchased from Life Technologies (Carlsbad, CA), and the iScript cDNA Synthesis kit and SYBR green from Bio-Rad (#1725150) (Hercules, CA). All other chemicals, reagents, and kits were purchased from Thermo Fisher Scientific (Pittsburgh, PA, USA).
Cell lines
The 4T1 cell line was purchased from ATCC (ATCC® CRL-2539™). Tumour cells were cultured in complete growth medium (RPMI, 10% heat-inactivated foetal bovine serum (FBS), 1% penicillin/ streptomycin) and incubated at 37 °C in 5% CO2.
Liposome preparation
Liposomes were prepared using a previously described film hydration method [13]. OPSS-liposomes were made by mixing DPPC/ DSPC/DSPE-PEG(2000)-PDP/DSPE-PEG(2000)/RhodaminePE in chloroform at a molecular ratio of 150:40:25:25:3. OPSS-liposomes are referred to as C3-liposomes when bound to complement C3. Control-liposomes were made following the same procedure, but substituting DSPE-PEG(2000)-PDP with DSPE-PEG(2000). Lipid mixtures were dried under nitrogen stream for 1–2 h, followed by rehydration of the film with 0.7 ml of filtered water for non-TLR agonist encapsulated liposomes. Liposomes containing TLR agonists were rehydrated with 0.7 ml TLR agonist solution. Control-and OPSS-liposomes containing TLR agonists, used in both in vitro and in vivo studies, encapsulated three different TLR agonists: CpG 1826, R848, and MPLA. MPLA (150 μl at 1 mg/mL) was incorporated into the liposomal bilayer, while CpG 1826 and R848 were encapsulated within the aqueous core with a final concentration of 5.97 mg/mL and 1.43 mg/mL, respectively. Liposomes were extruded 7–9 times through a 400 nm polycarbonate membrane filter (Avanti) at 47 °C. Extruded liposomes were column purified using a CL-4B sepharose column hydrated in 1x PBS, pH 7.4. The concentrations of control- and OPSS-liposome samples were normalised using a NanoDrop 2000 UV-Vis spectrophotometer, observing the rhodamine peak and diluting to a lipid concentration of 0.875 mg lipid/mL. Liposome size was determined using a Malvern Zetasizer Nano-S (Malvern Instruments, Malvern, UK); (control-liposomes: 89.57 ± 42.73 nm, OPSS-liposomes: 156.9 ± 203.1 nm).
Generation of bone marrow derived dendritic cells
Bone marrow was extracted from adult BALB/c mice and dendritic cells were generated as previously described [20,21]. Bone marrow-derived dendritic cells were plated at a density of 2 × 106 cells/mL culture medium [RPMI, 10% heat-inactivated foetal bovine serum (FBS), 1% penicillin/streptomycin]. Granulocyte-macrophage colony stimulating factor (GM-CSF) and Interleukin-4 (IL-4) were added at 40 ng/mL and 20 ng/mL, respectively. Cells were incubated at 37 °C, 5% CO2 and the culture medium (including cytokines) was replenished after 3 days. On day 6, non-adherent and loosely adherent cells were harvested by pipetting and re-suspended in culture medium (without cytokines) for use in experiments.
Flow cytometry analysis
Thawed bone marrow cells were distributed into a 96-well round-bottom plate (Corning), with 100,000 cells per well. Each well was treated with 1 μL of either PBS, control-liposomes, or C3-liposomes containing TLR agonists, followed by incubation at 37 °C and 5% CO2. Treatment groups were preincubated for 1 h in mouse serum that had not been heat-inactivated, allowing binding of complement C3 to the OPSS group on liposomes. Treatments were added to the 96-well plate so that the final serum concentration in the media was 10%. After 24 h, cells were rinsed with FACS buffer (1x PBS, 1% BSA), centrifuged at 400xg, and resuspended in 100 μL/ well of a master antibody mix for detecting myeloid cell activation markers. Cells were stained with CD11b, MHCII, CD40, CD80, and CD86 antibodies (Biolegend) diluted at 1:100 for 30 min in the dark at 4 °C, then centrifuged for 5 min at 1600 rpm, resuspended in 250 μL FACS buffer, and analysed using a Cytoflex flow cytometer (Beckman Coulter Life Sciences, Indianapolis, IN). The live cell population was selected using forward and side scatter, followed by myeloid cell selection with CD45 and CD11b. Activation of myeloid cells was determined by levels of MHCII, CD40, CD80, and CD86 expression. Cellular internalisation of rhodamine labelled liposomes was determined by mean fluorescence intensity (MFI) of rhodamine, detected on the PE channel.
RT-PCR analysis
Bone marrow derived dendritic cells were cultured as described above and distributed into a 24-well plate (Corning) at a concentration of 1 × 106 cells/mL. Treatment groups were preincubated in serum and added to the cells as described under the flow cytometry experiments. After treatment for 24 h, RNA was isolated from each well of cells using 200 μl TRIZOL reagent (Life Technologies), following the manufacturer instructions. RNA was resuspended in nuclease-free water, and RNA concentration and purity were determined using the Nanodrop 2000 UV-Vis spectrophotometer (Thermo-Scientific). cDNA was synthesised from RNA using the iScript cDNA Synthesis kit (Bio-Rad). For quantitative RT-PCR, cDNA was amplified with SYBR green (Bio-Rad) using forward and reverse primers. The following murine primers from Integrated DNA Technologies were used for the following cDNAs: IRF7, IP-10, IL-1β, IL-6, IL-12, and TNF-α (Table 1).
Table 1.
Primers used in RT-PCR analysis.
| Primer Sequence (5′–>3′) |
||
|---|---|---|
| Genes | Forward | Reverse |
|
| ||
| IRF7 | CTGGAGCCATGGGTATGCA | AAGCACAAGCCGAGACTGCT |
| IP-10 | TCATCCTGCTGGGTCTGAGTGG | CGCTTTCATTAAATTCTTGATGGTC |
| IL1 β | TGGAGAGTGTGGATCCCAAGAAAT | TGCTTGTGAGGTGCTGATGTACCA |
| IL6 | AAGAAATGATGGATGCTACC | GAGTTTCTGTATCTCTCTGAAG |
| IL12 | TGATGATGACCCTGTGCCTTGGTA | TTCAGCTCCTCCAGTGTACG |
| TNF α | TCTCATGCACCACCATCAAGGACT | ACCACTCTCCCTTTGCAGAACTCA |
4T1 tumour inoculation and mouse model
Female and male BALB/c mice were obtained from The Jackson Laboratory (Sacramento, CA, USA) and housed in the University of Alaska Anchorage (UAA) vivarium. All experiments were approved by the UAA Institutional Animal Care and Use Committee. Experiments utilising mice were designed and conducted to minimise the number of animals used, to reduce pain and discomfort, and to minimise anxiety and distress to mice, with mice monitored daily. Before injections, 4T1 cells were rinsed twice in PBS. Mice were shaved and received subcutaneous injections in their left and right flanks of 5 × 104 4T1 cells in 30 μl of PBS per injection. Injections were given under anaesthesia using isoflurane. Treatments were started when tumours became palpable (approximately 10–12 days, 2–4 mm). Mice were separated into groups of 3–4 and received local subcutaneous injections of 150 μL of either PBS, control-liposomes containing TLR agonists, or C3-liposomes containing TLR agonists. Liposomes were not preincubated with mouse C3-positive serum as endogenous C3 binds to the OPSS-liposomes after injection. Mice received treatment on one side only. The tumour on the opposite side was measured to document any systemic response to treatment. Injections were given every other day for 7 days. Tumour measurements were made before all injections, using a digital calliper, and volumes were reported as mm3.
Analysis of immune cell tumour infiltrate
Following treatment, mice were euthanised by CO2 asphyxiation followed by cervical dislocation. Both injected and distal tumours were collected and digested in collagenase (1 mg/mL) for 30 min at 37°C. Following digestion, samples were passed through 100 μm cell strainers and resuspended in complete media (RPMI, 10% heat-inactivated FBS, 1% penicillin/streptomycin). Samples were then spun at 400xg for 5 min in a Sorvall Legend X1R centrifuge and resuspended in FACS buffer (1x PBS, 1% BSA) for flow cytometry analysis, or resuspended in freezing media (90% FBS, 10% dimethyl sulfoxide) and stored in liquid nitrogen for later use.
Statistical analysis
Data is presented as mean ± standard error. Differences among groups were determined using an unpaired two-tailed Student’s t test. p Values of less than .05 were considered significant and are indicated by an asterisk over the data or over a bracket between samples.
Results
Liposomal delivery of TLR agonists increases levels of activation markers in myeloid cells
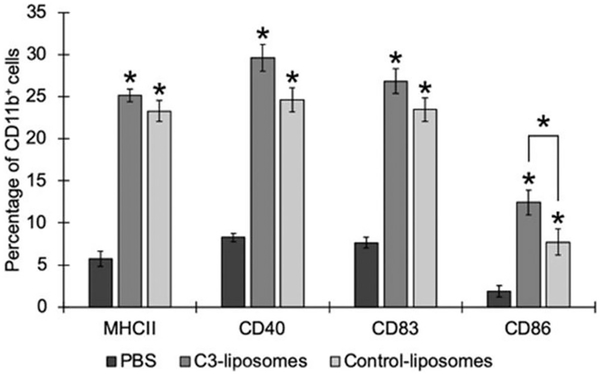
To determine if treatment with liposomes containing TLR agonists could activate monocytes, bone marrow cells were treated in vitro with targeted C3-liposomes versus control liposomes, with both containing TLR agonists. Bone marrow cells were isolated from healthy mice and treated with either PBS, C3-liposomes or control-liposomes, both containing the 3 TLR agonists, for 24 h. Following treatment, the levels of activation markers (MHCII, CD40, CD83, CD86) on the cells were analysed by flow cytometry (Figure 1). Treatment with either C3-liposomes or control-liposomes containing MPLA, R848, and CpG 1826 resulted in a significant upregulation of all activation markers, compared to PBS-treated cells. While results from C3-liposome treatment with the three TLR agonists trended towards a slightly greater increase in all markers, only the difference in CD86 expression was significant between the two liposome groups. Previous research has shown that monocytes upregulate CD86 expression in response to inflammatory stimuli and thereby overcome the regulatory/tolerance mechanisms in place [22]. These results indicate that delivery of targeted and non-targeted TLR agonists, encapsulated in liposomes, leads to monocyte activation and upregulation of activation markers, which are essential for an antigen-specific T cell response.
Figure 1.
Liposomal delivery of TLR agonists increases levels of activation markers in myeloid cells. Murine bone marrow cells were treated with PBS, C3-liposomes with 3 TLR agonists, or control-liposomes with 3 TLR agonists for 24 h before analysis by flow cytometry. Data are expressed as mean ± standard error (n = 3). *p Value < .05, compared to PBS. Bracket indicates significant difference between C3-liposomes and control-liposomes.
Targeted C3-liposomes with encapsulated TLR agonists induce expression of inflammatory cytokines in myeloid cells
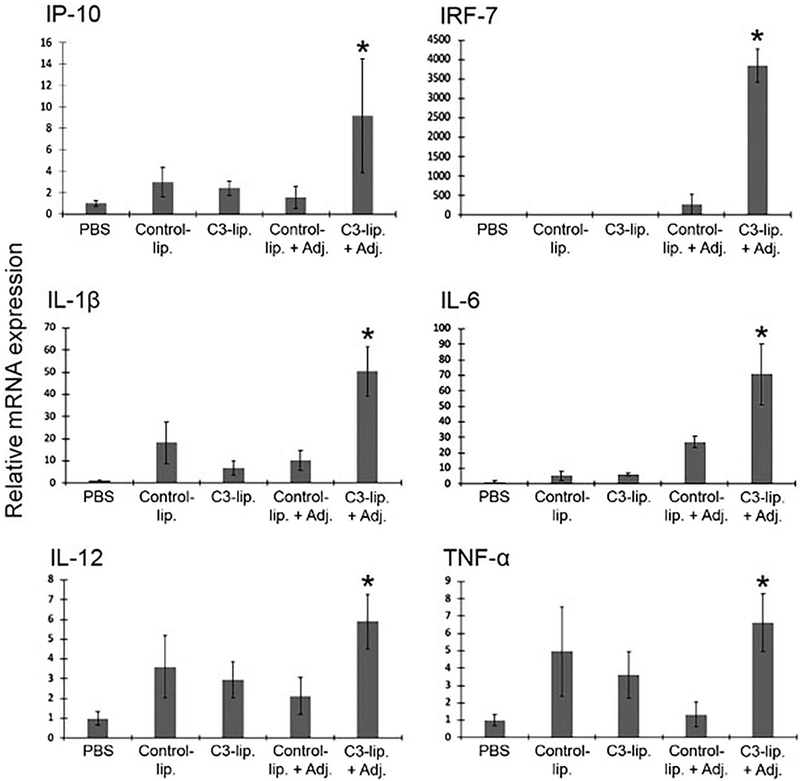
The ability of APCs to sense and amplify inflammatory signals leads to subsequent interactions with an array of immune cells. Here we investigated if C3-liposome encapsulation of TLR agonists resulted in increased expression of pro-inflammatory cytokines and factors in APCs in vitro. Bone marrow-derived cells were treated with either PBS, control-liposomes, C3-liposomes, control-liposomes with encapsulated TLR agonists, or C3-liposomes with encapsulated TLR agonists. Three different TLR agonists were encapsulated: CpG 1826 (specific for TLR9), R848 (specific for TLR7/8), and MPLA (specific for TLR4). After treatment of cells for 24 h, RNA was extracted, subjected to RT-PCR with specific primers, and the relative amplification of mRNAs analysed. Treatment with C3-liposomes encapsulating the three TLR agonists resulted in significant increased expression of IRF7, IP-10, IL-1β, IL-6, IL-12, and TNF-α (Figure 2), cytokines that are important for innate, adaptive and an antitumor immune response.
Figure 2.
C3-liposomes encapsulating TLR agonists increase expression of inflammatory cytokines in myeloid cells. Murine bone marrow derived cells were treated with PBS, control-liposomes, C3-liposomes, control-liposomes encapsulating TLR agonists (+Adj.), or C3-liposomes encapsulating TLR agonists (+Adj.) for 24 h. RT-PCR was used to analyse the relative expression of IRF7, IP-10, IL-1β, IL-6, IL-12, and TNF-α mRNAs in response to treatments. Data are expressed as mean ± standard error (n = 3) and normalised to PBS controls. *p Value < .05.
Treatment with C3-liposomes containing encapsulated TLR agonists leads to reduced tumour growth in mice
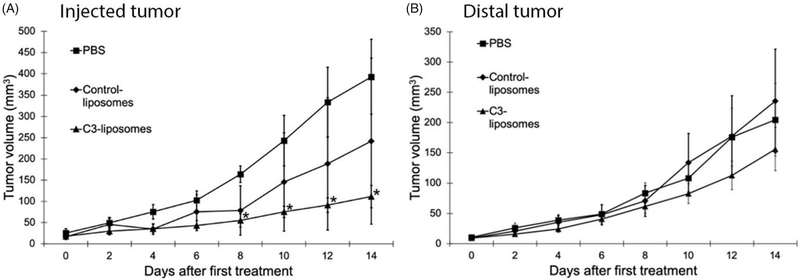
To determine if C3-liposomes activate APCs in vivo and induce an anti-tumour immune response, liposomes encapsulating TLR agonists (CpG 1826, R848, MPLA) were used to treat BALB/c mice with established 4T1 breast cancer tumours. Each mouse with two established tumours (about 2–4 mm) received a local subcutaneous injection of either PBS, control-liposomes with 3 TLR agonists, or C3-liposomes with 3 TLR agonists at one tumour site while the other tumour site, referred to as the distal tumour, was left untreated to evaluate the systemic response to therapy. Treatment with C3-liposomes resulted in reduced tumour growth in the injected tumours, but no observable reduction or slowed growth in the distal tumours (Figure 3). By day 8 and until the end of the study, only C3-liposome treatment resulted in a significant reduction in tumour volume compared to PBS (Figure 3(A)). In contrast, there was no significant difference in the volume of injected tumours between PBS and control-liposome treatments. Finally, as can be seen in Figure 3(B), neither C3-liposome nor control-liposome treatment of injected tumours had an effect on the volume of untreated distal tumours.
Figure 3.
C3-liposome treatment results in reduced tumour volume of treated established tumours. Established 4T1 tumours were treated by subcutaneous peritumoral injections of PBS or liposomes encapsulating TLR agonists starting on day 0, and every other day for a total of 7 injections. Tumour measurements were made before all injections, showing (A) injected tumour volume, and (B) distal non-injected tumour volume (mm3). By day 8, and through the end of the study, only C3-liposomes significantly reduced injected tumour volumes (*p Value < .05, compared to PBS). Data are expressed as mean ± standard error (n = 3–4).
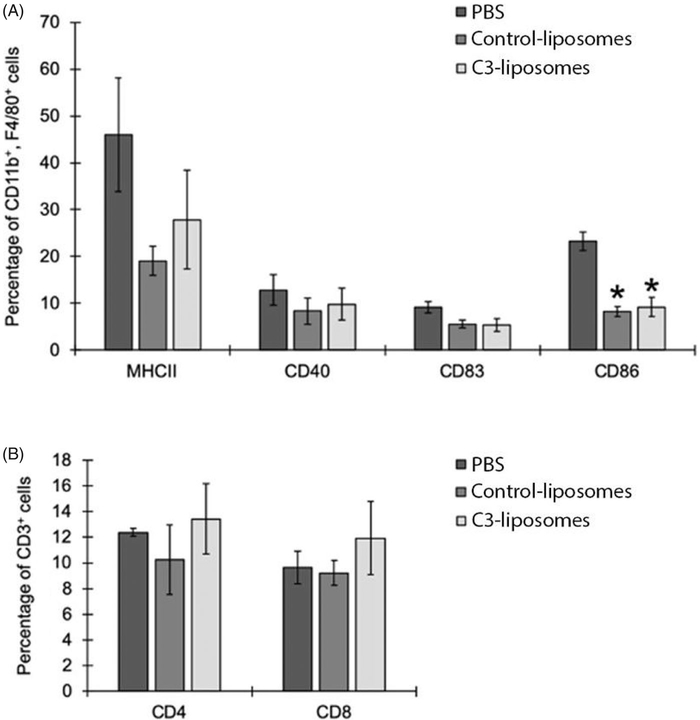
C3-liposome treatment does not result in differences in activation markers or T-cell levels in immune cell infiltrates of 4T1 tumours
Following the completion of the tumour study, mice were euthanised, and the tumours were collected for analysis of immune cell infiltrate. Tumours were digested and processed for flow cytometry analysis of immune activation markers and T cell markers (Figure 4). Because 4T1 tumours are known to have high levels of tumour-associated macrophages [18,19], activation markers on macrophages (CD11b+, F4/80+) within the tumours were specifically analysed. No significant differences were observed between treatment groups in the percentages of macrophages expressing activation markers MHCII, CD40, CD83 (Figure 4(A)). However, there was a significant decrease in CD86 expression in macrophages in both control- and C3-liposome-treated tumours, compared to PBS-treated tumours (Figure 4(A)). Finally, with respect to CD3+ T cells, there were no significant differences in the percentages of helper (CD4+) versus cytotoxic (CD8+) T cells found within the tumours injected with PBS, control-liposomes, or C3-liposomes (Figure 4(B)).
Figure 4.
Analysis of immune cell tumour infiltrate. Mice were euthanised, injected tumours digested, and cells analysed by flow cytometry. (A) Percentages of macrophages (CD11b+, F4/80+) expressing activation markers (MHCII, CD40, CD83, CD86), and (B) percentages of CD3+ T cells (either CD4+ or CD8+) in injected tumours treated with PBS, control-liposomes, or C3-liposomes. Cell populations are displayed as percentages of the total number of white blood cells. Data are expressed as mean ± standard error (n = 3–4). *p Value < .05, compared to PBS.
Discussion
TLR agonists have been shown to induce acute inflammation when used as adjuvants in treatments that may otherwise be non-immunogenic (e.g. peptide/protein-based cancer vaccines) [23]. TLR agonists mimic pathogen (PAMPs) and danger signals (DAMPs), thus activating a robust chain of inflammatory reactions that can often be detrimental to the immune response, surrounding tissues, and the overall organism (e.g. chronic/pathological inflammation and sepsis) [24]. By encapsulating TLR agonists within C3-liposomes, it may be possible to negate systemic/off-target effects and directly target the treatments to APCs, thereby inducing the expression of costimulatory molecules and cytokines that are essential for T cell activation and antitumor immunity.
Monocytes and macrophages are important immunomodulatory cells in the tumour microenvironment that are often distorted towards a suppressive phenotype. The presence of tumour-associated macrophages in the tumour microenvironment is generally correlated with a poor cancer prognosis, and their numbers are often elevated in solid tumours [19]. Delivery of TLR agonists in C3-liposomes to bone marrow derived APCs resulted in upregulated gene expression of IRF7, IP-10, IL-1β, IL-6, IL-12, and TNF-α. IRF7 and IP-10 are involved in type I and II interferon responses. These cytokines are important for both innate and adaptive cell activation and promote anti-tumour immune responses [25,26]. Increased levels of IL-1β, IL-6, IL-12, and TNF-α expression in cells treated with C3-liposomes containing TLR agonists indicate increased monocyte and macrophage activation [27–30]. Notably, the levels of cytokine expression were not significantly increased in non-targeted control-liposomes with TLR agonists, suggesting that increased uptake of C3-liposomes into bone marrow cells induced activation through binding of the TLR agonists to their endosomal TLRs. These results provide valuable information on specific targeting that may promote the differentiation of these influential myeloid cell types towards an antitumor phenotype.
In this study, mice were treated with C3-liposomes containing TLR agonists every other day for 14 days, resulting in regression of the injected tumour, but no response from the tumour distal to the injection site. The constant exposure and extensive treatment timeline may have been detrimental to the establishment of a systemic immune response, and it is possible that we may have induced tolerance to subsequent treatment. There is mounting evidence that continuous binding to TLRs may cause downregulation of costimulatory molecules and a decrease in antigen presentation [31–35]. TLR dysregulation has also been documented in multiple autoimmune diseases and Alzheimer’s disease [31,32]. TLRs activate APCs to produce proinflammatory cytokines, a process that is tightly regulated in order to prevent damage to surrounding tissues, and temporary tolerance to a second exposure is often seen [33–35]. A study examining systemic TLR agonist treatment and tolerance showed that constant TLR7 stimulation (R848 ligand) resulted in a state of tolerance and a significant decrease in cytokine production, which lasted for several days [33]. This same study showed that an initial stimulation of TLR3 followed by stimulation of TLR7 24 h later resulted in the highest level of cytokine production, repeated at five-day intervals. This highlights the importance of timing of treatment and an understanding of the underlying mechanisms of TLR stimulation. With the constant exposure of TLR agonists, as used in our injection pattern, it is possible that the tumour-infiltrating myeloid cells were pushed towards a TLR-tolerant phenotype. Therefore, it may be beneficial to stop TLR treatment earlier in the study and/or space out treatments in order to avoid tolerance.
In conclusion, targeted C3-liposomes with encapsulated TLR agonists provide a promising avenue for cancer treatment. Delivery of TLR agonists to APCs in vitro resulted in upregulation of activation markers and cytokines, indicating that APCs are reprogrammed to an activated phenotype. An activated phenotype is essential for promoting both innate and adaptive antitumor immune responses. Significantly, in vivo treatment of tumours with C3-liposomes containing TLR agonists resulted in a decrease in growth of injected tumours. However, the lack of efficacy at a non-injected tumour indicates that C3-liposome delivery of TLR agonists alone will not be sufficient for treatment of metastatic cancer. Optimisation of injection pattern and co-treatment with tumour antigens or checkpoint blockade could possibly improve the antitumor immune response seen with delivery of TLR agonists by C3-liposomes.
Acknowledgements
The authors thank Dr. Kristine Mann for her thoughtful discussion and critical thinking throughout this project.
Funding
This work was supported by grants from the Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number [P20GM103395], Alaska Run for Women, and the National Cancer Institute of the National Institutes of Health under grant number [CA227740].
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Geissmann F, Manz MG, Jung S, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol. 2017;17:349–362. [DOI] [PubMed] [Google Scholar]
- [3].Brutkiewicz RR. Cell signaling pathways that regulate antigen presentation. J Immunol. 2016;197:2971–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li H, Shao S, Cai J, et al. Artificial human antigen-presenting cells are superior to dendritic cells at inducing cytotoxic T-cell responses. Immunology. 2017;152:462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim JV, Latouche JB, Riviere I, et al. The ABCs of artificial antigen presentation. Nat Biotechnol. 2004;22:403–410. [DOI] [PubMed] [Google Scholar]
- [6].NealL R, Bailey SR, Wyatt MM, et al. The basics of artificial antigen presenting cells in T cell-based cancer immunotherapies. J Immunol Res Ther. 2017;2:68–79. [PMC free article] [PubMed] [Google Scholar]
- [7].Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388: 621–625. [DOI] [PubMed] [Google Scholar]
- [8].Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. [DOI] [PubMed] [Google Scholar]
- [9].Anwar MA, Shah M, Kim J, et al. Recent clinical trends in Toll-like receptor targeting therapeutics. Med Res Rev. 2019;39:1053–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Smith M, Garcia-Martinez E, Pitter MR, et al. Trial watch: toll-like receptor agonists in cancer immunotherapy. Oncoimmunology. 2018;7:e1526250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kaczanowska S, Joseph AM, Davila E. TLR agonists: our best frenemy in cancer immunotherapy. J Leukoc Biol. 2013;93: 847–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Merle NS, Noe R, Halbwachs-Mecarelli L, et al. Complement system part II: role in immunity. Front Immunol. 2015;6:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kullberg M, Martinson H, Mann K, et al. Complement C3 mediated targeting of liposomes to granulocytic myeloid derived suppressor cells. Nanomedicine. 2015;11:1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Francian A, Mann K, Kullberg M. Complement C3-dependent uptake of targeted liposomes into human macrophages, B cells, dendritic cells, neutrophils, and MDSCs. Int J Nanomedicine. 2017;12:5149–5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Francian A, Namen S, Stanley M, et al. Intratumoral delivery of antigen with complement C3-bound liposomes reduces tumor growth in mice. Nanomedicine. 2019;18:326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Prins RM, Craft N, Bruhn KW, et al. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J Immunol. 2006;176: 157–164. [DOI] [PubMed] [Google Scholar]
- [17].Whitmore MM, DeVeer MJ, Edling A, et al. Synergistic activation of innate immunity by double-stranded RNA and CpG DNA promotes enhanced antitumor activity. Cancer Res. 2004;64:5850–5860. [DOI] [PubMed] [Google Scholar]
- [18].Gatti-Mays ME, Balko JM, Gameiro SR, et al. If we build it they will come: targeting the immune response to breast cancer. NPJ Breast Cancer. 2019;5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Luo Y, Zhou H, Krueger J, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. [DOI] [PubMed] [Google Scholar]
- [21].Madaan A, Verma R, Singh AT, et al. A stepwise procedure for isolation of murine bone marrow and generation of dendritic cells. J Biol Methods. 2014;1:e1. [Google Scholar]
- [22].Sansom DM, Manzotti CN, Zheng Y. What’s the difference between CD80 and CD86? Trends Immunol. 2003;24: 314–319. [DOI] [PubMed] [Google Scholar]
- [23].Pan RY, Chung WH, Chu MT, Chen SJ, et al. Recent development and clinical application of cancer vaccine: targeting neoantigens. J Immunol Res. 2018;2018:4325874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hamerman JA, Pottle J, Ni M, et al. Negative regulation of TLR signaling in myeloid cells-implications for autoimmune diseases. Immunol Rev. 2016;269:212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ning S, Pagano JS, Barber GN. IRF7: activation, regulation, modification and function. Genes Immun. 2011;12:399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dufour JH, Dziejman M, Liu MT, et al. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–3204. [DOI] [PubMed] [Google Scholar]
- [27].Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011;22: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sun L, He C, Nair L, et al. Interleukin 12 (IL-12) family cytokines: Role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine. 2015;75:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Parameswaran N, Patial S. Tumor necrosis factor-a signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20: 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Anstadt EJ, Fujiwara M, Wasko N, et al. TLR tolerance as a treatment for central nervous system autoimmunity. J Immunol. 2016;197:2110–2118. [DOI] [PubMed] [Google Scholar]
- [32].Herrera-Rivero M, Santarelli F, Brosseron F, et al. Dysregulation of TLR5 and TAM ligands in the Alzheimer’s brain as contributors to disease progression. Mol Neurobiol. 2019;56:6539–6550. [DOI] [PubMed] [Google Scholar]
- [33].Hotz C, Treinies M, Mottas I, et al. Reprogramming of TLR7 signaling enhances antitumor NK and cytotoxic T cell responses. Oncoimmunology. 2016;5:e1232219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nahid MA, Benso LM, Shin JD, et al. TLR4, TLR7/8 agonist-induced miR-146a promotes macrophage tolerance to MyD88-dependent TLR agonists. J Leukoc Biol. 2016;100: 339–349. [DOI] [PubMed] [Google Scholar]
- [35].Butcher SK, O’Carroll CE, Wells CA, et al. Toll-like receptors drive specific patterns of tolerance and training on restimulation of macrophages. Front Immunol. 2018;9:933. [DOI] [PMC free article] [PubMed] [Google Scholar]