Abstract
Background
Cardiac arrhythmias have been identified as independent predictors of mortality in Coronavirus disease 2019 (COVID-19) patients. While some studies have reported poor prognosis with bradycardia in COVID-19 patients, others have not found any association between bradycardia and mortality in COVID-19 patients. This study aims to assess the relationship between bradycardia and mortality in COVID-19 patients by reviewing existing literature.
Main body
Articles were obtained by systematically searching the PubMed and Google scholar databases. Qualitative and quantitative analyses of the studies on bradycardia and mortality in COVID-19 were done. A pooled estimate, with a sample size of 1320 patients, comparing the effect of patients that were bradycardic during their admission with those that were not on mortality showed that bradycardia did not lead to increased mortality in COVID-19 patients (OR 1.25, 95% CI 0.41–3.84, p = 0.7).
Conclusions
This meta-analysis showed that bradycardia was not significantly associated with mortality in COVID-19 patients. However, this study is limited by the few studies on bradycardia and mortality in COVID-19 patients. Therefore, future studies should investigate this relationship so that clinicians can prognostically triage and treat COVID-19 patients appropriately.
Keywords: COVID-19, Bradycardia, Bradyarrhythmia, Mortality, Meta-analysis
Background
Coronavirus disease 2019 (COVID-19), a disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has affected over 260 million individuals and led to 5.2 million deaths worldwide as of late November 2021 [1]. COVID-19 is primarily considered a respiratory illness; however, it can affect multiple organ systems in the body through a systemic inflammatory response [2]. COVID-19 may result in fever, hypoxia from lung damage from the virus or the host inflammatory response, acute respiratory distress syndrome (ARDS), or multiple organ failure [3]. Additionally, COVID-19 has also been implicated in myocardial injury [4]. The myocardial damage is believed to be caused by direct virus invasion of the myocardium or by the body's inflammatory response to the virus [5]. Consequently, the fever, hypoxemia, and myocardial injury associated with COVID-19 can easily cause arrhythmia [4].
Clinicians worldwide have reported different arrhythmias in COVID-19 patients with atrial arrhythmias, sinus bradycardia, and complete heart block being the most common tachyarrhythmias and bradyarrhythmias reported [6–8]. Similarly, research studies have also reported cardiac arrhythmias, including atrial fibrillation [9, 10], ventricular arrhythmia [3], sinus tachycardia [3], and sinus bradycardia [11], as independent predictors of mortality in COVID-19 patients. While some studies have reported poor prognosis with bradycardia in COVID-19 patients [11, 12], others have not found any association between bradycardia and mortality in COVID-19 patients [10, 13]. This study aims to assess the relationship between bradycardia and mortality in COVID-19 patients by reviewing existing literature.
Main text
Study design and outcome
Our meta-analysis was designed according to the guidelines included in the PRISMA statement. Our study hypothesis is that bradycardia in COVID-19 patients is associated with mortality. A study protocol was not registered prior to the study.
Literature search and data extraction
Articles were obtained on April 10, 2022, by searching PubMed database and Google scholar titles with the keywords: bradycardia and COVID-19, bradycardia and COVID-19 mortality, bradyarrhythmia and COVID-19, bradyarrhythmia and COVID-19 mortality. The articles were manually reviewed by one of the authors and information on the number of patients that reported bradycardia and mortality was extracted into a datasheet in excel (Appendix 1). We excluded studies not written in English.
Methods for assessing the quality of studies and risk of bias
We assessed the quality of the primary studies using the National Institute of Health Study Quality Assessment Tools [14]. Two authors independently assessed the quality of the primary studies. In case of disagreement between the two authors, the matter was discussed and decided by consensus. The presence of publication bias for each outcome was assessed using funnel plots.
Data synthesis
We did qualitative data analysis by reviewing the studies, extracting essential information, and describing the similarities and differences in the study outcomes. Additionally, we calculated the odds ratio (OR) and 95% confidence interval (CI) for mortality in patients with and without bradycardia in each study and the combined composite odds ratio for mortality in the studies. We used the random effect model and tested the null hypothesis using Z-test. A p value of < 0.05 was interpreted as statistically significant. We tested heterogeneity in study outcomes using the Chi-square test and the I2 statistic. We did not perform a sensitivity analysis because of the limited number of studies in the analysis. The analysis was done using Comprehensive Meta-Analysis Version 3.
Identification of relevant studies
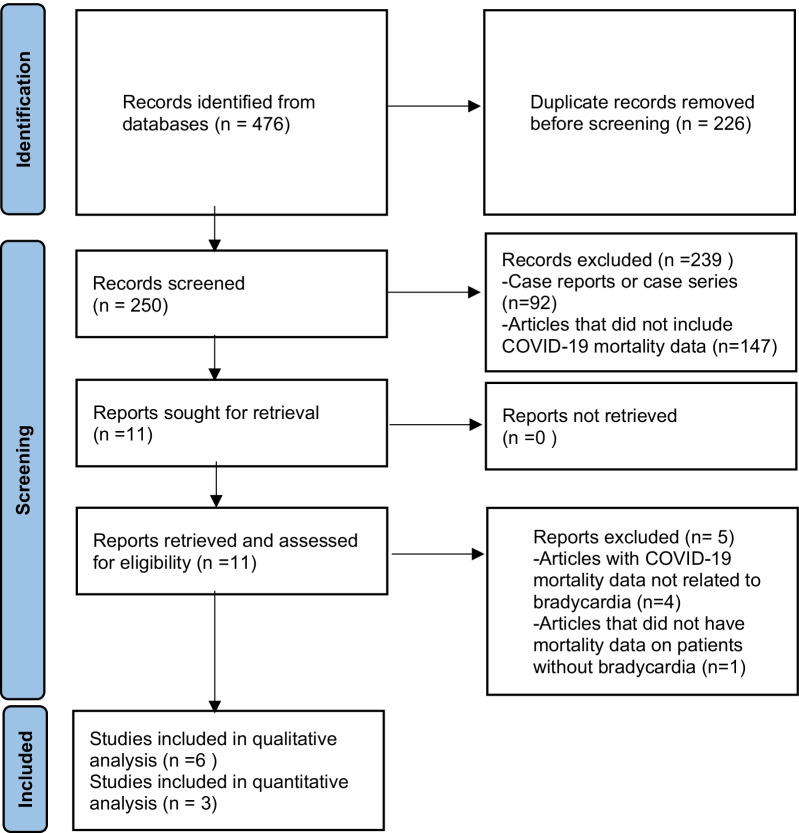
Our search produced 476 articles, of which 250 were unique articles after the removal of duplicate publications. We screened the 250 abstracts to assess if they met the inclusion and exclusion criteria in the review, after which we reviewed the full text of 11 articles. Articles excluded include case reports or case series (n = 92), articles that did not include COVID-19 mortality data (n = 147), articles with COVID-19 mortality data not related to bradycardia (n = 4), and articles that did not have mortality data on patients without bradycardia (n = 1) (Fig. 1).
Fig. 1.
Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines (PRISMA) flowchart of the selection process
In our qualitative analysis, we reviewed six studies on COVID-19 associated with bradycardia and mortality, including two conference abstracts and a study on relative bradycardia. However, our meta-analysis analyzed three studies on bradycardia and mortality in COVID-19 patients, excluding the conference abstracts and the study on relative bradycardia. The authors had access to the full text of all the relevant studies identified by the search strategy. We also made multiple unsuccessful attempts to get more data from the authors of the conference abstracts.
Risk of bias assessment
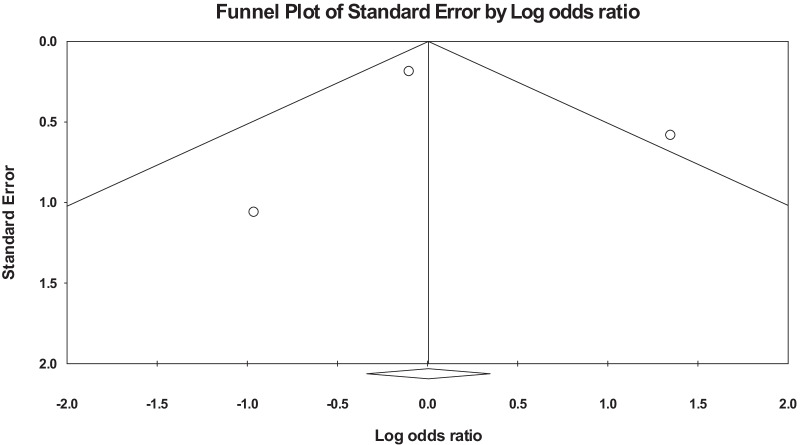
Table 1 shows the quality of each of the three primary studies in the meta-analysis using the National Institute of Health Study Quality Assessment (NIHSQA) Tool for observational cohort and cross-sectional studies. The primary studies in the meta-analysis were of good quality based on the NIHSQA tool for observational cohort and cross-sectional studies with a low risk of within-study bias. The heterogeneity between the studies was considerable with Tau2: 0.65; Chi2: 6.5; df: 2 (p = 0.038); and I2: 69.2, hence the use of the random effect model in our analysis. The funnel plot did not show any significant publication bias in the primary outcome assessed, given the meta-analysis's small number of primary studies (Fig. 2).
Table 1.
Shows the assessment of the quality of studies in the meta-analysis
| Criteria | Study | ||
|---|---|---|---|
| Chalkias et al. [16] | Kumar et al. [11] | Antwi-Amoabeng et al. [10] | |
| 1. Was the research question or objective in this paper clearly stated? | Yes | Yes | Yes |
| 2. Was the study population clearly specified and defined? | Yes | Yes | Yes |
| 3. Was the participation rate of eligible persons at least 50%? | Yes | Yes | Yes |
| 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Yes | Yes | Yes |
| 5. Was a sample size justification, power description, or variance and effect estimates provided? | NA | NA | NA |
| 6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | Yes | Yes | Yes |
| 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | Yes | Yes | Yes |
| 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | NA | NA | NA |
| 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes |
| 10. Was the exposure(s) assessed more than once over time? | NA | NA | NA |
| 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes |
| 12. Were the outcome assessors blinded to the exposure status of participants? | NR | No | NR |
| 13. Was loss to follow-up after baseline 20% or less? | Yes | Yes | Yes |
| 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | Yes | Yes | Yes |
*NA, not applicable; NR, not reported
Fig. 2.
Funnel plot of the primary studies in the meta-analysis
Qualitative result
Bradycardia and mortality in COVID-19 patients
We found three studies and two conference abstracts on bradycardia and mortality in COVID-19 patients. The two conference abstracts, Zaidi et al. and Farouji et al., and one of the observational studies, Antwi-Amoabeng et al., found no statistically significant association between bradycardia and mortality [10, 13, 15]. However, the large multicenter study by Kumar et al. found a significant association between bradycardia and mortality when bradycardic patients were compared with patients with normal heart rates [11]. Similarly, Chalkias et al. found that the onset of sinus bradycardia during their COVID-19 admission was associated with mortality in intensive care patients. [16]
Brief description of studies on bradycardia and mortality in COVID-19 patients
Kumar et al. studied bradycardia as a predictor of mortality in COVID-19 patients. The study was a multi-site retrospective observational study that included seven hospitals in Southern California and involved 1053 patients. Bradycardia was defined as a heart rate less than 60 beats per minute during continuous heart monitoring. Twenty-five percent of patients had bradycardia. The study found that patients with bradycardia were 6.59 times (95% CI [2.83–15.36]) more likely to die than a sub-set of patients with normal heart rates. Their study also showed no statistically significant correlation between hypoxia and bradycardia. However, the study did not report the odds of death of bradycardic patients compared to all those without bradycardia, including those with normal heart rate and those with tachycardia. [11]
Chalkias et al. studied the incidence of sinus bradycardia and its effect on mortality. The study was a single-center study involving 81 patients that required ICU level of care. After adjusting for different confounding factors, they found that the onset of sinus bradycardia was associated with mortality (p < 0.001). However, all the patients in the study eventually had bradycardia. Bradycardia was determined by a heart rate of less than 60 during continuous heart monitoring. They also found that sinus bradycardia was not associated with myocardial necrosis or the use of medication that induces bradycardia. [16]
Antwi-Amoabeng et al. studied the association between electrocardiographic features and mortality in 186 COVID-19 patients. An electrocardiogram (ECG) was done on the patients after testing positive for COVID-19. Only seven percent of the patients had sinus bradycardia on ECG. Bradycardia was not associated with mortality. However, A-Fib, atrial flutter, and ST-segment depression were found to be predictive of mortality. [10]
Zaidi et al. studied the association between sinus bradycardia and the severity of COVID-19 infection, including survival outcomes. The study was a multi-site retrospective observational study that included four hospitals and involved 1415 patients. They found that patients with incident bradycardia on three consecutive days were more likely to require ICU admission than those without these events (p = 0.001). However, there was no significant association between bradycardia and survival to hospital discharge (p = 0.761). Conversely, they found that tachycardia on day-3 of admission compared to normal heart rate was statistically significantly associated with 7-day mortality (p < 0.001).
Farouji et al. studied the relationship between transient sinus bradycardia on initial admission and clinical outcomes in 490 COVID-19 patients. They found that patients with transient sinus bradycardia on hospital admission were more likely to require intubation than those without it (p = 0.041). Additionally, transient sinus bradycardia on admission was associated with the risk of mortality (p = 0.052), though not statistically significant.
Relative bradycardia and mortality in COVID-19 patients
Oliva et al. studied the association between relative bradycardia and mortality in 101 COVID-19 patients with a fever of ≥ 38.3 °C. In the study, relative bradycardia was defined as heart rate < 90 bpm and concomitant fever (temperature ≥ 38.3 °C). Forty-two percent of these patients had relative bradycardia. They found that ICU admission and in-hospital mortality were not associated with relative bradycardia. [17]
Quantitative result
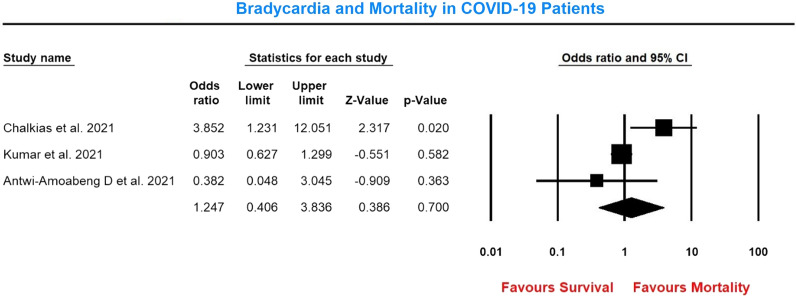
The pooled estimate of the effect of bradycardia on mortality in COVID-19 patients compared to patients who were not bradycardic with 1320 patients showed that bradycardia did not lead to increased mortality in COVID-19 patients (OR 1.25, 95% CI 0.41–3.84, p = 0.7) (Fig. 3).
Fig. 3.
Forest plot showing bradycardia and mortality in COVID-19 patients. Bradycardia group n/N: 99/339; Non-Bradycardia group n/N: 191/981. Heterogeneity: Tau2: 0.65; Chi2: 6.5; df: 2 (p = 0.038); I2: 69.2
Discussion
Our study found that bradycardia was not associated with mortality in COVID-19 patients. This result is contrary to the largest study on bradycardia and mortality by Kumar et al. that showed that bradycardia was predictive of mortality in COVID-19 patients. [11] The possible explanation for this is that Kumar et al. reported bradycardia being associated with mortality when bradycardic patients are compared to a subset of patients with normal heart rates. However, they excluded patients with tachycardia. Our study compared bradycardic patients with non-bradycardic patients, including patients with normal heart rates and tachycardic patients. Tachycardia has been associated with increased mortality in COVID-19 patients [3], and this might have explained why bradycardia was not associated with mortality in our study. Similarly, Olivia A et al. did not find any significant association between relative bradycardia, which is defined as heart rate < 90 bpm, and concomitant fever (temperature ≥ 38.3 °C) and mortality in COVID-19 patients. [17]
The average incidence of bradycardia in the three studies in our meta-analysis was 26%. The large variation in the incidence of bradycardia in the primary studies may be due to the difference in the study design. Kumar et al. and Chalkias et al. used continuous cardiac monitoring to determine bradycardia in all hospitalized COVID-19 patients and patients admitted to the ICU and reported a bradycardia incidence of 24.9% and 79%, respectively. [10, 16] Conversely, Antwi-Amoabeng et al. used ECG done after COVID-19 diagnosis to determine bradycardic patients and reported an incidence of seven percent [10]. Thus, using an ECG done after the COVID-19 diagnosis will likely underestimate the incidence of sinus bradycardia in COVID-19 patients. Conversely, Chalkias et al. measured bradycardia in only ICU patients and will likely lead to over-estimation of bradycardia. [16]
The mechanism of bradycardia in COVID-19 appears to be multifactorial. It could be caused by the direct pathogenic effect of the virus on the myocardium, hypoxia, drug toxicity, severe downregulation of myocardial angiotensin-converting enzyme 2 (ACE2) pathways resulting in myocardial inflammation, or the effect of the inflammatory cytokines on the heart [10, 18, 19]. However, the direct pathogenic effect of the COVID-19 virus on pacemaker cells and the effect of the systemic inflammation on cardiac pacemaker cells are the two most commonly proposed mechanisms of bradycardia [18, 19]. Beta-blockers and hypoxia were not associated with bradycardia in both Kumar et al. and Chalkias et al. studies. [11, 16]
Similarly, relative bradycardia has been reported in COVID-19 patients [17, 20, 21]. Physiologically, the heart rate is expected to increase by about ten beats for each degree increase in temperature in degrees Fahrenheit. Thus, a temperature of 101 °F (38.3 °C) is expected to have a corresponding heart rate of about 110 [22]. Relative bradycardia has been reported in infectious processes like typhoid and Q-fever and non-infectious processes like drug fever. The most common cause of relative bradycardia in patients with fever is the use of beta-blocker medications [22]. However, beta-blockers have not been associated with bradycardia in COVID-19 and are unlikely to cause relative bradycardia seen in COVID-19 patients [11, 16]. The direct pathogenic effect of the COVID-19 virus on cardiac pacemaker cells and/or the effect of inflammatory cytokines on cardiac pacemaker cells has been proposed as possible causes of relative bradycardia. [20, 23]
Limitations of the study
One of the major limitations is the few studies combined in the meta-analysis due to the limited studies that reported bradycardia and its association with mortality in COVID-19 patients. Thus, the small number of studies and participants may make it difficult to detect an effect, even if one exists, so the results of this analysis should be interpreted with caution. Furthermore, two conference abstracts identified during the literature search were not included in the meta-analysis because of incomplete data, which could potentially bias our study's result. However, both conference abstracts did not find any statistically significant relationship between bradycardia and COVID-19 mortality, which is consistent with the findings of our meta-analysis. Additionally, the heterogeneity of the studies in the meta-analysis is considerable and could have affected the conclusion of our study. However, we mitigated the effect of the heterogeneity in our analysis by using a random-effects model for the meta-analysis, which assumes that the primary studies are heterogeneous and gives a more conservative estimate of effect. Finally, our study is a combination of observational studies, and there could have been unmeasured or unadjusted confounders that affected the study outcome in the primary studies.
Implications of the results for practice, policy, and future research
Our study showed that bradycardia was not significantly associated with mortality in COVID-19 patients. The implication for practice is that there might be no mortality benefit in aggressively treating asymptomatic sinus bradycardia in COVID-19 patients.
The opportunities for future research include more studies to investigate the relationship between bradycardia and mortality in COVID-19 patients. Some studies have suggested that bradycardia in COVID-19 patients might be harbingers of a more severe disease. Therefore, understanding that association may help clinicians prognostically triage the patients appropriately. Additionally, it may be interesting to see the effect of bradycardia in different sub-groups of COVID-19 patients, such as symptomatic vs. asymptomatic patients and younger vs. older patients.
Conclusions
The result of this meta-analysis showed that bradycardia was not significantly associated with mortality in COVID-19 patients. However, this study is limited by the few studies on bradycardia and mortality in COVID-19 patients. Therefore, future studies should investigate this relationship so clinicians can prognostically triage and treat COVID-19 patients appropriately.
Acknowledgements
Not applicable.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- ACE2
Angiotensin-converting enzyme 2
- ARDS
Acute respiratory distress syndrome
- CI
Confidence interval
- COVID-19
Coronavirus disease 2019
- NIHSQA
National Institute of Health Study Quality Assessment
- OR
Odds ratio
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
Appendix
See Table 2.
Table 2.
Summary of studies included in the meta-analysis
| Study | Country of study | Definition of bradycardia | Patients analyzed in the study | Patients that had bradycardia | Patients without bradycardia | Number that died in bradycardia group | Number that died in non-bradycardia group |
|---|---|---|---|---|---|---|---|
| Chalkias et al. [16] | Greece | Heart rate ≤ 60 BPM on continuous heart rate monitoring | Consecutive patients requiring ICU admission | 64 | 17 | 52 | 9 |
| Kumar et al. [11] | USA | Heart rate < 60 BPM on two separate occasions, a minimum of 4 h apart, on continuous cardiac monitoring | All consecutive COVID-19 patients admitted during the study period | 262 | 791 | 46 | 151 |
| Antwi-Amoabeng et al. [10] | USA | Bradycardia on cardiologist-confirmed ECG report | All consecutive COVID-19 patients admitted during the study period | 13 | 173 | 1 | 31 |
BPM: beats per minute, ICU: intensive care unit, ECG: electrocardiogram
Author contributions
CU, SK, EW and PB were involved in conceptualizing the idea of the study. CU and EW extracted the data with inputs from SK and PB. CU analyzed and interpreted the data with input from SK, EW and PB. CU wrote the first draft of the paper. SK, EW and PB were involved in substantially reviewing and revising the paper. All authors read and approved the final manuscript.
Funding
No funding was received for conducting this study.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.CDC. U.S. Department of Health and Human Services. COVID Data Tracker. Accessed on November 24, 2021 from https://covid.cdc.gov/covid-data-tracker/#trends_totaldeaths
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Zhang S, He B, Chen X, Wang S, Zhao Q. Risk factors and electrocardiogram characteristics for mortality in critical inpatients with COVID-19. Clin Cardiol. 2020;43(12):1624–1630. doi: 10.1002/clc.23492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu L, Gong L, Jiang Z, et al. Clinical analysis of sinus bradycardia in patients with severe COVID-19 pneumonia. Crit Care. 2020;24:257. doi: 10.1186/s13054-020-02933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao SC, Shao SC, Cheng CW, Chen YC, Hung MJ. Incidence rate and clinical impacts of arrhythmia following COVID-19: a systematic review and meta-analysis of 17,435 patients. Crit Care. 2020;24(1):1–7. doi: 10.1186/s13054-019-2683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatla A, Mayer MM, Adusumalli S, Hyman MC, Oh E, Tierney A, Moss J, Chahal AA, Anesi G, Denduluri S, Domenico CM. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17(9):1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gopinathannair R, Merchant FM, Lakkireddy DR, Etheridge SP, Feigofsky S, Han JK, Kabra R, Natale A, Poe S, Saha SA, Russo AM. COVID-19 and cardiac arrhythmias: a global perspective on arrhythmia characteristics and management strategies. J Interv Card Electrophysiol. 2020;59:329–336. doi: 10.1007/s10840-020-00789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peltzer B, Manocha KK, Ying X, Kirzner J, Ip JE, Thomas G, Liu CF, Markowitz SM, Lerman BB, Safford MM, Goyal P. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J Cardiovasc Electrophysiol. 2020;31(12):3077–3085. doi: 10.1111/jce.14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antwi-Amoabeng D, Beutler BD, Singh S, et al. Association between electrocardiographic features and mortality in COVID-19 patients. Ann Noninvasive Electrocardiol. 2021;26(4):e12833. doi: 10.1111/anec.12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar S, Arcuri C, Chaudhuri S, et al. A novel study on SARS-COV-2 virus associated bradycardia as a predictor of mortality-retrospective multicenter analysis. Clin Cardiol. 2021;44(6):857–862. doi: 10.1002/clc.23622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinitz JS, Goyal R, Harding M, Veseli G, Gruberg L, Jadonath R, Maccaro P, Gandotra P, Ong L, Epstein LM. Bradyarrhythmias in patients with COVID-19: marker of poor prognosis? Pacing Clin Electrophysiol. 2020;43(10):1199–1204. doi: 10.1111/pace.14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaidi SA, Bollam R, Stalder M, Amanzai A, Mamauag F, Saleem K, Abdulmajeed F. Sinus bradycardia as a predictor of severity of covid-19 infection: tranquility before chaos. Chest. 2021;160(4):A577. doi: 10.1016/j.chest.2021.07.555. [DOI] [Google Scholar]
- 14.NIH. Study Quality Assessment Tools. Accessed on November 10, 2021 from https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 15.Farouji I, Chan KH, Damati A, Kiwan C, Battah A, Afaq S, Abed H, Habib M, Slim J. IS Bradycardia a prognostic indicator of COVID-19 mortality? A single center retrospective cohort analysis. J Am College Cardiol. 2021;77(18_Supplement_1):3085.
- 16.Chalkias A, Pantazopoulos I, Papagiannakis N, et al. Sinus bradycardia is associated with poor outcome in critically ill patients with COVID-19 due to the B.1.1.7 Lineage. Toxicol Rep. 2021;8:1394–1398. doi: 10.1016/j.toxrep.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliva A, Franchi C, Gatto MC, Galardo G, Pugliese F, Mastroianni C. Prevalence and clinical significance of relative bradycardia at hospital admission in patients with coronavirus disease 2019 (COVID-19) Clin Microbiol Infect. 2021;27:1185. doi: 10.1016/j.cmi.2021.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31(5):1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amaratunga EA, Corwin DS, Moran L, Snyder R. Bradycardia in patients with COVID-19: a calm before the storm? Cureus. 2020. 12(6). [DOI] [PMC free article] [PubMed]
- 20.Capoferri G, Osthoff M, Egli A, Stoeckle M, Bassetti S. Relative bradycardia in patients with COVID-19. Clin Microbiol Infect. 2021;27:295. doi: 10.1016/j.cmi.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeuchi K, Saito M, Yamamoto S, Nagai H, Adachi E. Relative bradycardia in patients with mild-to-moderate coronavirus disease, Japan. Emerg Infect Dis. 2020;26(10):2504. doi: 10.3201/eid2610.202648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunha BA. The diagnostic significance of relative bradycardia in infectious disease. Clin Microbiol Infect. 2000;6(12):633–634. doi: 10.1046/j.1469-0691.2000.0194f.x. [DOI] [PubMed] [Google Scholar]
- 23.Ye F, Winchester D, Stalvey C, Jansen M, Lee A, Khuddus M, Mazza J, Yale S. Proposed mechanisms of relative bradycardia. Med Hypotheses. 2018;1(119):63–67. doi: 10.1016/j.mehy.2018.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.