Abstract
Background
Ad26.COV2.S is a well-tolerated and effective vaccine against COVID-19. We evaluated durability of anti-SARS-CoV-2 antibodies elicited by single-dose Ad26.COV2.S and the impact of boosting.
Methods
In randomized, double-blind, placebo-controlled, phase 1/2a and phase 2 trials, participants received single-dose Ad26.COV2.S (5 × 1010 viral particles [vp]) followed by booster doses of 5 × 1010 vp or 1.25 × 1010 vp. Neutralizing antibody levels were determined by a virus neutralization assay (VNA) approximately 8–9 months after dose 1. Binding and neutralizing antibody levels were evaluated by an enzyme-linked immunosorbent assay and pseudotyped VNA 6 months after dose 1 and 7 and 28 days after boosting.
Results
Data were analyzed from phase 1/2a participants enrolled from 22 July–18 December 2020 (Cohort 1a, 18–55 years [y], N = 25; Cohort 2a, 18–55y, N = 17; Cohort 3, ≥65y, N = 22), and phase 2 participants from 14 to 22 September 2020 (18–55y and ≥ 65y, N = 73). Single-dose Ad26.COV2.S elicited stable neutralizing antibodies for at least 8–9 months and stable binding antibodies for at least 6 months, irrespective of age. A 5 × 1010 vp 2-month booster dose increased binding antibodies by 4.9- to 6.2-fold 14 days post-boost versus 28 days after initial immunization. A 6-month booster elicited a steep and robust 9-fold increase in binding antibody levels 7 days post-boost. A 5.0-fold increase in neutralizing antibodies was observed by 28 days post-boost for the Beta variant. A 1.25 × 1010 vp 6-month booster elicited a 3.6-fold increase in binding antibody levels at 7 days post-boost versus pre-boost, with a similar magnitude of post-boost responses in both age groups.
Conclusions
Single-dose Ad26.COV2.S elicited durable antibody responses for at least 8 months and elicited immune memory. Booster-elicited binding and neutralizing antibody responses were rapid and robust, even with a quarter vaccine dose, and stronger with a longer interval since primary vaccination.
Trial Registration:ClinicalTrials.gov Identifier: NCT04436276, NCT04535453.
Keywords: Ad26.COV2.S, Antibody, COVID-19, Enzyme-linked immunosorbent assay, Vaccine, Virus neutralization assay
1. Introduction
Janssen’s COVID-19 vaccine, Ad26.COV2.S [1], has been authorized for prevention of COVID-19 in adults and administered to > 35 million people worldwide as of November 2021 [2]. A single dose of Ad26.COV2.S confers durable efficacy lasting 6–8 months or longer [3] and high efficacy against severe/critical COVID-19, COVID-19–related hospitalization, and death [4], with variable but durable efficacy [4] against acquisition and moderate disease caused by SARS-CoV-2 variants [5], [6]. To counteract waning immunity and protection, the US Food and Drug Administration (FDA) recommends boosters after 6 months for 2 two-dose mRNA-based vaccines [7], [8], and after at least 2 months for the single-dose Ad26.COV2.S vaccine, whose protection has remained stable [9], to increase overall protection against COVID-19.
To study the immune responses underlying lasting protection [3] we assessed the durability of immunologic responses after 1 dose of Ad26.COV2.S at a 5 × 1010 viral particle (vp) dose level in phase 1/2a and phase 2 clinical trial participants [9]. We also evaluated humoral immune responses after a 5 × 1010 vp homologous dose administered 2 or 6 months after dose 1 and after a 4-fold lower Ad26.COV2.S dose administered at 6 months.
2. Material and methods
2.1. Study participants and immunogenicity assessment
Participants received a single dose of Ad26.COV2.S (5 × 1010 vp; Janssen Pharmaceuticals) in an ongoing phase 1/2a study (COV1001, NCT04436276; Cohort 1a, aged 18–55 years; Cohort 2a, aged 18–55 years; Cohort 3, aged ≥ 65 years; Supplementary Table 1) and an ongoing phase 2 study (COV2001, NCT04535453; aged 18–55 years and ≥ 65 years; Supplementary Table 2). Ad26.COV2.S or saline placebo was administered by intramuscular injection (1 mL in the phase 1/2a study; 0.5 mL in the phase 2 study) into the deltoid muscle. Participants received homologous Ad26.COV2.S booster doses of 5 × 1010 vp either 2 or 6 months after dose 1 or 1.25 × 1010 vp 6 months after dose 1 (Supplementary Tables 1 and 2). Samples collected after a participant experienced a SARS-CoV-2 infection during the study period were excluded from immunogenicity analyses. Both studies were reviewed and approved by local/regional ethics committees and institutional review boards. All participants provided written informed consent before enrollment. The trials adhere to the principles of the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice guidelines.
Spike-binding antibody levels were assessed by an enzyme-linked immunosorbent assay (ELISA) during a 6- to 9-month follow-up after dose 1 and following a booster dose 2 or 6 months after initial vaccination. Neutralizing antibody titers were evaluated by wild-type or pseudotyped virus neutralization assays (wtVNA or psVNA) in a subset of participants from each study. Per protocols and amendments, binding antibody geometric mean concentrations (GMCs) and neutralizing antibody geometric mean titers (GMTs) were measured periodically after dose 1. Binding and neutralizing antibody levels were evaluated 7 and 28 days after boosting. Geometric mean ratio (GMR) and geometric mean increase (GMI) were determined for GMCs and GMTs at various time points. See Supplementary Materials.
2.2. SARS-CoV-2 wild-type virus neutralization assay (wtVNA)
Neutralizing antibodies capable of inhibiting wild-type virus infections were quantified using the assay developed and qualified by Public Health England. Virus stocks were derived from the Victoria/1/2020 strain (see Supplementary Material s).
2.3. Recombinant lentivirus‐based pseudotyped virus neutralization assay (psVNA)
To measure the breadth of neutralization against SARS-CoV-2 spike variants (Supplementary Fig. 1 ), neutralizing antibody titers were measured in both validated and pre-qualified psVNA against several SARS-CoV-2 spike variants of concern as described previously (see Supplementary Material s) [10].
2.4. Spike protein ELISA (S-ELISA) for B.1
SARS-CoV-2 pre-fusion spike-specific binding antibody concentrations were determined using the human SARS-CoV-2 pre-spike IgG ELISA, an indirect ELISA based on antibody/antigen interactions. See Supplementary Materials.
2.5. S-ELISA for variants
IgG binding to SARS-CoV-2 variant spike protein was measured by ELISA using directly coated recombinant and stabilized trimeric spike protein antigen based on the Wuhan-Hu-1 SARS-CoV-2 strain [11]. See Supplementary Materials .
3. Results
3.1. Study participants
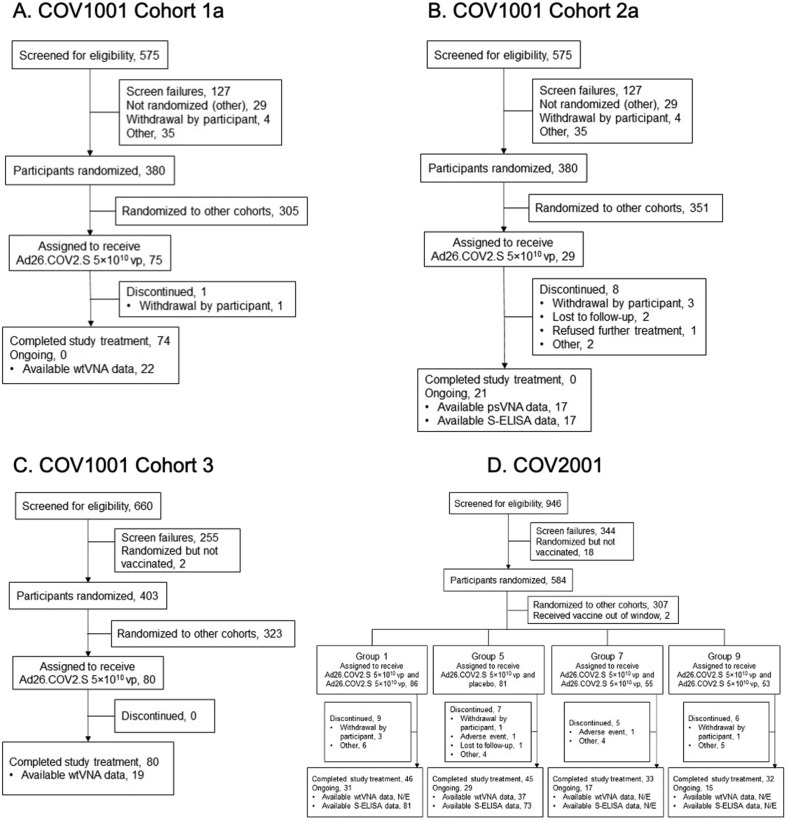
Participant disposition from each study is shown in Fig. 1; only cohorts/groups from which results were generated for this report are shown. Demographic data are in Supplementary Tables 3 and 4. In the phase 1/2a study, immunogenicity data were available for subsets of participants: wtVNA, aged 18–55 years, n = 25; ≥65 years, n = 24; psVNA and S-ELISA, 18–55 years, n = 17 (Fig. 2 .). In the phase 2 study, immunogenicity data were available for wtVNA (18–55 years, n = 22; ≥65 years, n = 15) and S-ELISA (18–55 years, n = 44–52 depending on group; ≥65 years, n = 29; Fig. 2 .); total N = 73 for serology analyses, N = 81 for safety assessments.
Fig. 1.
CONSORT diagrams for participants who received Ad26.COV2.S (phase 1/2a and phase 2 trials). Participants in (A) Cohort 1a, (B) Cohort 2a, and (C) Cohort 3 of COV1001 received a single dose of Ad26.COV2.S 5 × 1010 vp; participants in Cohort 2a received a booster dose of 5 × 1010 vp 6 months after the first vaccination. (D) Participants in COV2001 received a single dose of Ad26.COV2.S 5 × 1010 vp and a booster of 5 × 1010 vp at 2 months post-dose 1 (Group 1), placebo at 2 months post-dose 1 (Group 5), 5 × 1010 vp 1 month post-dose 1 (Group 7), and 5 × 1010 vp 3 months post-dose 1 (Group 9); all groups received a lower-dose booster of 1.25 × 1010 vp 6 months after dose 1. N/E, not evaluated; psVNA, pseudotyped virus neutralization assay; S-ELISA, spike protein enzyme-linked immunosorbent assay; vp, viral particles; wtVNA, wild-type virus neutralization assay.
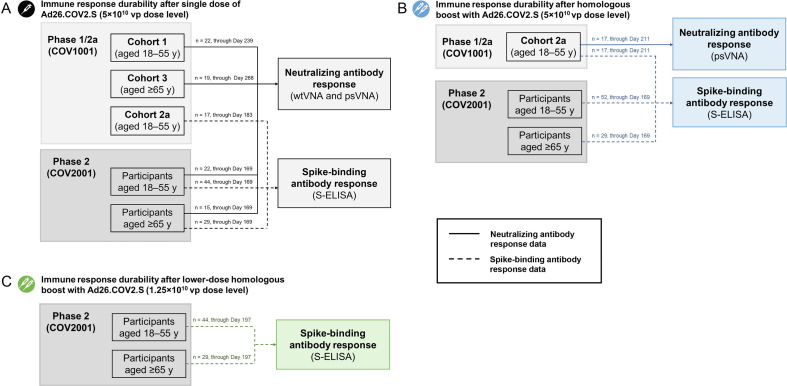
Fig. 2.
Summary of immunogenicity data according to cohort (phase 1/2a and phase 2 trials). The schematic shows available immune response durability data (neutralizing and/or binding antibody data) from each study cohort in COV1001 and COV2001. (A) For single-dose 5 × 1010 vp Ad26.COV2.S, neutralizing antibody response data were available from COV1001 Cohorts 1 and 3 and COV2001 participants aged 18–55 years and ≥ 65 years; spike-binding antibody response data were available for COV1001 Cohort 2a and both COV2001 age groups. (B) After a homologous boost with Ad26.COV2.S at the 5 × 1010 vp dose level, neutralizing antibody response data were available for COV1001 Cohort 2a; spike-binding data were available from COV1001 Cohort 2a and from both age groups of COV2001. (C) After a lower-dose boost with Ad26.COV2.S at the 1.25 × 1010 vp dose level, spike-binding antibody data were available from COV2001 participants in both age groups. The number of participants with available data (and corresponding time point) is shown above the line that indicates neutralizing or binding antibody data. psVNA, pseudotyped virus neutralization assay; S-ELISA, spike protein enzyme-linked immunosorbent assay; vp, viral particles; wtVNA, wild-type virus neutralization assay.
3.2. Durability of humoral immunity after single-dose Ad26.COV2.S (5 × 1010 vp)
Phase 1/2a
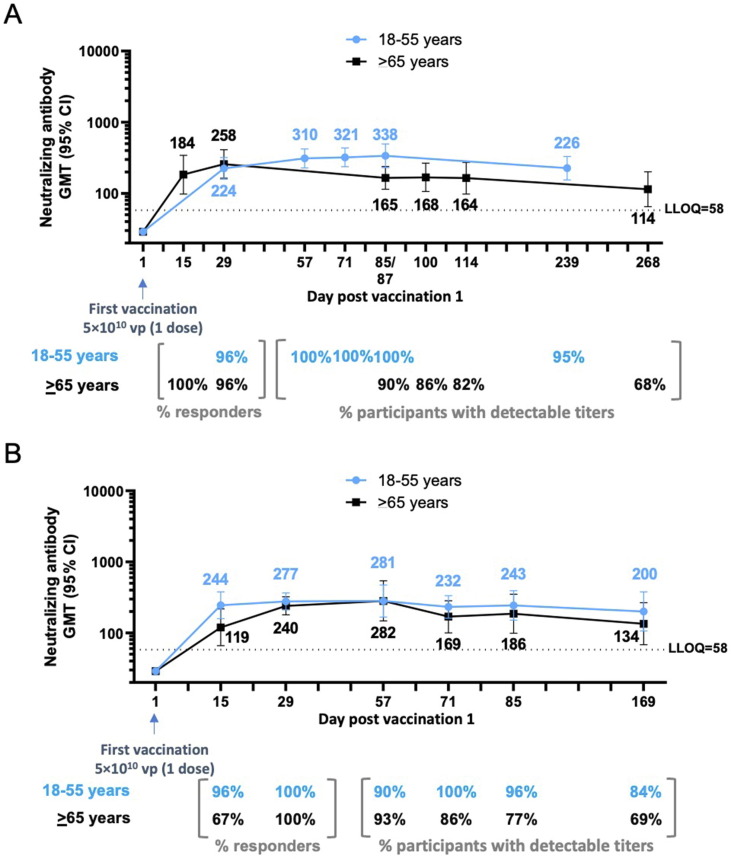
We previously reported short-term follow-up of immune responses after single-dose vaccination with Ad26.COV2.S [12]. Here, we report neutralizing antibody levels after longer follow-up in Cohort 1a and Cohort 3 (8 and 9 months follow-up, respectively). In Cohort 1a, B.1 neutralizing antibody responses were detectable up to at least Day 239 (8 months), with 21/22 (95%) of participants having detectable titers (GMT, 226; 95% confidence interval [CI], 154–331), which was similar to Day 29 after dose 1 (GMT of 224 [158–319] and 96% responders; Fig. 3 A).
Fig. 3.
Durability of neutralizing antibody responses following a single dose of Ad26.COV2.S (5 × 1010 vp) (phase 1/2a and phase 2 trials). Phase 1/2a participants, aged 18–55 years (Cohort 1a) and aged ≥ 65 years (Cohort 3), were administered a single dose of Ad26.COV2.S (5 × 1010 vp) at Day 1. (A) Serum neutralizing antibody responses against SARS-CoV-2 were evaluated by wtVNA up to 8–9 months after the dose 1, in subsets of participants aged 18–55 years (N = 25; 22 with available data at Day 239; blue line) and ≥ 65 years (N = 24; 19 with available data at Day 268; black line). (B) Phase 2 participants, aged 18–55 years and ≥ 65 years, were administered a single dose of Ad26.COV2.S (5 × 1010 vp) at Day 1. Serum neutralizing antibody responses against SARS-CoV-2 were evaluated by wtVNA up to 6 months after dose 1 in subsets of participants aged 18–55 years (N = 22; blue line) and ≥ 65 years (N = 15; black line). GMTs are depicted above each time point, and response rates are illustrated at the bottom of each panel. In all panels, error bars represent 95% CIs. CI, confidence interval; GMT, geometric mean titer; LLOQ, lower limit of quantification; vp, viral particles; wtVNA, wild-type virus neutralization assay.
In Cohort 3, B.1 neutralizing antibodies were still detectable in 13/19 (68%) participants by Day 268 (9 months) after 1 Ad26.COV2.S dose, with GMT of 114 (65–201). This represents a 2.3-fold decrease in GMTs versus Day 29 after dose 1 (GMT, 258 [163–410] and 96% responders; Fig. 3 A).
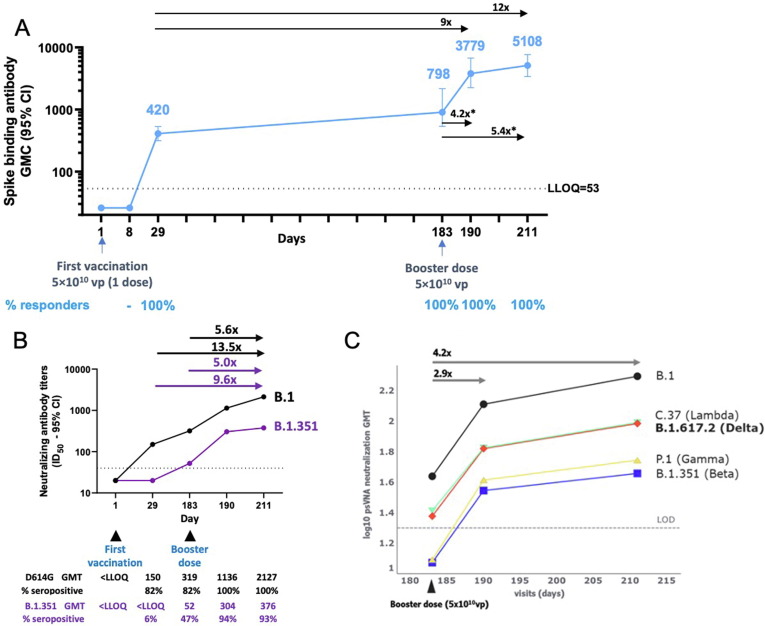
From Cohort 2a (described previously [12]), 17 participants (18–55 years of age) had detectable binding antibody levels by Day 29 post-dose 1 (GMC, 418 [322–554], with 100% responders; Fig. 4 A). By Day 183 (6 months), GMC in Cohort 2a had increased to 798 (441–1443) with 100% of participants with detectable binding antibodies (Fig. 4 A).
Fig. 4.
Durability of spike-binding antibody responses 6 months after first vaccination and impact of a 6-month booster dose on binding and neutralizing antibodies (phase 1/2a trial). Phase 1/2a participants aged 18–55 years (N = 27) were administered a single dose of Ad26.COV2.S (5 × 1010 vp) at Day 1, and 20 participants received a booster dose of Ad26.COV2.S (5 × 1010 vp) at approximately 6 months (Day 183) after the first vaccination; 17 participants had data available at Day 190. Only participants with booster data available were included in these analyses. (A) Serum spike-binding antibodies against SARS-CoV-2 were evaluated in a validated S-ELISA up to 28 days post-boost (Day 211). Participants aged 18–55 years are represented with a blue line. GMCs are depicted above each time point (error bars represent 95% CIs), and response rates are illustrated at the bottom of the panel. The asterisk denotes geometric mean increase. (B) Serum (N = 17) neutralizing antibody titers were evaluated via validated psVNA against the B.1 (D614G) reference strain and B.1.351 (Beta) variant at Days 1, 29, 183, 190, and 211. Arrows above the graph indicate GMI for Day 211 versus Day 183 and GMR for Day 211 versus Day 29. (C) Serum (N = 17) neutralizing antibody titers against the B.1 (D614G), B.1.617.2 (Delta), C.37 (Lambda), P.1 (Gamma), and Beta variants were evaluated via pre-qualified psVNA at Days 183, 190, and 211. The log10 GMTs per visit per strain were estimated in a Tobit model with subject, visit, strain, and interactions as factors. One participant who was missing data for Day 211 was excluded. With heavy censoring, the estimated GMT is below the LOD. Adjusting for censoring revealed good proportionality. The average GMT fold increase for all variants is indicated above the graph for Days 183–211 and Days 183–190. In all panels, error bars represent 95% CIs. CI, confidence interval; GMC, geometric mean concentration; GMT, geometric mean titer; ID50, serum dilution conferring 50% inhibition; LLOQ, lower limit of quantification; LOD, limit of detection; psVNA, pseudotyped virus neutralization assay; S-ELISA, spike protein enzyme-linked immunosorbent assay; vp, viral particles.
Phase 2
A single dose of Ad26.COV2.S elicited B.1 neutralizing antibody responses by Day 15 in 21/22 participants aged 18–55 years (96% responders; GMT, 244 [158–277]) and in 10/15 participants aged ≥ 65 years (67% responders; GMT, 119 [66–217]; Fig. 3 B). These responses further increased by Day 29 in both age groups (18–55 years, 100% responders and GMT, 277 [211–365]; ≥65 years, 100% responders and GMT, 240 [179–322]; Fig. 3 B).
Up to Day 85, B.1 neutralizing antibody responses remained stable in participants aged 18–55 years while they decreased modestly in participants aged ≥ 65 years. Six months after vaccination, neutralizing antibody levels in participants aged 18–55 years (GMT, 200 [106–378]; 84% with detectable titers) were in a similar range as Day 29 levels. In adults aged ≥ 65 years, the GMT of neutralizing antibody at 6 months after dose 1 was 134 (68–266), with 69% having detectable titers. These results are consistent with phase 1/2a results.
Binding antibody levels also gradually increased from baseline and remained stable up to Day 85 in both age groups (18–55 years: GMC, 572 [420–780], ≥65 years: GMC, 313 [201–486], with 98% and 96% above the LLOQ of the assay in the respective groups; Fig. 5 ). The GMC at Day 29 for those aged 18–55 years is in the same range as observed in adults aged 18–55 years in the phase 1/2a study (Fig. 4 A). GMCs in participants aged ≥ 65 years were slightly lower at all time points compared to those aged 18–55 years.
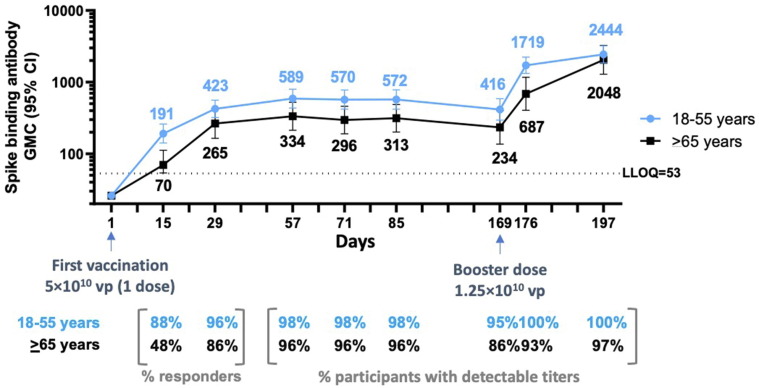
Fig. 5.
Durability of spike-binding antibody responses 6 months after first vaccination and responses 7 and 28 days after boosting (phase 2 trial). Phase 2 participants aged 18–55 years (N = 44; blue line) and ≥ 65 years (N = 29; black line) were administered a single dose of Ad26.COV2.S (5 × 1010 vp) at Day 1 and 73 received a booster dose of Ad26.COV2.S (1.25 × 1010 vp) at approximately 6 months (Day 169) after dose 1. Serum spike-binding antibody responses against SARS-CoV-2 were evaluated by validated S-ELISA up to 28 days after the booster dose (Day 197). GMCs are depicted above each time point (error bars represent 95% CIs), and response rates are illustrated at the bottom of each panel. CI, confidence interval; GMC, geometric mean concentration; LLOQ, lower limit of quantification; S-ELISA, spike protein enzyme-linked immunosorbent assay; vp, viral particles.
At 6 months after dose 1, GMCs of binding antibodies had declined to 416 (294–588) and 234 (136–403), with 96% and 86% of participants still having titers above the LLOQ of the assay in those aged 18–55 years and ≥ 65 years, respectively (Fig. 5).
3.3. Humoral immune responses after homologous boosting (5 × 1010 vp dose level)
Phase 1/2a
Participants in Cohort 2a (aged 18–55 years; N = 17) who had received 1 dose of Ad26.COV2.S at a 5 × 1010 vp dose level were given a homologous booster 6 months later. By Days 8 and 29 post-boost, all participants demonstrated respective increases (GMI) in binding antibody levels of 4.2-fold (GMC, 3779 [2583–5529]) and 5.4-fold (GMC, 5108 [3402–7669]) compared to immediate pre-boost binding antibody levels and a 9.0- and 12.0-fold GMR, respectively, compared to Day 29 binding antibody levels after the initial immunization (Fig. 4 A).
Neutralizing antibody GMTs, assessed by validated psVNA for the B.1 (D614G) reference strain and B.1.351 (Beta) variant at Day 29 after vaccination in Cohort 2a, were 150 (77–294) and < LLOQ, respectively, and increased respectively to 319 (131–779) and 52 (<LLOQ–107) by Day 183. By Day 211 (28 days post-boost), antibody levels increased 5.6- and 5.0-fold (GMI) versus pre-boost for B.1 and B.1.351, respectively (Fig. 4 B). Compared with Day 29 after dose 1, neutralizing antibodies rose 6.7- and 7.7-fold (GMR), respectively, by 7 days post-boost, and 13.5- and 9.6-fold, respectively, by 28 days post-boost.
Similar observations were made in a pre-qualified psVNA for B.1 and the B.1.351 variant. In the pre-qualified psVNA for all tested variants of concern, titers increased proportionally by 4.2-fold within 28 days post-boost (Fig. 4 C). Proportionality analyses for fold-change between time points for each variant relative to the B.1 reference demonstrated equivalence within a 1.4-fold margin (Supplementary Fig. 2 A). However, absolute titer levels were lower for some variants, including B.1.351 and P.1 (Gamma). Neutralizing (pre-qualified) and binding antibody (pre-qualified) titers correlated strongly (R = 0.92; P < 0.001) for the B.1 reference strain (Supplementary Fig. 3).
Pre-qualified S-ELISA analyses using the B.1.351 and B.1.617.2 (Delta) variants demonstrated that a booster dose 6 months after dose 1 elicited robust increases in B.1.351 and B.1.617.2 binding antibodies by 28 days after boosting. Increases relative to Day 29 after dose 1 were similar for the reference strain and these two variants (B.1, 10.5-fold; B.1.351 and B.1.617.2, both 11-fold; Supplementary Fig. 4 A–C).
Correlation analyses for the B.1 reference strain and the B.1.351 and B.1.617.2 variants at all time points for S-ELISA relative potency showed strong positive correlation across variants tested, indicating that vaccine-induced antibodies are cross-binding (Supplementary Fig. 4 D–F). Binding antibody levels and neutralizing titers for B.1.617.2 increased by > 10-fold and 3.2-fold, respectively, by 28 days after boosting, similar to increases observed for the B.1 reference strain (Supplementary Fig. 5).
In psVNA neutralization assays, GMTs for variants B.1.351, B.1.617.2, P.1, and C.37 (Lambda) were approximately 1.4- to 1.8-fold lower at Day 183, 2.0- to 3.4-fold lower at Day 190, and 2.0- to 4.2-fold lower at Day 211 versus the B.1 reference strain (Supplementary Fig. 2 B–E).
Phase 2
Participants who received 1 dose of Ad26.COV2.S (5 × 1010 vp) received a booster at 2 months at the same dose level (18–55 years of age, N = 52; ≥65 years of age, N = 29). By 14 days post-boost, binding antibody levels (validated assay) increased 3.5-fold versus immediate pre-boost levels and 4.9-fold versus Day 29 levels after dose 1 in participants aged 18–55 years (Supplementary Fig. 6 A). Binding antibody levels also increased following a booster in those aged ≥ 65 years, with an increase of 5.4-fold 14 days post-boost compared with immediate pre-boost levels and 6.2-fold compared with Day 29 levels after dose 1 (Supplementary Fig. 6 B). Responses in both age groups were durable through approximately 6 months of follow-up.
3.4. Humoral immune responses after lower-dose homologous boosting (1.25 × 1010 vp dose level)
Phase 2
A lower dose of 1.25 × 1010 vp was given at 6 months in 44 participants aged 18–55 years and 29 participants aged ≥ 65 years (Fig. 5). This lower dose also elicited a rapid 3.6-fold increase (GMR) in binding antibody levels 7 days post-boost (GMC, 1719 [1321–2236]) compared to immediate pre-boost binding antibody levels. Antibody levels further increased by 28 days post-boost (GMC, 2444 [1855–3219]), representing 6.8- and 7.3-fold increases compared to Day 29 after dose 1 and immediate pre-boost antibody levels, respectively. While the kinetics after boosting with the 1.25 × 1010 vp dose level were slower in adults aged ≥ 65 years, the magnitude of the response by 28 days later was similar in younger and older adults (Fig. 5).
3.5. Safety of a booster dose of Ad26.COV2.S
In Cohort 2 of the phase 1/2a study in 17 participants, post-dose 1 and post-booster reactogenicity appeared similar to previously reported reactogenicity [12]. In the phase 2 study, after 81 participants received dose 1 (5 × 1010 vp) and a booster dose (1.25 × 1010 vp) 6 months later, solicited adverse events (AEs) were reported, respectively, by 67.9% versus 54.1% of participants, and, for grade ≥ 3 solicited AEs, by 1.2% versus 0%. The frequencies of solicited local AEs after the first dose versus after the booster were 51.9% versus 47.3%; for solicited local AEs of grade ≥ 3, 0% versus 0%; for solicited systemic AEs, 61.7% versus 37.8%; and for solicited systemic AEs of grade ≥ 3, 1.2% versus 0%.
4. Discussion
We previously reported that a single dose of Ad26.COV2.S is immunogenic and provides robust efficacy against severe/critical COVID-19 and COVID-19–related hospitalization and death, including in areas where the Beta variant had high prevalence [4], [12]. Additionally, data from several real-world studies [13], [14], [15], [16], [17] demonstrated effectiveness of a single dose of Ad26.COV2.S against COVID-19–related hospitalization and death during a period of high prevalence of the Delta variant.
In our phase 1/2a study, after 1 dose of Ad26.COV2.S (5 × 1010 vp), neutralizing and binding antibody levels were durable in most participants. In younger participants, antibody levels at 6 months were similar to Day 29 levels, while in older adults, antibody levels showed an approximately 2-fold decline between Day 29 and Month 6 after vaccination. This durability is consistent with previous observations in a sub-cohort of our phase 1/2a study demonstrating stable humoral immune responses for 8 months after Ad26.COV2.S vaccination in adults aged 18–55 years, including against Beta and Delta variants [5].
Neither an immune correlate nor a threshold of protection is established for COVID-19 vaccines, but antibody levels have been associated with vaccine efficacy [18], [19], [20], and a vaccine manufacturer recently proposed tentative neutralizing antibody thresholds related to protection [21]. A single dose of Ad26.COV2.S in our phase 3 study induced protection against severe/critical COVID-19 by Day 8, when antibody levels were considerably lower than at Days 15–29 when protection against hospitalization and death (by Day 15), and symptomatic disease (by Days 15–29) occurred [4]. Protection against severe/critical COVID-19 may therefore require lower levels of vaccine-induced neutralizing antibodies, possibly combined with Fc functionality [22] and/or cellular immunity [4]. Antibody titers 6–8 months following immunization were similar (or slightly lower in participants aged ≥ 65 years) to those at 28 days after immunization, suggesting durable protection for at least 6–8 months, which is consistent with durable efficacy observed in our phase 3 study with longer follow-up [3], [4]. This contrasts with the waning immunity that correlated with lower efficacy observed by 6 months for mRNA-based vaccines [21], [23], [24], [25], [26].
As reported here and previously [12], a homologous booster with Ad26.COV2.S (5 × 1010 vp) at 2 months after single-dose primary vaccination elicited a rapid, approximately 3–6-fold increase in SARS-CoV-2–specific binding antibody levels and a 4-fold increase in neutralizing antibodies versus Day 29 post-prime [12]. As recently announced, in a phase 3 study, these rises were associated with a 20–25 percentage point increase in the point estimate of vaccine efficacy against symptomatic COVID-19 (including against variants), with 94% efficacy in the United States where the blinded portion of the study ended in early July 2021 [3]. A homologous booster 6 months after dose 1 produced even stronger increases in immune responses. Importantly, even in the few older adults in whom SARS-CoV-2–specific antibody titers had declined to unquantifiable levels 6 months after dose 1, the 1.25 × 1010 vp booster elicited rapid increases in antibodies to similar levels as seen in the younger age group. These anamnestic responses indicate durable memory and may explain the durable protection seen against symptomatic and severe/critical COVID-19 in our phase 3 trial [3].
A homologous booster dose given 6 months after dose 1 elicited strong increases in neutralizing antibodies against variants of concern, including Delta. Antibody levels for variants increased proportionally to the reference strain, although titers were lower overall versus the reference. Our previous analyses showed that Ad26.COV2.S-elicited spike-specific antibody binding levels that strongly correlated with SARS-CoV-2 neutralizing antibody levels [12]. The current data extend these observations and clearly demonstrate that S-ELISA and psVNA titers correlated significantly for the reference and variants tested. This robust correlation supports the notion that binding is associated with protection, as S-ELISA correlations between B.1 and variants indicate vaccine-induced antibodies are cross-binding, and psVNA is correlated with efficacy (protection).
Our data also confirm earlier observations [12], [27], [28] that repeated administration of an Ad26-based vaccine boosts immune responses, despite the possible induction of anti-vector antibodies. Ad26.COV2.S can boost immune responses after 1 dose of Ad26.COV2.S [7], [8] or either mRNA vaccine [29], [30]. Based on these data, the FDA authorized homologous and heterologous boosting with Ad26.COV2.S.
In this study, the assays used to determine the neutralization of the SARS-CoV-2 reference strain, neutralization of SARS-CoV-2 variants, and antibody binding to the SARS-CoV-2 spike protein were carefully selected based on specific criteria, such as specificity, linearity, sensitivity and repeatability. Because Ad26.COV2.S is used as a single-dose vaccine (primary plus booster), sensitivity of these assays was a key factor in their selection. For neutralization assays, the use of both wtVNA and psVNA increased confidence in the selected assays due to the concordance and correlation of their respective results.
The results presented here confirm that a wider interval between vaccine doses increases the magnitude of post-booster immune responses, as previously reported [31]. A homologous booster at 2 months with an Ad26-vectored Zika vaccine demonstrated longevity of immune responses of at least 1 year [28]. This could be similar for a homologous Ad26.COV2.S booster, especially if longer intervals between priming and boosting lead to not only higher, but even more durable responses [28].
As previously reported [12], solicited local and systemic AEs were transient and generally mild following Ad26.COV2.S (5 × 1010 vp) when given 2 months after the first dose, with less severity versus the initial dose. In the current study, boosting at 6 months at either dose level was similarly well tolerated with primarily transient mild systemic and local AEs. Grade ≥ 3 solicited local and systemic AEs were rare following dose 1, with none seen at boosting. Solicited local AEs were similar after dose 1 versus the booster (51.9% vs 47.3%), while solicited systemic AEs were lower after the booster (61.7% vs 37.8%), possibly due to dose level.
Thrombosis with thrombocytopenia syndrome (TTS) cases [32], [33] were not observed in these studies. For another adenovirus-based COVID-19 vaccine for which TTS has been reported as a side effect, ChAdOx1 nCoV-19, the risk for TTS after a second dose of that vaccine was significantly lower than after dose 1 (no longer above background incidence) [34].
5. Conclusions
Overall, our data demonstrate that a single dose of Ad26.COV2.S elicits durable immunity for at least 8 months and immune memory supporting robust anamnestic responses after boosting. The recently observed increased efficacy after a booster dose of Ad26.COV2.S with a 2-month interval [3] supports boosting after at least 2 months. However, a longer interval after primary single-dose vaccination resulted in higher immune responses after boosting. This finding, combined with the durability of protection after a single dose of Ad26.COV2.S, supports flexibility in the timing of a booster dose at least 2 months after dose 1. Additionally, such boosted immune responses translate into sustained protective efficacy, including against variants of concern. The FDA and Advisory Committee on Immunization Practices recently approved and adopted, respectively, an Ad26.COV2.S booster dose at least 2 months after primary vaccination and approved Ad26.COV2.S boosting of other COVID-19 vaccines licensed in the United States [35], [36]. Longer follow-up of immune responses after boosting will show whether durability following boosting is expected to be as long or longer than after a single dose.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: All authors are employees of Janssen Pharmaceuticals, a Johnson & Johnson company, and may hold shares in Johnson & Johnson.
Acknowledgments
Acknowledgements
The wild-type virus neutralization assays were kindly conducted by the MNA Testing Team, UK Health Security Agency, Porton Down, Salisbury, UK. Writing and editorial assistance were provided by Jill E. Kolesar, PhD, and Catherine DeBrosse, PhD, of Cello Health Communications/MedErgy, and were funded by Janssen Global Services, LLC.
Funding Statement
This study was supported by Janssen Vaccines and Prevention B.V. in collaboration with the Biomedical Advanced Research and Development Authority, the National Institutes of Health (NIH), the Department of Defense, and the COVID-19 Prevention Network. This project has been funded in whole or in part with Federal funds from the Biomedical Advanced Research and Development Authority, part of the Office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services (HHS), under contract No. HHSO100201700018C.
Author Contributions
JS, MLG, BB, VC, GS, NV, DH, CT, GS, JH, JR-G, FS, JVH, MD, and HS contributed to the study design. JS, MLG, VC, GS, and AMdG collected data. JS, MLG, BB, NV, DH, CT, MJ, KK, and JT analyzed the data. JS, MLG, BB, NV, DH, CT, MJ, KK, and JT conducted statistical analyses. All authors contributed to drafting/critically revising the manuscript for intellectual content, provided final approval of the version to be published, and agreed to be accountable for all aspects of the work. All authors had full access to all the data in the study, and BB, NV, DH, CT, MJ, and JT take responsibility for the integrity of the data and the accuracy of the data analysis.
Data Sharing Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Authorship
All authors attest they meet the ICMJE criteria for authorship.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.05.047.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Bos R., Rutten L., van der Lubbe J.E.M., Bakkers M.J.G., Hardenberg G., Wegmann F., et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines. 2020;5(1) doi: 10.1038/s41541-020-00243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Our World in Data. Statistics and research: coronavirus (COVID-19) vaccinations, https://ourworldindata.org/covid-vaccinations; [accessed 30 November 2021].
- 3.FDA Briefing Document: Janssen Ad26.COV2.S Vaccine for the Prevention of COVID-19. Vaccines and Related Biological Products Advisory Committee. https://www.fda.gov/media/146217/download. [Accessed 27 October 2021].
- 4.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barouch D.H., Stephenson K.E., Sadoff J., Yu J., Chang A., Gebre M., et al. Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccination. N Engl J Med. 2021;385(10):951–953. doi: 10.1056/NEJMc2108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jongeneelen M., Kaszas K., Veldman D., Huizingh J., van der Vlugt R., Schouten T., et al. Ad26.COV2.S elicited neutralizing activity against Delta and other SARS-CoV-2 variants of concern. bioRxiv. 2021 doi: 10.1101/2021.07.01.450707. Preprint. [DOI] [Google Scholar]
- 7.Pfizer-BioNTech. Fact sheet for healthcare providers administering vaccine (vaccination providers). Emergency use authorization (EUA) of the Pfizer-Biontech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19), https://www.fda.gov/media/153715/download; 2021 [accessed 27 October 2021].
- 8.Moderna. Fact sheet for healthcare providers administering vaccine (vaccination providers). Emergency use authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19), https://www.fda.gov/media/144637/download; 2021 [accessed 27 October 2021].
- 9.Sadoff J., Le Gars M., Cardenas V., Shukarev G., Vaissiere N., Heerwegh D., et al. Durability of antibody responses elicited by a single dose of Ad26.COV2.S and substantial increase following late boosting. medRxiv. 2021 doi: 10.1101/2021.08.25.21262569. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solforosi L., Kuipers H., Jongeneelen M., Rosendahl Huber S.K., van der Lubbe J.E.M., Dekking L., et al. Immunogenicity and efficacy of one and two doses of Ad26.COV2.S COVID vaccine in adult and aged NHP. J Exp Med. 2021;218(7):e20202756. doi: 10.1084/jem.20202756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juraszek J., Rutten L., Blokland S., Bouchier P., Voorzaat R., Ritschel T., et al. Stabilizing the closed SARS-CoV-2 spike trimer. Nat Commun. 2021;12(1) doi: 10.1038/s41467-020-20321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., de Groot A.M., et al. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021;384(19):1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grannis S.J., Rowley E.A., Ong T.C., Stenehjem E., Klein N.P., DeSilva M.B., et al. Interim estimates of COVID-19 vaccine effectiveness against COVID-19–associated emergency department or urgent care clinic encounters and hospitalizations among adults during SARS-CoV-2 B.1.617.2 (Delta) variant predominance — nine states, June–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(37):1291–1293. doi: 10.15585/mmwr.mm7037e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polinski J.M., Weckstein A.R., Batech M., Kabelac C., Kamath T., Harvey R., et al. Effectiveness of the single-dose Ad26.COV2.S COVID vaccine. medRxiv. 2021 doi: 10.1101/2021.09.10.21263385. Preprint. [DOI] [Google Scholar]
- 15.Bekker L.G., Garrett N., Goga A., Fairall L., Reddy T., Yende-Zuma N., et al. Effectiveness of the Ad26.COV2.S vaccine in health-care workers in South Africa (the Sisonke study): results from a single-arm, open-label, phase 3B, implementation study. Lancet. 2022;399:1141–1153. doi: 10.1016/S0140-6736(22)00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Gier B., Kooijman M., Kemmeren J., de Keizer N., Dongelmans D., van Iersel S.C.J.L., et al. COVID-19 vaccine effectiveness against hospitalizations and ICU admissions in the Netherlands, April-August 2021. medRxiv. 2021 doi: 10.1101/2021.09.15.21263613. Preprint. [DOI] [Google Scholar]
- 17.Rosenberg E.S., Dorabawila V., Easton D., Bauer U.E., Kumar J., Hoen R., et al. Covid-19 vaccine effectiveness in New York State. N Engl J Med. 2022;386(2):116–127. doi: 10.1056/NEJMoa2116063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 19.Earle K.A., Ambrosino D.M., Fiore-Gartland A., Goldblatt D., Gilbert P.B., Siber G.R., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39(32):4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert P.B., Montefiori D.C., McDermott A., Fong Y., Benkeser D.C., Deng W., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy trial. medRxiv. 2021 doi: 10.1101/2021.08.09.21261290. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moderna. Fact sheet for healthcare providers administering vaccine (vaccination providers): emergency use authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). https://www.fda.gov/media/144637/download; [accessed 31 January 2022]
- 22.Alter G., Yu J., Liu J., Chandrashekar A., Borducchi E.N., Tostanoski L.H., et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature. 2021;596(7871):268–272. doi: 10.1038/s41586-021-03681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pegu A., O’Connell S.E., Schmidt S.D., O’Dell S., Talana C.A., Lai L., et al. Durability of mRNA-1273 vaccine–induced antibodies against SARS-CoV-2 variants. Science. 2021;373(6561):1372–1377. doi: 10.1126/science.abj4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Widge A.T., Rouphael N.G., Jackson L.A., Anderson E.J., Roberts P.C., Makhene M., et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collier A.-RY., Yu J., McMahan K., Liu J., Chandrashekar A., Maron J.S., et al. Differential kinetics of immune responses elicited by Covid-19 vaccines. N Engl J Med. 2021;385(21):2010–2012. doi: 10.1056/NEJMc2115596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfizer. BNT162b2 [Comirnaty (COVID-19 vaccine, mRNA)]: evaluation of a booster dose (third dose). Vaccines and related biological products advisory committee briefing document 2021. https://www.fda.gov/media/152161/download; [accessed 27 October 2021]
- 27.Stephenson K.E., Wegmann F., Tomaka F., Walsh S.R., Tan C.S., Lavreys L., et al. Comparison of shortened mosaic HIV-1 vaccine schedules: a randomised, double-blind, placebo-controlled phase 1 trial (IPCAVD010/HPX1002) and a preclinical study in rhesus monkeys (NHP 17–22) Lancet HIV. 2020;7(6):e410–e421. doi: 10.1016/S2352-3018(20)30001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salisch N.C., Stephenson K.E., Williams K., Cox F., van der Fits L., Heerwegh D., et al. A double-blind, randomized, placebo-controlled phase 1 study of Ad26.ZIKV.001, an Ad26-vectored anti–Zika virus vaccine. Ann Intern Med. 2021;174(5):585–594. doi: 10.7326/M20-5306. [DOI] [PubMed] [Google Scholar]
- 29.Iketani S., Liu L., Nair M.S., Mohri H., Wang M., Huang Y., et al. A third COVID-19 vaccine shot markedly boosts neutralizing antibody potency and breadth. medRxiv. 2021 doi: 10.1101/2021.08.11.21261670. Preprint. [DOI] [Google Scholar]
- 30.Atmar R.L., Lyke K.E., Deming M.E., Jackson L.A., Branche A.R., El Sahly H.M., et al. Heterologous SARS-CoV-2 booster vaccinations – preliminary report. medRxiv. 2021 doi: 10.1101/2021.10.10.21264827. Preprint. [DOI] [Google Scholar]
- 31.Pollard A.J., Launay O., Lelievre J.-D., Lacabaratz C., Grande S., Goldstein N., et al. Safety and immunogenicity of a two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): a randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2021;21(4):493–506. doi: 10.1016/S1473-3099(20)30476-X. [DOI] [PubMed] [Google Scholar]
- 32.See I., Su J.R., Lale A., Woo E.J., Guh A.Y., Shimabukuro T.T., et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448–2456. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shay D.K., Gee J., Su J.R., Myers T.R., Marquez P., Liu R., et al. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 vaccine — United States, March–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:680–684. doi: 10.15585/mmwr.mm7018e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhuyan P., Medin J., da Silva H.G., Yadavalli M., Shankar N.K., Mullerova H., et al. Very rare thrombosis with thrombocytopenia after second AZD1222 dose: a global safety database analysis. Lancet. 2021;398(10300):577–578. doi: 10.1016/S0140-6736(21)01693-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mbaeyi S., Oliver S.E., Collins J.P., Godfrey M., Goswami N.D., Hadler S.C., et al. The Advisory Committee on Immunization Practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines — United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(44):1545–1552. doi: 10.15585/mmwr.mm7044e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.US Food and Drug Administration. Fact sheet for healthcare providers administering vaccine (vaccination providers): emergency use authorization (EUA) of the Janssen COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19), https://www.fda.gov/media/146304/download; 2021 [accessed 20 September 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.