Abstract
Opportunistic infections are serious complications in critically ill COVID-19 patients, especially co-infections with bacterial and fungal agents. Here we report a rare case of bloodstream co-infection by Trichosporon asahii, an emerging yeast, and Acinetobacterbaumannii, an opportunistic nosocomial pathogen, both multidrug resistant, in a tertiary hospital from southern Brazil. A review of the literature regarding similar cases is also included. Treatment with multiple antimicrobials failed, and the patient progressed to death four days after the diagnosis of bacteremia and fungemia.
Keywords: Trichosporon spp., Emerging pathogens, Opportunistic disease, Fungemia, COVID-19 co-infections, Multi-drug resistance, Sepsis
Introduction
The current Coronavirus Disease 2019 (COVID-19) pandemic caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) has already affected more than 365 million people, resulting in more than 5 million deaths worldwide [1]. Brazil ranks third among countries in the number of cases of the disease (24.5 million), behind only the United States (72.3 million) and India (40,6 million). Moreover, the number of deaths in Brazil (more than 600 thousand) is the second largest in the world ranking [1]. Cases of COVID-19 that progress to severe acute respiratory syndrome (SARS) and to the necessity for intensive care unit (ICU) admission imply multiple risk factors for co-infections [2]. Bacterial and fungal sepsis in these patients is not uncommon [3], and contributes to a poor prognosis and increased mortality [2, 3].
Fungal diseases more frequently described in COVID-19 patients are caused by Aspergillus spp. [2, 3], Candida spp. and mucormycetes [3–5]. Although other fungal agents have also been reported, Trichosporon spp. is rare in this scenario [4, 6]. In the last two decades, Trichosporon asahii, a yeast-like basidiomycete that may be present in the intestinal microbiota, has emerged from a rare opportunistic pathogen [7] to a global worry, especially owing to its profile of resistance to antifungal agents [4, 8]
Among bacterial co-infections, the most prevalent etiological agents are coagulase-negative Staphylococcus (CNS), probably associated with the use of catheters, followed by anaerobic gram negative bacilli, present in the intestinal microbiota [3]. Non-fermenting Gram-negative bacilli such as carbapenem-resistent A. baumanii (CRAB) is an opportunistic and emerging pathogen that is an urgent health problem, worsening the actual pandemic [2].
Here we report the first case of sepsis due to Trichosporon asahii and Acinetobacter baumannii, in a critically ill COVID-19 patient, from a tertiary hospital in southern Brazil. In addition, a brief review of the literature regarding Trichosporon spp. infection in COVID-19 patients is included.
Case
A woman, 58 years old, obese, hypertensive and diabetic, sought the emergency service owing to “flu-like” symptoms with respiratory distress. She was hospitalized due to a drop in oxygen saturation (90–91% SO2), on May 7th (D0), and the diagnosis of COVID-19 was confirmed by RT-PCR on May 8th (D + 1). The patient was conscious and wearing an oxygen mask, with saturation levels ranging from 93 to 98%. On May 10th (D + 3), mechanical ventilation was instituted owing to an abrupt drop in her oxygen saturation (53%). Tomography confirmed about 70% lung damage, and therapy with ceftriaxone 2 g/day, dexamethasone 10 mg/day and heparin 5000 u/3 times a day was started.
On the next day (D + 4), the patient showed cyanosis and cold extremities, cardiogenic shock, and 60% saturation. The oxygen fraction was increased to 100% (FIO2), and treatment with sodium piperacillin + sodium tazobactam (2.0 g + 0.5 g every six hours) was started. Laboratory tests and arterial blood gases showed respiratory acidosis (pH 7.2 and pCO2 93.3 mm Hg), with acute renal failure (urea = 115 mg/dL, creatinine = 3.8 mg/dL). Decompensated diabetes, despite insulin, (glucose 172 mg/dL), and liver profile deterioration (AST 160 U; ALT 149 U) were also detected. On May 13 (D + 6), hemodialysis was started without response, and worsening of her renal function was confirmed after 3 days (urea = 200 mg/dL, creatinine = 5.8 mg/dL).
On May 17 (D + 9), she was admitted to the COVID-Intensive Care Unit (ICU), where she remained in critical condition with no improvement in respiratory or renal function and with recurrent episodes of diarrhea. After six days (D + 15), Acinetobacter baumannii complex producing carbapenemases was isolated in a peri-anal surveillance swab and tracheal aspirate, and treatment was started with vancomycin 500 mg/day and meropenem 1 g/day. In the next two days (D + 17), blastoconidia and arthroconidia were seen in the microscopy of the patient's blood culture, which resulted in the growth of Acinetobacter baumannii and Trichosporon asahii (BACTEC™ 9050™) (Fig. 1). The yeast was identified phenotypically and the species confirmed by MALDI-TOF. Treatment with antibacterials was maintained, but later bacterial identification and antimicrobial susceptibility analysis were performed via the automated system BD Phoenix®. The isolate was resistant to third-generation cephalosporins (ceftazidime and cefepime), fluoroquinolones (ciprofloxacin and levofloxacin), aminoglycosides (gentamicin), trimethoprim + sulfamethoxazole and carbapenems (imipenem and meropenem) and was susceptible only to ampicillin-sulbactam and amikacin.
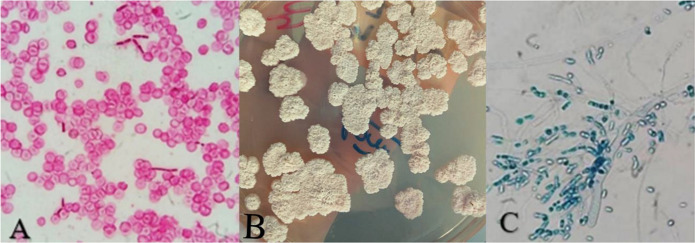
Fig. 1.
A. Blastoconidia and arthroconidia in Gram stain of blood culture sample. B. Isolation of T. asahii from a blood culture sample on Sabouraud Agar medium. C. Microscopy (lactophenol blue stain) of this sample showing the presence of blastoconidia, hyaline hyphae and arthroconidia. (color figure)
Treatment with intravenous amphotericin B deoxycholate 50 mg/day was started the same day, but the patient clinically deteriorated, showing episodes of desaturation, worsening in ventilatory function and CO2 retention, instability in systemic blood pressure, marked edema of the limbs and cyanotic extremities. With refractory hypoxia and a consistent drop in blood pressure, the patient died on May 29 (D + 20) (Fig. 2). Postmortem results of the antifungal susceptibility test, performed by broth microdilution technique, showed susceptibility to fluconazole (MIC = 2 µg/mL) but resistance of the isolate to amphotericin B (MIC = 4 µg/mL).
Fig. 2.
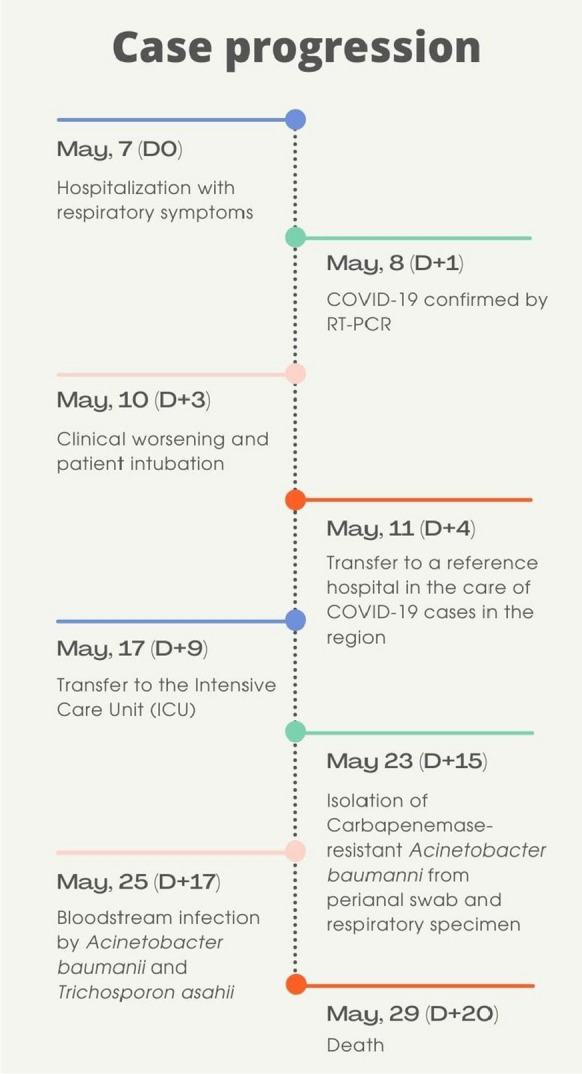
Timeline of the case reported, showing the progression of a critically ill Covid-19 patient with bloodstream infection by T. asahii and A. baumanni, from her symptoms onset to her fatal outcome
Literature Review
A search for reports of Trichosporon spp. infections on COVID-19 patients was carried out in the main scientific databases, Pubmed, Scielo, Lilacs, Bireme, using the descriptor “Trichosporon AND COVID-19”. Cases reports, series of cases, or prevalence studies published in English, Portuguese and/or Spanish were included. Review articles, letters to the editor, survey of colonized patients, or non-concomitant infections were excluded from the review (Fig. 3).
Fig. 3.

Flowchart showing the selection of the articles included in the literature review of Trichosporon spp. co-infection with SARS-CoV-2
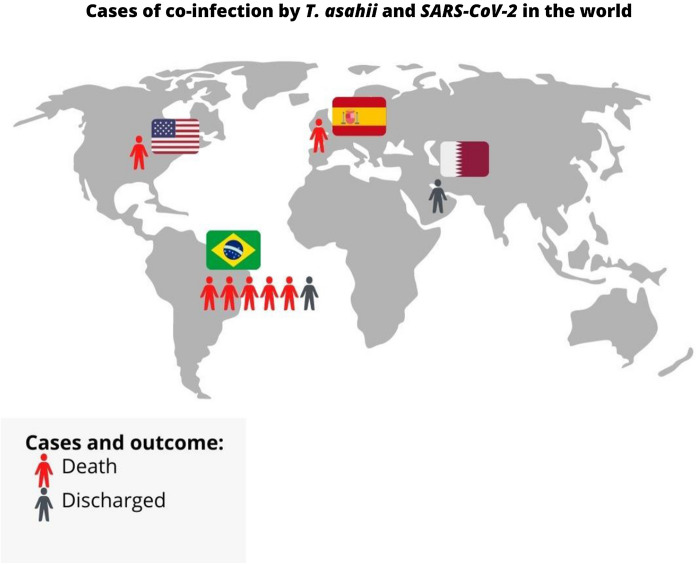
Only four studies reporting a total of 9 cases of Trichosporon spp. and SARS-CoV-2 co-infection were described in the scientific literature until January 2022. Cases have been reported in different continents, including North America (n = 1) [10], South America (n = 6 cases including this report [4]), Europe (n = 1) [6] and the Middle East (n = 1) [9] (Fig. 4), presenting as nosocomial pneumonia [6], fungemia [4, 9] or urinary tract infection [10]. All cases described were caused by Trichosporon asahii (Table 1).
Fig. 4.
Cases and outcomes of co-infection by T. asahii and SARS-CoV-2 in the world reported until January, 2022
Table 1.
Cases of Trichosporon asahii infection in COVID-19 patients reported in the literature from the onset of the COVID-19 pandemic to January 2022
| Conditions | Cases | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ref | Our case report | 4 | 4 | 4 | 4 | 4 | 6 | 9 | 10 |
| T. asahii disease | Invasive | Invasive | Invasive | Invasive | Invasive | Invasive | Pulmonarya | Invasive | Urinary |
| Local | Brazil | Brazil | Brazil | Brazil | Brazil | Brazil | Spain | Qatar | USA |
| Age | 58 | 57 | 74 | 75 | 73 | 72 | 58 | 58 | 73 |
| Sex | Female | Male | Male | Female | Male | Male | Male | Male | Male |
| Obesity | Yes | No | No | Yes | Yes | No | No | No | No |
| Diabetes | Yes | Yes | No | No | Yes | No | No | No | No |
| Days in ICU | 8 | 30 | 31 | 27 | 15 | 11 | 26 | 24 | > 60 |
| Mechanical ventilation time (days) | 19 | 30 | 27 | 27 | 15 | 11 | 26 | 24 | > 60 |
| Previous receipt of broad-spectrum antibiotics | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Previous receipt of echinocandins | No | Yes | Yes | Yes | Yes | Yes | No | No | No |
| Previous receipt of steroids | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hemodialysis | Yes | No | Yes | Yes | Yes | Yes | No | No | No |
| Diarrhea episodes | Yes | Yes | Yes | Yes | No | No | No | No | No |
| Bacteremia (pathogen) |
Yes AB |
No |
Yes EN |
No | No |
Yes EN |
Yes PAM/SM |
No |
Yes SM/CK |
| Previous candidemia | No | Yes | Yes | Yes | No | No | No | No | No |
| Absolute lymphopenia | No | No | Yes | No | Yes | Yes | NI | Yes | No |
| Antifungal therapy | AMB | VOR | No treatC | VOR + AMB-L | VOR + AMB-L | VOR + AMB-L | VOR | VOR |
FLUd VOR |
| Outcome | Death | Aliveb | Death | Death | Death | Death | Death | Discharged | Death |
aPatient died from septic shock, however the agent found in the bloodstream was not identified.
bFollowed for 30 days after diagnosis of fungemia.
cPatient died before the antifungal therapy.
dBefore the results of susceptibility tests. AB: Acinetobacter baumannii, EN: Enterococcus spp., PAM: Pseudomonas aeruginosa MDR, SM: Stenotrophomonas maltophila, CK: Citrobacter koser NI, not informed. AMB: Amphotericin B, AMB-L: Amphotericin B—liposomal, VOR: Voriconazole, FLU: fluconazole
The infections occurred predominantly in men (77.7%), aged 57 years or more (mean age 66.4 and median age 72 years), with severe presentation of COVID-19. All patients (9/9) were given broad-spectrum antibiotic therapy and systemic corticoids, and 55.5% (5/9) had previously received antifungal drugs. The length of stay in the ICU ranged from 8 to > 60 days (median 26 days), and mechanical ventilation from 11 to > 60 days (median 26 days). More than 40% of the patients (4/9) had episodes of diarrhea and 55.5% co-infection with bacteremia (5/9). The mortality rate was 77.7% (7/9), despite the different treatment regimens used by patients (Table 1).
There were no reports of bloodstream co-infection by Trichosporon spp. and Acinetobacter spp. in patients with or without COVID-19 published in the literature up to January, 2022.
Discussion
Critically ill COVID-19 patients often have prolonged hospital stays, mechanical ventilation and multiple catheters, as well as being “challenged” by immunosuppressive drugs and broad-spectrum antibiotics, promoting intense dysbiosis and rupture of immune barriers, and increasing their risk of developing opportunistic diseases [3, 4, 10]. Secondary bacterial and fungal infections prevalence ranges from 5 to 30% and are associated with a higher mortality rate in COVID-19 patients [2, 3, 5]. Our study is the first to report a fatal bloodstream co-infection by two emerging opportunistic pathogens, Trichosporon asahii and Acinetobacter baumannii, highlighting the difficulty in managing a patient infected by multidrug-resistant fungi and bacteria [2, 3].
Trichosporon spp. is one of the most important yeasts causing fungemia, only Candida spp. and Cryptococcus spp are more common [11, 12]. Although invasive trichosporonosis represents from 1 to 6% of all cases of fungal sepsis [7, 8], we found fewer than ten cases of fungemia by Trichosporon spp. in COVID-19 patients described in the scientific literature. Interestingly, the majority of them are from Brazil, with five cases from an outbreak in a hospital from the Northeast region [4], and here we report the first case in the Southern region of Brazil. The total number of cases registered in Brazil is higher than all cases together described worldwide. The more plausible explanation is the outbreak reported, where five cases occurred in a short period of time (3 months) in a single center, suggesting an uncommon horizontal transmission of the pathogen [4].
In the last two decades, the incidence of trichosporonosis has increased significantly, affecting mainly immunosuppressed patients with hematological diseases [13], however the current COVID-19 pandemic seems to be an additional factor for the re-emerging of this yeast [4, 12]. The main concern is its resistance to antifungal drugs, including echinocandins and amphotericin B [8]. Although the combined therapy with Amphotericin B and 5-Flucitosine has been suggested, few data are available [13]. In vitro susceptibility profile shows that voriconazole is the antifungal of choice against Trichosporon spp. [8, 13, 14], being the treatment with this azole recommended by the ESCMID/ECMM guidelines [15].
Systemic infection by this emergent yeast frequently leads to an acute fatal condition, as described in our patient. The high mortality rate (86%) of invasive Trichosporon asahii disease in COVID-19 patients found in our brief review of the literature is in agreement with the rates reported in other groups of patients, not infected by SARS-CoV-2, which ranges from 45 to 90% [4, 8]. Most of the cases of systemic trichosporonosis caused by T. asahii in COVID-19 patients described in the literature had also bacteremia episodes associated, mainly with bacterial species of the gastrointestinal microbiota [4, 6]. Dysbiosis increases the possibility of microorganism translocation as a source of opportunistic pathogen infections in critically ill patients [2, 7, 11].
It is estimated that the cumulative risk of developing a bloodstream infection increases by 25% after 48 h of ICU stay [2, 3, 16]. Our patient was > 10 days in the ICU in mechanical ventilation, and eight days after sepsis by A. baumannii, the T. asahii fungemia was confirmed. Patients with extreme obesity, diabetes or who have a delay between the onset of symptoms and admission to the ICU have a higher risk of mortality [3]. Our patient had these risk factors, aggravated by multiple co-infections, by SARS-CoV-2 and two multi-resistant pathogens.
Despite the institution of an aggressive treatment (vancomycin and meropenem for bacteremia and amphotericin B for fungemia) our patient died 72 h after the diagnosis of bloodstream infection. Unfortunately, A. baumannii was multidrug resistant and T. asahii was resistant to amphotericin, but the susceptibility profile information was only available to the physicians when the patient had already died. A shorter turnaround to obtain the microorganism susceptibility profile is still a limitation for a rational use of antimicrobials [4, 6, 10].
Voriconazole seems to be the best option to treat fungemia by Trichosporon spp. [8, 17]. This drug is approved by ANVISA (National Health Surveillance Agency) in Brazil for the treatment of serious invasive infections, but it is not included in the Pharmaceutical Assistance list of the Brazilian Unified Health System (SUS) [19], which makes it promptly access unavailable, and often makes timely treatment unfeasible.
The COVID-19 pandemic brought a further challenge to the rational use of medicines, and already shows a negative impact on antimicrobial resistance [2, 5, 16]. The difficulty in treating infections caused by multidrug resistant pathogens is reflected in the low survival rate of patients [5, 16]. The management of critically ill patients in COVID-19 must consider the rational use of antibiotics and the increased risk of secondary infections [3, 4, 7], even by uncommon emergent pathogens [11, 12, 18]. There is an urgent need to create and implement diagnostic algorithms for the early detection and treatment of secondary infections in COVID-19 patients [19]. Nebreda-Mayoral and collaborators reflect that despite the use of personal protective equipment, difficulties of overcrowding, the possibility of poorly trained teams and other unfavorable factors that contribute to the emergence of nosocomial infection outbreaks, ongoing review of hospital protocols for infection prevention is mandatory for the control and diagnosis of secondary infections [19].
We report the first case of co-infection by multiresistant T. asahii and A. baumannii in a COVID-19 patient, reinforcing the importance of screening for secondary infections in severe COVID-19 patients. Multiple co-infections of different etiologies cannot be ruled out [11, 12, 18]. The early diagnosis of these co-infections is essential to start the most appropriate therapy for each case, to improve the prognosis of the patient.
Acknowledgements
The authors are grateful to Graduate Program in Health Sciences (PPGCS), Faculty of Medicine, Federal University of Rio Grande (FURG), Rio Grande, Brazil; to University Hospital Dr. Miguel Riet Correa (HU-FURG/EBSERH), Rio Grande. Brazil; to Coordination for the Improvement of Higher Education Personnel (CAPES) Brazil and the National Research Council (CNPq), and to the Institutional Internationalization Project of the Federal University of Rio Grande (PrInt-FURG).
Authors Contributions
All authors contributed to the study conception and design, according description below: JLB: Study design, development, collection and data interpration, diagnostic evaluation, case discussion, and writing all. RPB: Collection and data interpration, case discussion, manuscript revision. TWG: Patient follow-up and case discussion, diagnostic evaluation and therapeutic decisions, collection of data, manuscript revision. VRP: Study design, development, data interpration, writing sections and manuscript revision. LSM: Collection and data interpration, case discussion, manuscript revision. KBM: Collection and data interpration, case discussion, writing sections and manuscript revision. HEZ: Collection and data interpration, case discussion, writing sections and manuscript revision. AVG: Collection and data interpration, case discussion, writing sections and manuscript revision. CBS: data interpretation, diagnostic evaluation, case discussion, manuscript revision. DA.S: Study design, case discussion, writing all, manuscript revision and review of writing in english by native speaker. MOX: Study design, development, collection and data interpration, diagnostic evaluation, case discussion, writing all and manuscript revision.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics Approval
This project was approved by the Health Research Ethics Committee of the Federal University of Rio Grande (FURG), under number 234/2018, which dispenses with the need to sign the consent form in retrospective case reports.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Acessed January 29, 2022.
- 2.Lai CC, Chen SY, Ko WC, Hsueh PR. Increased antimicrobial resistance during the COVID-19 pandemic. Int J Antimicrob Agents. 2021;57(4):1–6. doi: 10.1016/j.ijantimicag.2021.106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ripa M, Galli L, Poli A, Oltolini C, Spagnuolo V, Mastrangelo A, et al. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect. 2021;27(3):451–457. doi: 10.1016/j.cmi.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nobrega de Almeida Jr J, Moreno L, Francisco EC, Noronha Marques G, Mendes AV, et al. Trichosporon asahii superinfections in critically ill COVID-19 patients overexposed to antimicrobials and corticosteroids. Mycoses. 2021;64(8):817–822. doi: 10.1111/myc.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meawed TE, Ahmed SM, Mowafy SMS, Samir GM, Anis RH. Bacterial and fungal ventilator associated pneumonia in critically ill COVID-19 patients during the second wave. J Infect Public Health. 2021;14(10):1375–1380. doi: 10.1016/j.jiph.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segrelles-Calvo G, Araújo GRS, Llopis-Pastor E, Frasés S. Trichosporon asahii as cause of nosocomial pneumonia in patient with COVID-19: a triple co-infection. Arch Bronconeumol. 2021;1(57):46–48. doi: 10.1016/j.arbres.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamagata E, Kamberi P, Yamakami Y, Hashimoto A, Nasu M. Experimental model of progressive disseminated Trichosporonosis in mice with latent Trichosporonemia. J Clin Microbiol. 2000;38(9):3260–3266. doi: 10.1128/JCM.38.9.3260-3266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo SH, Lu PL, Chen YC, et al. The epidemiology, genotypes, antifungal susceptibility of Trichosporon species, and the impact of voriconazole on Trichosporon fungemia patients. J Formos Med Assoc. 2021;120(9):1686–1694. doi: 10.1016/j.jfma.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Ali GA, Husain A, Salah H, Goravey W. Trichosporon Asahii fungemia and Covid-19 co-infection: an emerging fungal pathogen; case report and review of the literature. IDCases. 2021;25:e01244. doi: 10.1016/j.idcr.2021.e01244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cronyn V, Howard J, Chiang L, Le L, Tims-Cook Z, Gertz AM. Trichosporon asahii urinary tract infection in a patient with severe COVID-19. Case Rep Infect Dis. 2021;2021:6841393. doi: 10.1155/2021/6841393.PMID:34925928;PMCID:PMC8683162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming RV, Walsh TJ, Anaissie EJ. Emerging and less common fungal pathogens. Infect Dis Clin North Am. 2002;16(4):915–933. doi: 10.1016/S0891-5520(02)00041-7. [DOI] [PubMed] [Google Scholar]
- 12.de Almeida Júnior JN, Hennequin C. Invasive Trichosporon infection: a systematic review on a re-emerging fungal pathogen. Front Microbiol. 2016 doi: 10.3389/fmicb.2016.01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Guo M, Wang C, et al. Epidemiological Study of Trichosporon asahii infections over the past 23 years. Epidemiol Infect. 2020;148:e169. doi: 10.1017/S0950268820001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta V, Chander J, Gulati N, et al. Epidemiological profile and antifungal susceptibility pattern of Trichosporon species in a tertiary care hospital in Chandigarh India. Curr Med Mycol. 2021;7(1):19–24. doi: 10.18502/cmm.7.1.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arendrup MC, Boekhout T, Akova M, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect. 2014;20(Suppl 3):76–98. doi: 10.1111/1469-0691.12360. [DOI] [PubMed] [Google Scholar]
- 16.Palanisamy N, Vihari N, Meena DS, Kumar D, Midha N, Tak V, Sharma A, et al. Clinical profile of bloodstream infections in COVID-19 patients: a retrospective cohort study. BMC Infect Dis. 2021;21(1):933. doi: 10.1186/s12879-021-06647-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ministério da Saúde, MS, Brazil. Nota Técnica N° 63, 2012 -Voriconazol. Available in: https://www.gov.br/saude/pt-br/composicao/conjur/demandas-judiciais/notas-tecnicas/notas-tecnicas-medicamentos/notas-tecnicas/v/voriconazol-atualizada-em-15-10-2013.pdf. Acessed on January, 29, 2022.
- 18.Walsh TJ, Groll A, Hiemenz J, Fleming R, Roilides E, Anaissie E. Infections due to emerging and uncommon medically important fungal pathogens. Clin Microbiol Infect. 2004;1:48–66. doi: 10.1111/j.1470-9465.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- 19.Nebreda-Mayoral T, Miguel-Gómez MA, March-Rosselló GA, Puente-Fuertes L, Cantón-Benito E, Martínez-García AM, et al. Bacterial/fungal infection in hospitalized patients with COVID-19 in a tertiary hospital in the Community of Castilla y León, Spain. Enferm Infecc Microbiol Clin (Engl Ed) 2020 doi: 10.1016/j.eimc.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]