Abstract
Aims and objectives
Statins have been proposed as potentially useful agents for modulating the host response in COVID-19. However, solid evidence-based recommendations are still lacking. Our aim was to study the association between statin use and clinical outcomes in a large cohort of hospitalized patients with SARS-CoV-2 infection, as well as the specific consequences of chronic treatment withdrawal during hospital admission.
Material and methods
Retrospective observational study including 2191 hospitalized patients with confirmed SARS-CoV-2 infection.
Results
Mean age was 68.0±17.8 years and 597 (27.3%) patients died during follow-up. A total of 827 patients (37.7% of the whole sample), received chronic treatment with statins. Even though they underwent more frequent admissions in critical care units, chronic treatment with statins was not independently associated with all-cause mortality [HR 0.95 (0.72-1.25)]. During the whole hospital admission, 371 patients (16.9%) received at least one dose of statin. Although these patients had a significantly worse clinical profile, both treatment with statins during admission [HR 1.03 (0.78-1.35)] and withdrawal of chronic statin treatment [HR 1.01 (0.78-1.30)] showed a neutral effect in mortality. However, patients treated with statins presented more frequently hepatic cytolysis, rhabdomyolysis and thrombotic/hemorrhagic events.
Conclusions
In this large cohort of hospitalized COVID-19 patients, statins were not independently associated with all-cause mortality during follow-up. Clinically relevant statin-associated adverse effects should be carefully monitored during hospital admission.
Keywords: Statins, COVID-19, Mortality, Morbidity, Adverse effects, Treatment with drawal
Abstract
Antecedentes y objetivos
Se ha especulado que las estatinas pueden ser de utilidad en el tratamiento de pacientes con COVID-19, pero no existen evidencias clínicas sólidas. El objetivo de este trabajo es conocer su utilidad en una cohorte de gran tamaño de pacientes hospitalizados por COVID-19, así como si su retirada se asocia con un peor pronóstico.
Material y métodos
Estudio retrospectivo observacional. Se incluyeron 2.191 pacientes hospitalizados con infección confirmada con SARS-CoV-2.
Resultados
La edad media fue de 68,0 ± 17,8 años y fallecieron un total de 597 (27,3%) pacientes. Un total de 827 pacientes (37,7% de la muestra) estaban tratados previamente con estatinas. Aunque precisaron con mayor frecuencia de ingreso en camas de críticos, dicho grupo terapéutico no resultó un factor predictor independiente de muerte en el seguimiento [HR 0,95 (0,72-1,25)]. Un total de 371 pacientes (16,9%) recibió al menos una dosis de estatina durante el ingreso. A pesar de ser una población con un perfil clínico más desfavorable, tanto su uso [HR 1,03 (0,78-1,35)] como la suspensión durante el ingreso en pacientes que las recibían crónicamente [HR 1,01 (0,78-1,30)] presentaron un efecto neutro en la mortalidad. No obstante, el grupo con estatinas desarrolló con mayor frecuencia datos de citolisis hepática, rabdomiolisis y más eventos trombóticos y hemorrágicos.
Conclusiones
En nuestra muestra, las estatinas no se asociaron de forma independiente a una menor mortalidad en pacientes con COVID-19. En aquellos pacientes que tengan indicación de recibirlas por su patología previa es necesario monitorizar estrechamente sus potenciales efectos adversos durante el ingreso hospitalario.
Palabras clave: Estatinas, COVID-19, Mortalidad, Morbilidad, Efectos adversos, Retirada medicación
Introduction
The COVID-19 pandemic has caused more than three million deaths in the world's population over the past year1 and numerous authors2, 3 have identified risk factors and established cardiovascular disease as poor prognostic factors. It is precisely in these patients that therapeutic guidelines4 recommend the use of high-dose statins because of the prognostic improvement and excellent tolerability demonstrated in a multitude of randomised clinical trials.
In addition to improving the lipid profile, statins act in a multidimensional way through what are known as pleiotropic effects5. These include anti-inflammatory, antioxidant, endothelium-protective and anti-thrombotic action.6 Some of these pleiotropic actions, especially the immunomodulatory properties,7 could be the theoretical foundation of its possible usefulness in patients with COVID-19.8 The idea is attractive, especially considering its good tolerability, availability and affordability, but there is as yet no solid clinical evidence, based on properly designed clinical trials, on its usefulness in SARS-CoV-2 infections.
The aim of this study is to analyse the implications of statin treatment in patients hospitalised for COVID-19, and to assess whether its withdrawal in patients receiving it chronically is associated with a worse prognosis.
Methods
Study design and patients included: All patients with clinical suspicion of COVID-19 seen in the emergency department of a tertiary hospital from 1 March 2020 to 20 April 2020 were assessed. Only those patients with SARS-CoV-2 infection confirmed through PCR by means of nasopharyngeal swab specimens, and who ultimately required hospital admission, were included in the analysis. The Clinical Research Ethics Committee of our site approved this study. An informed consent document was not required to be completed based on national legislation for health alert scenarios. In order to avoid a selection bias as much as possible in cases of severe disease, medication withdrawal was defined as the absence of statin administration during the entire hospital stay in those patients who were chronically treated with drugs from this therapeutic group. The in-hospital administration of drugs was verified by analysing the centralized records of the Hospital Pharmacy Department of our site.
Analysed data: Epidemiological, demographic, clinical, laboratory information was collected, as well as the treatment and clinical outcomes from the electronic medical record prepared during the index admission and successive hospitalizations. In addition, the data available in the Horus system, which brings together health information from the entire network of public hospitals and primary care centres in the Community of Madrid, was analysed. All data were reviewed in detail by a team of 13 cardiologists. Particular attention was paid to the identification of cardiovascular characteristics at admission and clinical outcomes.
Statistical analysis: Categorical variables are presented as counts and percentages, while continuous variables are described as mean ± standard deviation or median (interquartile range) depending on the characteristics of the distribution. Means of continuous variables were analysed using Student's t test or the Mann-Whitney test. The normality of the distributions was analysed using the Shapiro-Wilk test. Categorical variables were analysed using the χ2 test or Fisher's exact test. Survival analysis was performed using Kaplan-Meier techniques and the log-rank test. The association of chronic treatment with statins and its withdrawal with all-cause mortality during follow-up was analysed using univariate and multivariate Cox regression techniques, selecting potential clinically relevant confounding variables (age, sex, previous cardiovascular disease, other comorbidities, clinical status at admission and treatment received during hospitalisation). All data were analysed using Stata v14.2 statistical software (StataCorp, College Station, TX, USA). A two-sided p-value < 0.05 was considered statistically significant for all analyses.
Results
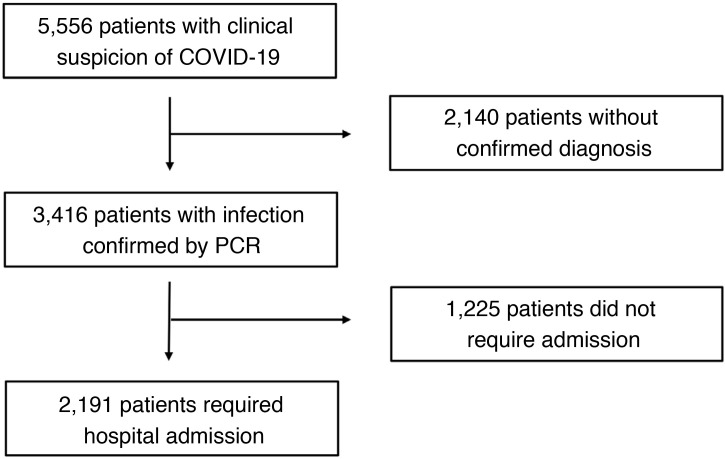
During the study period, 2,191 hospitalised patients with confirmed SARS-CoV-2 infection met the inclusion criteria and were included in the present analysis (Fig. 1 ). The mean age was 68.0 ± 17.8 years and 1,246 (56.9%) were male. During a median follow-up of 57 (27-64) days, a total of 597 (27.3%) patients died.
Fig. 1.
Flow of patients during the study period.
Patients hospitalized for COVID-19 with and without previous chronic statin treatment
A total of 827 patients previously treated with statins (37.7% of hospitalised patients with positive PCR for SARS-CoV-2) were incorporated in the analysis. The baseline characteristics of the study population are shown in Table 1 . Patients previously treated with statins were older (74.4± 12.4 vs. 64.1± 19.4; p < 0.001), had a significantly higher cardiovascular risk profile and a significantly higher rate of cardiovascular disease history (coronary, cerebrovascular and peripheral arterial disease). As a result, they received significantly more antiplatelet, anticoagulant and secondary cardiovascular prevention drug treatment. Regarding the prescription of specific drugs for COVID-19, there were significant differences in favour of greater use of glucocorticoids in the group treated with statins.
Table 1.
Baseline characteristics, cardiovascular treatment, vital signs, laboratory data and clinical outcomes of patients with and without statin treatment prior to hospital admission for COVID-19.
| Variable | All patients (n = 2191) | Without chronic statin treatment (n = 1364) | With chronic statin treatment (n = 827) | p-value |
|---|---|---|---|---|
| Baseline characteristics and comorbidities | ||||
| Age (years) | 68,0 ± 17,8 | 64,1 ± 19,4 | 74,4 ± 12,4 | < 0.001 |
| Males (%) | 1,246 (56.9) | 734 (54.2) | 507 (61.3) | < 0.001 |
| Hypertension (%) | 1,150 (52.5) | 567 (41.6) | 583 (70.5) | < 0.001 |
| Diabetes (%) | 486 (22.2) | 184 (13.5) | 302 (33.5) | < 0.001 |
| Dyslipidemia (%) | 942 (43.0) | 183 (13.4) | 759 (91.8) | < 0.001 |
| Smoking (%) | 239 (10.9) | 120 (8.8) | 119 (14.4) | < 0.001 |
| Obesity (%) | 347 (15.8) | 197 (14.4) | 150 (18.1) | 0,022 |
| Peripheral arterial disease (%) | 181 (8.3) | 35 (2,6) | 146 (17.7) | < 0.001 |
| Ischemic stroke (%) | 171 (7.8) | 66 (4.8) | 105 (12.7) | < 0.001 |
| Coronary artery disease (%) | 183 (8.4) | 26 (1.9) | 157 (19.0) | < 0.001 |
| Atrial fibrillation/flutter (%) | 250 (11.4) | 112 (8.2) | 138 (16.7) | < 0.001 |
| COPD (%) | 217 (9.9) | 104 (7.6) | 113 (13.7) | < 0.001 |
| Chronic kidney diseasea (%) | 166 (7.6) | 70 (5.1) | 96 (11.6) | < 0.001 |
| Cancer (%) | 255 (11.6) | 140 (10.3) | 115 (13.9) | 0,010 |
| Cardiovascular treatment prior to hospital admission | ||||
| Anticoagulation (%) | 283 (12.9) | 126 (9.2) | 157 (19.0) | < 0.001 |
| Antiplatelet agent(%) | 392 (17.9) | 123 (9.0) | 269 (32.5) | < 0.001 |
| ACEI or ARB (%) | 865 (39.5) | 385 (28.2) | 480 (58.0) | < 0.001 |
| Aldosterone antagonist (%) | 87 (4.0) | 31 (2.3) | 56 (6.8) | < 0.001 |
| Sacubitril/valsartan (%) | 12 (0.6) | 1 (0.1) | 11 (1.3) | < 0.001 |
| Beta-blocker (%) | 355 (16.2) | 129 (9.5) | 226 (27.3) | < 0.001 |
| Diuretics (%) | 569 (26.0) | 263 (19.3) | 306 (37.0) | < 0.001 |
| iSGLT-2 (%) | 34 (1.6) | 8 (0.6) | 26 (3.4) | < 0.001 |
| Digoxin (%) | 21 (1.0) | 10 (0.7) | 11 (1.3) | 0,164 |
| First measurement of vital signs | ||||
| SBP (mmHg) | 129.1 ± 21.7 | 128,4 ± 21,2 | 130,1 ± 22,3 | 0,091 |
| Heart rate (bpm) | 92,7 ± 19,6 | 94,8 ± 20,3 | 89,4 ± 18,0 | < 0.001 |
| First SpO2 (%) | 91,2 ± 6,5 | 91,8 ± 6,0 | 90,2 ± 7,1 | < 0.001 |
| O2 support at time of first saturation (%) | 265 (12.1%) | 146 (10.7%) | 119 (14.4%) | 0,012 |
| First chest x-ray | ||||
| Without pneumonia (%) | 298 (13.8) | 176 (12.9) | 122 (14.8) | 0,506 |
| Unilateral pneumonia (%) | 438 (20.3) | 275 (20.2) | 163 (19.7) | |
| Bilateral pneumonia (%) | 1,422 (65.9) | 889 (65.2) | 533 (64.5) | |
| Laboratory data | ||||
| Median GFR (mL/min/1.73 m2) | 74,2 ± 21,6 | 78,1 ± 19,2 | 68,0 ± 23,7 | < 0.001 |
| Haemoglobin median (g/dL) | 13,3 ± 1,8 | 13,5 ± 1,8 | 13,1 ± 1,9 | < 0.001 |
| Maximum ferritin value (ng/dL) | 729 (338-1427) | 707 (313-1421) | 762 (372-1453) | 0,010 |
| Maximum Dimer D value (ng/mL) | 1,170 (600-3,875) | 1,062 (553-3,330) | 1,560 (746-4,407) | < 0.001 |
| Maximum Troponin value (ng/L) | 8.0 (2.8-34.9) | 6.2 (2.5-39.7) | 12.6 (5.2-48.3) | < 0.001 |
| Maximum NT-proBNP value (pg/mL) | 1149 (287-4831) | 874 (178-3651) | 1,722 (440-6254) | < 0.001 |
| Maximum lactate value (mmol/L) | 2,45 ± 1,16 | 2,54 ± 1,24 | 2,36 ± 1,06 | 0,239 |
| Peak AST value (IU/L) | 50 (33-83) | 47 (29-74) | 60 (39-93) | 0,023 |
| Maximum ALT value (IU/L) | 44 (25-87) | 45 (25-91) | 42 (24-78) | 0,276 |
| Maximum LDH value (IU/L) | 372 (299-486) | 365 (294-475) | 383 (308-505) | 0,004 |
| Maximum CPK value (IU/L) | 113 (66-230) | 90 (50-186) | 122 (77-251) | < 0.001 |
| Lowest value of prothrombin activity (%) | 79,4 ± 24,5 | 81,5 ± 23,8 | 75,9 ± 25,3 | < 0.001 |
| Maximum fibrinogen value (mg/dL) | 862,5 ± 264,1 | 841,9 ± 269,6 | 896,3 ± 251,4 | < 0.001 |
| Maximum CRP value (mg/L) | 124.6 (58.0-214.4) | 117.1 (51.6-207.8) | 140.2 (69.7-223.9) | < 0.001 |
| Maximum IL-6 value (pg/mL) | 58.1 (17.9-373.0) | 50.7 (18.1-330.0) | 72.7 (17.6-373.0) | 0,040 |
| Specific treatment for COVID-19 | ||||
| Hydroxychloroquine (%) | 1,997 (91.2) | 1,236 (90.6) | 761 (92.0) | 0,262 |
| Lopinavir/ritonavir (%) | 307 (14.0) | 193 (14.2) | 114 (13.8) | 0,812 |
| Azithromycin (%) | 1,252 (57.1) | 783 (57.4) | 469 (56.7) | 0,750 |
| Tocilizumab (%) | 223 (10.2) | 133 (9.8) | 90 (10.9) | 0,396 |
| Glucocorticoids (%) | 435 (19.9) | 240 (17.6) | 195 (23.6) | < 0.001 |
| Clinical outcomes | ||||
| Acute heart failure (%) | 76 (3.5) | 34 (2.5) | 42 (5.1) | < 0.001 |
| Pulmonary embolism (%) | 73 (3.3) | 45 (3.3) | 28 (3.4) | 0,913 |
| Thrombotic eventb (%) | 112 (5.1) | 63 (4.6) | 49 (5.9) | 0,178 |
| Any bleeding (%) | 86 (3.9) | 49 (3.6) | 37 (4.5) | 0,294 |
| Major bleedingc (%) | 22 (1.0) | 10 (0.7) | 12 (1.5) | 0,102 |
| Atrial fibrillation/flutter during admission (%) | 83 (3.8) | 31 (2.3) | 52 (6.3) | < 0.001 |
| Ventricular arrhythmias during admission (%) | 11 (0.5) | 7 (0.5) | 4 (0.5) | 1.000 |
| Critical care admission (%) | 177 (8.1) | 93 (6.8) | 84 (10.2) | 0,004 |
| Mechanic ventilation (%) | 169 (7.7) | 86 (6.4) | 83 (10.2) | < 0.001 |
| Death (%) | 597 (27.3) | 303 (22.2) | 294 (35.5) | < 0.001 |
COPD: chronic obstructive pulmonary disease; ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker; SGLT2i: sodium-glucose cotransporter 2 inhibitors; SBP: systolic blood pressure; SpO2: oxygen saturation; O2: oxygen; GFR: glomerular filtration rate; NT-proBNP: N-terminal prohormone of brain natriuretic peptide; AST: aspartate aminotransferase; ALT: alanine aminotransferase; CPK: creatine phosphokinase; CRP: C-reactive protein; IL-6 interleukin 6.
Values express n (%) for qualitative variables or mean ± standard deviation or median (interquartile range) for continuous variables based on the characteristics of the distribution.
Chronic kidney disease was defined as a GFR < 60 mL/min/1.73 m2 for three months or more.
Thrombotic event was defined as the incidence of stroke, acute coronary syndrome, acute arterial ischemia in lower limbs, deep vein thrombosis and pulmonary thromboembolism.
Indicates major bleeding according to the TIMI classification.
The presentation in patients on statins was slightly more severe, with a lower initial oxygen saturation (90.2 ± 7.1% vs. 91.8 ± 6.0%; p < 0.001), although with a similar prevalence of bilateral pneumonia (64.5 vs. 65.2%; p = 0.506).
Regarding laboratory values, the cohort treated with statins had a lower glomerular filtration rate (68.0 ± 23.7 vs. 78.1 ± 19.2 mL/min; p < 0.001) and lower haemoglobin values. Inflammatory markers (ferritin, fibrinogen, C-reactive protein and IL-6) as well as cardiac biomarkers of stress and myocardial damage (NT-proBNP, troponin) and D-dimer were significantly higher. Laboratory parameters that may be related to adverse reactions of statins were also significantly higher in patients previously treated with these drugs (AST, LDH, CPK, and lower prothrombin activity).
The statin group had a higher number of episodes of acute heart failure (5.1 vs. 2.5%; p < 0.001) and previously non-existent atrial fibrillation/flutter (6.3 vs. 2.3%; p < 0.001) and the same number of thrombotic events (defined as the sum of deep vein thrombosis, pulmonary embolism, acute coronary syndrome, stroke and acute lower limb arterial ischemia) compared to the cohort without statins (5.9 vs. 4.6%; p = 0.178).
Patients on statins required admission to critical care (10.2 vs. 6.8%; p = 0.004) and mechanical ventilation more frequently and had a higher mortality rate (35.5 vs. 22.2%; p < 0.001). However, when this medical history was adjusted for other potential confounders (Supplementary Table 1), it was not an independent predictor of death during follow-up [HR 0.95 (0.72-1.25)].
Patients hospitalised for COVID-19 with and without inpatient statin therapy
A total of 371 patients (16.9%) received at least one dose of statin therapy (69.5% of them atorvastatin with a mean dose of 38.6 ± 23.5 mg and 34% with simvastatin with a mean dose of 23.0 ± 12.2 mg). A total of 13 patients (3.5%) received non-simultaneously both types of statins during admission.
The mean age of the group that received statins was 73.6 ± 11.9 years. Cardiovascular risk factors, systemic atherosclerosis, manifested as chronic ischaemic heart disease, ischaemic stroke or peripheral arterial disease, associated comorbidities, inflammatory markers, cardiac biomarkers, as well as cardiovascular treatments and COVID-19 specific drugs were significantly higher than in hospitalised COVID-19 patients who did not receive statins (Table 2 ). Again, laboratory parameters linked to potential statin-associated adverse reactions were also significantly higher (AST, LDH, CPK, lower prothrombin activity).
Table 2.
Baseline characteristics, cardiovascular treatment, vital signs, laboratory data and clinical outcomes of the patients according to hospital treatment for COVID-19 with and without statins.
| Variable | All patients (n = 2191) | No statins during hospitalization (n = 1820) | Hospital treatment with statins (n = 371) | p-value |
|---|---|---|---|---|
| Baseline characteristics and comorbidities | ||||
| Age (years) | 68.0 ± 17.8 | 66.9 ± 18.5 | 73.6 ± 11.9 | < 0.001 |
| Males (%) | 1.246 (56.9) | 1.016 (55.8) | 230 (62.0) | 0.029 |
| Hypertension (%) | 150 (52.5) | 892 (49.0) | 258 (69.5) | < 0.001 |
| Diabetes (%) | 486 (22.2) | 360 (19.8) | 26 (34.0) | < 0.001 |
| Dyslipidemia (%) | 942 (43.0) | 620 (34.1) | 322 (86.8) | < 0.001 |
| Smoking (%) | 239 (0.9) | 184 (10.1) | 55 (14.8) | 0.008 |
| Obesity (%) | 347 (15.8) | 275 (15.1) | 72 (19.4) | 0.039 |
| Peripheral arterial disease (%) | 181 (8.3) | 114 (6.3) | 67 (18.1) | < 0.001 |
| Ischemic stroke (%) | 171 (7.8) | 131 (7.2) | 40 (10.8) | 0.017 |
| Coronary artery disease (%) | 183 (8.4) | 105 (5.8) | 78 (21.0) | < 0.001 |
| Atrial fibrillation/flutter (%) | 250 (11.4) | 184 (10.1) | 66 (17.8) | < 0.001 |
| COPD (%) | 217 (9.9) | 168 (9.2) | 49 (13.2) | 0.019 |
| Chronic kidney diseasea (%) | 166 (7.6) | 127 (7.0) | 39 (10.5) | 0.019 |
| Cancer (%) | 255 (11.6) | 212 (11.7) | 43 (11.6) | 0.975 |
| Cardiovascular treatment prior to hospital admission | ||||
| Anticoagulation (%) | 283 (12.9) | 202 (11.1) | 81 (21.8) | < 0.001 |
| Antiplatelet agent(%) | 392 (17.9) | 277 (15.2) | 115 (31.0) | < 0.001 |
| ACEI or ARB (%) | 865 (39.5) | 653 (35.9) | 212 (57.1) | < 0.001 |
| Aldosterone antagonist (%) | 87 (4.0) | 60 (3.3) | 27 (7.3) | < 0.001 |
| Sacubitril/valsartan (%) | 12 (0.6) | 6 (0.3) | 6 (1.6) | 0.008 |
| Beta-blocker (%) | 355 (16.2) | 245 (13.5) | 110 (29.7) | < 0.001 |
| Diuretics (%) | 569 (26.0) | 420 (23.1) | 149 (40.2) | < 0.001 |
| iSGLT-2 (%) | 34 (1.6) | 22 (1.2) | 12 (3.2) | 0.004 |
| Digoxin (%) | 21 (1.0) | 15 (0.8) | 6 (1.6) | 0.153 |
| Statins (%) | 769 (35.1) | 456 (25.1) | 313 (84.4) | < 0.001 |
| First measurement of vital signs | ||||
| SBP (mmHg) | 129.1 ± 21.7 | 128.8 ± 21.6 | 130.1 ± 22.1 | 0.327 |
| Heart rate (bpm) | 92.7 ± 19.6 | 93.5 ± 19.7 | 89.1 ± 18.5 | < 0.001 |
| First SpO2 (%) | 91.2 ± 6.5 | 91.4 ± 6.3 | 90.5 ± 7.4 | 0.019 |
| O2 support at time of first saturation (%) | 265 (12.1) | 204 (11.2) | 61 (16.4) | 0.007 |
| First chest x-ray | ||||
| Without pneumonia (%) | 298 (13.8) | 240 (13.4) | 58 (15.8) | 0.108 |
| Unilateral pneumonia (%) | 438 (20.3) | 377 (21.1) | 61 (16.6) | |
| Bilateral pneumonia (%) | 422 (65.9) | 173 (65.5) | 249 (67.7) | |
| Laboratory data | ||||
| Median GFR (mL/min/1.73m2) | 74.2 ± 21.6 | 75.3 ± 21.2 | 69.4 ± 22.9 | < 0.001 |
| Haemoglobin median (g/dL) | 13.3 ± 1.8 | 13.4 ± 1.8 | 12.9 ± 1.9 | < 0.001 |
| Maximum ferritin value (ng/dL) | 729 (338-1427) | 675 (310-1368) | 971 (508-1573) | 0.027 |
| Maximum D-dimer value (ng/mL) | 1.170 (600-3875) | 1.084 (571-3337) | 1912 (809-10400) | 0.011 |
| Maximum Troponin value (ng/L) | 8.0 (2.8-34.9) | 6.5 (2.5-24.3) | 20.4 (5.9-91.6) | 0.003 |
| Maximum NT-proBNP value (pg/mL) | 1.149 (287-4831) | 1.016 (249-4243) | 1.762 (499-7917) | 0.002 |
| Maximum lactate value (mmol/L) | 2.45 ± 1.16 | 2.54 ± 1.22 | 2.20 ± 0.9 | 0.066 |
| Peak AST (IU/L) | 50 (33-83) | 49 (32-81) | 54.4 (38-95) | < 0.001 |
| Maximum ALT value (IU/L) | 44 (25-87) | 40 (22-81) | 49 (31-93) | < 0.001 |
| Maximum LDH value (IU/L) | 372 (299-486) | 363 (294-475) | 407 (332-528) | < 0.001 |
| Maximum CPK value (IU/L) | 113 (66-230) | 110 (65-215) | 132 (69-342) | < 0.001 |
| Lowest value of prothrombin activity (%) | 79.4 ± 24.5 | 80.5 ± 24.1 | 74.1 ± 6.1 | < 0.001 |
| Maximum fibrinogen value (mg/dL) | 862.5 ± 264.1 | 852.4 ± 265.6 | 911.2 ± 251.3 | < 0.001 |
| Maximum CRP value (mg/L) | 124.6 (58.0-214.4) | 120.0 (54.2-209.1) | 149.3 (70.2-240.1) | < 0.001 |
| Maximum IL-6 value (pg/mL) | 58.1 (17.9-373.0) | 54.6 (17.7-326.0) | 69.9 (18.6-426.5) | 0.003 |
| Specific treatment for COVID-19 | ||||
| Hydroxychloroquine (%) | 1.997 (91.2) | 1.641 (90.2) | 356 (96.0) | < 0.001 |
| Lopinavir/ritonavir (%) | 307 (14.0) | 267 (14.7) | 40 (10.8) | 0.049 |
| Azithromycin (%) | 1.252 (57.1) | 1.021 (56.1) | 231 (62.3) | 0.029 |
| Tocilizumab (%) | 223 (10.2) | 174 (9.6) | 49 (13.2) | 0.034 |
| Glucocorticoids (%) | 435 (19.9) | 334 (18.4) | 101 (27.2) | < 0.001 |
| Clinical outcomes | ||||
| Acute heart failure (%) | 76 (3.5) | 62 (3.4) | 14 (3.8) | 0.725 |
| Pulmonary embolism (%) | 73 (3.3) | 52 (2.9) | 21 (5.7) | 0.006 |
| Thrombotic eventb (%) | 112 (5.1) | 78 (4.3) | 34 (9.2) | < 0.001 |
| Any bleeding (%) | 86 (3.9) | 62 (3.4) | 24 (6.5) | 0.004 |
| Major bleedingc (%) | 22 (1.0) | 11 (0.6) | 11 (3.0) | < 0.001 |
| Fibrillation/flutter atrial during admission (%) | 83 (3.8) | 57 (3.1) | 26 (7.0) | < 0.001 |
| Ventricular arrhythmias during admission (%) | 11 (0.5) | 9 (0.5) | 2 (0.5) | 0.500 |
| Critical care admission (%) | 177 (8.1) | 122 (6.7) | 55 (14.8) | < 0.001 |
| Mechanic ventilation (%) | 169 (7.7) | 115 (6.3) | 54 (14.6) | < 0.001 |
| Death (%) | 597 (27.3) | 470 (25.8) | 127 (34.2) | 0.001 |
COPD: chronic obstructive pulmonary disease; ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker; SGLT2i: sodium-glucose cotransporter 2 inhibitors; SBP: systolic blood pressure; SpO2: oxygen saturation; O2: oxygen; GFR: glomerular filtration rate; NT-proBNP: N-terminal prohormone of brain natriuretic peptide; AST: aspartate aminotransferase; ALT: alanine aminotransferase; CPK: creatine phosphokinase; CRP: C-reactive protein; IL-6 interleukin 6.
Values express n (%) for qualitative variables or mean ± standard deviation or median (interquartile range) for continuous variables based on the characteristics of the distribution.
Chronic kidney disease was defined as a GFR < 60 mL/min/1.73 m2 for three months or more.
Thrombotic event was defined as the incidence of stroke, acute coronary syndrome, acute arterial ischemia in lower limbs, deep vein thrombosis and pulmonary thromboembolism.
Indicates major bleeding according to the TIMI classification.
There was a greater tendency to be admitted to the critical care unit (14.8 vs. 6.7%; p < 0.001) and mechanical ventilation (14.6 vs. 6.3%; p < 0.001). Regarding the analysis of mortality in the group with COVID-19 infection and statin administration, it was very high and significantly higher than those who did not receive statins (34.2 vs. 25.8%; p = 0.001).
A multivariate analysis adjusted for all relevant covariates (Supplementary Table 2) showed that statin therapy during admission was not an independent factor for mortality [HR 1.03 (0.78-1.35)].
Impact of statin withdrawal in patients with COVID-19
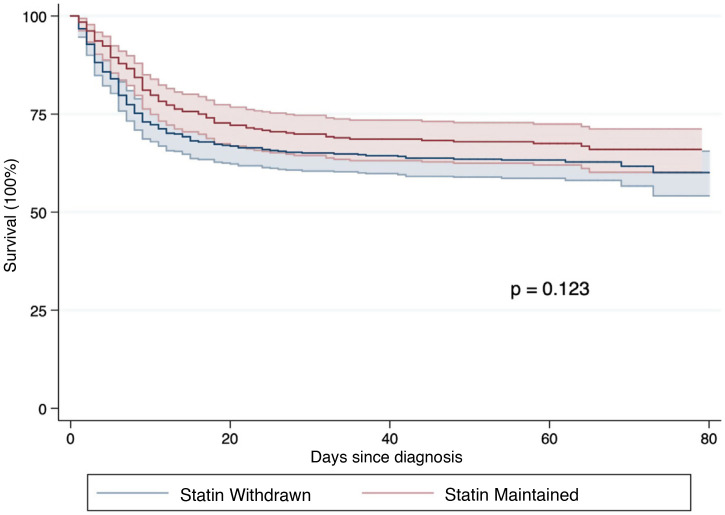
Regarding the withdrawal of statins, a total of 456 (55.1%) patients previously medicated with these drugs did not receive them during admission. The differences between patients who discontinued and those who maintained treatment with statins are shown in Table 3 . There were no relevant differences in the baseline characteristics of the two groups. Laboratory differences do show significant variations in parameters linked to the adverse reactions to statins (AST, ALT, LDH, CPK) in the group receiving statins during hospital admission. Patients who were maintained on statin therapy had more thrombotic events, including pulmonary embolism and more bleeding complications. There was also a greater tendency to be admitted to the critical care unit (14.8 vs. 6.4%; p < 0.001), but with no difference in mortality (34.2 vs. 36.6%; p = 0.469). Survival analysis using Kaplan-Meier techniques (p = 0.123, according to the log-rank test, Fig. 2 ) and Cox regression (Supplementary Table 3), showed that its discontinuation was not associated with a higher incidence of mortality [HR 1.01 (0.78-1.30)].
Table 3.
Baseline characteristics, cardiovascular treatment, vital signs, laboratory data and clinical outcomes of patients according to withdrawal or non-withdrawal of statins during hospital admission by COVID-19.
| Variable | All patients with chronic statin treatment (n = 827) | Statins withdrawn on admission (n = 456) | Statins maintained at admission (n = 371) | p |
|---|---|---|---|---|
| Baseline characteristics and comorbidities | ||||
| Age (years) | 74.4 ± 12.4 | 75.1 ± 12.7 | 73.6 ± 11.9 | 0.081 |
| Male (%) | 507 (61.3) | 277 (60.8) | 230 (62.0) | 0.714 |
| Hypertension (%) | 583 (70.5) | 325 (71.3) | 258 (69.5) | 0.560 |
| Diabetes (%) | 302 (36.5) | 176 (38.6) | 126 (34.0) | 0.119 |
| Dyslipidemia (%) | 759 (91.8) | 437 (95.8) | 322 (86.8) | < 0.001 |
| Smoking (%) | 119 (14.4) | 64 (14.0) | 55 (14.8) | 0.748 |
| Obesity (%) | 150 (18.1) | 78 (7.1) | 72 (19.4) | 0.393 |
| Peripheral arterial disease (%) | 146 (17.7) | 79 (17.3) | 67 (18.1) | 0.795 |
| Ischemic stroke (%) | 105 (12.7) | 65 (14.3) | 40 (10.8) | 0.136 |
| Coronary artery disease (%) | 157 (19.0) | 79 (17.3) | 78 (21.0) | 0.183 |
| Atrial fibrillation/flutter (%) | 138 (16.7) | 72 (15.8) | 66 (17.8) | 0.443 |
| COPD (%) | 113 (13.7) | 64 (14.0) | 49 (13.2) | 0.730 |
| Chronic kidney diseasea (%) | 96 (11.6) | 57 (12.5) | 39 (10.5) | 0.375 |
| Cancer (%) | 115 (13.9) | 72 (15.8) | 43 (11.6) | 0.083 |
| Cardiovascular treatment prior to hospital admission | ||||
| Anticoagulation (%) | 157 (19.0) | 76 (16.7) | 81 (21.8) | 0.056 |
| Antiplatelet agent(%) | 269 (32.5) | 154 (33.8) | 115 (31.0) | 0.397 |
| ACEI or ARB (%) | 480 (58.0) | 268 (58.8) | 212 (57.1) | 0.637 |
| Aldosterone antagonist (%) | 56 (6.8) | 29 (6.4) | 27 (7.3) | 0.601 |
| Sacubitril/valsartan (%) | 11 (1.3) | 5 (1.1) | 6 (1.6) | 0.555 |
| Beta-blocker (%) | 226 (27.3) | 116 (25.4) | 110 (29.7) | 0.177 |
| Diuretic (%) | 306 (37.0) | 157 (34.4) | 149 (40.2) | 0.090 |
| iSGLT-2 (%) | 26 (3.1) | 14 (3.1) | 12 (3.2) | 0.893 |
| Digoxin (%) | 11 (1.3) | 5 (1.1) | 6 (1.6) | 0.555 |
| First measurement of vital signs | ||||
| SBP (mmHg) | 130.1 ± 22.3 | 130.1 ± 22.5 | 130.1 ± 22.1 | 0.995 |
| Heart rate (bpm) | 89.4 ± 18.0 | 89.7 ± 17.6 | 89.1 ± 18.5 | 0.681 |
| First SpO2 (%) | 90.2 ± 7.1 | 90.0 ± 6.9 | 90.5 ± 7.4 | 0.293 |
| O2 support at time of first saturation (%) | 119 (14.4) | 58 (12.7) | 61 (16.4) | 0.154 |
| First chest x-ray | ||||
| Without pneumonia (%) | 122 (14.8) | 64 (14.0) | 58 (15.6) | |
| Unilateral pneumonia (%) | 163 (19.7) | 102 (22.4) | 61 (16.4) | 0.094 |
| Bilateral pneumonia (%) | 533 (64.5) | 284 (62.3) | 249 (67.1) | |
| Laboratory data | ||||
| Median GFR (mL/min/1.73m2) | 68.0 ± 23.7 | 66.8 ± 24.3 | 69.4 ± 22.9 | 0.125 |
| Median haemoglobin (g/dL) | 13.1 ± 1.9 | 13.3 ± 1.8 | 12.9 ± 1.9 | < 0.001 |
| Maximum ferritin value (ng/dL) | 816 (391-1482) | 699 (311-1297) | 971 (508-1573) | < 0.001 |
| Maximum D-dimer value (ng/mL) | 1.629 (761-4.989) | 1.383 (682-4.030) | 1912 (809-10.400) | 0.003 |
| Maximum Troponin value (ng/L) | 14.0 (5.3-59.5) | 8.6 (4.2-30.8) | 20.4 (5.9-91.6) | < 0.001 |
| Maximum NT-proBNP value (pg/mL) | 1857 (513-6984) | 1952 (513-6254) | 1.762 (499-7.917) | 0.991 |
| Maximum lactate value (mmol/L) | 2.36 ± 1.06 | 2.54 ± 1.20 | 2.20 ± 0.91 | 0.087 |
| Maximum AST value (IU/L) | 52 (35-87) | 49 (31-82) | 55 (38-95) | 0.002 |
| Maximum ALT value (IU/L) | 43 (24-79) | 40 (23-69) | 46 (28-88) | < 0.001 |
| Maximum LDH value (IU/L) | 386.5 (312.0-512.0) | 370.5 (302.5-492.9) | 406.5 (332.0-528.0) | 0.002 |
| Maximum CPK value (IU/L) | 122 (69-277) | 116 (69-229) | 132 (69-342) | 0.035 |
| Lowest prothrombin activity value (%) | 75.9 ± 25.3 | 77.5 ± 24.6 | 74.1 ± 26.1 | 0.062 |
| Maximum fibrinogen value (mg/dL) | 896.3 ± 251.4 | 883.9 ± 251.1 | 911.2 ± 251.3 | 0.124 |
| Maximum CRP value (mg/L) | 145.3 (71.4-228.0) | 140.4 (71.6-219.1) | 149.3 (70.2-240.1) | 0.134 |
| Maximum IL-6 value (pg/mL) | 75.9 (18.6-409.5) | 79.6 (18.8-404.5) | 69.9 (18.6-426.5) | 0.119 |
| Specific treatment for COVID-19 | ||||
| Hydroxychloroquine (%) | 761 (92.0) | 405 (88.8) | 356 (96.0) | < 0.001 |
| Lopinavir/ritonavir (%) | 114 (13.8) | 74 (16.2) | 40 (10.8) | 0.024 |
| Azithromycin (%) | 469 (56.7) | 238 (52.2) | 231 (62.3) | 0.004 |
| Tocilizumab (%) | 90 (10.9) | 41 (9.0) | 49 (13.2) | 0.053 |
| Glucocorticoids (%) | 195 (23.6) | 94 (20.6) | 101 (27.2) | 0.026 |
| Clinical outcomes | ||||
| Acute heart failure (%) | 42 (5.1) | 28 (6.1) | 14 (3.8) | 0.123 |
| Pulmonary embolism (%) | 28 (3.4) | 7 (1.5) | 21 (5.7) | < 0.001 |
| Thrombotic eventb (%) | 49 (5.9) | 15 (3.3) | 34 (9.2) | < 0.001 |
| Any bleeding (%) | 37 (4.5) | 13 (2.9) | 24 (6.5) | 0.010 |
| Major bleedingc (%) | 12 (1.5) | 1 (0.2) | 11 (3.0) | 0.002 |
| Atrial fibrillation/flutter during admission (%) | 52 (6.3) | 26 (5.7) | 26 (7.0) | 0.441 |
| Ventricular arrhythmias during admission (%) | 4 (0.5) | 2 (0.4) | 2 (0.5) | 1.000 |
| Admission to critical care (%) | 84 (10.2) | 29 (6.4) | 55 (14.8) | < 0.001 |
| Mechanical ventilation (%) | 83 (10.0) | 29 (6.4) | 50 (14.6) | < 0.001 |
| Death (%) | 294 (35.6) | 167 (36.6) | 127 (34.2) | 0.469 |
COPD: chronic obstructive pulmonary disease; ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker; SGLT2i: sodium-glucose cotransporter 2 inhibitors; SBP: systolic blood pressure; SpO2: oxygen saturation; O2: oxygen; GFR: glomerular filtration rate; NT-proBNP: N-terminal prohormone of brain natriuretic peptide; AST: aspartate aminotransferase; ALT: alanine aminotransferase; CPK: creatine phosphokinase; CRP: C-reactive protein; IL-6 interleukin 6.
Values express n (%) for qualitative variables or mean ± standard deviation or median (interquartile range) for continuous variables based on the characteristics of the distribution.
Chronic kidney disease was defined as a GFR < 60 mL/min/1.73 m2 for three months or more.
Thrombotic event was defined as the incidence of stroke, acute coronary syndrome, acute arterial ischemia in lower limbs, deep vein thrombosis and pulmonary thromboembolism.
Indicates major bleeding according to the TIMI classification.
Fig. 2.
Survival analysis according to the discontinuation or not of statins during hospital admission for COVID-19 according to the Kaplan-Meier method in patients who had chronic treatment with these drugs. Their discontinuation was not associated with a higher incidence of mortality [HR 1.01 (0.78-1.30)].
Safety of statins in patients with COVID-19
During the study period, excluding those patients with acute arterial ischaemia, 19 patients (0.9%) had CPK elevation 10 times above the upper limit of normal, which is the classic cut-off point for defining the presence of rhabdomyolysis. The proportion of patients with this entity was significantly higher in the group of patients treated with statins (1.9 vs. 0.66%, p < 0.020). On the other hand, 282 patients (12.9%) had clinically relevant elevation of AST (usually defined as a three-fold increase in AST above the upper limit of normal). Similarly, this adverse reaction was more prevalent in patients who received statins during admission (17.8 vs. 11.9%, p < 0.002).
Discussion
The hypothesis that statins through their immunomodulatory effects7, 8, 9 may counteract the deleterious effects of the exacerbated inflammatory response known as cytokine storm10 that characteristically occur in COVID-19 patients with worse clinical outcomes is a thought-provoking idea but has not been clinically demonstrated in randomised clinical trials.
This exaggerated organ-damaging immune response is the main cause of respiratory distress10 and of damage to the vascular system in these patients.11 This pathophysiology is not new and has many similarities with that of other bacterial and viral infections causing pneumonia.12
Observational studies in patients admitted for pneumonia and/or influenza suggest that statin therapy is associated with fewer cardiovascular complications and reduced disease severity compared to patients not taking statins.9, 13 We might think that there is no compelling reason to imagine that with COVID-19 it will be different, however, the situation is not completely analogous. In fact, the most characteristic and leading cause of death in patients with COVID-19 is the severe respiratory distress it causes.3 A randomised study evaluating rosuvastatin for its anti-inflammatory potential in patients with acute respiratory distress, mostly caused by pneumonia, concluded that it did not reduce mortality compared to placebo.14
The authors of a meta-analysis15 that combined the results of four observational studies on the effects of statins in patients with COVID-19 concluded that they could reduce the risk of mortality or severe complications by 30% and that their tolerability is acceptable. On the other hand, the SEMI-COVID registry of the Spanish Society of Internal Medicine identified that continuation of statin therapy was associated with lower all-cause mortality compared to those whose therapy was withdrawn during hospitalisation after SARS-CoV-2 infection.16
However, it is necessary to consider that throughout the pandemic, several therapeutic alternatives have been proposed on the basis of encouraging data obtained in multiple observational studies,17, 18, 19 which were later disproved in the corresponding randomised clinical trials. Our analysis introduces a discordant note with the findings previously described, since the statin-treated group, although presenting a higher number of admissions to critical care units and more need for mechanical ventilation, did not show significant differences in mortality [HR 1.03 (0.78-1.35), p = 0.850] when a multivariate analysis adjusted for all potential confounders was performed. This analysis suggests that the use of statins during the acute phase of infection may have a neutral effect on the clinical endpoint of all-cause death.
In addition, our data do not show signs that the use of statins may result in a reduction of cardiovascular complications from SARS-CoV-2 infection. Although several studies have shown the influence of endothelial20 and immune thrombotic phenomena in these patients,21, 22, 23 those who received statins (drugs considered endothelium-protective) during admission had more thrombotic and bleeding events during the course of the disease. This is probably related to the fact that these patients have atherosclerotic cardiovascular disease, the first stage of which is endothelial dysfunction. There is reason to believe that atherosclerosis may represent a situation of cardiovascular vulnerability to SARS-CoV-2 infection that could not be modified by receiving statins during the acute phase, in contrast to the significant benefits observed in secondary prevention in other clinical settings.24 However, the low number of thrombotic arterial complications recorded during follow-up limits the ability to draw definitive conclusions.
In relation to the above, patients on statins had higher levels of inflammatory markers such as CRP, ferritin, fibrinogen or IL-6. This raises the hypothesis that the anti-inflammatory effect of statins may not be potent enough to regulate the COVID-19 cytokine storm at least partially. An analogous situation occurs in other entities characterized mainly by inflammation, such as rheumatoid arthritis. In this disease this therapeutic group modestly decreases inflammation parameters7 and yet they are not considered useful for its treatment.
Statins are well-tolerated drugs with few adverse reactions. Of these, myopathy is the most clinically relevant. The mildest form of this disorder is myalgia without elevated creatine phosphokinase (CPK), which we have not been able to specifically assess as it overlaps with symptoms typical of patients with COVID-19 (in a large cohort of COVID-19 patients in our setting, 26.8% of patients reported symptoms suggestive of myalgia on admission).25 In our sample, both significant elevation of transaminases and rhabdomyolysis were more common in statin-treated patients.
In relation to the above, prothrombin activity was also significantly lower in this group of patients which, associated with a higher incidence of thrombotic and bleeding events, raises the hypothesis that this therapeutic group may increase the likelihood of coagulopathy throughout the clinical course of the disease.
Throughout the pandemic, several scientific societies26, 27 have recommended maintaining statin therapy in those patients receiving it on the basis of a clinically correct indication. In this sense, the neutral results of our study are compatible with this recommendation, although they do not constitute a rationale for introducing the drug de novo with the aim of improving the prognosis of COVID-19, except in research studies specifically designed to test its efficacy. Moreover, the uncertain clinical course of the disease, interactions with other treatments and indications of potential adverse reactions to statins in this clinical setting call for careful use of this therapeutic group and close monitoring of possible complications.
This is a retrospective observational study, with the usual limitations and biases of this type of design, conducted in a single centre with a high volume of patients with COVID-19. This observational study cannot in any way replace a randomized clinical trial that clarifies whether or not statins are effective in the context of SARS-CoV-2 infection. The results are therefore not conclusive. In addition, the presence of myalgias (the most common adverse reaction of statins) was not systematically recorded in all the patients of the cohort, nor were CPK and transaminase values determined in all of them, which may somehow condition a selection bias.
In conclusion, in our cohort, statins were not independently associated with lower mortality in patients with COVID-19. Those patients who received statins during hospital admission showed higher inflammatory parameters, in addition to a greater number of thrombotic and bleeding events.
Ethical considerations
The study was approved by the Clinical Research Ethics Committee of our site in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its subsequent amendments. An informed consent document was not required to be completed based on national legislation for health alert scenarios.
Funding
This paper has not received any type of funding.
Conflict of interests
José Luis Merino has received fees from Bayer, Correvio, Daiichi-Sankyo and Sanofi, unrelated to this paper. Esteban López-de-Sá has received fees from Zoll Medical Corporation, Boehringer Ingelheim, Servier, Daiichi Sankyo, Rovi, BARD and AstraZeneca, unrelated this paper.
Footnotes
Please cite this article as: Rey JR, Merino Llorens JL, Iniesta Manjavacas ÁM, Rosillo Rodríguez SO, Castrejón-Castrejón S, Arbas-Redondo E, et al. Influencia del tratamiento con estatinas en una cohorte de pacientes ingresados por COVID-19. Med Clin (Barc). 2022;158:586–595.
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.medcle.2022.05.004.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.COVID-19 Map. Johns Hopkins Coronavirus Resource Center. (consultado 19 Abr 2020). Disponible en https://coronavirus.jhu.edu/map.htlm.
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 5.Oesterle A., Laufs U., Liao J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ Res. 2017;120:229–243. doi: 10.1161/CIRCRESAHA.116.308537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corsini A., Bellosta S., Baetta R., Fumagalli R., Paoletti R., Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther. 1999;84:413–428. doi: 10.1016/s0163-7258(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.McCarey D.W., McInnes I.B., Madhok R., Hampson R., Scherbakov O., Ford I., et al. Trial of Atorvastatin in Rheumatoid Arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet. 2004;363:2015–2021. doi: 10.1016/S0140-6736(04)16449-0. [DOI] [PubMed] [Google Scholar]
- 8.Castiglione V., Chiriacò M., Emdin M., Taddei S., Vergaro G. Statin therapy in COVID-19 infection. Eur Hear J Cardiovasc Pharmacother. 2020;6:258–259. doi: 10.1093/ehjcvp/pvaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fedson D.S. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417–435. doi: 10.1016/j.antiviral.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Elia R.V., Harrison K., Oyston P.C., Lukaszewski R.A., Clark G.C. Targeting the "cytokine storm" for therapeutic benefit. Clin Vaccine Immunol. 2013;20:319–327. doi: 10.1128/CVI.00636-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pertzov B., Eliakim-Raz N., Atamna H., Trestioreanu A.Z., Yahav D., Leibovici L. Hydroxymethylglutaryl-CoA reductase inhibitors (statins) for the treatment of sepsis in adults - A systematic review and meta-analysis. Clin Microbiol Infect. 2019;25:280–289. doi: 10.1016/j.cmi.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Truwit J.D., Bernard G.R., Steingrub J., Matthay M.A., Liu K.D., Albertson T.E., et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370:2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kow C.S., Hasan S.S. Meta-analysis of Effect of Statins in Patients with COVID-19. Am J Cardiol. 2020;134:153–155. doi: 10.1016/j.amjcard.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres-Peña J.D., Pérez-Belmonte L.M., Fuentes-Jiménez F., López Carmona M.D., Pérez-Martinez P., López-Miranda J., et al. Prior Treatment with Statins is Associated with Improved Outcomes of Patients with COVID-19: Data from the SEMI-COVID-19 Registry. Drugs. 2021 doi: 10.1007/s40265-021-01498-x. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R., et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients with Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Libby P., Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGonagle D., O’Donnell J., Sharif K., Emery P., Bridgewood C. Immune Mechanisms of Pulmonary Intravascular Coagulopathy (PIC) in COVID-19 Pneumonia. Lancet Rheumatol. 2020;2019:1–9. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caro-Codón J., Lip G.Y.H., Rey J.R., Iniesta A.M., Rosillo S.O., Castrejon-Castrejon S., et al. Prediction of thromboembolic events and mortality by the CHADS2 and the CHA2DS2-VASc in COVID-19. EP Europace. 2021;23:937–947. doi: 10.1093/europace/euab015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rey J.R., Caro-Codón J., Poveda Pineda D., Merino J.L., Iniesta ÁM, López-Sendón J.L. Arterial thrombotic complications in hospitalized patients with COVID-19. Rev Esp Cardiol (English Edition) 2020;73:769–782. doi: 10.1016/j.rec.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Libby P. Mechanisms of Acute Coronary Syndromes and Their Implications for Therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 25.Borobia A.M., Carcas A.J., Arnalich F., Álvarez-Sala R., Monserrat-Villatoro J., Quintana M., et al. A cohort of patients with COVID-19 in a major teaching hospital in Europe. J Clin Med. 2012;53:1689–1699. doi: 10.3390/jcm9061733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iqbal Z., Ho J.H., Adam S., France M., Syed A., Neely D., et al. Managing hyperlipidaemia in patients with COVID-19 and during its pandemic: An expert panel position statement from HEART UK. Atherosclerosis. 2020;313:126–136. doi: 10.1016/j.atherosclerosis.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.European Society of cardiology. ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. [accessed 3 jul 2021]. Available from: https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.