Abstract
CONTEXT:
Immune system dysfunction is poorly represented in pediatric organ dysfunction definitions.
OBJECTIVE:
To evaluate evidence for criteria that define immune system dysfunction in critically ill children and associations with adverse outcomes and develop consensus criteria for the diagnosis of immune system dysfunction in critically ill children.
DATA SOURCES:
We conducted electronic searches of PubMed and Embase from January 1992 to January 2020, using medical subject heading terms and text words to define immune system dysfunction and outcomes of interest.
STUDY SELECTION:
Studies of critically ill children with an abnormality in leukocyte numbers or function that is currently measurable in the clinical laboratory in which researchers assessed patient-centered outcomes were included. Studies of adults or premature infants, animal studies, reviews and commentaries, case series (≤10 subjects), and studies not published in English with inability to determine eligibility criteria were excluded.
DATA EXTRACTION:
Data were abstracted from eligible studies into a standard data extraction form along with risk of bias assessment by a task force member.
RESULTS:
We identified the following criteria for immune system dysfunction: (1) peripheral absolute neutrophil count <500 cells/μL, (2) peripheral absolute lymphocyte count <1000 cells/μL, (3) reduction in CD4+ lymphocyte count or percentage of total lymphocytes below age-specific thresholds, (4) monocyte HLA-DR expression <30%, or (5) reduction in ex vivo whole blood lipopolysaccharide-induced TNFα production capacity below manufacturer-provided thresholds.
LIMITATIONS:
Many measures of immune system function are currently limited to the research environment.
CONCLUSIONS:
We present consensus criteria for the diagnosis of immune system dysfunction in critically ill children.
The immune system is central to host defense against pathogens as well as remodeling and healing of injured tissues. Acute defects in innate and adaptive immunity place critically ill children at high risk for adverse outcomes from critical illness across the spectrum of ICU diagnoses. The immune system is highly complex and includes cellular elements (leukocytes), noncellular elements (eg, cytokines, chemokines, complement), and barrier defenses (eg, skin, mucociliary clearance). Abnormalities in leukocyte numbers or function represent immune system defects that are quantifiable and to which adverse ICU outcomes can be attributed. Studies of pediatric sepsis, pediatric oncology, and HIV have consistently shown associations between reduced absolute cell counts, infection risk, and mortality. In recent decades, severe critical illness–induced immune suppression (“immunoparalysis”) has been identified as risk factor for these adverse outcomes as well.1 Other components of the immune system are more challenging to incorporate into current definitions but represent important research priorities. Cytokines and chemokines serve to make the local environment favorable for host defense through vasodilation, increased capillary permeability, and immune cell recruitment. Elevations in numerous systemic cytokine and chemokine levels have been associated with adverse outcomes from critical illness in children.2–5 Although these inflammatory mediators are produced by activated leukocytes, they are also produced by other cell types including stressed or injured endothelial or parenchymal cells. It is therefore difficult to ascribe abnormalities in inflammatory biomarker profiles specifically to immune system dysfunction rather than to concurrent tissue injury. The degree to which immune dysfunction due to barrier disruption in burn injury, for example, contributes to increased risk for adverse outcomes versus the direct effects of tissue injury is similarly difficult to quantify.
Lastly, there are numerous aspects of leukocyte function that can be abnormal in the setting of primary (chronic) immunodeficiency. Examples include defects in the ability of leukocytes to migrate to a site of infection, ingest and kill pathogens, produce antibody, effect extracellular killing, and proliferate. Although many of these defects can be identified in a specialized immunology laboratory through cellular or genetic testing, most of these tests are not practical for the pediatric intensivist to use for realtime diagnosis and management. Also, specific thresholds of these tests that are associated with adverse ICU outcomes have not been identified. For these reasons, measures of primary immunodeficiency (other than cell counts) have not been included in these definitions.
METHODS
The PODIUM collaborative sought to develop evidence-based criteria for organ dysfunction in critically ill children. In this article, we report on the systematic review on immune system dysfunction scoring tools performed as part of PODIUM, provide a critical evaluation of the available literature, and propose evidence-based criteria for immune system dysfunction in critically ill children, as well as recommendations for future research. The PODIUM Executive Summary details Population, Interventions, Comparators, and Outcomes questions, search strategies, study inclusion and exclusion criteria, and processes for risk of bias assessment, data abstraction and synthesis and for drafting and developing agreement for criteria indicating immune system dysfunction.6
RESULTS
Of 7566 unique citations published between 1992 and 2020, 39 studies were eligible for inclusion, as shown in the PRISMA flowchart (Fig 1). Data tables (Supplemental Tables 1 and 2) and risk of bias assessment summaries (Supplemental Fig 1) are detailed in the Supplemental Information. The 2 groups of studies that informed the immune system dysfunction criteria focus on severe leukopenia and critical illness–induced innate immune suppression (“immunoparalysis”). This important form of secondary immunodeficiency can be diagnosed with small blood volume, rapid turnaround time assays. Although these are not yet available in the clinical laboratory in the United States, they are clinically available in other regions. These assays include the measurement of monocyte HLA-DR expression (a measure of antigen presenting capacity) and ex vivo stimulation assays (measures of leukocyte responsiveness). Criteria for immune system dysfunction in critically ill children informed by the evaluated evidence are listed in Table 1.
FIGURE 1.
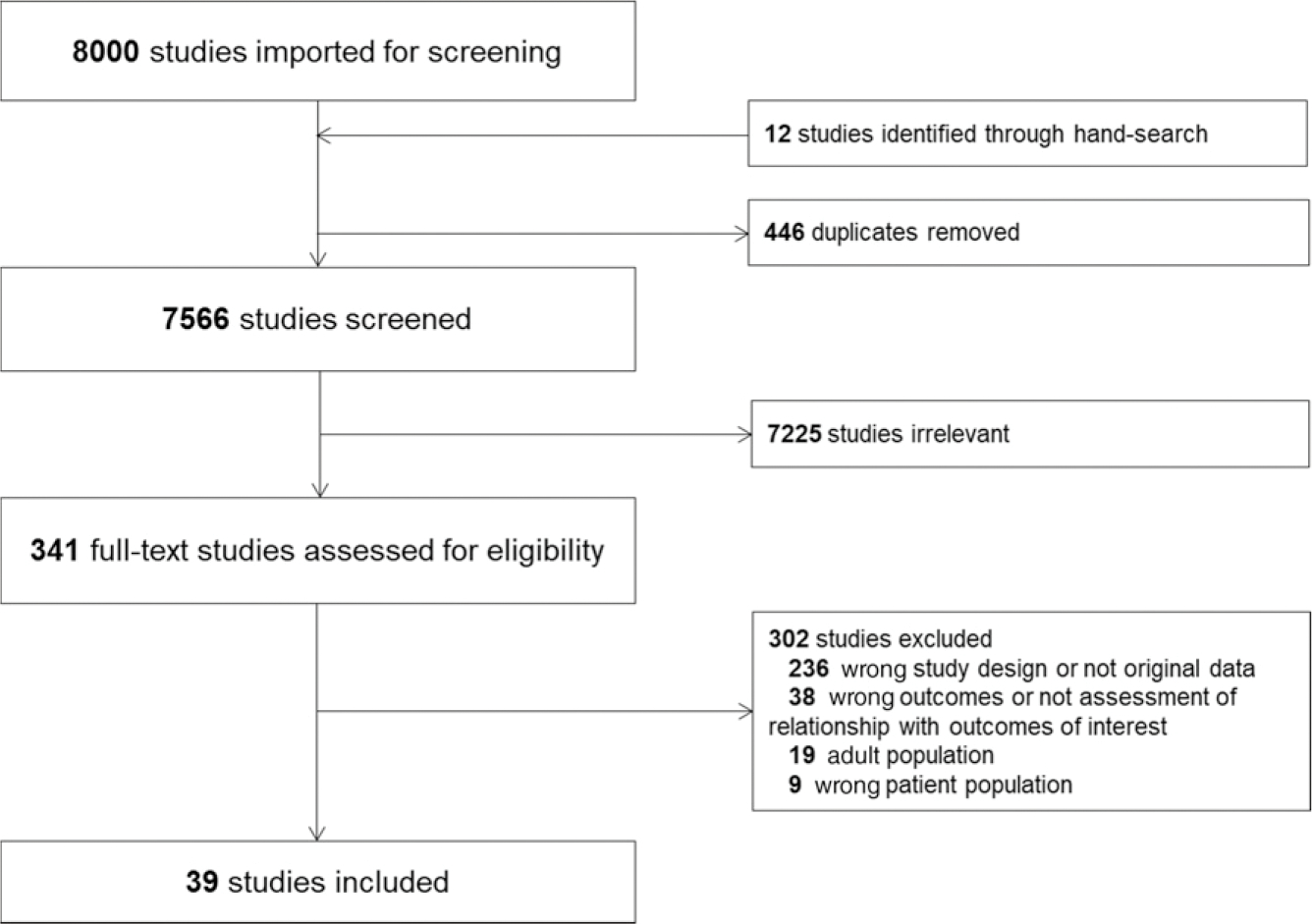
Study flow diagram according to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols recommendations.
TABLE 1.
Criteria for Immune System Dysfunction in Pediatric Critical Illness
| Organ System | Criterion For Organ Dysfunction | Suggested Thresholds | Conditions | Severity |
|---|---|---|---|---|
|
| ||||
| Immune | Peripheral absolute neutrophil count | <500 cells/μL | None | Not graded |
| Immune | Peripheral ALC | <1000 cells/μL | None | Not graded |
| Immune | CD4+ T-lymphocyte count | <750 cells/μL | Age <1 y | Not graded |
| <500 cells/μL | Age 1–5 y | Not graded | ||
| <200 cells/μL | Age ≥6 y | Not graded | ||
| Immune | CD4+ T-lymphocyte percentage of total lymphocytes | <26% | Age <1 y | Not graded |
| <22% | Age 1–5 y | Not graded | ||
| <14% | Age ≥6 y | Not graded | ||
| Where clinically availablea | ||||
| Immune | Monocyte HLA-DR expression | <30% | None | Not graded |
| Immune | Ex vivo LPS-induced TNFα production capacity | Below manufacturer-provided thresholds | None | Not graded |
These tests may be clinically available outside the United States.
Severe Neutropenia
Data from the oncology literature has long indicated that an absolute neutrophil count ([% neutrophils 1 % band forms] × total white blood cell count) <500 cells per mm3 confers significantly increased risk for the development of new infection and death.7–11 A 10-year single-center survey of patients receiving cytolytic chemotherapy revealed that persistent neutropenia was strongly associated with mortality.9 In a recent study of 270 children after hematopoietic stem cell transplant, prolonged severe neutropenia (>7 days) was strongly associated with an increased risk for Gram-negative bacteremia (odds ratio 19.5, 95% confidence interval 2.6–148).7 Similarly, among 120 children undergoing allogeneic hematopoietic stem cell transplant, the duration of severe neutropenia was a primary risk factor for invasive fungal infection.10 In a 2019 study of 126 children with malignant bone tumors, the only infection-related mortality occurred in children with severe neutropenia.7 Although not all infections and deaths in these studies occurred in a critical care environment, the strong association between severe neutropenia and these adverse outcomes warrants inclusion of severe neutropenia in these criteria.
Severe Lymphopenia
Lymphopenia has been associated with increased risks for adverse outcomes from pediatric burn injury, appendicitis, and surgery to repair congenital heart disease.12–14 The presence of an absolute lymphocyte count (ALC) (% lymphocytes × total white blood cell count) <1000 cells per μL for a least 7 days was associated with increased odds of nosocomial infection and death in a 113-subject cohort of critically ill children.15 In a 22-subject cohort of children with septic shock, lower ALC within 48 hours of sepsis onset was associated with greater risk of new or persistent infection, with the median value of <1000 cells per μL in the group with infectious complications.16 Among 52 children with critical illness due to acute influenza infection, the ALC within 48 hours of ICU admission was lower in nonsurvivors: 364 (192–748) vs 1670 (955–2055) cells per μL (P = .005).4 Among children undergoing cardiac surgery, preoperative lymphopenia was associated with increased mortality risk, with each drop in the ALC of 1000 cells per μL being associated with an increase in the risk of mortality by 2.67-fold.14 Although transient lymphopenia is common in the setting of acute infection, the strength of association between an ALC <1000 cells per μL and adverse ICU outcomes suggests that this degree of lymphopenia merits inclusion as part of the definition of immune system dysfunction.
Severe Reduction in CD4+ T Cells
Data from children with HIV suggest that the absolute and relative counts of CD4+ T cells are important predictors of adverse outcomes including mortality. Current guidelines from the Centers for Disease Control and Prevention provide age-specific definitions of severe immune system dysfunction in children on the basis of CD4+ T-cell count or CD4+ T-cell percentage of total lymphocytes.17 Although there is a paucity of data regarding CD4+ T-cell counts in critically ill children without HIV, the strength of association with mortality outcomes in children with HIV suggests that this metric also merits inclusion as part of the definition of immune system dysfunction.
Reduced Monocyte HLA-DR Expression
Children with immunoparalysis demonstrate a reduction in the expression of the class II major histocompatibility complex molecule HLA-DR on the surface of circulating monocytes. Severe reduction in monocyte HLA-DR expression, and/or failure to increase monocyte HLA-DR expression over time, has been associated with increased risks for nosocomial infection and death from pediatric sepsis, cardiopulmonary bypass, and multiple organ dysfunction syndrome.18–22 Thresholds of HLA-DR expression that are associated with adverse outcomes depend on the method of quantitation that is used. If <30% of circulating monocytes are positive for HLA-DR by flow cytometry, this has been shown to confer increased risks of nosocomial infection and death in children with multiple organ dysfunction syndrome (MODS).18,23 An alternative method permits the quantitation of HLA-DR expression in terms of molecules per cell. Although adult data24 and some pediatric data25 suggest that a threshold of <8000 molecules per cell confers increased risk, other pediatric data have suggested that failure to increase monocyte HLA-DR expression by at least 1000 molecules per cell over the first week of sepsis, not the absolute level of expression, is associated with increased mortality risk.20 Consensus has not yet been achieved on how best to use the molecules/cell measure of HLA-DR expression in the PICU; therefore, the 30% expression threshold was used for these definitions.
Reduced ex vivo LipopolysaccharideInduced Tumor Necrosis Factor-α Response
The ability of whole blood to produce cytokines when stimulated ex vivo is also reduced in the setting of immunoparalysis. Reduced production of the cytokine tumor necrosis factor (TNF)-α by whole blood on ex vivo stimulation with lipopolysaccharide (LPS) has been consistently shown to be associated with adverse outcomes from pediatric critical illness. These outcomes include nosocomial infection, prolonged organ dysfunction, and death in patient populations including children with cardiopulmonary bypass,26,27 sepsis,28 MODS,18,29 trauma,30 and critical influenza.4 Standardization of the LPS stimulation reagent, blood volumes used, incubation duration, and TNFα quantitation platform are essential for clinical use given that clinically relevant thresholds of TNFα response will vary depending on the characteristics of the assay. Like measurement of monocyte HLA-DR expression, measurement of the TNFα response is not available for clinical use in the United States, although is available elsewhere.
CONCLUSIONS
Impairment of the immune system is common in pediatric critical illness and is associated with adverse outcomes including increased risks for nosocomial infection, prolonged organ dysfunction, and death. Although many biomarkers of the inflammatory response are not specific to the immune system, it is likely that proteomic profiling, transcriptomic profiling, or a combination of both31 may allow us to identify children with immune system dysfunction in the PICU. For now, this is limited to the research environment, but the transition of this approach to the clinical laboratory is a key priority in the field (see Supplemental Information for additional detail on research priorities) Severe reductions in neutrophil and lymphocyte numbers (including CD4+ T cells) can predict adverse outcomes in critically ill children and are easily measurable in the clinical laboratory. Measures of immunoparalysis including severe reductions in monocyte HLA-DR expression and/or the ex vivo TNFα response represent additional indicators of immune system dysfunction in critically ill children. Expanding the availability of these tests to US clinical laboratories is another key priority in the field.
Supplementary Material
ABBREVIATIONS
- ALC
absolute lymphocyte count
- LPS
lipopolysaccharide
- MODS
multiple organ dysfunction syndrome
- TNF
tumor necrosis factor
Footnotes
POTENTIAL CONFLICT OF INTEREST: Consultant work by LaJolla Pharmaceuticals for service on a Data and Safety Monitoring Board, not related to this work (MWH).
Drs Hall, Carcillo, and Cornell performed abstract and manuscript reviews, identified studies of inclusion, and drafted consensus definitions; Dr Hall drafted the initial manuscript with subsequent editing and revision by Drs Carcillo and Cornell; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Greathouse KC, Hall MW. Critical illness-induced immune suppression: current state of the science. Am J Crit Care. 2016;25(1):85–92 [DOI] [PubMed] [Google Scholar]
- 2.Wong HR, Cvijanovich NZ, Anas N, et al. Pediatric sepsis biomarker risk model-ii: redefining the pediatric sepsis biomarker risk model with septic shock phenotype. Crit Care Med. 2016;44(11):2010–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finnerty CC, Jeschke MG, Herndon DN, et al. ; Investigators of the Inflammation and the Host Response Glue Grant. Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med. 2008;14(9–10):553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall MW, Geyer SM, Guo CY, et al. ; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network PICFlu Study Investigators. Innate immune function and mortality in critically ill children with influenza: a multicenter study. Crit Care Med. 2013;41(1):224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doughty LA, Kaplan SS, Carcillo JA. Inflammatory cytokine and nitric oxide responses in pediatric sepsis and organ failure. Crit Care Med. 1996;24(7): 1137–1143 [DOI] [PubMed] [Google Scholar]
- 6.Bembea MM, Agus M, Akcan-Arikan A, et al. Pediatric organ dysfunction information update mandate (PODIUM) contemporary organ dysfunction criteria: executive summary. Pediatrics. 2022; 149(suppl 1):e2021052888B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinker-Shuster M, Stepensky P, Temper V, Shayovitz V, Masarwa R, Averbuch D. Gram-negative bacteremia in children with hematologic malignancies and following hematopoietic stem cell transplantation: epidemiology, resistance, and outcome. J Pediatr Hematol Oncol. 2019;41(8):e493–e498 [DOI] [PubMed] [Google Scholar]
- 8.Czyzewski K, Galazka P, Zalas-Wiecek P, et al. Infectious complications in children with malignant bone tumors: a multicenter nationwide study. Infect Drug Resist. 2019;12:1471–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wehl G, Allerberger F, Heitger A, Meister B, Maurer K, Fink FM. Trends in infection morbidity in a pediatric oncology ward, 1986–1995. Med Pediatr Oncol. 1999;32(5):336–343 [DOI] [PubMed] [Google Scholar]
- 10.Dvorak CC, Steinbach WJ, Brown JM, Agarwal R. Risks and outcomes of invasive fungal infections in pediatric patients undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2005;36(7): 621–629 [DOI] [PubMed] [Google Scholar]
- 11.Cesaro S, Tridello G, Castagnola E, et al. Retrospective study on the incidence and outcome of proven and probable invasive fungal infections in high-risk pediatric onco-hematological patients. Eur J Haematol. 2017;99(3):240–248 [DOI] [PubMed] [Google Scholar]
- 12.Thakkar RK, Diltz Z, Drews JD, et al. Abnormal lymphocyte response after pediatric thermal injury is associated with adverse outcomes. J Surg Res. 2018;228:221–227 [DOI] [PubMed] [Google Scholar]
- 13.Lodwick DL, Cooper JN, Kenney B, Deans KJ, Minneci PC, Thakkar RK. Lymphocyte depression as a predictor of postoperative intraabdominal abscess after appendectomy in children. J Pediatr Surg. 2017;52(1):93–97 [DOI] [PubMed] [Google Scholar]
- 14.Cabrera AG, Dyamenahalli U, Gossett J, et al. Preoperative lymphopenia is a predictor of postoperative adverse outcomes in children with congenital heart disease. J Thorac Cardiovasc Surg. 2009;138(5):1172–1179 [DOI] [PubMed] [Google Scholar]
- 15.Felmet KA, Hall MW, Clark RS, Jaffe R, Carcillo JA. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol. 2005;174(6):3765–3772 [DOI] [PubMed] [Google Scholar]
- 16.Muszynski JA, Nofziger R, Greathouse K, et al. Early adaptive immune suppression in children with septic shock: a prospective observational study. Crit Care. 2014;18(4):R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC). Revised surveillance case definition for HIV infection–United States, 2014. MMWR Recomm Rep. 2014;63(RR-03): 1–10 [PubMed] [Google Scholar]
- 18.Hall MW, Knatz NL, Vetterly C, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011; 37(3):525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allen ML, Peters MJ, Goldman A, et al. Early postoperative monocyte deactivation predicts systemic inflammation and prolonged stay in pediatric cardiac intensive care. Crit Care Med. 2002; 30(5):1140–1145 [DOI] [PubMed] [Google Scholar]
- 20.Manzoli TF, Troster EJ, Ferranti JF, Sales MM. Prolonged suppression of monocytic human leukocyte antigen-DR expression correlates with mortality in pediatric septic patients in a pediatric tertiary intensive care unit. J Crit Care. 2016;33:84–89 [DOI] [PubMed] [Google Scholar]
- 21.Boeddha NP, Kerklaan D, Dunbar A, et al. HLA-DR expression on monocyte subsets in critically ill children. Pediatr Infect Dis J. 2018;37(10):1034–1040 [DOI] [PubMed] [Google Scholar]
- 22.Genel F, Atlihan F, Ozsu E, Ozbek E. Monocyte HLA-DR expression as predictor of poor outcome in neonates with late onset neonatal sepsis. J Infect. 2010;60(3):224–228 [DOI] [PubMed] [Google Scholar]
- 23.Hoffman JA, Weinberg KI, Azen CG, et al. Human leukocyte antigen-DR expression on peripheral blood monocytes and the risk of pneumonia in pediatric lung transplant recipients. Transpl Infect Dis. 2004;6(4):147–155 [DOI] [PubMed] [Google Scholar]
- 24.Döcke WD, Höflich C, Davis KA, et al. Monitoring temporary immunodepression by flow cytometric measurement of monocytic HLA-DR expression: a multicenter standardized study. Clin Chem. 2005;51(12):2341–2347 [DOI] [PubMed] [Google Scholar]
- 25.Remy S, Kolev-Descamps K, Gossez M, et al. Occurrence of marked sepsis-induced immunosuppression in pediatric septic shock: a pilot study. Ann Intensive Care. 2018;8(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen ML, Hoschtitzky JA, Peters MJ, et al. Interleukin-10 and its role in clinical immunoparalysis following pediatric cardiac surgery. Crit Care Med. 2006;34(10):2658–2665 [DOI] [PubMed] [Google Scholar]
- 27.Cornell TT, Sun L, Hall MW, et al. Clinical implications and molecular mechanisms of immunoparalysis after cardiopulmonary bypass. J Thorac Cardi ovasc Surg. 2012;143(5):1160–1166.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muszynski JA, Nofziger R, Moore-Clingenpeel M, et al. Early immune function and duration of organ dysfunction in critically ill children with sepsis. Am J Respir Crit Care Med. 2018;198(3): 361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall MW, Gavrilin MA, Knatz NL, Duncan MD, Fernandez SA, Wewers MD. Monocyte mRNA phenotype and adverse outcomes from pediatric multiple organ dysfunction syndrome. Pediatr Res. 2007;62(5):597–603 [DOI] [PubMed] [Google Scholar]
- 30.Muszynski JA, Nofziger R, Greathouse K, et al. Innate immune function predicts the development of nosocomial infection in critically injured children. Shock. 2014;42(4):313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong HR, Cvijanovich NZ, Anas N, et al. Improved risk stratification in pediatric septic shock using both protein and mRNA biomarkers. PERSEVERE-XP. Am J Respir Crit Care Med. 2017;196(4):494–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


