Abstract
Introduction
Black and Hispanic/Latinx cisgender men who have sex with men (MSM), transgender women, transgender men, and gender nonbinary (TGNB) individuals have been historically underrepresented in HIV pre-exposure prophylaxis (PrEP) clinical trials. There is an urgent need for ongoing engagement with communities that have been the most impacted by HIV and diverse representation in clinical trials. Here we describe strategic approaches undertaken in the PURPOSE 2 trial to optimize engagement of underrepresented individuals.
Methods and results
PURPOSE 2 is an ongoing Phase 3 trial evaluating the safety and efficacy of lenacapavir as PrEP in cisgender MSM and TGNB individuals. In PURPOSE 2, we used a multipronged approach aimed at enriching participation of underrepresented individuals. We conducted a review to identify evidence-informed recommendations from literature, engaged with stakeholders, and established the Global Community Advisory and Accountability Group (GCAG) to represent the needs of the community. Insights from stakeholders and GCAG members resulted in an expansion of the study population to include transgender men, gender nonbinary persons, and adolescents, and evaluation of population-specific outcomes. Feedback from stakeholders and GCAG members also informed investigator and site selection; these were selected based on prior experience working with persons from diverse racial, ethnic and gender identities, and estimates of local HIV incidence. Site selection was also expanded to include community-based clinics with services tailored towards Black, Hispanic/Latinx, and TGNB populations. We established a study-wide recruitment goal of 50% Black MSM and 20% Hispanic/Latinx MSM in US sites and 20% transgender women globally. Site-specific recruitment goals were also developed based on local demographics and HIV incidence. Mandatory trainings included Good Participatory Practice guidelines, gender inclusivity, and antiracism.
Conclusion
While further work is needed to achieve equitable representation, the strategies we describe may serve as a framework for future clinical trials.
Trial registration
Clinical Trial Number: NCT04925752.
Introduction
Historically underrepresented individuals, particularly Black and Hispanic/Latinx cisgender gay, bisexual, and other men who have sex with men (MSM) and transgender women, transgender men, and gender nonbinary (TGNB) individuals, have a disproportionate burden of HIV [1, 2]. In the US, HIV incidence among Black and Hispanic/Latinx MSM is estimated to be eight and four times higher than their White counterparts, respectively [3]. In 2019, over half of all new HIV diagnoses were in Southern states, of which 47% were among Black MSM and 28% among Hispanic/Latinx MSM [4]. Estimates suggest HIV prevalence among transgender women is as high as 40% in some US cities, with Black and Hispanic/Latinx transgender women bearing the greatest burden [5]. Globally, transgender women have 49-fold greater odds of having HIV compared with cisgender individuals [6]. Less is known about the HIV burden in transgender men, but available data from US cohorts suggest an HIV prevalence of up to 11% among transgender MSM [7, 8].
Adolescents, particularly those in racial, ethnic, gender, and sexual minority groups, similarly experience high rates of HIV infection and face additional challenges to accessing healthcare services [9]. An estimated 1.75 million adolescents are living with HIV globally [10], and among US adolescents diagnosed with HIV, 5% were in persons aged 15–17 years [4]. Despite significant disparities in ongoing HIV incidence, uptake of pre-exposure prophylaxis (PrEP) in these key populations have been limited. This is noteworthy as PrEP is highly effective in reducing the risk of acquiring HIV [11], but estimates suggest that only approximately 20% of the 1.2 million estimated people who would benefit from PrEP in the US are currently using it [12]. Globally, the proportion of persons on PrEP is even lower [13, 14] and among individuals who initiate PrEP, many discontinue within a few months of starting the regimen [15, 16].
The factors driving inequities in PrEP uptake and persistence in key populations are complex and multifactorial. However, underrepresentation of these communities in clinical research, as well as stigma, discrimination, and transphobia in research and medical settings have perpetuated mistrust [17–19]. Engagement with communities that have the highest HIV burden, as well as advocates, providers, and investigators who represent these communities, is critical, [20, 21] and there is renewed focus on designing clinical trials that have diverse representation. In this paper, we provide a detailed description of the strategic evidence-informed approaches aimed at optimizing engagement of historically underrepresented individuals undertaken ahead of the initiation of a recently launched Phase 3 trial of a novel PrEP agent.
Methods
PURPOSE 2 (GS-US-528-9023; NCT04925752) is an ongoing Phase 3 clinical trial evaluating the safety and efficacy of lenacapavir (LEN) as PrEP for preventing HIV-1 infection in cisgender MSM and TGNB individuals sponsored by Gilead Sciences. A separate study, PURPOSE 1 (GS-US-412-5624; NCT04994509), is evaluating LEN in cisgender women. LEN is a first-in-class capsid inhibitor, which disrupts HIV capsid and viral replication in multiple steps including nuclear entry and capsid disassembly prior to HIV integration and is administered subcutaneously every six months [22]. In PURPOSE 2, participants are randomized in a 2:1 ratio to receive subcutaneous LEN every 26 weeks plus daily oral placebo or daily oral emtricitabine/tenofovir disoproxil fumarate plus a placebo subcutaneous injection every 26 weeks. The trial started in June 2021 with study sites in Brazil, Peru, South Africa, and the US, and will enroll approximately 3,000 individuals.
In planning for PURPOSE 2, the study team (defined as the sponsor, Gilead Sciences, and global collaborators) used a multipronged approach aimed at intentionally enriching participation of historically underrepresented individuals. We conducted a review of publications to identify effective strategies to increase enrollment of Black and Hispanic/Latinx MSM and TGNB individuals, particularly in HIV treatment and prevention studies. We also engaged with community and patient stakeholders in the US and globally through several community forums, roundtable discussions, and individual meetings to understand community preferences, concerns, and challenges prior to protocol development. Input from these meetings were summarized and incorporated along with recommendations from the literature. The Global Community Advisory and Accountability Group (GCAG) was subsequently established to provide ongoing input on the needs of the community, accountability, and to serve as a resource to the study team and site investigators and staff. In collaboration with members of the GCAG, we developed criteria for site selection and demographic inclusion goals. We also established mandatory trainings for all individuals involved in the study that encompassed Good Participatory Practice (GPP) guidelines, which provides a framework for building effective partnerships with key stakeholders in research [23]; gender inclusivity; and antiracism. The PURPOSE 2 study protocol was approved by institutional review boards/ethics committees (IRB/EC) at each site and by relevant regulatory agencies in each country where recruitment has started, and IRB/EC and regulatory review are continuing in pending countries and sites. All participants provide written informed consent prior to enrollment and when adolescent enrollment initiates, adolescents will provide assent and/or parental consent, as appropriate.
Results
Evidence-informed recommendations from literature
The study team reviewed publications on approaches to address disparities in enrollment and retention in clinical trials, including global consensus documents related to the conduct of HIV prevention clinical trials [23, 24]. Key elements to inform new trials included intentional engagement with community stakeholders and advocates, including the establishment of a trial-specific community advisory and accountability group [25–29]. Recommendations also underscored judicious site selection by prioritizing study sites with culturally-sensitive investigators and staff, with prioritization for sites that have local community advisory boards and strong relationships with community-based organizations focused on HIV prevention in key populations [30–32]. Of particular importance, site investigators and/or staff should include individuals from the communities represented in the study [30, 32–34]. Lastly, findings from our review also emphasized the importance of culturally-relevant study design and implementation, including gender affirming practices [18, 35]. These strategies include TGNB-inclusive recruitment materials; gender identity screening and organ inventory; inclusive, trauma-informed sexual history taking guidelines; assessment of intimate partner violence and substance use, and referral to appropriate support services; and more accurately describing TGNB participant inclusion and exclusion criteria. These recommendations from the literature were incorporated into the design and implementation of the PURPOSE 2 study.
Stakeholder engagement and establishing the GCAG
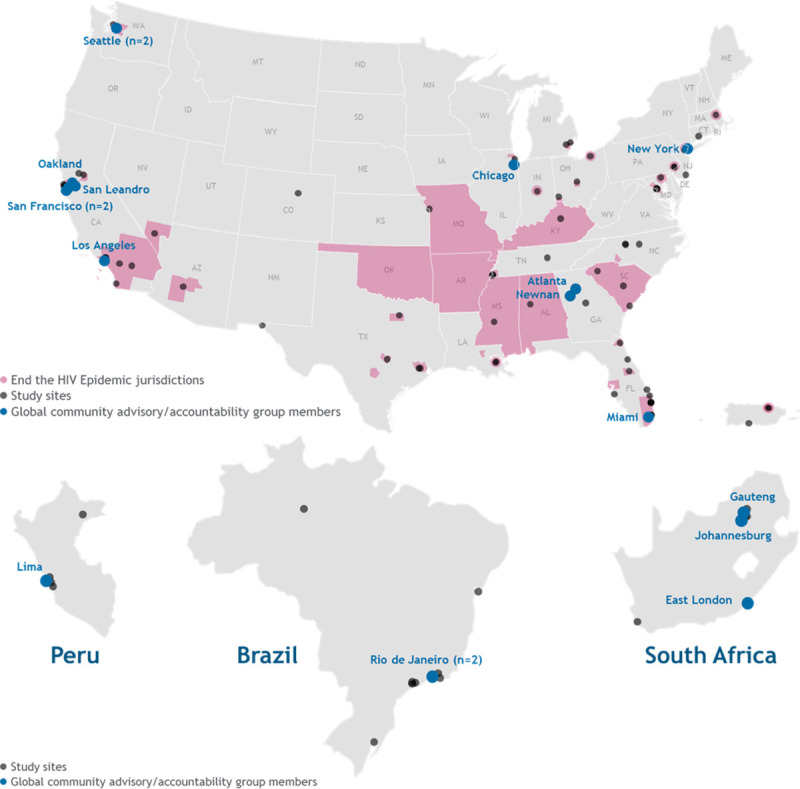
We utilized a two-pronged approach to engage with stakeholders. First, the study team reached out to global advocates, scientists, community representatives, policymakers, and others to request initial feedback on the trial design. We also worked with these initial broader stakeholders to identify and form relationships with advocates who had clinical trial expertise and were from the communities we wished to represent in the study. Building on these relationships, we then established the GCAG to provide ongoing community input on the study. Patient and community advocates who wished to participate in the GCAG were asked to complete an online application. Members were selected based on their personal lived experience as well as HIV research and clinical trial advisory expertise, experience in community engagement with the priority demographic populations for the study, and from recommendations from key community leaders, patient advocates, and site investigators. Prior studies that examined the implementation community engagement across various HIV prevention trials emphasized early involvement and broad representation of community stakeholders as critical to building trust and meaningful collaboration [20, 21]. Thus, GCAG members were involved in each step of the study, including protocol development, site selection, development of participant-facing materials, and study implementation. Additionally, we ensured that the individuals selected to join the GCAG reflected the priority recruitment race, ethnic, and gender demographic goals of the study as well as the geography of the trial sites (Fig 1). The group is compensated for their time and is currently comprised of 18 members.
Fig 1. PURPOSE 2 clinical trial study sites and locations of Global Community Advisory and Accountability Group (GCAG) members.
Insights from meetings with broader stakeholders and the GCAG resulted in meaningful changes to the study (Table 1). For example, the trial’s population was expanded from MSM and transgender women to include transgender men and gender nonbinary individuals who have sex with men. GCAG members made recommendations regarding site and investigator selection, and the inclusion of adolescents in the clinical trial. GCAG members also highlighted concerns that TGNB individuals may have regarding drug-drug interactions between gender-affirming hormone therapy (GAHT) and drugs for HIV prevention, and strongly recommended conducting evaluations of exposure in persons on GAHT within the trial. Moreover, stakeholders recommended specific language and descriptions to improve our inclusion criteria, conduct an organ inventory to assess pregnancy potential, and revise our sexual behavior questionnaires to better reflect the practices of our TGNB participants. Stakeholders also recommended allowing those participants who were assigned female sex at birth and had reproductive potential to remain in the study if they became pregnant, consistent with the evolving consensus on the inclusion of pregnant people in PrEP trials [36] and our plans for the accompanying PURPOSE 1 study.
Table 1. Changes to the PURPOSE 2 study design and protocol based on stakeholder and Global Community Advisory and Accountability (GCAG) feedback.
| Stakeholder and GCAG Input | Changes Made |
|---|---|
| Transgender men and gender nonbinary people have been excluded from HIV prevention research | • Inclusion of transgender men and gender nonbinary Individuals • Selection of research sites with experience in engaging transgender men in prevention research |
| Proactive efforts are needed to ensure inclusion of Black MSM | • Implementation of site-specific recruitment plans designed to facilitate a goal of enrolling 50% Black MSM in the US |
| Inclusion of diverse study staff is important and often neglected | • Outreach to and selection of investigators and study sites with staff who are representative of the study’s priority participant populations |
| Many adolescents, especially transgender adolescents, are particularly vulnerable and in need of HIV prevention options | • Inclusion of adolescents ages 16–17 in the study protocol |
| Participants assigned female sex at birth should not be removed from the study if they become pregnant | • Modification of the protocol to allow participants who become pregnant to remain on study drug after re-consent |
| Participants who acquire HIV need active connection to HIV care | • Protocol written to facilitate rapid ART start • Continued follow-up in the study until virologic suppression is achieved |
| Gender-affirming surgical history must be collected in a manner that is accurate and affirming | • Addition of an organ inventory to medical history |
| Information on gender and sexual behaviors must be obtained in a way that is inclusive and affirming | De novo development of sexual behavior questionnaires with skip logic based on gender identity and organ inventory |
| Concerns about interactions between PrEP and GAHT continue to be a barrier to PrEP for some transgender people | • Discussion about lack of interactions expected between PrEP drugs and GAHT in the protocol and consent • Rigorous collection of concomitant GAHT • Inclusion of LEN and GAHT drug-drug interaction analyses |
MSM = men who have sex with men; ART = antiretroviral therapy; PrEP = pre-exposure prophylaxis; GAHT = gender-affirming hormone therapy; LEN = lenacapavir
Site selection
The PURPOSE 2 trial uses a novel counterfactual study design. In this design, efficacy is estimated by comparing HIV incidence among participants on the study drug with the background HIV incidence, which is calculated using a recent infection testing algorithm from persons who test positive on a recency assay for HIV in the cohort screened for randomization into the study [37, 38]. This design necessitates identifying sites in locations with high rates of new HIV infection. However, input from stakeholders and the GCAG emphasized the importance of identifying investigators within those settings who have expertise in engaging with individuals from our key populations. Thus, there were two main factors that the study team considered in the selection of study sites: 1) their prior work with individuals from diverse racial, ethnic and gender backgrounds and 2) local estimates of HIV incidence.
Site selection began with a standard feasibility survey that was enhanced to include questions about the site’s staff demographics; cultural sensitivity; and expertise in working with racial, ethnic, and gender-diverse groups. Sites were asked to name the community-based organizations and groups they would work with to support the recruitment of these key populations. Sites were also asked about their prior work with community advisors and advocates, and whether they had their own standing community advisory groups. Site selection consideration was expanded beyond HIV clinical treatment trial sites to include community-based clinical research sites; Federally Qualified Health Centers; and other community-based clinics with services tailored to Black, Hispanic/Latinx, and TGNB populations. We focused on increasing the number of sites that provided gender-affirming care, as well as sites and investigators with expertise in HIV prevention for adolescent populations.
We also considered geographic regions that were the most disproportionately affected by HIV so we could engage with persons who could benefit from PrEP. Site selection in the US aimed to reflect the high-priority municipalities and regions identified in the Ending the HIV Epidemic Initiative, where more than 50% of new HIV infections occurred in 2016–2017 (Fig 1) [39, 40]. Selection of sites in the southern US was prioritized, given the high burden of HIV in the region, and potential sites outside the US were identified using historical HIV incidence data.
Study-wide demographic recruitment goals and site-specific recruitment plans
The study team identified study-wide demographic recruitment goals to support robust representation of key populations. In consultation with stakeholders and GCAG members, we set a goal of enrolling 50% Black MSM and 20% Hispanic/Latinx MSM across US study sites. These benchmarks were informed by recommendations from the HPTN Black Caucus Scientific Report [41] and HIV incidence surveillance data in Hispanic/Latinx MSM [4]. Globally, we set a goal of enrolling 20% transgender women, based on HIV prevalence estimates among transgender women in the countries included in the PURPOSE 2 trial [42–44]. We did not specify goals for transgender men or gender nonbinary individuals due to a lack of granular data regarding HIV incidence in these populations at the local level.
To facilitate recruitment of diverse study participants at each site and achieve our study-wide recruitment goals, we also developed site-specific recruitment plans in collaboration with each site investigator and their research team. Sites have individualized recruitment goals that reflect the demographics of each locale and the HIV incidence in MSM in the local jurisdiction. Sites with declining HIV incidence in MSM (i.e., <1.5 per 100 person-years) will focus on the recruitment of TGNB individuals and those with expertise in recruiting adolescents are encouraged to focus on this age group; however, no specific age goals were set.
During the screening and enrollment phase of the trial, the study team will review the overall metrics for each demographic goal weekly. If needed, the site-specific recruitment plans and the order of site activation (regionally and globally) will be adjusted to achieve overall recruitment goals.
Training modules on GPP, gender inclusivity, and antiracism
The PURPOSE 2 study team wanted to ensure that all internal team members, external site investigators and staff, and the contract research organization and other vendors were trained on cross-cultural humility and were competent on key issues. We developed three trainings modules focused on 1) GPP; 2) cultural humility and competence in research with TGNB individuals; and 3) racism and implicit bias in clinical trials. These modules were delivered live to internal study teams and recorded and distributed broadly to all investigators, staff, and vendors. The modules were also uploaded to the study’s online portal for on-demand viewing. Completion of trainings is mandatory in the onboarding of new internal and external research staff.
Clinical trial website and online approaches
Stakeholders provided feedback on the importance of a centralized online location for participants, site investigators and staff, GCAG members, medical professionals, and the general public to access clinical trial information. The study team created a website (http://www.purposestudies.com) to address these needs and house information about the PURPOSE clinical trials. The website includes a site locator, allowing individuals with interest in participating to enter their ZIP code or location to find clinical trial sites in their area. The website will also host study updates, data presentations at scientific conferences, regulatory updates and communications, media coverage, and other study-related information. The study team developed the website to increase awareness, promote recruitment, support continued engagement, and provide updated information for all those interested in HIV prevention clinical trials.
Discussion
Equitable representation of Black and Hispanic/Latinx MSM and TGNB individuals in HIV PrEP clinical trials is imperative. These populations are the most disproportionately affected by HIV but have been historically underrepresented in clinical trials. Regulatory and funding agencies have implemented various national-level policies to improve participation of marginalized communities in research [45, 46], but these efforts have not resulted in meaningful changes [47–50]. To address the limitations of prior PrEP trials, the PURPOSE 2 study team used novel and intentional approaches to plan for engagement of these key populations.
Historical abuses and unethical treatment of minorities continue to influence people’s perceptions of research [20, 51, 52]. Individuals have described apprehensions around being experimented on and others have expressed skepticism towards investigators’ motives and whether the interventions will truly benefit their communities [20, 49, 50, 53]. In the DISCOVER trial, which demonstrated the non-inferior efficacy of emtricitabine/tenofovir alafenamide to emtricitabine/tenofovir disoproxil fumarate, the enrollment of underrepresented and disproportionately affected populations was suboptimal [48, 54]. For example, the percentage of US participants in the study who identified as Black was only 13%, while transgender women comprised less than 1% of the total study sample [48]. The disappointing number of individuals from demographically diverse backgrounds included in DISCOVER and in HIV research overall [47, 48, 55] underscore the need for a multifactorial approach to support actualized engagement with key communities.
However, focusing solely on racism, discrimination, and mistrust oversimplifies the inequities and challenges faced by racial, ethnic, gender, and sexual minorities [32]. Persons from these groups often face competing priorities, including safety, housing, employment, transportation, and mental health [53]. TGNB individuals may face these challenges in addition to others, such as access to gender-affirming care [56]. Advocates have underscored the incorporation of a social justice framework in the design and conduct of research where we acknowledge and address the intersectional oppression that drives disparities and underrepresentation [32, 57]. This motivated our team to rework our approach, collaborate with various stakeholders, and identify strategies to support better representation of communities with a disproportionate HIV burden. Numerous other frameworks that focus on different aspects of community engagement exist, including frameworks for addressing power dynamics between researchers and community members and methods for involving community in establishing research priorities [58]. These strategies to support meaningful stakeholder engagement can be leveraged and adapted to better address the needs and context of future HIV trials and support effective collaboration with community.
The study team followed the principles of GPP [23] and incorporated evidence-informed strategies identified in the literature. In PURPOSE 2, we worked closely with community and patient advocates in the GCAG, as well as with broader stakeholders, to ensure that the study design and procedures were culturally-relevant, inclusive, and responsive to the needs of the populations we wanted to engage. We set enrollment goals that are monitored weekly and shared with site investigators so that recruitment strategies and the order of site activation can be modified as needed. The study team meets internally weekly, with the site investigators and staff monthly, and with the GCAG approximately every two months to discuss study progress, including progress towards the overall demographic goals.
Beyond diversity benchmarks, we aimed to address structural and social factors by selecting research sites in high HIV incidence regions. We partnered with clinics embedded within these areas to increase access to the clinical trial and reduce the potential burdens of participation, such as transportation costs. We were also intentional in selecting sites that had established trust within their communities and with staff demographics reflective of participants. Studies examining participation of underrepresented groups in HIV studies found that individuals were more likely to feel comfortable and engage when research staff were from the same communities [32, 50]. Strategies to increase representation of TGNB individuals and equity in HIV prevention research highlighted the need to evaluate outcomes important to the TGNB community, such as drug-drug interactions between LEN and GAHT, which have been added to the study so the data will be available to guide providers and potential users of PrEP.
Our efforts do have limitations. We acknowledge that requiring diversity trainings for investigators, vendors, internal and external research staff does not always translate to cultural sensitivity and competence. Relatedly, being an investigator or research staff member who shares characteristics with a priority population does not automatically equate to site inclusivity. Most importantly, as enrollment is currently ongoing, we are not yet able to characterize the results of our efforts; but we plan to share the full demographic characteristics of the enrolled PURPOSE 2 trial and whether we achieved our demographic recruitment goals in the future.
Conclusion
Insights from implementation studies that examined barriers to PrEP uptake have underscored the importance of building trust with communities disproportionately affected by HIV [17]. Underrepresentation of racial, ethnic, and gender minorities in PrEP research have limited our ability to examine outcomes important to these communities and understand potential issues that may be unique to certain populations. Further, inadequate engagement with global stakeholders and community members that have been disproportionately impacted by HIV have hampered PrEP scale-up in key populations and limited its public health impact. Learning from past lessons, we have carefully chosen with whom, where, and how we work to increase diversity, equity, and inclusion in the PURPOSE 2 trial. While these are initial steps and further work is needed to achieve equitable representation, our efforts to increase engagement of Black and Hispanic/Latinx MSM and TGNB individuals in the PURPOSE 2 study may serve as framework for future PrEP and other clinical trials.
Acknowledgments
We thank the individuals who participated in this study and their families, the PURPOSE 2 Global Community Advisory and Accountability Group and all the PURPOSE 2 site investigators and research staff. Some of the work in this manuscript was previously presented at the 2021 IDWeek Conference (Virtual). The PURPOSE 2 study is sponsored by Gilead Sciences.
Data Availability
The manuscript does not contain any data. All relevant information are within the manuscript.
Funding Statement
This study is funded by Gilead Sciences. The funder was involved in the preparation of the manuscript and in the decision to publish through authorship by members of the study team, MD, JCH, AE, ACD, LC, CCW, CC, AK, and JMB, who are employees and shareholders of Gilead Sciences. The specific roles of the authors are articulated in the author contributions.
References
- 1.McCree DH, Williams AM, Chesson HW, Beer L, Jeffries WL, Lemons A, et al. Changes in Disparities in Estimated HIV Incidence Rates Among Black, Hispanic/Latino, and White Men Who Have Sex With Men (MSM) in the United States, 2010–2015. J Acquir Immune Defic Syndromes. 2019;81(1):57–62. [DOI] [PubMed] [Google Scholar]
- 2.Kanny D, Jeffries WL, Chapin-Bardales J, Denning P, Cha S, Finlayson T, et al. Racial/ethnic disparities in HIV preexposure prophylaxis among men who have sex with men—23 urban areas, 2017. MMWR MorbMortal Wkly Rep. 2019;68(37):801–6. doi: 10.15585/mmwr.mm6837a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Estimated HIV incidence and prevalence in the United States, 2015–2019 [Internet]. 2021. Available from: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html
- 4.Centers for Disease Control and Prevention. HIV Surveillance Report, 2019 [Internet]. 2021. Available from: https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html
- 5.Centers for Disease Control and Prevention. HIV Infection, Risk, Prevention, and Testing Behaviors Among Transgender Women—National HIV Behavioral Surveillance, 7 U.S. Cities, 2019–2020. HIV Surveillance Special Report 27. 2021. Available from: https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html
- 6.Baral SD, Poteat T, Strömdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: A systematic review and meta-analysis. Lancet Infect Dis. 2013. Mar;13(3):214–22. doi: 10.1016/S1473-3099(12)70315-8 [DOI] [PubMed] [Google Scholar]
- 7.Poteat T, Scheim A, Xavier J, Reisner S, Baral S. Global Epidemiology of HIV Infection and Related Syndemics Affecting Transgender People. J Acquir Immune Defic Syndr. 2016;72(Suppl 3):S210–9. doi: 10.1097/QAI.0000000000001087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radix A, Goldstein Z, Harris A, Vail R. HIV prevalence among transgender men at a NYC community health center. Oral abstract presented at: Conference on Retroviruses and Opportunistic Infections. 2020 March 8–11; Boston, MA. Available from: https://www.croiconference.org/abstract/hiv-prevalence-among-transgender-men-at-an-nyc-community-health-center/
- 9.Slogrove AL, Mahy M, Armstrong A, Davies M. Living and dying to be counted: What we know about the epidemiology of the global adolescent HIV epidemic. J Int Aids Soc. 2017. May 16;20(Suppl 3):21520. doi: 10.7448/IAS.20.4.21520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UNAIDS. UNAIDS Data 2021 [Internet]. Available from: https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf
- 11.Riddell J, Amico KR, Mayer KH. HIV Preexposure Prophylaxis: A Review. JAMA. 2018. Mar 27;319(12):1261–1268. doi: 10.1001/jama.2018.1917 [DOI] [PubMed] [Google Scholar]
- 12.Sullivan PS, Sanchez TH, Zlotorzynska M, Chandler CJ, Sineath R, Kahle E, et al. National trends in HIV pre‐exposure prophylaxis awareness, willingness and use among United States men who have sex with men recruited online, 2013 through 2017. J Int Aids Soc. 2020. Mar;23(3):e25461. doi: 10.1002/jia2.25461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaefer R, Schmidt H-MA, Ravasi G, Mozalevskis A, Rewari BB, Lule F, et al. Adoption of guidelines on and use of oral pre-exposure prophylaxis: a global summary and forecasting study. Lancet HIV. 2021. Aug;8(8):e502–e510. doi: 10.1016/S2352-3018(21)00127-2 Epub 2021 Jul 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pyra MN, Haberer JE, Hasen N, Reed J, Mugo NR, Baeten JM. Global implementation of PrEP for HIV prevention: setting expectations for impact. J Int Aids Soc. 2019;22(8):e25370. doi: 10.1002/jia2.25370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serota DP, Rosenberg ES, Sullivan PS, Thorne AL, Rolle C-PM, Rio C del, et al. Pre-exposure prophylaxis uptake and discontinuation among young black men who have sex with men in Atlanta, Georgia: A prospective cohort study. Clin Infect Dis. 2019;67:1147. doi: 10.1093/cid/ciz894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hojilla JC, Hurley LB, Marcus JL, Silverberg MJ, Skarbinski J, Satre DD, et al. Characterization of HIV Preexposure Prophylaxis Use Behaviors and HIV Incidence Among US Adults in an Integrated Health Care System. AMA Netw Open. 2021. Aug 2;4(8):e2122692. doi: 10.1001/jamanetworkopen.2021.22692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer KH, Agwu A, Malebranche D. Barriers to the Wider Use of Pre-exposure Prophylaxis in the United States: A Narrative Review. Adv Ther. 2020. May;37(5):1778–1811. doi: 10.1007/s12325-020-01295-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sevelius JM, Deutsch MB, Grant R. The future of PrEP among transgender women: the critical role of gender affirmation in research and clinical practices. J Int AIDS Soc. 2016;19(7(Suppl 6)):21105. doi: 10.7448/IAS.19.7.21105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrasik MP, Chandler C, Powell B, Humes D, Wakefield S, Kripke K, et al. Bridging the Divide: HIV Prevention Research and Black Men Who Have Sex With Men. Am J Public Health. 2014;104(4):708–14. doi: 10.2105/AJPH.2013.301653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman PA, Rubincam C, Slack C, Essack Z, Chakrapani V, Chuang D-M, et al. Towards a Science of Community Stakeholder Engagement in Biomedical HIV Prevention Trials: An Embedded Four-Country Case Study. PloS One. 2015;10(8):e0135937. doi: 10.1371/journal.pone.0135937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reza-Paul S, Lazarus L, Jana S, Ray P, Mugo N, Ngure K, et al. Community Inclusion in PrEP Demonstration Projects: Lessons for Scaling Up. Gates Open Res. 2019;3:1504. doi: 10.12688/gatesopenres.13042.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbasi J. Promising Early Results for Potent, Long-Acting HIV Injection. JAMA. 2020. Aug 11;324(6):539. doi: 10.1001/jama.2020.14031 [DOI] [PubMed] [Google Scholar]
- 23.UNAIDS. Good participatory practice: Guidelines for biomedical HIV prevention trials. [Internet]. 2011. Available from: https://www.unaids.org/en/resources/documents/2011/20110629_JC1853_GPP_Guidelines_2011%20OK
- 24.UNAIDS. Ethical considerations in HIV prevention trials [Internet]. 2021. Available from: https://www.unaids.org/en/resources/documents/2021/ethical-considerations-in-hiv-prevention-trials
- 25.Kubicek K, Robles M. Resource for integrating community voices into a research study: Community advisory board toolkit [Internet]. 2011. Available from: https://sc-ctsi.org/uploads/resources/CommunityAdvisoryBoard_Toolkit.pdf#asset:789 [Google Scholar]
- 26.International AIDS Vaccine Initiative. Guidance tool for community advisory boards [Internet]. 2012. Available from: https://www.iavi.org/images/Clinical_Research_Centers/IAVI_Community_Advisory_Board_Guidance_Tool.pdf
- 27.HIV Prevention Trials Network. Community advisory board fact sheet [Internet]. Available from: https://www.hptn.org/sites/default/files/2016-05/Community%20Advisory%20Board%20Fact%20Sheet.pdf
- 28.Zhao Y, Fitzpatrick T, Wan B, Day S, Mathews A, Tucker JD. Forming and implementing community advisory boards in low- and middle-income countries: A scoping review. BMC Med Ethics. 2019. Oct 17;20(1):73. doi: 10.1186/s12910-019-0409-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrasik MP, Broder GB, Wallace SE, Chaturvedi R, Michael NL, Bock S, et al. Increasing Black, Indigenous and People of Color participation in clinical trials through community engagement and recruitment goal establishment. PLoS One. 2021. Oct 19;16(10):e0258858. doi: 10.1371/journal.pone.0258858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Getz K, Faden L. Racial Disparities Among Clinical Research Investigators. Am J Ther. Jan-Feb 2008;15(1):3–11. doi: 10.1097/MJT.0b013e31815fa75a [DOI] [PubMed] [Google Scholar]
- 31.FitzGerald C, Hurst S. Implicit bias in healthcare professionals: A systematic review. BMC Med Ethics. 2017. Mar 1;18(1):19. doi: 10.1186/s12910-017-0179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dubé K, Kanazawa J, Campbell C, Boone CA, Maragh-Bass AC, Campbell DM, et al. Considerations for Increasing Racial, Ethnic, Gender, and Sexual Diversity in HIV Cure-Related Research with Analytical Treatment Interruptions: A Qualitative Inquiry. AIDS Res Hum Retroviruses. 2022. Jan;38(1):50–63. doi: 10.1089/AID.2021.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark LT, Watkins L, Piña IL, Elmer M, Akinboboye O, Gorham M, et al. Increasing Diversity in Clinical Trials: Overcoming Critical Barriers. Curr Probl Cardiol. 2019. May;44(5):148–172. doi: 10.1016/j.cpcardiol.2018.11.002 Epub 2018 Nov 9. [DOI] [PubMed] [Google Scholar]
- 34.National Academies of Sciences, Engineering, and Medicine. Strategies for Ensuring Diversity, Inclusion, and Meaningful Participation in Clinical Trials: Proceedings of a Workshop. In: Roundtable on the Promotion of Health Equity and the Elimination of Health Disparities [Internet]. Washington, DC: National Academies Press; 2016. Available from: https://www.ncbi.nlm.nih.gov/books/NBK384599/ [PubMed] [Google Scholar]
- 35.Rodriguez-Diaz CE, Martinez O, Bland S, Crowley JS. Ending the HIV epidemic in US Latinx sexual and gender minorities. Lancet. 2021. Mar 20;397(10279):1043–1045. doi: 10.1016/S0140-6736(20)32521-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The PHASES Working Group. Ending the evidence gap for pregnant women around HIV & co-infections: A call to action. [Internet]. Chapel Hill, NC; July 2020. Available from: http://www.hivpregnancyethics.org/
- 37.Glidden DV. Statistical approaches to accelerate the development of long-acting antiretrovirals for HIV pre-exposure prophylaxis. Curr Opin HIV AIDS. 2020;15(1):56–60. doi: 10.1097/COH.0000000000000589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller V. Innovative Clinical Trial Designs to Accelerate Increase in PrEP Choices. In: 11th IAS Conference on HIV Science [Internet]. 2021 Jul 18; Virtual. Available from: https://theprogramme.ias2021.org/Programme/Session/164
- 39.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV Epidemic: A Plan for the United States. JAMA. 2019. Mar 5;321(9):844–845. doi: 10.1001/jama.2019.1343 [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Ending the HIV Epidemic in the U.S. [Internet]. 2021. Available from: https://www.cdc.gov/endhiv/jurisdictions.html
- 41.Watson CC, Lucas JP, Fields SD, Wheeler DP. Identifying research gaps for Black men who have sex with men: A way forward [Internet]. 2014. Available from: https://www.hptn.org/sites/default/files/2016-05/BMSMSciGenMtgRpt%20%281%29.pdf [Google Scholar]
- 42.Castillo R, Konda KA, Leon SR, Silva-Santisteban A, Salazar X, Klausner JD, et al. HIV and Sexually Transmitted Infection Incidence and Associated Risk Factors Among High-Risk MSM and Male-to-Female Transgender Women in Lima, Peru. J Acquir Immune Defic Syndromes 1999. 2015;69(5):567–75. doi: 10.1097/QAI.0000000000000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grinsztejn B, Jalil EM, Monteiro L, Velasque L, Moreira RI, Garcia ACF, et al. Unveiling of HIV dynamics among transgender women: a respondent-driven sampling study in Rio de Janeiro, Brazil. Lancet HIV. 2017. Apr;4(4):e169–e176. doi: 10.1016/S2352-3018(17)30015-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poteat T, Ackerman B, Diouf D, Ceesay N, Mothopeng T, Odette K-Z, et al. HIV prevalence and behavioral and psychosocial factors among transgender women and cisgender men who have sex with men in 8 African countries: A cross-sectional analysis. PloS Med. 2017;14(11):e1002422. doi: 10.1371/journal.pmed.1002422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Institutes of Health. Amendment: NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research [Internet]. 2017 [cited 2021 Nov 10]. Available from: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-014.html
- 46.White House Office of National AIDS Policy. National HIV/AIDS strategy for the United States: Updated to 2020 [Internet]. 2015. Available from: https://www.hiv.gov/sites/default/files/nhas-2020-action-plan.pdf
- 47.Castillo-Mancilla JR, Cohn SE, Krishnan S, Cespedes M, Floris-Moore M, Schulte G, et al. Minorities remain underrepresented in HIV/AIDS research despite access to clinical trials. HIV Clin Trials. Jan-Feb 2014;15(1):14–26. doi: 10.1310/hct1501-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayer KH, Molina J-M, Thompson MA, Anderson PL, Mounzer KC, Wet JJD, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet. 2020. Jul 25;396(10246):239–254. doi: 10.1016/S0140-6736(20)31065-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez‐Brumer A, Naz‐McLean S, Huerta L, Salazar X, Lama JR, Sanchez J, et al. The wisdom of mistrust: qualitative insights from transgender women who participated in PrEP research in Lima, Peru. J Int Aids Soc. 2021;24(9):e25769. doi: 10.1002/jia2.25769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bass SB, D’Avanzo P, Alhajji M, Ventriglia N, Trainor A, Maurer L, et al. Exploring the Engagement of Racial and Ethnic Minorities in HIV Treatment and Vaccine Clinical Trials: A Scoping Review of Literature and Implications for Future Research. AIDS Patient Care STDS. 2020. Sep;34(9):399–416. doi: 10.1089/apc.2020.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scharff DP, Mathews KJ, Jackson P, Hoffsuemmer J, Martin E, Edwards D. More than Tuskegee: understanding mistrust about research participation. J Health Care Poor Underserved. 2010. Aug;21(3):879–97. doi: 10.1353/hpu.0.0323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fisher JA, Kalbaugh CA. Challenging assumptions about minority participation in US clinical research. Am J Public Health. 2011;101(12):2217–22. doi: 10.2105/AJPH.2011.300279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.George S, Duran N, Norris K. A Systematic Review of Barriers and Facilitators to Minority Research Participation Among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104(2):e16–31. doi: 10.2105/AJPH.2013.301706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ogbuagu O, Ruane PJ, Podzamczer D, Salazar LC, Henry K, Asmuth DM, et al. Long-term safety and efficacy of emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV-1 pre-exposure prophylaxis: week 96 results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet HIV. 2021. Jul;8(7):e397–e407. doi: 10.1016/S2352-3018(21)00071-0 [DOI] [PubMed] [Google Scholar]
- 55.Escudero DJ, Kerr T, Operario D, Socías ME, Sued O, Marshall BDL. Inclusion of trans women in pre-exposure prophylaxis trials: a review. AIDS Care. 2015;27(5):637–41. doi: 10.1080/09540121.2014.986051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poteat T, Wirtz A, Malik M, Cooney E, Cannon C, Hardy WD, et al. A Gap Between Willingness and Uptake: Findings From Mixed Methods Research on HIV Prevention Among Black and Latina Transgender Women. J Acquir Immune Defic Syndr. 2019. Oct 1;82(2):131–140. doi: 10.1097/QAI.0000000000002112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wesp LM, Malcoe LH, Elliott A, Poteat T. Intersectionality Research for Transgender Health Justice: A Theory-Driven Conceptual Framework for Structural Analysis of Transgender Health Inequities. Transgend Health. 2019. Oct 29;4(1):287–296. doi: 10.1089/trgh.2019.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenhalgh T, Hinton L, Finlay T, Macfarlane A, Fahy N, Clyde B, et al. Frameworks for supporting patient and public involvement in research: Systematic review and co‐design pilot. Health Expect. 2019;22(4):785–801. doi: 10.1111/hex.12888 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The manuscript does not contain any data. All relevant information are within the manuscript.


