Abstract
Background
People with MS treated with anti-CD20 therapies and fingolimod often have attenuated responses to initial COVID-19 vaccination. However, uncertainties remain about the benefit of a 3rd (booster) COVID-19 vaccine in this group.
Methods
PwMS without a detectable IgG response following COVID-19 vaccines 1&2 were invited to participate. Participants provided a dried blood spot +/- venous blood sample 2–12 weeks following COVID-19 vaccine 3. Humoral and T cell responses to SARS-CoV-2 spike protein and nucleocapsid antigen were measured.
Results
Of 81 participants, 79 provided a dried blood spot sample, of whom 38 also provided a whole blood sample; 2 provided only whole blood. Anti-SARS-CoV-2-spike IgG seroconversion post-COVID-19 vaccine 3 occurred in 26/79 (33%) participants; 26/40 (65%) had positive T-cell responses. Overall, 31/40 (78%) demonstrated either humoral or cellular immune response post-COVID-19 vaccine 3. There was no association between laboratory evidence of prior COVID-19 and seroconversion following vaccine 3.
Conclusions
Approximately one third of pwMS who were seronegative after initial COVID-19 vaccination seroconverted after booster (third) vaccination, supporting the use of boosters in this group. Almost 8 out of 10 had a measurable immune response following 3rd COVID-19 vaccine.
Keywords: Multiple sclerosis (MS), COVID-19, Vaccination, Disease modifying therapies (DMTs), Immune response
1. Introduction
To date, the COVID-19 pandemic has resulted in over 5 million deaths worldwide (WHO, 2022). Vaccines have reduced overall COVID-19 associated morbidity and mortality, but uncertainties remain about the benefit of COVID-19 vaccination for people with primary and secondary immunocompromise. We and others have demonstrated that many people with multiple sclerosis (pwMS) treated with anti-CD20 and Sphingosine-1-phosphate (S1P) modulators have an attenuated immune response to the first two doses of COVID-19 vaccination (Tallantyre et al., 2022; Achiron et al., 2021). Studies have so far largely focused on humoral immune responses. However, the impact of booster vaccination in pwMS who mount an inadequate response to initial COVID-19 vaccination is not known. We set out to report humoral and T-cell responses following a third COVID-19 vaccination in pwMS who were known to be seronegative after their second COVID-19 vaccine.
2. Methods
A subgroup of pwMS taking part in a seroprevalence study (Tallantyre et al., 2022), were invited to participate. Selection criteria were IgG negative anti-spike COVID-19 antibody status at week 4–8 post-COVID-19 vaccine 2, and willingness to provide a further blood sample 2–12 weeks following COVID-19 vaccine 3. All participants were invited to provide a dried blood spot and a whole blood sample. Dried blood spot samples were collected as previously described (Tallantyre et al., 2022) during November 2021-January 2022. Where participants were willing to attend for blood sampling, a whole blood sample was collected on the same day. Data on demographics, MS type and treatment, and COVID-19 infection/vaccine dates were derived from the medical notes during January-February 2022. All participants provided written informed consent to take part in this study. This study has Research Ethics Committee approval (REC 19/WA/0058 (Wales REC 3 – covering samples processed in Cardiff), 05/WSE03/111 (South East Wales REC – covering samples processed in Cardiff) and 20/NE/0176 (Newcastle North Tyneside REC – covering QMUL samples).
Humoral responses to SARS-CoV-2 (S1 subunit of the spike protein) following COVID-19 vaccine 3 were measured on dried blood spots using the FDA-approved EuroImmun (PerkinElmer) enzyme-linked immunosorbent assay (ELISA) as per manufacturer instructions. Validation of the EuroImmun assay demonstrates that plasma/serum and DBS specimens produce equivalent results (Zava and Zava, 2021). Results are expressed as a ratio of the optical density (OD) of the participant sample over the OD of the calibrator: ratio <0.8 negative, </=0.8 to <1.1 borderline, >/=1.1 positive. This assay provided good agreement with the assays used in our previous study (Kantaro vs EuroImmun, n = 23, R2=0.94, kappa=1.0; Globody vs EuroImmun, n = 23, R2=0.81, kappa=0.83).
In the subset of 40 participants who provided whole blood samples, humoral responses were also measured using the Bio-Plex Pro-Human SARS-CoV-2 (N/RBD/S1/S2 subunits of spike protein) alongside the VIROTROL SARS-CoV-2 single-level control (Bio-Rad) and performed according to manufacturer's instructions on a Bio-Plex 200 (Bio-Rad). T-cell responses to SARS-CoV-2 were measured in these participants, using a commercially available whole blood assay (ImmunoServ Ltd), as previously described (Scurr et al., 2022). Briefly, 10 ml heparinised venous blood from each patient was collected and processed within 24 h of blood draw. Whole blood samples were stimulated with peptides (Miltenyi-Biotec) spanning the entire spike (RBD/S1/S2) protein (S), nucleocapsid phosphoprotein (NP) and membrane glycoprotein (M). Additional tubes containing phytohaemagglutinin-L (Sigma) or nothing were run alongside as positive and negative controls, respectively. Whole blood samples were incubated at 37 °C for 20–24 h before harvesting plasma from each sample to quantify IFN-g by ELISA (BioLegend). SARS-CoV-2-specific T-cell responses were identified as positive using previously defined criteria for differentiating naïve controls from prior COVID-19 vaccinated and/or infected individuals (sensitivity 96.0% and specificity 84.4%) (Scurr et al., 2022).
2.1. Statistical analysis
Humoral and T-cell response rates to COVID-19 vaccine 3 are expressed using descriptive statistics. Fisher's Exact test and a two-sample t-test were used to explore the relationship between prior COVID-19 infection and immune response to COVID-19 vaccine 3. Statistical analysis was performed in Stata v16 (Stata corp ltd).
3. Results
Of 188 potential participants invited, 81 participated. 79 participants provided a dried blood spot sample, of whom 38 also provided a whole blood sample; 2 provided only whole blood. Fifty-eight (72%) were women, mean age 45.8 years, 55 were receiving ocrelizumab and 15 fingolimod, 9 were on other immunosuppressants (in some cases for co-morbidity rather than MS – see Table 1 for further details) and 2 received no DMT (Table 1). Vaccine type for initial (1st and 2nd) and booster (3rd) vaccination is given in Table 1; the majority received mRNA-based vaccines as their booster (3rd) dose. Mean interval from vaccine 3 to blood draw was 5.9 (SD 1.4) weeks.
Table 1.
Clinical and demographic features of the study population.
| Entire cohort (n = 81) | |||
|---|---|---|---|
| Age (mean; SD) | 45.8; 11.1 | ||
| Gender (F:M;%F) | 58:23, 72% | ||
| Vaccine 1 and 2 type (n [n,% seroconverted] | |||
| BNT162b2 (Pfizer-BioNTech – 2 doses) | 27 (5, 19%) | ||
| CHAdOx1 nCoV-19 (Oxford/AstraZeneca) | 45 (21, 47%) | ||
| Unknown | 9 (0, 0%) | ||
| Vaccine 3 type (available for n = 57) | BNT162b2 (Pfizer-BioNTech) n = 54 mRNA-1273 (Moderna) n = 2 CHAdOx1 nCoV-19 (Oxford/AstraZeneca) n = 1 |
||
| Interval between vaccination 2 and 3 (weeks) (median, IQR) | 27.0 (24.0–29.4) | ||
| DMT | n (%) | DMT duration (mean (SD) years) | Interval from last infusion to vaccine 3 (mean (SD) weeks) |
| Ocrelizumab | 55 (67.9%) | 2.2 (0.64) | 20.9 (24.2) |
| Fingolimod | 15 (18.5%) | 5.9 (1.7) | – |
| Alemtuzumab | 2 (2.5%) | 7.5 (n/a) | – |
| Natalizumab | 2 (2.5%) | 2.4 (2.0) | – |
| Rituximab | 1 (1.3%) | 4.5 (n/a) | – |
| Other/none* | 6 (7.3%) | – | – |
Abatacept for co-existent seronegative arthritis n = 1, Copaxone generic formulation n = 1, Oral Cladribine n = 1, prednisolone 15 mg od for a person with MS who was previously considered to have neuromyelitis optica (Aquaporin 4 and MOG negative) n = 1, none n = 2.
Anti-spike IgG data from dried blood spot samples (EuroImmun assay) demonstrated that 26 of 79 (33%) participants seroconverted post-COVID-19 vaccine 3. Eight of 52 (15%) people receiving ocrelizumab seroconverted, 7 of 15 (47%) people taking fingolimod, and all 11 (100%) of those on other/no DMT. Six people had borderline results: 3 on ocrelizumab and 3 on fingolimod. People who received the CHAdOx1 nCoV-19 (Oxford/AstraZeneca) vaccine for their initial (1st and 2nd) vaccination were more likely to seroconvert that those who received the BNT162b2 (Pfizer-BioNTech – 2 doses) for their initial vaccine course (odds ratio, OR 3.85, 95% confidence interval 1.24–11.97, p = 0.02) (Table 1).
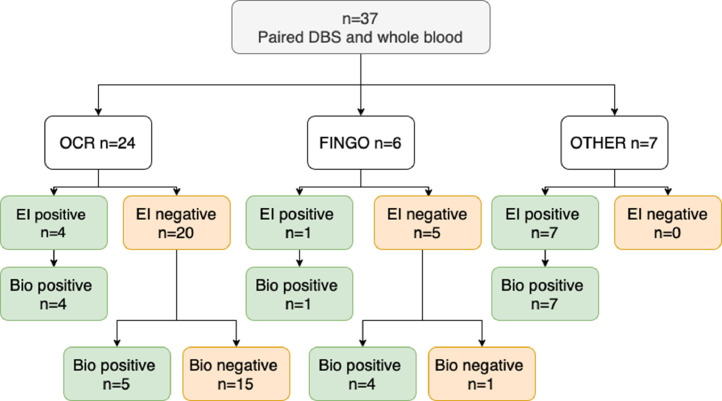
Of the 38 participants who had paired whole blood samples (Bio-rad assay), concordance for Euroimmun seropositivity was 100%, but an additional 9 (24%) people seronegative on DBS analysis were found to be anti-spike positive (Fig. 1 ), giving a Kappa coefficient for agreement of 0.76. The assays appeared to have good correlation (r 2=0.89), which persisted when only samples with a positive result on at least one assay were included (r 2=0.86) (Supplementary Figs. 1 and 2).
Fig. 1.
Flow diagram illustrating seropositive / seronegative status to two anti-spike IgG assays. Bio: Bio-Rad anti-spike IgG assay performed on whole blood, DBS: dried blood spot, EI: EuroImmun anti-spike IgG assay performed on dried blood spots, Fingo: fingolimod, OCR: ocrelizumab, OTHER: see Table 1 for range of other immune-modulating treatments.
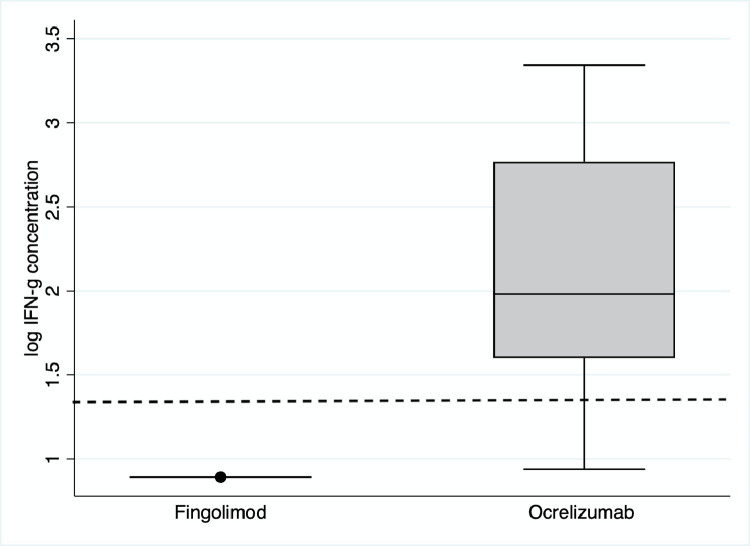
Anti-spike T-cell responses were positive in 26/40 (65%) participants, including 24/27 (86%) on ocrelizumab and 0/6 (0%) on fingolimod (Fig. 2 ). Overall, 31/40 (78%) demonstrated either humoral or cellular immune response post-COVID-19 vaccine 3; this rose to 35/40 (88%) when including IgG results from whole blood.
Fig. 2.
Box and whisker plots of quantitative anti-spike T-cell responses (IFN-g concentration in plasma following stimulation) according to disease modifying therapy (fingolimod [n = 6] vs ocrelizumab [n = 28]). The box represents the interquartile range intersected by the median; whiskers the range. The dotted line represents the positive/negative cut off value.
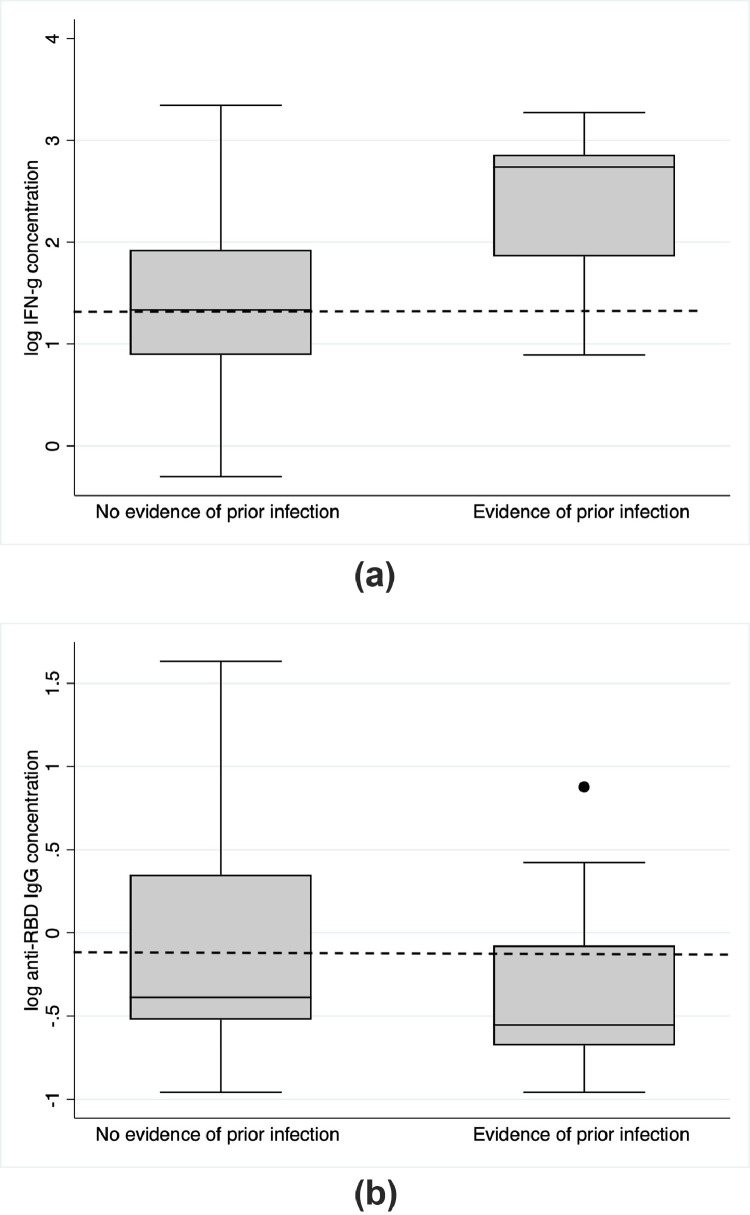
Laboratory evidence of COVID-19 infection (either antibody or T-cell response to nucleocapsid) was present in 14/40 (35%), of whom 2 had prior laboratory-confirmed COVID-19. There was no association between presence or absence of laboratory evidence of prior COVID-19 infection and either T-cell response or anti-spike seroconversion following COVID-19 vaccine 3 as measured by Fishers exact test. However, evidence of prior infection appeared to be associated with a higher magnitude T-cell response (p < 0.0001, 2 sample t-test) (Fig. 3 a). Following the exclusion of 2 outliers with very low IgG responses, there was no significant difference in quantitative IgG response between those with and without evidence of prior infection (p = 0.33) (Fig. 3b). Six of 81 (7.5%) participants experienced PCR-confirmed COVID-19 infection subsequent to vaccine 3 and blood sampling. Four of these six had either T-cell or antibody response to SARS-CoV-2 spike when tested prior to developing COVID; all recovered without antivirals or hospital admission.
Fig. 3.
Box and whisker plots of (a) quantitative anti-spike T cell responses (IFN-g concentration in plasma following stimulation) and (b) quantitative anti-spike IgG titres measured using the EuroImmun ELISA on dried blood spots according to evidence of prior COVID-19 infection (defined by either T-cell or antibody responses directed against the nucleocapsid antigen). N = 26 samples had no evidence of prior infection, and n = 12 had evidence of prior infection. The box represents the interquartile range intersected by the median; whiskers the range. The dotted line represents the positive/negative cut off value.
4. Discussion
We show that a third of pwMS who were seronegative after initial COVID-19 vaccination seroconverted after booster (3rd) vaccination, and between 8 and 9 out of 10 had a measurable immune response to COVID-19, depending on the IgG assay used. These findings support the use of booster vaccinations in this group. Many pwMS exposed to disease modifying therapies (DMTs) have an attenuated response to initial COVID-19 vaccination. This has been most profound when measuring humoral immunity in those exposed to anti-CD20s and fits with data showing an increased vulnerability to COVID-19 among this group (Simpson-Yap et al., 2021, Sormani et al., 2021, Garjani et al., 2022).
Our findings are aligned with the mechanistic action of the MS DMTs. Seroconversion after COVID-19 vaccine 3 occurred in a high proportion of those on fingolimod, but only around 1 in 6 of those on anti-CD20 therapy, in keeping with another recent study of humoral response (König et al., 2022). The profound and selective B-cell depletion seen in pwMS on anti-CD20s is likely to explain this tendency towards an inability to generate class-switched SARS-CoV-2 specific memory B-cells and subsequent anti-SARS-CoV2 IgG production. In contrast to humoral response, T-cell responses were not demonstrated in any of the 6 people we studied on fingolimod but were present in the majority of those on ocrelizumab. Other studies have demonstrated robust or even augmented T-cell responses to either COVID-19 vaccines 1&2 (Gadani et al., 2021; Brill et al., 2021; Apostolidis et al., 2021), or COVID-19 infection in pwMS on antiCD20 therapy (Asplund Högelin et al., 2021). Our study used a whole-blood IFN-γ release assay to measure SARS-CoV2-specific T-cell responses. The finding that none of the participants taking fingolimod generated a detectable T-cell response requires further thought. It may be that this response is truly absent, or that there are too few circulating T-cells to allow detectable responses. However, another study using an ELISpot assay (which corrects for lymphopenia) has recently reported results consistent with our findings (Achiron et al., 2022), suggesting that the attenuated T-cell response in pwMS on fingolimod is not simply a consequence of lymphopenia. Further explanations could be that the relevant T-cells are trapped in secondary lymphoid organs due to the mode of action of fingolimod, or that circulating fingolimod in whole blood samples blocks in vitro responses.
We found that the proportion of people who seroconvert following vaccine 3 depends to some degree on the assay used. For many pathogens, epidemiological study and consensus on assays has evolved over years to provide quantifiable laboratory correlates (typically IgG concentrations) that infer protection against infection and/or severe outcomes of infection (Plotkin, 2010). Due to its recency, the clinical correlates of measurable antibody and T-cell responses to COVID-19 are not yet established, and there is no consensus on the best-performing assay in terms of sensitivity, specificity and clinical correlate. The two assays we used appeared well correlated, suggesting that they are reliably measuring the same response, but the cut-off for EuroImmun appeared more conservative than Bio-Plex (Supplementary Figs. 1 and 2). This highlights the importance of assay selection, and the consideration of borderline values when interpreting results, until laboratory correlates of protection are better established.
Although precise immune correlates of COVID protection are lacking, measurable antibody and T-cell responses are almost certainly associated with milder subsequent disease. Virus-specific CD4+ and CD8+ T cell responses produce effector cytokines and exert cytotoxic activity. Antibodies can directly neutralize virus and reduce viral load, or effect functions including antibody-dependent cellular cytotoxicity and complement deposition. Recent studies have demonstrated that newer SARS-CoV-2 variants appear less susceptible to the neutralizing activity of COVID-19 vaccine-elicited antibodies (Chen et al., 2021; Wang et al., 2021a, 2021b). On the other hand, SARS-CoV-2-specific T-cell epitopes appear to be conserved among emerging variants (Tarke et al., 2021), suggesting that T-cell responses may be particularly important in attenuating current COVID-19 severity. In a recent population study following COVID-19 vaccine roll out, the risk of COVID-19 was shown to be 1.79 (1.57–2.03) times higher in PwMS on ocrelizumab and 1.40 (1.20–1.63) higher in those on fingolimod compared to the general population (Garjani et al., 2022), supporting clinical relevance of our findings. In our small cohort, three participants had laboratory confirmed COVID-19 after vaccine 3 but all cases were mild.
We found that people who had received CHAdOx1 nCoV-19 (Oxford/AstraZeneca) for their initial vaccine course had a higher chance of seroconverting than those who had originally received BNT162b2 (Pfizer-BioNTech). Evidence suggests that the BNT162b2 is more immunogenic than CHAdOx1 nCoV-19 (Munro et al., 2021), which likely explains this observation. It must be noted that our cohort was selected based on seronegative status after initial vaccine course, and so may have been relatively enriched with people who initially received CHAdOx1 nCoV-19, and subsequently seroconverted in response to a more immunogenic vaccine.
This work is subject to several limitations. Firstly, while higher incidence and/or worse outcomes of COVID-19 infection have been observed in people receiving anti-CD20 (Simpson-Yap et al., 2021; Sormani et al., 2021), and fingolimod (Garjani et al., 2022), some (but not all Garjani et al. (2022)) of these studies were performed prior to the widespread availability of booster vaccines, and the clinical implications of our laboratory findings remain uncertain. Secondly, our small sample size is a limitation to statistical analysis, in particular subgroup analyses. Finally, it is possible that the T-cell responses seen following booster vaccination were initially generated following the initial vaccine course, however the improvement in the proportion who mount a serological response to vaccination still provides a rationale for people with MS to take up booster vaccinations.
5. Conclusion
The main practical implication of this work is that a third dose of COVID-19 vaccination appears to provide additional benefit to people with MS who have failed to respond to the initial vaccine course. All people with MS should therefore be encouraged to follow vaccination schedules in order to obtain maximal possible protection. T-cell and antibody testing of pwMS on certain DMTs may allow more individualised counselling on infection risk. Those with detectable IgG antibodies against the spike protein are likely to have greater clinical protection that those with borderline or absent responses; breakthrough infections have been linked to antibody titre in an Italian cohort (Sormani et al.,). This highlights the importance of ensuring full vaccination prior to treatment initiation with fingolimod and anti-CD20 monoclonal antibodies in particular.
Given the time taken to generate an immune response, vaccination should occur a minimum of 2, and preferably 4 weeks prior to administration of immunosuppressive agents. This should also be considered where people with MS are switching between disease modifying therapy for any reason. However, uncertainties remain over whether DMTs should be interrupted in an attempt to augment immune response to vaccination; this study is not powered nor designed to answer these questions. These questions are highly relevant to in clinical practice, and along with the clinical correlates and durability of these immune responses, they will require further longitudinal studies.
Supplementary Fig. 1.Correlation between IgG titres as measured by EuroImmun ELISA and using the Bio-Plex Pro-Human SARS-CoV-2 – all samples.
Supplementary Fig. 2.Correlation between IgG titres as measured by EuroImmun ELISA and using the Bio-Plex Pro-Human SARS-CoV-2 – samples testing positive on Bio-Plex Pro-Human SARS-CoV-2 only
Potential Conflicts of Interest
Biogen, Merck, Novartis, Roche, Sanofi/Genzyme, Teva all manufacture multiple sclerosis disease modifying therapies that were used in this study, or which could be affected by the study. The following authors have received speaker fees, consultancy fees and/ or travel expenses to attend educational meetings from one or more of these companies: ECT, DB, RD, GI, KEH, NE, GG, AK, NPR, KS, SJ. MS and AG are co-founders of and holds equity in ImmunoServ Ltd, which provided T-cell analysis. NV, AR, VA, RC, KG, AG, AH, MJ, ASK, SL, SJM, MW have no conflicts of interest.
CRediT authorship contribution statement
Emma C Tallantyre: Conceptualization, Visualization, Writing – original draft, Methodology. Martin J Scurr: Funding acquisition, Formal analysis, Writing – original draft, Methodology. Nicola Vickaryous: Funding acquisition, Formal analysis, Conceptualization, Visualization, Writing – original draft, Methodology. Aidan Richards: Funding acquisition, Formal analysis. Valerie Anderson: Funding acquisition, Formal analysis. David Baker: Conceptualization, Visualization. Randy Chance: Funding acquisition, Formal analysis. Nikos Evangelou: Funding acquisition, Formal analysis. Katila George: Funding acquisition, Formal analysis. Gavin Giovannoni: Conceptualization, Visualization. Katharine E Harding: Funding acquisition, Formal analysis. Aimee Hibbert: Funding acquisition, Formal analysis. Gillian Ingram: Funding acquisition, Formal analysis. Stephen Jolles: Conceptualization, Visualization. Meleri Jones: Funding acquisition, Formal analysis. Angray S Kang: Funding acquisition, Formal analysis. Samantha Loveless: Funding acquisition, Formal analysis. Stuart J Moat: Conceptualization, Visualization, Funding acquisition, Formal analysis. Neil P Robertson: Conceptualization, Visualization. Francesca Rios: . Klaus Schmierer: Conceptualization, Visualization. Mark Willis: Conceptualization, Visualization. Andrew Godkin: Funding acquisition, Formal analysis, Conceptualization, Visualization, Writing – original draft, Methodology. Ruth Dobson: Conceptualization, Visualization, Writing – original draft, Methodology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The T cell work was funded by BMA Foundation for Medical Research (Vera Down award) to RD and ECT.
The authors would like to acknowledge the help of Aliye Nazli Asardag, Sita Navin Shah, Swee Vickie Nixon, Ray Wynford-Thomas, Marija Cauchi, Catherine McConnell, Cynthia Butcher, Zin Min Htet, Miranda Maria Piana Parra, Joseph Bishop and Raees Samli in the running of the study.
Additional study support was provided as follows:
UHW: Support for equipment and consumables was provided by Cardiff and Vale UHB and Cardiff University. Salary for Samantha Loveless was partly provided by the BRAIN Unit Infrastructure Award (Grant no: UA05). The BRAIN Unit is funded by Welsh Government through Health and Care Research Wales.
QMUL: Work performed as part of this study at QMUL is funded by Merck Serono Ltd., Feltham, UK, an affiliate of Merck KGaA and Biogen UK. There was additional support from the MS community via crowdfunding. This work was performed within the PNU, which is funded by Barts Charity.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2022.103937.
Appendix. Supplementary materials
References
- WHO Coronavirus (COVID-19) Dashboard. cited 6 Feb 2022 Available: https://covid19.who.int.
- Tallantyre E.C., Vickaryous N., Anderson V., Asardag A.N., Baker D., Bestwick J., et al. COVID-19 vaccine response in people with multiple sclerosis. Ann. Neurol. 2022;91:89–100. doi: 10.1002/ana.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A., Mandel M., Dreyer-Alster S., Harari G., Magalashvili D., Sonis P., et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021;14 doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zava T.T., Zava D.T. Validation of dried blood spot sample modifications to two commercially available COVID-19 IgG antibody immunoassays. Bioanalysis. 2021:13–28. doi: 10.4155/bio-2020-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scurr M.J., Zelek W.M., Lippiatt G., Somerville M., Burnell S.E.A., Capitani L., et al. Whole blood-based measurement of SARS-CoV-2-specific T cells reveals asymptomatic infection and vaccine immunogenicity in healthy subjects and patients with solid-organ cancers. Immunology. 2022;165:250–259. doi: 10.1111/imm.13433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson-Yap S., De Brouwer E., Kalincik T., Rijke N., Hillert J.A., Walton C., et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. 2021;97:e1870–e1885. doi: 10.1212/WNL.0000000000012753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Salvetti M., Labauge P., Schiavetti I., Zephir H., Carmisciano L., et al. DMTs and Covid-19 severity in MS: a pooled analysis from Italy and France. Ann. Clin. Transl. Neurol. 2021;8:1738–1744. doi: 10.1002/acn3.51408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garjani A., Patel S., Bharkhada D., Rashid W., Coles A., Law G.R., et al. Impact of mass vaccination on SARS-CoV-2 infections among multiple sclerosis patients taking immunomodulatory disease-modifying therapies in England. Mult. Scler. Relat. Disord. 2022 doi: 10.1016/j.msard.2021.103458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König M., Torgauten H.M., Tran T.T., Holmøy T., Vaage J.T., Lund-Johansen F., et al. Immunogenicity and safety of a third SARS-CoV-2 vaccine dose in patients with multiple sclerosis and weak immune response after COVID-19 vaccination. JAMA Neurol. 2022;79:307–309. doi: 10.1001/jamaneurol.2021.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani S.P., Reyes-Mantilla M., Jank L., Harris S., Douglas M., Smith M.D., et al. Discordant humoral and T cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. EBioMedicine. 2021;73 doi: 10.1016/j.ebiom.2021.103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill L., Rechtman A., Zveik O., Haham N., Oiknine-Djian E., Wolf D.G., et al. Humoral and T-cell response to SARS-CoV-2 vaccination in patients with multiple sclerosis treated with ocrelizumab. JAMA Neurol. 2021;78:1510–1514. doi: 10.1001/jamaneurol.2021.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27:1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asplund Högelin K., Ruffin N., Pin E., Månberg A., Hober S., Gafvelin G., et al. Development of humoral and cellular immunological memory against SARS-CoV-2 despite B cell depleting treatment in multiple sclerosis. iScience. 2021;24 doi: 10.1016/j.isci.2021.103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achiron A., Mandel M., Gurevich M., Dreyer-Alster S., Magalashvili D., Sonis P., et al. Immune response to the third COVID-19 vaccine dose is related to lymphocyte count in multiple sclerosis patients treated with fingolimod. J. Neurol. 2022;269:2286–2292. doi: 10.1007/s00415-022-11030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A.T., Altschuler K., Zhan S.H., Chan Y.A., Deverman B.E. COVID-19 CG enables SARS-CoV-2 mutation and lineage tracking by locations and dates of interest. eLife. 2021;10 doi: 10.7554/eLife.63409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C.O., Finkin S., et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- Tarke A., Sidney J., Methot N., Yu E.D., Zhang Y., Dan J.M., et al. Impact of SARS-CoV-2 variants on the total CD4 and CD8 T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021 doi: 10.1016/j.xcrm.2021.100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro A.P.S., Janani L., Cornelius V., Aley P.K., Babbage G., Baxter D., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Schiavetti I., Inglese M., Carmisciano L., Laroni A., Lapucci C., et al. Breakthrough SARS-CoV-2 infections after COVID-19 mRNA vaccination in MS patients on disease modifying therapies during the Delta and the Omicron waves in Italy. EBioMedicine. 2022;80 doi: 10.1016/j.ebiom.2022.104042. May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.