Abstract
Background
Cannabis products, including the cannabinoids CBD and THC, are rising in popularity and increasingly used for medical purposes. While there is some evidence that cannabinoids improve cancer-associated symptoms, understanding regarding appropriate use remains incomplete.
Purpose
To describe patient experiences with medical cannabis with focus on use contexts and patients’ reported benefits and harms.
Methods
A standardized intake form was implemented in a dedicated medical cannabis clinic at an NCI-designated cancer center; data from this form was abstracted for all initial visits from October 2019 to October 2020. We report descriptive statistics, chi-square analysis, and multivariate logistic regression.
Results
Among 163 unique new patients, cannabis therapy was commonly sought for sleep, pain, anxiety, and appetite. Twenty-nine percent expressed interest for cancer treatment; 40% and 46% reported past use of CBD and THC, respectively, for medical purposes. Among past CBD users, the most commonly reported benefits were less pain (21%) or anxiety (17%) and improvement in sleep (15%); 92% reported no side effects. Among those with past THC use, reported benefits included improvement in appetite (40%), sleep (32%), nausea (28%), and pain (17%); side effects included feeling “high.” Seeking cannabis for anti-neoplastic effects was associated with receipt of active cancer treatment in both univariate and multivariate analysis.
Conclusion
Cancer patients seek medical cannabis to address a wide variety of concerns despite insufficient evidence of benefits and harms. As more states move to legalize medical and recreational cannabis, cancer care providers must remain aware of emerging data and develop knowledge and skills to counsel their patients about its use.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00520-022-07170-8.
Keywords: Cannabis, Cancer symptom management, Cannabinoids, Cannabidiol
Introduction
Cannabis, sometimes pejoratively known as marijuana, contains chemical compounds called cannabinoids, including delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD); THC is intoxicating while CBD is not. Cannabis, in multiple forms that include varied levels of THC and CBD, is rising in popularity and availability [1]. Although cannabis use remains illegal at the federal level, as of October 2021, 36 states in the USA have passed laws allowing access to medical cannabis, with 18 states and the District of Columbia also allowing adult use of recreational cannabis [2], and Canada legalized all use in October 2018. CBD-only products derived from hemp have an undetermined regulatory status but are widely available in most states [1]. In the 2013–2014 National Survey on Drug Use and Health, 19.8 million respondents aged 12 years and older (7.5%) reported using cannabis in the past month, and use by older Americans has been growing [3, 4]. Among adult users, 9.8% reported use for medical purposes [4].
In medical cannabis programs, cancer is a qualifying condition in nearly all states. There is some evidence that cannabinoids may be effective in the management of symptoms that are commonly associated with cancer, including sleep, pain, anxiety, appetite, and nausea and vomiting [5–9]. The few studies that have evaluated cannabinoids specifically in cancer populations—for chemotherapy-related nausea and vomiting and cancer pain—have focused on THC-containing products and are limited by methodological issues [10, 11]. There is even less evidence of the potential benefits and harms of CBD-only products, which are widely available and growing in popularity [12, 13]. Thus, our understanding of the safety and efficacy of cannabinoids for the management of cancer-related symptoms remains incomplete.
The frequency of and indications for cannabis use in cancer populations are not well established. The variable legal standing of cannabis, as well as challenges in administering randomized controlled trials for plant-based interventions, has contributed to a dearth of high-quality studies, though a few studies have described use patterns. A review of data from a medical cannabis dispensary in New York State found that among patients with cancer who were given cannabis, the most common indication was pain, with sublingual tincture as the preferred method of administration [14]. Another anonymous survey study of patients at a cancer center in Washington State found that inhalation or consuming edibles was the most common method of administration with use primarily for physical (pain, nausea, and appetite) and neuropsychiatric (stress, depression, and sleep) symptoms [15]. Studies in Canada prior to recreational legalization show widespread use for symptoms of pain, insomnia, nausea, and anxiety, with many hoping for anti-cancer effect [16, 17].
Amidst growing interest in cannabinoids in the medical community and the general population and shifting attitudes toward cannabis [18], clinicians must understand current cannabis use patterns among cancer patients to inform appropriate counseling and guidance. While systemic factors create barriers to performing randomized controlled trials of cannabis in the USA, observational data can help to inform practice. We set out to describe patient experiences with medical cannabis, including CBD, at a dedicated clinic at a National Cancer Institute (NCI)-designated cancer center with a focus on use contexts and patients’ reported benefits and harms.
Methods
Clinical population
The Integrative Medicine Service of the Department of Medicine at Memorial Sloan Kettering Cancer Center (MSK) established the CBD and medical cannabis clinic to counsel patients on the use of these products. The clinic began in May 2019 staffed by an Integrative Medicine physician (NJR), with most patients referred by physicians or other advanced practice providers at MSK (< 5% self-referred). The clinic visit includes symptom evaluation, education on cannabis and cannabinoids, guidance on types of products and dosing, referral to multidisciplinary services, as appropriate, and interval follow-up. At the time of the study, medical cannabis was legal in New York State; it was and remains illegal at the federal level.
Data collection
We implemented a patient-completed standardized intake form in October 2019 (Appendix A). The form requested information on use patterns, beliefs, and experiences with cannabis, differentiating THC-containing from CBD-only products. From October 2019 to March 2020, patients self-completed the form at the time of their initial visit to the medical cannabis clinic, with the information confirmed during the visit by the physician (NJR). The recall period for the form was lifetime with use for medical symptoms specified for the cancer and cancer survivorship period. In the context of the COVID-19 pandemic, starting April 2020, all visits were converted to telemedicine, and the same information was collected verbally at the beginning of each visit and documented by the physician.
Data abstraction and analysis
Data from self-completed intake forms was input into the clinical note; these data were abstracted from the notes from the first visit in the cannabis clinic for all patients seen from October 1, 2019, through October 30, 2020. We collected demographic and cancer treatment data for each participant by querying our institutional database. Data on cannabis use, experience, and beliefs was manually abstracted from clinical charts by VS and JB and independently verified by NJR. All data were entered into a REDCap database, with export to Microsoft Excel, from which we report descriptive statistics.
We performed statistical analysis using STATA software (Windows version 15.0, StataCorp LP, College Station, TX). Descriptive statistics of the demographic and clinical variables of the study participants were summarized using number and percentage. Chi-square test of independence was used to explore factors potentially associated with interest in using CBD for cancer treatment. A multivariate logistic regression model was then used to identify predictors of interest in using CBD for cancer treatment. Variables with p < 0.10 in the chi-square analyses were included in the multivariate analysis. We chose to include cancer stage into the model due to the importance of this variable. All analyses were two-sided with a p < 0.05 indicating significance. This retrospective study was approved by the MSK IRB as an exempt protocol (#17–481).
Results
Patient demographics
There were 321 clinic visits during the study period with 163 unique new patients, all of whom completed initial intake forms. Of the 163 participants, the mean age was 48 years, and roughly half were men (52%, n = 84, Table 1). Patients were predominately White (83%, n = 135) and Non-Hispanic (90%, n = 147)) with two-thirds having private insurance (67%, n = 109). Among cancer diagnoses, the largest group had sarcoma (22%, n = 36), followed by GI malignancies (16%, n = 26), with 56% (n = 91) actively receiving cancer treatment and 58% (n = 95) with stage IV or advanced disease at the time of intake form completion. The most common cancer treatment received was chemotherapy (87%). Table 1 shows full participant demographics.
Table 1.
Characteristics of Clinic Population (N = 163)
| Characteristic | N | % |
|---|---|---|
| Age | ||
| <40 | 63 | 38% |
| 40–65 | 63 | 38% |
| >65 | 37 | 23% |
| Sex | ||
| Male | 84 | 52% |
| Female | 79 | 48% |
| Race | ||
| White | 135 | 83% |
| Black or African American | 11 | 7% |
| Asian | 11 | 7% |
| Other | 1 | 1% |
| More than one race | 5 | 3% |
| Ethnicity | ||
| Hispanic | 16 | 10% |
| Non-Hispanic | 147 | 90% |
| Health insurance | ||
| Private | 109 | 67% |
| Public | 13 | 8% |
| Medicaid | 10 | 6% |
| Medicare | 31 | 19% |
| Cancer type | ||
| Breast | 20 | 12% |
| Gastrointestinal | 26 | 16% |
| Genitourinary | 20 | 12% |
| Gynecologic | 3 | 2% |
| Hematologic | 21 | 13% |
| Sarcoma | 36 | 22% |
| Thoracic | 6 | 4% |
| Prostate | 11 | 7% |
| Head and neck | 9 | 6% |
| Other | 11 | 7% |
| Cancer stage | ||
| Non-stage IV/non-advanced | 63 | 39% |
| Stage IV/advanced | 95 | 58% |
| Unknown/N/A | 5 | 3% |
| Active treatment | ||
| Yes | 91 | 56% |
| No | 72 | 44% |
| Treatment received (current or prior) | ||
| Chemotherapy | 142 | 87% |
| Radiation | 78 | 48% |
| Surgery | 96 | 59% |
| Stem cell transplant | 9 | 6% |
| Targeted/immunotherapy | 19 | 12% |
| CBD use (current or prior) | ||
| Total | 66 | 40% |
| Sublingual oil/tincture | 32 | 20% |
| Inhaled/vaping | 3 | 2% |
| Oral/edible | 19 | 12% |
| Topical | 18 | 11% |
| Unknown/other | 11 | 7% |
| Medical cannabis use (current or prior) | ||
| Total | 75 | 46% |
| Sublingual oil/tincture | 20 | 12% |
| Inhaled/vaping | 30 | 18% |
| Smoking | 11 | 7% |
| Oral/edible | 29 | 18% |
| Topical | 0 | 0% |
| Unknown/other | 10 | 6% |
Reasons for current interest in cannabinoids
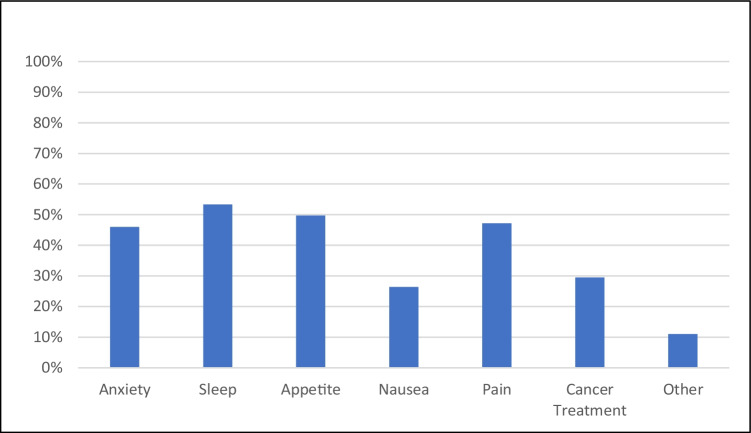
Patients reported current interest in cannabis to manage a variety of symptoms, most commonly sleep (53%), appetite (46%), pain (47%), and anxiety (46%). In addition, 48 (29%) were interested in cannabis as a treatment for their underlying cancer (Fig. 1).
Fig. 1.
Patient-reported reasons for current interest in medical cannabis
Interest in using CBD for cancer treatment
Among study participants, 48 (29.5%) were interested in using CBD for cancer treatment. There was no significant association between interest in using CBD for cancer treatment and gender, race, and cancer stage. However, age (19.1% < 40 vs. 39.7% 40–60 years old vs. 29.7% ≥ 60 years old; p = 0.040) and active treatment (37.4% in active treatment vs. 19.4% not in active treatment; p = 0.013) were found to be significantly associated with expressing interest in using CBD for cancer treatment.
In multivariate analysis, age and active treatment (yes/no) status remained significantly associated with interest in using CBD for cancer treatment. Compared to younger patients (< 40 years old), patients between 40 and 60 years old were 3.1 times more likely to be interested in using CBD for cancer treatment (OR, 3.1; 95% CI, 1.32–7.25; p = 0.009, Table 2); compared to those not currently in active treatment, those who are in active treatment were 2.27 times more likely to be interested in using CBD for cancer treatment (OR, 2.27; 95% CI, 1.06–4.86; p = 0.036).
Table 2.
Multivariate analysis of factors associated with interest in CBD use for cancer treatment
| OR | 95% CI | p value | |
|---|---|---|---|
| Age | |||
| <40 | 1 | ||
| ≥40 and <60 | 3.1 | 1.32–7.25 | 0.009 |
| ≥60 | 1.7 | 0.65–4.66 | 0.27 |
| Active treatment | |||
| No | 1 | ||
| Yes | 2.27 | 1.06–4.86 | 0.036 |
| Cancer stage | |||
| Non-stage IV/not advanced | 1 | ||
| Stage IV/advanced | 1.35 | 0.63–2.92 | 0.44 |
OR odds ratio, CI confidence interval. Bolded values indicated p < 0.05
Past experiences with cannabinoids
Forty percent (n = 66) of respondents reported use of CBD, and 46% (n = 75) reported use of THC for medical purposes prior to attending the medical cannabis clinic. The most common method of administration was sublingual tincture for CBD (n = 32, 20%) and inhalation (n = 30, 18%) and edibles (n = 29, 18%) for THC.
Of the 66 patients who reported past CBD use, the most commonly reported benefits were decrease in pain (21%) followed by decrease in anxiety (17%) and improvement in sleep (15%) (Appendix B). There was no report of improvement in appetite with the use of CBD. Twenty patients (30%) indicated experiencing no symptom benefit. While the large majority (92%) reported no side effects, the few described side effects included dry mouth, headache, hot flashes, difficulty sleeping, and feeling high.
Of the 75 patients who reported past THC use, reported benefits included improvement in appetite (40%), sleep (32%), nausea (28%), and decrease in pain (17%). Fourteen patients (19%) reported no symptomatic benefit with THC. Among past THC users, 38 (51%) had experienced side effects, most commonly feeling high (n = 30, 40%).
Discussion
This study describes past experiences with cannabis and reasons for seeking current treatment in consecutive patients seen at a cancer center medical cannabis clinic. Patients pursued cannabis treatment to relieve symptoms of insomnia, poor appetite, pain, anxiety, and others reflecting the well-described burden of symptoms in cancer patients, many of which may be undertreated [19–25]. Many patients also expressed interest in cannabis as a treatment for their underlying cancer, with active treatment status associated with more interest. There was a relationship between age and seeking cannabis as an anti-cancer therapy, with the highest rate in patients aged 40–65. This finding should be interpreted in the context of the mean age of patients in our study, which was 48, compared to a higher median age of cancer diagnosis of 66 in the USA [26]. Among past THC users, the most common reported benefits were in appetite, sleep, nausea, and pain, with about half reporting side effects. We also explored past experiences with CBD, of which the most common benefits were in pain, anxiety, and sleep, with few reported side effects. To our knowledge, this is the first study specifically describing CBD use and experience in cancer patients.
Evidence that cannabinoids improve symptoms for which patients sought care is scant. Several mostly observational studies suggest that THC-containing cannabis can improve chemotherapy-induced nausea and vomiting [5, 7, 10], though studies are generally of poor quality with few comparisons to effective anti-emetics. Studies of THC for pain have shown some effectiveness as an adjunct to opioids [27–31], with similar methodological limitations, and to our knowledge there is no evidence supporting THC use for cancer-related anxiety [32]. The mechanism of action for CBD is mediated through serotonin and cannabinoid receptor activity, possibly acting as an anxiolytic [33]. Data in support of CBD use for symptom management is even more scant [9, 32, 34], but the product’s lack of psychoactive effects make it an attractive potential therapy [35]. Notably, nearly all patients who had tried CBD reported an absence of side effects; this perceived low side-effect profile may motivate those seeking current use.
Close to one-third of the patients sought treatment to improve cancer outcomes. While public messaging has advocated cannabis as a treatment for cancer, any benefit is still theoretical, based on animal models basic science studies and incomplete evidence in humans [36–38]. The extent to which cancer patients use cannabinoids to treat their cancer has not been well described. While our study reflects a biased sample of patients referred to a cannabis clinic, the high number of patients seeking cannabinoids to treat their cancer suggests this is a common and perhaps unrecognized phenomenon more broadly. Indeed, we found an association between active treatment and interest in cannabis as an anti-cancer strategy, despite few studies of potential pharmacokinetic interactions between cannabis and conventional cancer treatments [1]. Clinicians caring for cancer patients should be aware of the popularity of cannabis to treat cancer and should ask patients about the use of these and other complementary therapies.
The wide range of symptoms for which patients sought cannabinoid treatment represents a challenge to clinicians. Surveys of cancer patients in a variety of settings have found that between 9 and 46% use medical cannabis [39–42]. High rates among patients in states that have legalized cannabis suggest that these numbers may soon grow [15]. Further, while most patients want information about cannabis from providers, very few receive such information [15]. Surveys of clinicians in the USA suggest that they are unprepared or unwilling to answer patient questions about medical cannabis, and the majority want to learn more [15, 43, 44]. The disconnect between patient interest and provider insight may drive patients to other, less reliable, sources of information [37].
Limitations
This study has important limitations. The patient population we describe was specifically referred to discuss cannabis with a physician, so their beliefs and experiences may not reflect all cannabis users with cancer. We are also limited by not having more nuanced data for patients with advanced disease (recurrence rates, referral patterns). However, we believe that the robust referral to this clinic reflects broad patient interest in cannabis as part of the management of cancer and cancer symptoms and perhaps the challenges providers face in addressing patient interest. Further, patient reports of symptom control and side effects from past use were based on recollection and self-report and may be subject to recall bias; they are also fundamentally subjective. Finally, our findings regarding the proportion of patients with past use are likely to shift over time in an evolving legal environment. Further prospective study is warranted to validate our findings and evaluate future shifts in attitudes and behaviors.
Conclusion
Cancer patients seek cannabinoid therapy in a specialized clinic for a wide variety of indications including control of common cancer-related symptoms and treatment of underlying cancer, despite insufficient evidence of benefits and harms. As more states move to legalize medical and recreational cannabis, cancer care providers must remain aware of emerging data and develop knowledge and skills to counsel their patients about its use.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Dr. Raghunathan, Dr. Mao, and Dr. Korenstein contributed to the study conception and design. Material preparation, data abstraction, and analysis were performed by Dr. Raghunathan, Swetha Vemuri, Dr. Brens, and Qing Li. The first draft of the manuscript was written by Dr. Raghunathan and Dr. Brens, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Support for this research was provided by the National Institutes of Health/National Cancer Institute grant to Memorial Sloan Kettering Cancer Center (P30 CA008748), the Translational and Integrative Medicine Research Fund at Memorial Sloan Kettering Cancer Center, and the Herbal Education and Research in Oncology Program made possible by the Laurance S. Rockefeller Fund.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. This retrospective study was approved by the MSK IRB as an exempt protocol (#17–481).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
Dr. Korenstein discloses that her spouse serves on the scientific advisory board of Vedanta Biosciences and has equity interest, serves on the scientific advisory board of PFL-NYC, and provides consulting for Fibrion. Dr. Mao reports research funding provided to MSK from Tibet Cheezheng Tibetan Medicine Company, Ltd. Dr. Raghunathan, Jessica Brens, Swetha Vemuri, and Qing Li have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pdq Integrative A, Complementary Therapies Editorial B (2002) Cannabis and cannabinoids (PDQ®): Health Professional VersionPDQ Cancer Information Summaries. National Cancer Institute (US), Bethesda (MD).
- 2.Berke J, Gal S, Lee YJ (2021) Marijuana legalization is sweeping the US: see every state where cannabis is legal.Business Insider https://www.businessinsider.com/legal-marijuana-states-2018-1. Accessed April 14, 2021.
- 3.Maxwell CJ, Jesdale BM, Lapane KL. Recent trends in cannabis use in older Americans. Ann Intern Med. 2021;174:133–135. doi: 10.7326/M20-0863. [DOI] [PubMed] [Google Scholar]
- 4.Compton WM, Han B, Hughes A, Jones CM, Blanco C. Use of marijuana for medical purposes among adults in the United States. JAMA. 2017;317:209–211. doi: 10.1001/jama.2016.18900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mersiades AJ, Tognela A, Haber PS, Stockler M, Lintzeris N, Simes J, McGregor I, Olver I, Allsop DJ, Gedye C, Kirby AC, Morton RL, Fox P, Clarke S, Briscoe K, Aghmesheh M, Wong N, Walsh A, Hahn C, Grimison P. Oral cannabinoid-rich THC/CBD cannabis extract for secondary prevention of chemotherapy-induced nausea and vomiting: a study protocol for a pilot and definitive randomised double-blind placebo-controlled trial (CannabisCINV) BMJ Open. 2018;8:e020745. doi: 10.1136/bmjopen-2017-020745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimison P, Mersiades A, Kirby A, Lintzeris N, Morton R, Haber P, Olver I, Walsh A, McGregor I, Cheung Y, Tognela A, Hahn C, Briscoe K, Aghmesheh M, Fox P, Abdi E, Clarke S, Della-Fiorentina S, Shannon J, Gedye C, Begbie S, Simes J, Stockler M. Oral THC:CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting: a randomised, placebo-controlled, phase II crossover trial. Ann Oncol. 2020;31:1553–1560. doi: 10.1016/j.annonc.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Strasser F, Luftner D, Possinger K, Ernst G, Ruhstaller T, Meissner W, Ko YD, Schnelle M, Reif M, Cerny T. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: a multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol. 2006;24:3394–3400. doi: 10.1200/JCO.2005.05.1847. [DOI] [PubMed] [Google Scholar]
- 8.Shannon S, Lewis N, Lee H, Hughes S. Cannabidiol in Anxiety and Sleep: A Large Case Series. Perm J. 2019;23:18–041. doi: 10.7812/TPP/18-041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, Kapczinski F, Quevedo J, Roesler R, Schröder N, Nardi AE, Martín-Santos R, Hallak JE, Zuardi AW, Crippa JA. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology. 2011;36:1219–1226. doi: 10.1038/npp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith LA, Azariah F, Lavender VT, Stoner NS, Bettiol S (2015) Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. The Cochrane database of systematic reviews 2015: Cd009464 [DOI] [PMC free article] [PubMed]
- 11.Meng H, Dai T, Hanlon JG, Downar J, Alibhai SMH, Clarke H. Cannabis and cannabinoids in cancer pain management. Curr Opin Support Palliat Care. 2020;14:87–93. doi: 10.1097/SPC.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 12.Mead A. The legal status of cannabis (marijuana) and cannabidiol (CBD) under U.S. law. Epilepsy Behav. 2017;70:288–291. doi: 10.1016/j.yebeh.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 13.VanDolah HJ, Bauer BA, Mauck KF. Clinicians' guide to cannabidiol and hemp oils. Mayo Clin Proc. 2019;94:1840–1851. doi: 10.1016/j.mayocp.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Kim A, Kaufmann CN, Ko R, Li Z, Han BH. Patterns of medical cannabis use among cancer patients from a medical cannabis dispensary in New York State. J Palliat Med. 2019;22:1196–1201. doi: 10.1089/jpm.2018.0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pergam SA, Woodfield MC, Lee CM, Cheng GS, Baker KK, Marquis SR, Fann JR. Cannabis use among patients at a comprehensive cancer center in a state with legalized medicinal and recreational use. Cancer. 2017;123:4488–4497. doi: 10.1002/cncr.30879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martell K, Fairchild A, LeGerrier B, Sinha R, Baker S, Liu H, Ghose A, Olivotto IA, Kerba M. Rates of cannabis use in patients with cancer. Curr Oncol. 2018;25:219–225. doi: 10.3747/co.25.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawley P, Gobbo M. Cannabis use in cancer: a survey of the current state at BC Cancer before recreational legalization in Canada. Curr Oncol. 2019;26:e425–e432. doi: 10.3747/co.26.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Green T (2021) Americans overwhelmingly say marijuana should be legal for recreational or medical use. In: Editor (ed)^(eds) Book Americans overwhelmingly say marijuana should be legal for recreational or medical use. Pew Research Center, City.
- 19.Batra A, Yang L, Boyne DJ, Harper A, Cheung WY, Cuthbert CA. Associations between baseline symptom burden as assessed by patient-reported outcomes and overall survival of patients with metastatic cancer. Support Care Cancer. 2021;29:1423–1431. doi: 10.1007/s00520-020-05623-6. [DOI] [PubMed] [Google Scholar]
- 20.Bubis LD, Davis L, Mahar A, Barbera L, Li Q, Moody L, Karanicolas P, Sutradhar R, Coburn NG. Symptom burden in the first year after cancer diagnosis: an analysis of patient-reported outcomes. J Clin Oncol. 2018;36:1103–1111. doi: 10.1200/JCO.2017.76.0876. [DOI] [PubMed] [Google Scholar]
- 21.Cuthbert CA, Boyne DJ, Yuan X, Hemmelgarn BR, Cheung WY. Patient-reported symptom burden and supportive care needs at cancer diagnosis: a retrospective cohort study. Support Care Cancer. 2020;28:5889–5899. doi: 10.1007/s00520-020-05415-y. [DOI] [PubMed] [Google Scholar]
- 22.Merchant SJ, Brogly SB, Booth CM, Goldie C, Nanji S, Patel SV, Lajkosz K, Baxter NN. Palliative care and symptom burden in the last year of life: a population-based study of patients with gastrointestinal cancer. Ann Surg Oncol. 2019;26:2336–2345. doi: 10.1245/s10434-019-07320-z. [DOI] [PubMed] [Google Scholar]
- 23.Miaskowski C, Paul SM, Snowberg K, Abbott M, Borno H, Chang S, Chen LM, Cohen B, Hammer MJ, Kenfield SA, Kober KM, Levine JD, Pozzar R, Rhoads KF, Van Blarigan EL, Van Loon K. Stress and symptom burden in oncology patients during the COVID-19 pandemic. J Pain Symptom Manage. 2020;60:e25–e34. doi: 10.1016/j.jpainsymman.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandya C, Magnuson A, Flannery M, Zittel J, Duberstein P, Loh KP, Ramsdale E, Gilmore N, Dale W, Mohile SG. Association between symptom burden and physical function in older patients with cancer. J Am Geriatr Soc. 2019;67:998–1004. doi: 10.1111/jgs.15864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou T, Yang K, Thapa S, Liu H, Wang B, Yu S. Differences in symptom burden among cancer patients with different stages of cachexia. J Pain Symptom Manage. 2017;53:919–926. doi: 10.1016/j.jpainsymman.2016.12.325. [DOI] [PubMed] [Google Scholar]
- 26.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data, 9 Registries, Nov 2020 Sub (1975–2018) - Linked To County Attributes - Time Dependent (1990–2018) Income/Rurality, 1969–2019 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2021, based on the November 2020 submission.
- 27.Inglet S, Winter B, Yost SE, Entringer S, Lian A, Biksacky M, Pitt RD, Mortensen W. Clinical data for the use of cannabis-based treatments: a comprehensive review of the literature. Ann Pharmacother. 2020;54:1109–1143. doi: 10.1177/1060028020930189. [DOI] [PubMed] [Google Scholar]
- 28.Johnson JR, Lossignol D, Burnell-Nugent M, Fallon MT. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manage. 2013;46:207–218. doi: 10.1016/j.jpainsymman.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Lichtman AH, Lux EA, McQuade R, Rossetti S, Sanchez R, Sun W, Wright S, Kornyeyeva E, Fallon MT. Results of a double-blind, randomized, placebo-controlled study of nabiximols oromucosal spray as an adjunctive therapy in advanced cancer patients with chronic uncontrolled pain. J Pain Symptom Manage. 2018;55:179–188.e171. doi: 10.1016/j.jpainsymman.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 2014;47:166–173. doi: 10.1016/j.jpainsymman.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, McQuade R, Wright S, Fallon MT. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13:438–449. doi: 10.1016/j.jpain.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Sarris J, Sinclair J, Karamacoska D, Davidson M, Firth J. Medicinal cannabis for psychiatric disorders: a clinically-focused systematic review. BMC Psychiatry. 2020;20:24. doi: 10.1186/s12888-019-2409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JLC, Bertoglio LJ, Guimarães FS, Stevenson CW. Cannabidiol regulation of emotion and emotional memory processing: relevance for treating anxiety-related and substance abuse disorders. Br J Pharmacol. 2017;174:3242–3256. doi: 10.1111/bph.13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin-Santos R, Crippa JA, Batalla A, Bhattacharyya S, Atakan Z, Borgwardt S, Allen P, Seal M, Langohr K, Farré M, Zuardi AW, McGuire PK. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Des. 2012;18:4966–4979. doi: 10.2174/138161212802884780. [DOI] [PubMed] [Google Scholar]
- 35.Good P, Haywood A, Gogna G, Martin J, Yates P, Greer R, Hardy J. Oral medicinal cannabinoids to relieve symptom burden in the palliative care of patients with advanced cancer: a double-blind, placebo controlled, randomised clinical trial of efficacy and safety of cannabidiol (CBD) BMC Palliat Care. 2019;18:110. doi: 10.1186/s12904-019-0494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seltzer ES, Watters AK, MacKenzie D, Jr., Granat LM, Zhang D (2020) Cannabidiol (CBD) as a promising anti-cancer drug. Cancers (Basel) 12 [DOI] [PMC free article] [PubMed]
- 37.Abraham A, Zhang AJ, Ahn R, Woodbridge A, Korenstein D, Keyhani S. Media Content Analysis of Marijuana's Health Effects in News Coverage. J Gen Intern Med. 2018;33:1438–1440. doi: 10.1007/s11606-018-4492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guzmán M, Duarte MJ, Blázquez C, Ravina J, Rosa MC, Galve-Roperh I, Sánchez C, Velasco G, González-Feria L. A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br J Cancer. 2006;95:197–203. doi: 10.1038/sj.bjc.6603236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macari DM, Gbadamosi B, Jaiyesimi I, Gaikazian S. Medical cannabis in cancer patients: a survey of a community hematology oncology population. Am J Clin Oncol. 2020;43:636–639. doi: 10.1097/COC.0000000000000718. [DOI] [PubMed] [Google Scholar]
- 40.Podda M, Pagani Bagliacca E, Sironi G, Veneroni L, Silva M, Angi M, Massimino M, Ferrari A, Clerici CA. Cannabinoids use in adolescents and young adults with cancer: a single-center survey. Tumori. 2020;106:281–285. doi: 10.1177/0300891620912022. [DOI] [PubMed] [Google Scholar]
- 41.Reblin M, Sahebjam S, Peeri NC, Martinez YC, Thompson Z, Egan KM. Medical cannabis use in glioma patients treated at a comprehensive cancer center in Florida. J Palliat Med. 2019;22:1202–1207. doi: 10.1089/jpm.2018.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cousins MM, Jannausch ML, Coughlin LN, Jagsi R, Ilgen MA (2021) Prevalence of cannabis use among individuals with a history of cancer in the United States. Cancer [DOI] [PubMed]
- 43.Kaplan L, Klein T, Wilson M, Graves J. Knowledge, practices, and attitudes of Washington State health care professionals regarding medical cannabis. Cannabis Cannabinoid Res. 2020;5:172–182. doi: 10.1089/can.2019.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Philpot LM, Ebbert JO, Hurt RT. A survey of the attitudes, beliefs and knowledge about medical cannabis among primary care providers. BMC Fam Pract. 2019;20:17. doi: 10.1186/s12875-019-0906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.