This cohort study examines the association of fractures, cardiovascular events, and mortality with untreated primary hyperparathyroidism and with surgical treatment.
Key Points
Question
Is the risk of fractures and cardiovascular events higher in patients with untreated primary hyperparathyroidism (pHPT) and lower in patients who undergo parathyroidectomy (PTX)?
Findings
In this cohort study of 16 374 Swedish patients with pHPT, the risk of hip fracture was significantly increased by 51%, of any fracture by 39%, and of cardiovascular events by 45% in patients with pHPT compared with a matched control group. The risk of these outcomes was significantly reduced with PTX.
Meaning
Findings from this study suggest an association between pHPT and increased risk of fractures, cardiovascular events, and death as well as an association between PTX and reduced risk of these outcomes.
Abstract
Importance
Patients with primary hyperparathyroidism (pHPT) appear to have an increased risk of fractures and other comorbidities, such as cardiovascular disease, although results from previous studies have been inconsistent. Evidence of the association of parathyroidectomy (PTX) with these outcomes is also limited because of the lack of large well-controlled trials.
Objective
To investigate whether untreated pHPT was associated with an increased risk of incident fractures and cardiovascular events (CVEs) and whether PTX was associated with a reduced risk of these outcomes.
Design, Setting, and Participants
This cohort study included all patients who were diagnosed with pHPT at hospitals in Sweden between July 1, 2006, and December 31, 2017. Each patient was matched with 10 control individuals from the general population by sex, birth year, and county of residence. The patients were followed up until December 31, 2017. Data analyses were performed from October 2021 to April 2022.
Main Outcomes and Measures
The primary outcomes were fractures, CVEs, and death. Cumulative incidence of events was estimated using the 1-minus Kaplan-Meier estimator of corresponding survival function. Cox proportional hazards regression models were used to calculate hazard ratios (HRs).
Results
A total of 16 374 patients with pHPT were identified (mean [SD] age, 67.5 [12.9] years; 12 806 women [78.2%]), with 163 740 control individuals. The follow-up time was 42 310 person-years for the pHPT group and 803 522 person-years for the control group. Compared with the control group, the pHPT group had a higher risk of any fracture (unadjusted HR, 1.39; 95% CI, 1.31-1.48), hip fracture (unadjusted HR, 1.51; 95% CI, 1.35-1.70), CVEs (unadjusted HR, 1.45; 95% CI, 1.34-1.57), and death (unadjusted HR, 1.72; 95% CI, 1.65-1.80). In a time-dependent Poisson regression model, PTX was associated with a reduced risk of any fracture (HR, 0.83; 95% CI, 0.75-0.93), hip fracture (HR, 0.78; 95% CI, 0.61-0.98), CVEs (HR, 0.84; 95% CI, 0.73-0.97), and death (HR, 0.59; 95% CI, 0.53-0.65).
Conclusions and Relevance
Results of this study suggest that pHPT is associated with increased risk of fractures, CVEs, and death, highlighting the importance of identifying patients with this condition to prevent serious unfavorable outcomes. The reduced risk of these outcomes associated with PTX suggests a clinical benefit of surgery.
Introduction
Primary hyperparathyroidism (pHPT) is a common endocrine disorder characterized by elevated serum calcium combined with normal or high blood levels of parathyroid hormone.1 The reported prevalence in the general population has varied between countries and over time. The prevalence of pHPT was 0.86% in the general population in a large US study2 and was 3.4% in a population-based screening program of Swedish postmenopausal women.3
In most high-income countries wherein biochemical screening of serum calcium is common, pHPT usually presents as an asymptomatic disorder of older adults.4 Disturbed calcium homeostasis can potentially have long-term adverse outcomes, including tissue damage in known target organs such as the kidneys, bone, and cardiovascular system.5 Observational studies of pHPT, especially severe cases, have reported a higher prevalence of osteoporosis, hypertension, diabetes, lipid abnormalities, endothelial dysfunction, arrhythmias, and left ventricular hypertrophy in patients with the disorder than in control individuals.6,7
Some population-based studies have observed a higher risk of cardiovascular events (CVEs) and death in patients with pHPT,8,9,10 a conclusion supported by a meta-analysis of prospective studies.11 International guidelines on pHPT management do not include cardiovascular disease among the criteria for parathyroidectomy (PTX)12 because of insufficient evidence regarding the association of PTX with cardiovascular risk.13,14,15
Increased bone loss and an elevated fracture risk at most skeletal sites, including the spine, wrist, ribs, and pelvis, are known concerns in pHPT.16 For hip fracture, the most severe fracture associated with substantially higher morbidity and mortality, evidence of increased risk is insufficient and inconsistent.17,18,19,20 The American Association of Endocrine Surgeons guidelines for management of pHPT recommend PTX in patients with symptomatic pHPT, hypercalcemia (>1 mg/dL above the normal range), kidney involvement, or osteoporosis or fragility fracture.10
Because pHPT is easily diagnosed and diagnosis may be important for identifying the future risk of severe and debilitating outcomes, including hip fractures, CVEs, and mortality, additional well-powered and representative population-based studies with incident outcome data are warranted. In this retrospective cohort study, we aimed to investigate whether untreated pHPT was associated with an increased risk of incident fractures (including hip fractures) and CVEs compared with the risk of the sex, birth year, and county–matched control group. We also sought to investigate whether PTX was associated with a reduced risk of these outcomes vs nonoperatively managed pHPT.
Methods
Study Design
The cohort study was approved by the regional ethical review board of Gothenburg, Sweden, which issued a waiver of the patient informed consent requirement because all of the data used were collected from registers without the investigators having direct contact with participants. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
All patients who were diagnosed with pHPT (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] code E210) at hospitals in Sweden between July 1, 2006, and December 31, 2017, were included. To study untreated patients with pHPT exclusively, we excluded patients who were previously treated with antiparathyroid agents (Anatomical Therapeutic Chemical code H05BX [eg, cinacalcet]) or PTX (Klassifikation av kirurgiska åtgärder codes BBA3-BBA5) before the pHPT diagnosis or study period. Each patient with pHPT was matched to 10 control individuals from the general population by sex, birth year, and county of residence using sampling with replacement to avoid bias.21 Control individuals were assigned the baseline diagnosis date of their matched patient; control individuals with a previous pHPT diagnosis who underwent PTX or who had treatment with antiparathyroid agents were excluded.
The risk of fractures, injurious falls, CVEs, kidney stones, and overall and cardiovascular deaths was analyzed. Follow-up time was censored at the end of study (December 31, 2017), emigration, or death (whichever occurred first) and also at the start of antiparathyroid agents or PTX and osteoporosis medication (for fracture outcomes). Control individuals were also censored at incident pHPT, PTX, or use of antiparathyroid agents. Associations between PTX and different outcomes were investigated separately in patients with pHPT, with PTX serving as a time-dependent variable.
Data Sources
Swedish national registers were combined to serve as sources of clinical characteristics and outcomes of patients with pHPT. All Swedish residents are assigned a unique personal identification number at birth or at the time of immigration, enabling data linkage across the national registers. The Swedish Patient Register was used to retrieve data on pHPT diagnosis, comorbidities, CVEs, kidney stones, fractures, and injurious falls that were identified during inpatient and outpatient visits. Medication data were retrieved from the Swedish Prescribed Drug Register, and data on socioeconomic factors and deaths were obtained from Statistics Sweden. Race and ethnicity data were not available in the Swedish registers used.
Variable Definitions
Data on fractures, cardiovascular outcomes, and comorbidities were collected from hospital visits and were defined using ICD-10 codes (eTable 1 in the Supplement). All nonmalignant fracture diagnoses regardless of type of trauma were included, and the data were refined in several steps. Fracture diagnoses with a simultaneous code indicating a revisit (ICD-10 codes Z09, Z47, and Z48) and hip fracture diagnoses without a simultaneous code for surgical procedure (Klassifikation av kirurgiska åtgärder codes NFB, NFC, or NFJ) were discarded. Furthermore, a washout period of 5 months was used; that is, if a fracture diagnosis referring to the same skeletal site was repeated within 5 months, the latter diagnosis was excluded, minimizing the role of revisits and maximizing the accuracy of true fractures.22 Incident hip fracture was defined as a fracture in the femoral head, neck, trochanter, or subtrochanteric region of the femur. Incident fracture of any kind included all fractures except of the head, fingers, and toes. Incident major osteoporotic fracture was defined as a fracture of the hip, vertebrae, pelvis, upper arm, or lower arm.
To avoid overlap with the fracture outcomes, we specified injurious fall as any event with an injury code (ICD-10 codes S00-T14, with a 5-month washout period) and a fall code (ICD-10 codes W00-W19) but without a fracture code. Incident CVE included admissions with myocardial infarction (ICD-10 codes I21-I22), hemorrhagic stroke (ICD-10 code I63), or ischemic stroke (ICD-10 code I61), with a 7-year washout period for each diagnosis.23 For incident kidney stone (ICD-10 codes N20-N22), a washout period of 1 year was used.24
A number of covariates representing previous illnesses and medications with presumed implications for a patient’s comorbidity and risk of the studied outcomes were selected (Table 1). Charlson Comorbidity Index was calculated to summarize and quantify comorbidity.25 This index ranged from 0 to 15, with the lowest score indicating very low 1-year mortality rates.
Table 1. Baseline Characteristics.
| Variablea | Individuals, No. (%) | SMD | |
|---|---|---|---|
| Control group (n = 163 740) | pHPT group (n = 16 374) | ||
| Age, mean (SD), y | 67.5 (12.9) | 67.5 (12.9) | 0.000 |
| Sex | |||
| Female | 128 060 (78.2) | 12 806 (78.2) | 0.000 |
| Male | 35 680 (21.8) | 3568 (21.8) | 0.000 |
| Urban residency, ≥200 per km2 | 44 627 (27.3) | 4774 (29.2) | 0.042 |
| Sickness benefits | 9340 (5.7) | 2209 (13.5) | 0.267 |
| Non-Nordic citizenship at birth | 13 450 (8.2) | 1442 (8.8) | 0.021 |
| Charlson Comorbidity Index | |||
| Mean (SD) | 0.61 (1.32) | 0.93 (1.57) | 0.218 |
| 0 | 118 665 (72.5) | 9823 (60.0) | −0.266 |
| 1 or 2 | 34 032 (20.8) | 4636 (28.3) | 0.176 |
| ≥3 | 11 043 (6.7) | 1915 (11.7) | 0.172 |
| Diagnosesb | |||
| Osteoporosis | 3775 (2.3) | 1204 (7.4) | 0.237 |
| Previous alcohol-related disease | 1740 (1.1) | 188 (1.1) | 0.008 |
| Rheumatoid arthritis | 2523 (1.5) | 378 (2.3) | 0.056 |
| Previous fracture | |||
| Any | 17 405 (10.6) | 2484 (15.2) | 0.136 |
| Recent (past year) | 4672 (2.9) | 994 (6.1) | 0.156 |
| Multiple (≥2 occasions) | 3194 (2.0) | 499 (3.0) | 0.070 |
| Previous injurious fall | |||
| Any | 10 267 (6.3) | 1412 (8.6) | 0.090 |
| Recent (past year) | 2722 (1.7) | 477 (2.9) | 0.084 |
| Multiple (≥2 occasions) | 1172 (0.7) | 176 (1.1) | 0.038 |
| Dementia | 2862 (1.7) | 325 (2.0) | 0.018 |
| Ischemic heart disease | 10 953 (6.7) | 1646 (10.1) | 0.122 |
| Myocardial infarction | 3025 (1.8) | 455 (2.8) | 0.062 |
| Cerebrovascular disease | |||
| Any | 6459 (3.9) | 927 (5.7) | 0.080 |
| Previous hemorrhagic stroke | 388 (0.2) | 64 (0.4) | 0.028 |
| Previous ischemic stroke | 3091 (1.9) | 457 (2.8) | 0.060 |
| CHD | 5545 (3.4) | 1036 (6.3) | 0.137 |
| Diabetes | 10 563 (6.5) | 1720 (10.5) | 0.146 |
| Kidney failure | 1890 (1.2) | 716 (4.4) | 0.197 |
| Previous kidney stone | 1731 (1.1) | 984 (6.0) | 0.271 |
| COPD | 7010 (4.3) | 1154 (7.0) | 0.120 |
| Hyperthyroidism | 1207 (0.7) | 369 (2.3) | 0.125 |
| Medications used in past yearc | |||
| Osteoporosis drugs | 7758 (4.7) | 1079 (6.6) | 0.080 |
| Calcium and vitamin D | 10 288 (6.3) | 662 (4.0) | −0.101 |
| Prednisolone | 11 286 (6.9) | 1637 (10.0) | 0.112 |
| Opioids | 10 896 (6.7) | 1769 (10.8) | 0.147 |
| Antiepileptics | 3859 (2.4) | 576 (3.5) | 0.069 |
| Antiparkinson drugs | 2234 (1.4) | 273 (1.7) | 0.025 |
| Antidepressants | 18 818 (11.5) | 2467 (15.1) | 0.105 |
| Antidementia drugs | 2022 (1.2) | 147 (0.9) | −0.033 |
| Thiazid diuretics | 7816 (4.8) | 873 (5.3) | 0.025 |
| β-blockers | 35 053 (21.4) | 4536 (27.7) | 0.147 |
| Calcium antagonist | 20 999 (12.8) | 2960 (18.1) | 0.146 |
| RAS inhibitors | 37 649 (23.0) | 5319 (32.5) | 0.213 |
Abbreviations: CHD, congestive heart disease; COPD, chronic obstructive pulmonary disease; pHPT, primary hyperparathyroidism; RAS, renin-angiotensin system; SMD, standardized mean difference.
Detailed definitions of variables are presented in eTable 1 in the Supplement.
A 5-year historical window was used for diagnoses, if not otherwise stated.
Medications used in the past year were defined by a prescription during the past year, were repeated (≥2 prescriptions), and were ongoing (most recent prescription collected <120 days before baseline).
Statistical Analysis
To assess the differences in baseline characteristics, standardized mean differences were calculated. Incident rates per 1000 person-years were calculated to enable comparison of periods with different follow-up lengths and were presented with exact Poisson 95% CIs. The cumulative incidence of events was estimated using the 1-minus Kaplan-Meier estimator of the corresponding survival function and then presented graphically with 95% CIs. Cox proportional hazards regression models were used to calculate hazard ratios (HRs). Cox assumption of proportional hazards was tested using graphical methods. Cox proportional hazards regression models were adjusted for age, sex, Charlson Comorbidity Index, and prevalence of investigated outcome (previous fracture, injurious fall, CVE, or kidney stone).
A sensitivity analysis was performed, with the follow-up time censored after 1 year, reducing the difference in follow-up time between the pHTP group and the control group. Interaction and subgroup analyses stratified by sex and Charlson Comorbidity Index were performed. The potential association between PTX and outcomes was investigated using an extension of a Poisson regression model,26,27 which was adjusted for current time since diagnosis, current age, sex, calendar year, Charlson Comorbidity Index, and baseline prevalence of the investigated outcome. An alternative sensitivity analysis was conducted to compare the incidence of outcomes before and after surgery in patients who underwent PTX. To assess the potential implications of death as a competing risk, we estimated the cumulative incidence function (or subdistribution function) of fracture or injurious fall, with death as a competing risk, using the Aalen-Johansen estimator. The subdistribution hazard for fracture was compared between the pHPT group and control group (matched 1:1) using the Fine and Gray proportional hazards model, with death as the competing risk.28
Statistical analyses were performed from October 2021 to April 2022, using SPSS, version 26 (IBM SPSS), and RStudio, version 1.4.1106 (R Foundation for Statistical Computing). Two-sided P < .05 (P = .10 for interactions) was considered to be significant.
Results
In total, 16 374 hospital patients with pHPT were identified, mostly during outpatient visits. These patients included 12 806 women (78.2%) and 3568 men (21.8%) with a mean (SD) age of 67.5 (12.9) years. Each patient was matched to 10 control individuals by sex, birth year, and county of residence (n = 163 740). Baseline characteristics of the pHPT and control groups are presented in Table 1.
Patients in the pHPT group had more comorbidities than the control group (Charlson Comorbidity Index ≥3: 11.7% vs 6.7%). Similarly, the proportion with registered sickness benefits was higher among the pHPT group than among the control group (13.5% vs 5.7%). Both previous fracture (15.2% vs 10.6%) and injurious fall (8.6% vs 6.3%) were more common among patients with pHPT than among control individuals. Previous diagnosis of osteoporosis was documented in 7.4% of patients with pHPT compared with 2.3% of the control individuals. A smaller percentage of patients in the pHPT group vs control group had received a prescription for calcium and vitamin D supplements (4.0% vs 6.3%), but use of other medications for a variety of conditions was consistently higher in the pHPT group than in the control group. A greater proportion of patients with pHPT than control individuals had experienced a previous myocardial infarction (2.8% vs 1.8%) and were more likely to have been diagnosed with ischemic heart disease (10.1% vs 6.7%) and cerebrovascular disease (5.7% vs 3.9%). The prevalence of kidney stones (6.0% vs 1.1%) and kidney failure (4.4% vs 1.2%) was higher in the pHPT group vs the control group (Table 1).
The total follow-up time for the study was 845 832 person-years, with 42 310 person-years for the pHPT group and 803 522 person-years for the control group. The median (IQR) follow-up time was 1.15 (0.40-4.06) years for the pHPT group and 4.62 (2.08-7.51) years for the control group. More than half of patients with pHPT were censored before the end of the study or death from either parathyroidectomy (n = 6934 [42.3%]; median [IQR] time, 0.50 [0.25-0.94] years) or cinacalcet treatment (n = 943 [5.8%]; median [IQR] time, 0.71 [0.17-2.74] years) or, for fracture outcomes, from osteoporosis treatment (n = 3283 [20.1%]; median [IQR] time, 0.38 [0.11-2.01] years).
During follow-up, the incidence rates per 1000 person-years for any fracture (35.52 [95% CI, 33.50-37.64] vs 25.36 [95% CI, 24.98-25.74]), major osteoporotic fracture (23.46 [95% CI, 21.85-25.17] vs 16.35 [95% CI, 16.05-16.65]), hip fracture (9.30 [95% CI, 8.31-10.38] vs 6.26 [95% CI, 6.08-6.45]), injurious fall (28.59 [95% CI, 26.95-30.31] vs 18.97 [95% CI, 18.66-19.28]), any CVE (17.22 [95% CI, 15.97-18.54] vs 11.98 [95% CI, 11.74-12.22]), kidney stone (9.27 [95% CI, 8.37-10.25] vs 2.47 [95% CI, 2.36-2.58]), and death (51.83 [95% CI, 49.68-54.05] vs 30.95 [95% CI, 30.57-31.34]) were significantly higher in patients with pHPT than in control individuals (Table 2). The risk was also significantly higher for all observed outcomes, as demonstrated by unadjusted HRs for the pHPT group vs the control group (Table 2; Figure). Patients with pHPT had significantly higher risk of any fracture (unadjusted HR, 1.39; 95% CI, 1.31-1.48), major osteoporotic fracture (unadjusted HR, 1.43; 95% CI, 1.33-1.54), hip fracture (unadjusted HR, 1.51; 95% CI, 1.35-1.70), and injurious fall (unadjusted HR, 1.51; 95% CI, 1.42-1.60) compared with control individuals. The risk of fractures was significantly increased at different peripheral sites (eTable 2 in the Supplement): wrist (unadjusted HR, 1.34; 95% CI, 1.18-1.52), upper arm (unadjusted HR, 1.46; 95% CI, 1.25-1.71), and lower leg (unadjusted HR, 1.31; 95% CI, 1.12-1.54). For any CVE, patients with pHPT had an overall increased risk (unadjusted HR, 1.45; 95% CI, 1.34-1.57), as well as increased risk for acute myocardial infarction (unadjusted HR, 1.39; 95% CI, 1.24-1.56) and for ischemic stroke (unadjusted HR, 1.51; 95% CI, 1.36-1.68), whereas there was no significantly increased risk for hemorrhagic stroke (unadjusted HR, 1.09; 95% CI, 0.82-1.45) (eTable 3 in the Supplement). The risk of overall deaths (unadjusted HR, 1.72; 95% CI, 1.65-1.80) and cardiovascular-related deaths was higher among the pHPT group than among the control group (eTable 4 in the Supplement). The risk of kidney stones during follow-up was almost 4 times higher in patients with pHPT than in control individuals (unadjusted HR, 3.65; 95% CI, 3.27-4.08). All associations remained significant, although attenuated after adjustment for age, sex, Charlson Comorbidity Index, and prevalence of investigated outcomes.
Table 2. Clinical Outcomes.
| Variable | Control group (n = 163 740) | pHPT group (n = 16 374)a | P value |
|---|---|---|---|
| Time at risk, median (IQR), y | 4.62 (2.08-7.51) | 1.15 (0.40-4.06) | NA |
| Any fracture | |||
| Events, No. (%) | 17 326 (10.6) | 1150 (7.0) | NA |
| Per 1000 person-years (95% CI) | 25.36 (24.98-25.74) | 35.52 (33.50-37.64) | |
| Cox proportional hazards regression model, HR (95% CI)b | |||
| Unadjusted | 1 [Reference] | 1.39 (1.31-1.48) | <.001 |
| Adjusted | 1 [Reference] | 1.22 (1.15-1.30) | <.001 |
| Major osteoporotic fracture | |||
| Events, No. (%) | 11 472 (7.0) | 780 (4.8) | NA |
| Per 1000 person-years (95% CI) | 16.35 (16.05-16.65) | 23.46 (21.85-25.17) | |
| Cox proportional hazards regression model, HR (95% CI)b | |||
| Unadjusted | 1 [Reference] | 1.43 (1.33-1.54) | <.001 |
| Adjusted | 1 [Reference] | 1.23 (1.15-1.33) | <.001 |
| Hip fracture | |||
| Events, No. (%) | 4519 (2.8) | 319 (1.9) | NA |
| Per 1000 person-years (95% CI) | 6.26 (6.08-6.45) | 9.30 (8.31-10.38) | |
| Cox proportional hazards regression model, HR (95% CI)b | |||
| Unadjusted | 1 [Reference] | 1.51 (1.35-1.70) | <.001 |
| Adjusted | 1 [Reference] | 1.20 (1.07-1.35) | .002 |
| Injurious fall | |||
| Events, No. (%) | 14 436 (8.8) | 1130 (6.9) | NA |
| Per 1000 person-years (95% CI) | 18.97 (18.66-19.28) | 28.59 (26.95-30.31) | |
| Cox proportional hazards regression model, HR (95% CI)b | |||
| Unadjusted | 1 [Reference] | 1.51 (1.42-1.60) | <.001 |
| Adjusted | 1 [Reference] | 1.30 (1.22-1.38) | <.001 |
| Any CVE | |||
| Events, No. (%) | 9350 (5.7) | 703 (4.3) | NA |
| Per 1000 person-years (95% CI) | 11.98 (11.74-12.22) | 17.22 (15.97-18.54) | |
| Cox proportional hazards regression model, HR (95% CI)b | |||
| Unadjusted | 1 [Reference] | 1.45 (1.34-1.57) | <.001 |
| Adjusted | 1 [Reference] | 1.22 (1.13-1.32) | <.001 |
| Kidney stone | |||
| Events, No. (%) | 1967 (1.2) | 384 (2.3) | NA |
| Per 1000 person-years (95% CI) | 2.47 (2.36-2.58) | 9.27 (8.37-10.25) | |
| Cox proportional hazards regression model, HR (95% CI)b | |||
| Unadjusted | 1 [Reference] | 3.65 (3.27-4.08) | <.001 |
| Adjusted | 1 [Reference] | 2.60 (2.32-2.91) | <.001 |
| Death | |||
| Events, No. (%) | 24 869 (15.2) | 2193 (13.4) | NA |
| Per 1000 person-years (95% CI) | 30.95 (30.57-31.34) | 51.83 (49.68-54.05) | |
| Cox proportional hazards regression model, HR (95% CI)b | |||
| Unadjusted | 1 [Reference] | 1.72 (1.65-1.80) | <.001 |
| Adjusted | 1 [Reference] | 1.27 (1.22-1.33) | <.001 |
| Cardiovascular-related deaths | |||
| Events, No. (%) | 8106 (5.0) | 722 (4.4) | NA |
| Per 1000 person-years (95% CI) | 10.09 (9.87-10.31) | 17.06 (15.84-18.36) | |
| Cox proportional hazards regression model, HR (95% CI)b | |||
| Unadjusted | 1 [Reference] | 1.73 (1.60-1.86) | <.001 |
| Adjusted | 1 [Reference] | 1.24 (1.15-1.34) | <.001 |
Abbreviations: CVE, cardiovascular event; HR, hazard ratio; NA, not applicable; pHPT, primary hyperparathyroidism.
All patients in the pHPT group were included and censored before the end of the study period or death from either parathyroidectomy or cinacalcet treatment or, for the fracture outcomes, from osteoporosis treatment.
Cox proportional hazards regression models were adjusted for age, sex, Charlson Comorbidity Index, and the prevalence of the investigated outcome (previous fracture, injurious fall, CVE, or kidney stone).
Figure. Cumulative Incidences for Patients With Primary Hyperparathyroidism (pHPT) and Control Individuals.
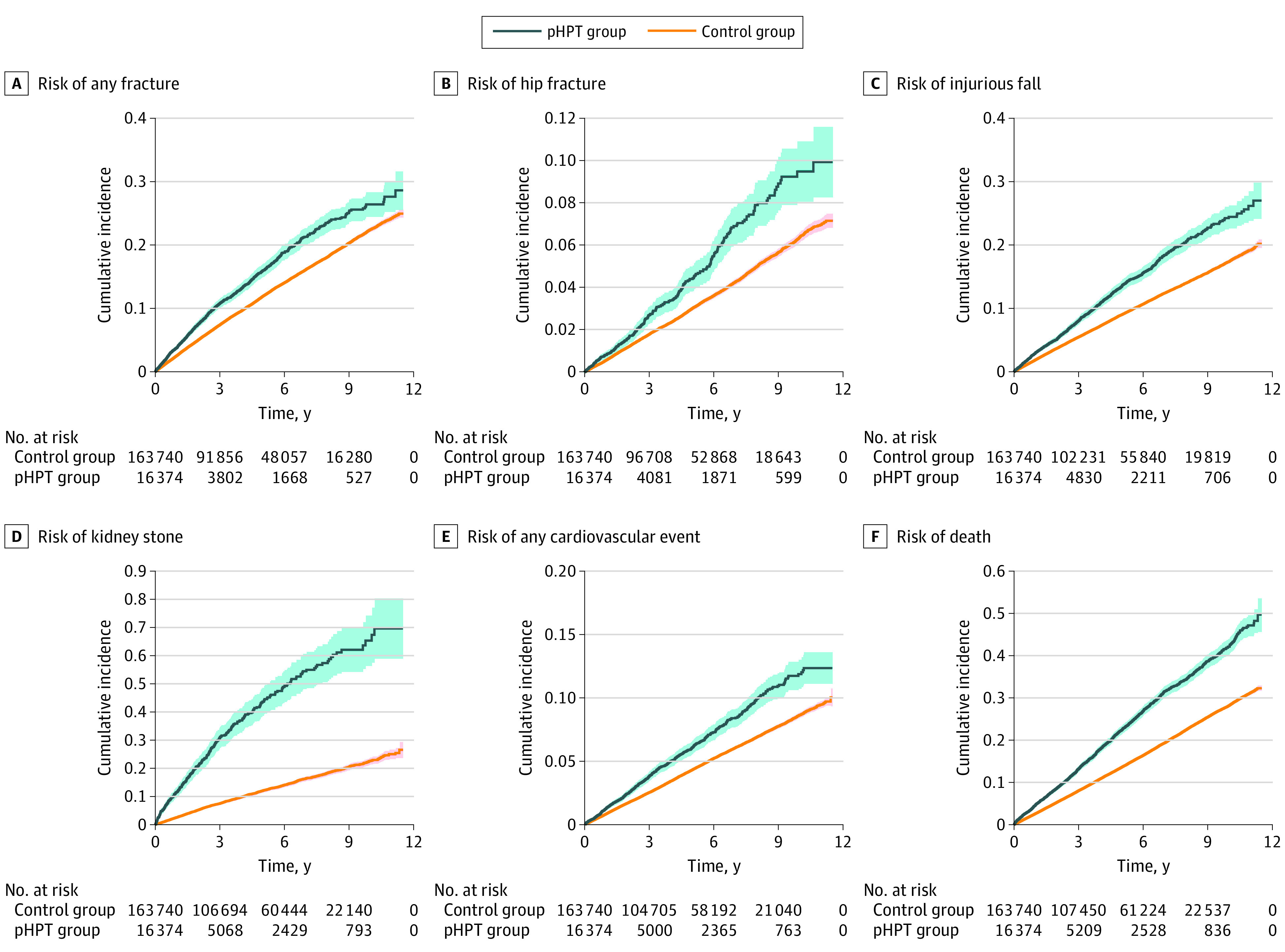
The cumulative incidence of events for untreated pHPT and control groups was estimated using 1-minus Kaplan-Meier estimate of the corresponding survival function and presented with 95% CIs (indicated by shaded areas).
There was no interaction between pHPT diagnosis and sex for any outcome, and the HRs for all reported outcomes in unadjusted analyses were significant in both men and women with pHPT compared with control individuals (eTable 5 in the Supplement). There was no interaction between pHPT diagnosis and Charlson Comorbidity Index for any fracture or CVE.
To account for the difference in follow-up time between groups, we performed a sensitivity analysis in which the Cox proportional hazards regression models were truncated after 1 year. The increased risk of all outcomes in the pHPT group was significant and similar to that found in the main analysis (eTable 6 in the Supplement). For example, the risk of hip fracture was 51% higher both in the analysis with truncated follow-up (HR, 1.51; 95% CI, 1.21-1.87) and in the analysis using the entire follow-up (HR, 1.51; 95% CI, 1.35-1.70).
Visualization of the cumulative incidence functions of each outcome, with death as a competing risk, revealed similar incidence differences in the studied associations (eFigure in the Supplement). Fine and Gray analyses with death as a competing risk demonstrated that the associations between pHPT and all outcomes remained (eTable 7 in the Supplement). For example, the risk of hip fracture was increased after adjustment for competing risk (sub-HR, 1.37; 95% CI, 1.19-1.58).
Of the 16 374 patients with pHPT, 6934 (42.3%) underwent PTX a median (IQR) of 0.50 (0.25-0.94) years after diagnosis. These patients were considerably younger, had substantially fewer comorbidities, and had lower prevalence of fracture and CVE compared with patients who received conservative treatment (Table 3).
Table 3. Baseline Characteristics per Conservative Treatment or Future Parathyroidectomy.
| Variablea | Individuals, No. (%) | SMD | |
|---|---|---|---|
| Conservative treatment | Parathyroidectomy | ||
| No. of patients | 9440 | 6934 | NA |
| Age, mean (SD), y | 71.1 (12.5) | 62.6 (11.6) | −0.710 |
| Female sex | 7418 (78.6) | 5388 (77.7) | −0.021 |
| Male sex | 2022 (21.4) | 1546 (22.3) | 0.021 |
| Urban residency, ≥200 per km2 | 2768 (29.3) | 2006 (28.9) | −0.009 |
| Sickness benefits | 815 (8.6) | 1394 (20.1) | 0.331 |
| Non-Nordic citizenship at birth | 829 (8.8) | 613 (8.8) | 0.002 |
| Charlson Comorbidity Index | |||
| Mean (SD) | 1.18 (1.76) | 0.58 (1.19) | −0.403 |
| 0 | 4869 (51.6) | 4954 (71.4) | 0.417 |
| 1 or 2 | 3060 (32.4) | 1576 (22.7) | −0.218 |
| ≥3 | 1511 (16.0) | 404 (5.8) | −0.331 |
| Diagnosesb | |||
| Osteoporosis | 836 (8.9) | 368 (5.3) | −0.139 |
| Previous alcohol-related disease | 111 (1.2) | 77 (1.1) | −0.006 |
| Rheumatoid arthritis | 251 (2.7) | 127 (1.8) | −0.056 |
| Previous fracture | |||
| Any | 1641 (17.4) | 843 (12.2) | −0.148 |
| Recent (past year) | 688 (7.3) | 306 (4.4) | −0.123 |
| Multiple (≥2 occasions) | 354 (3.8) | 145 (2.1) | −0.099 |
| Previous injurious fall | |||
| Any | 920 (9.7) | 492 (7.1) | −0.096 |
| Recent (past year) | 352 (3.7) | 125 (1.8) | −0.118 |
| Multiple (≥2 occasions) | 128 (1.4) | 48 (0.7) | −0.066 |
| Dementia | 306 (3.2) | 19 (0.3) | −0.227 |
| Ischemic heart disease | 1225 (13.0) | 421 (6.1) | −0.237 |
| Myocardial infarction | 359 (3.8) | 96 (1.4) | −0.153 |
| Cerebrovascular disease | |||
| Any | 728 (7.7) | 199 (2.9) | −0.218 |
| Previous hemorrhagic stroke | 52 (0.6) | 12 (0.2) | −0.063 |
| Previous ischemic stroke | 356 (3.8) | 101 (1.5) | −0.145 |
| CHD | 891 (9.4) | 145 (2.1) | −0.319 |
| Diabetes | 1228 (13.0) | 492 (7.1) | −0.198 |
| Kidney failure | 552 (5.8) | 164 (2.4) | −0.176 |
| Previous kidney stone | 437 (4.6) | 547 (7.9) | 0.135 |
| COPD | 844 (8.9) | 310 (4.5) | −0.179 |
| Hyperthyroidism | 254 (2.7) | 115 (1.7) | −0.071 |
| Medications used in past yearc | |||
| Osteoporosis drugs | 711 (7.5) | 368 (5.3) | −0.091 |
| Calcium and vitamin D | 502 (5.3) | 160 (2.3) | −0.158 |
| Prednisolone | 1124 (11.9) | 513 (7.4) | −0.153 |
| Opioids | 1184 (12.5) | 585 (8.4) | −0.134 |
| Antiepileptics | 389 (4.1) | 187 (2.7) | −0.079 |
| Antiparkinson drugs | 207 (2.2) | 66 (1.0) | −0.100 |
| Antidepressants | 1568 (16.6) | 899 (13.0) | −0.103 |
| Antidementia drugs | 128 (1.4) | 19 (0.3) | −0.121 |
| Thiazide diuretics | 565 (6.0) | 308 (4.4) | −0.069 |
| β-blockers | 3072 (32.5) | 1464 (21.1) | −0.260 |
| Calcium antagonist | 1906 (20.2) | 1054 (15.2) | −0.131 |
| RAS inhibitors | 3465 (36.7) | 1854 (26.7) | −0.215 |
Abbreviations: CHD, congestive heart disease; COPD, chronic obstructive pulmonary disease; NA, not applicable; RAS, renin-angiotensin system; SMD, standardized mean difference.
Detailed definitions of variables are provided in eTable 1 in the Supplement.
A 5-year historical window was used for diagnoses, if not otherwise stated.
Medications used in the past year were defined by a prescription during the past year, were repeated (≥2 prescriptions), and were ongoing (most recent prescription collected <120 days before baseline).
When we studied the pHPT group separately using an extension of the Poisson regression model, with incident PTX as a time-dependent variable, we found an association between PTX and reduced risk of hip fracture (HR, 0.78; 95% CI, 0.61-0.98), any fracture (HR, 0.83; 95% CI, 0.75-0.93), injurious fall (HR, 0.83; 95% CI, 0.74-0.92), CVE (HR, 0.84; 95% CI, 0.73-0.97), and death (HR, 0.59; 95% CI, 0.53-0.65) after adjustment for time since diagnosis, current age, sex, and calendar year. These associations remained similar after further adjustment for prevalent disease and Charlson Comorbidity Index (Table 4).
Table 4. Association Between Parathyroidectomy and Outcomesa.
| HR (95% CI) | P value | |
|---|---|---|
| Any fracture | ||
| Adjusted for time since diagnosis, current age, sex, and calendar year | 0.83 (0.75-0.93) | .001 |
| Further adjusted for Charlson Comorbidity Index and previous fracture | 0.84 (0.76-0.94) | .003 |
| Major osteoporotic fracture | ||
| Adjusted for time since diagnosis, current age, sex, and calendar year | 0.82 (0.71-0.94) | .004 |
| Further adjusted for Charlson Comorbidity Index and previous fracture | 0.83 (0.72-0.95) | .007 |
| Hip fracture | ||
| Adjusted for time since diagnosis, current age, sex, and calendar year | 0.78 (0.61-0.98) | .04 |
| Further adjusted for Charlson Comorbidity Index and previous fracture | 0.80 (0.63-1.02) | .07 |
| Injurious fall | ||
| Adjusted for time since diagnosis, current age, sex, and calendar year | 0.83 (0.74-0.92) | <.001 |
| Further adjusted for Charlson Comorbidity Index and previous injurious fall | 0.85 (0.76-0.95) | .003 |
| Any CVE | ||
| Adjusted for time since diagnosis, current age, sex, and calendar year | 0.84 (0.73-0.97) | .02 |
| Further adjusted for Charlson Comorbidity Index and previous CVE | 0.87 (0.75-1.00) | .05 |
| Acute myocardial infarction | ||
| Adjusted for time since diagnosis, current age, sex, and calendar year | 0.84 (0.68-1.04) | .10 |
| Further adjusted for Charlson Comorbidity Index and previous CVE | 0.87 (0.70-1.08) | .20 |
| Ischemic stroke | ||
| Adjusted for time since diagnosis, current age, sex, and calendar year | 0.90 (0.74-1.09) | .28 |
| Further adjusted for Charlson Comorbidity Index and previous CVE | 0.92 (0.76-1.12) | .30 |
| Kidney stone | ||
| Adjusted for time since diagnosis, current age, sex, and calendar year | 0.89 (0.76-1.06) | .19 |
| Further adjusted for Charlson Comorbidity Index and previous kidney stone | 0.77 (0.65-0.91) | .003 |
| Death overall | ||
| Adjusted for time since diagnosis, current age, sex, and calendar year | 0.59 (0.53-0.65) | <.001 |
| Further adjusted for Charlson Comorbidity Index | 0.64 (0.58-0.71) | <.001 |
| Cardiovascular-related death | ||
| Adjusted for time since diagnosis, current age, sex, and calendar year | 0.60 (0.50-0.71) | <.001 |
| Further adjusted for Charlson Comorbidity Index and previous CVE | 0.71 (0.59-0.85) | <.001 |
Abbreviations: CVE, cardiovascular event; HR, hazard ratio.
Parathyroidectomy was used as a time-dependent variable in the Poisson regression model, with adjustments for time since diagnosis, current age, sex, and calendar year in the primary models as well as outcome-specific additional adjustments as indicated.
When we investigated the outcomes before and after surgery of the 6934 patients who underwent PTX, using a Cox proportional hazards regression model adjusted for age and sex, we found that the risk of any fracture (HR, 0.77; 95% CI, 0.63-0.95) and kidney stone (HR, 0.61; 95% CI, 0.48-0.78) was lower after vs before PTX, whereas no risk difference was seen for injurious fall (HR, 1.02; 95% CI, 0.80-1.31) (eTable 8 in the Supplement).
Discussion
Patients with pHPT had a significantly higher risk of hip fracture, CVE, and all-cause mortality vs their control counterparts. In patients with pHPT, PTX was associated with reduced risk of all of these outcomes.
Although pHPT is a relatively common disorder,1 especially among postmenopausal women, and its clinical consequences for affected organ systems have long been known, there is still controversy regarding the clinical importance of these outcomes. Most studies of fracture risk in pHPT are relatively small, and a recent meta-analysis of all potential publications between 1966 and 2019 revealed only 12 studies involving 5233 patients with pHPT and 13 154 control individuals.20 The meta-analysis reported an increased risk of any fracture, but the analysis of hip fracture risk was limited to 3 studies that included relatively young patients with pHPT; thus, few cases of hip fractures emerged during the follow-up period. This limitation likely affected the observed lack of association between pHPT and risk of hip fracture.18,19,29
To our knowledge, in terms of the number of patients with pHPT and observed outcomes, the present cohort study is the largest analysis performed thus far. The 51% increased risk of hip fracture in patients with pHPT (unadjusted HR, 1.51) compared with control individuals was based on 319 and 4519 events, respectively. Hip fracture is the most serious osteoporosis-related fracture and is associated with substantially higher morbidity, mortality, and functional decline,30 emphasizing the importance of identifying patients at high risk and including pHPT in the risk assessment.
Previous studies found impaired bone properties in both trabecular and cortical bone31 in patients with pHPT. This finding was supported by a recent meta-analysis that identified an increased risk of any fracture, vertebral fracture, and forearm fracture in these patients.20 Results of the present study, which used a substantially larger data set, are in agreement with these previous analyses. We found an increased risk of any fracture, major osteoporotic fracture, and peripheral fracture in patients with pHPT.
Randomized clinical trials in patients with mild pHPT found that PTX improved bone mineral density and reduced serum calcium, but its role in the risk of fracture could not be ascertained owing to the small number of patients included in these trials.32 Observational studies found that the risk of fracture appeared to be lower after rather than before surgery,17 but this finding was confounded by the observation that those who underwent PTX had lower risk than those who were treated conservatively.33 In the time-dependent analysis in the present study, PTX was associated with a decrease in fracture risk. This finding was supported by the lower fracture rate and fracture risk seen after rather than before surgery, further supporting that PTX was associated with lower fracture risk. This finding also agrees with results from a recent observational study of 183 433 Medicare beneficiaries in the US.34 A lower fracture rate was observed in patients with pHPT (87.3% women) who underwent PTX compared with those who had conservative treatment.34 Because control individuals without pHPT were not included, the risk of fracture associated with pHPT was not analyzed.34
Previous studies of surgical cases and untreated patients with pHPT found an increased risk of myocardial events and cardiovascular mortality8,35,36,37 in both of these groups. This finding was in contrast to the lack of elevated mortality risk reported by a US study of patients with pHPT in the community.9 In a Danish cohort of 674 patients with pHPT who underwent PTX and an equal number of sex- and age-matched control individuals, the risk of myocardial infarction, stroke, congestive heart failure, and hypertension was higher before surgery; more than a year after surgery, this increased risk was no longer seen for myocardial infarction and stroke but remained for the other outcomes. However, the Danish study was limited by few events for several outcomes.38 Because of the conflicting results, especially regarding mild pHPT, and the absence of large randomized clinical trials, there is insufficient evidence to recommend PTX based on risk of cardiovascular disease.10 In the present study, the risk of myocardial infarction, stroke (both ischemic and hemorrhagic), and death was higher in untreated patients with pHPT and the risk was inversely associated with PTX. These findings support the inclusion of cardiovascular risk in the indications for surgery, but large randomized clinical trials are needed to identify any benefits of PTX.
With advancing age, the risks of functional decline, reduced muscle performance, and falls increase.39 Falls are the leading cause of injury-related morbidity and mortality in older men and women in the US.40 Nearly 29% of the population 65 years or older reported falling in the past year.40 The risk of falls or injurious falls in patients with pHPT has not been investigated. In this study, this risk was found to be inversely associated with PTX, indicating that falls may be prevented by surgery in some patients. We did not confirm this finding by analyzing the risk before and after PTX, but survival bias could have affected this analysis.
Strengths and Limitations
This study has several strengths. To our knowledge, it is the largest study yet to investigate multiple outcomes in all untreated patients with pHPT identified in hospitals in Sweden as well as matched control individuals. A time-dependent model, which accounted in part for bias, was used to study the association between PTX and outcomes.
This study also has several limitations. First, the observational design could not establish causality, although the time-dependent analysis suggested an association between PTX and outcomes. Second, data on pHPT-related symptoms, serum calcium, or parathyroid hormone were not available, preventing the assessment of pHPT severity. Third, patients were included on the basis of hospital diagnoses; thus patients who were diagnosed in a primary care setting were not included. Fourth, the use of control individuals from the general population with less comorbidity than patients who were diagnosed with pHPT at hospitals could introduce a bias. However, the absence of a significant interaction between pHPT and Charlson Comorbidity Index for any fracture or CVE indicates an increased risk, regardless of other comorbidities.
Conclusions
The increased risk of fractures, CVEs, and death observed in patients with pHPT suggests that it is important to identify patients with this condition to prevent serious outcomes. The reduced risk of these outcomes associated with PTX suggests a clinical benefit of surgery.
eTable 1. Definitions
eTable 2. Other Fracture Outcomes
eTable 3. Cardiovascular Outcomes
eTable 4. Overall Death and Cardiovascular Deaths
eTable 5. Clinical Outcomes per Sex
eTable 6. Clinical Outcomes Within One Year
eFigure. Cumulative Incidence Function in PHPT Patients vs. Controls
eTable 7. Subhazard Ratios for PHPT Patients vs Population Controls, With Consideration of Competing Risk of Death
eTable 8. Outcomes for PTX Patients After Surgery Using the Same PTX Patients Before Surgery as Controls
References
- 1.Bilezikian JP. Primary hyperparathyroidism. J Clin Endocrinol Metab. 2018;103(11):3993-4004. doi: 10.1210/jc.2018-01225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Press DM, Siperstein AE, Berber E, et al. The prevalence of undiagnosed and unrecognized primary hyperparathyroidism: a population-based analysis from the electronic medical record. Surgery. 2013;154(6):1232-1237. doi: 10.1016/j.surg.2013.06.051 [DOI] [PubMed] [Google Scholar]
- 3.Lundgren E, Hagström EG, Lundin J, et al. Primary hyperparathyroidism revisited in menopausal women with serum calcium in the upper normal range at population-based screening 8 years ago. World J Surg. 2002;26(8):931-936. doi: 10.1007/s00268-002-6621-0 [DOI] [PubMed] [Google Scholar]
- 4.Silverberg SJ, Walker MD, Bilezikian JP. Asymptomatic primary hyperparathyroidism. J Clin Densitom. 2013;16(1):14-21. doi: 10.1016/j.jocd.2012.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu CY, Sturgeon C, Yeh MW. Diagnosis and management of primary hyperparathyroidism. JAMA. 2020;323(12):1186-1187. doi: 10.1001/jama.2020.0538 [DOI] [PubMed] [Google Scholar]
- 6.Pepe J, Cipriani C, Sonato C, Raimo O, Biamonte F, Minisola S. Cardiovascular manifestations of primary hyperparathyroidism: a narrative review. Eur J Endocrinol. 2017;177(6):R297-R308. doi: 10.1530/EJE-17-0485 [DOI] [PubMed] [Google Scholar]
- 7.Assadipour Y, Zhou H, Kuo EJ, Haigh PI, Adams AL, Yeh MW. End-organ effects of primary hyperparathyroidism: a population-based study. Surgery. 2019;165(1):99-104. doi: 10.1016/j.surg.2018.04.088 [DOI] [PubMed] [Google Scholar]
- 8.Yu N, Donnan PT, Flynn RW, et al. ; The Parathyroid Epidemiology and Audit Research Study (PEARS) . Increased mortality and morbidity in mild primary hyperparathyroid patients. Clin Endocrinol (Oxf). 2010;73(1):30-34. doi: 10.1111/j.1365-2265.2009.03766.x [DOI] [PubMed] [Google Scholar]
- 9.Wermers RA, Khosla S, Atkinson EJ, et al. Survival after the diagnosis of hyperparathyroidism: a population-based study. Am J Med. 1998;104(2):115-122. doi: 10.1016/S0002-9343(97)00270-2 [DOI] [PubMed] [Google Scholar]
- 10.Wilhelm SM, Wang TS, Ruan DT, et al. The American Association of Endocrine Surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg. 2016;151(10):959-968. doi: 10.1001/jamasurg.2016.2310 [DOI] [PubMed] [Google Scholar]
- 11.van Ballegooijen AJ, Reinders I, Visser M, Brouwer IA. Parathyroid hormone and cardiovascular disease events: a systematic review and meta-analysis of prospective studies. Am Heart J. 2013;165(5):655-664, 664.e1-664.e5. doi: 10.1016/j.ahj.2013.02.014 [DOI] [PubMed] [Google Scholar]
- 12.Khan AA, Hanley DA, Rizzoli R, et al. Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management—a Canadian and international consensus. Osteoporos Int. 2017;28(1):1-19. doi: 10.1007/s00198-016-3716-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bollerslev J, Rosen T, Mollerup CL, et al. ; SIPH Study Group . Effect of surgery on cardiovascular risk factors in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2009;94(7):2255-2261. doi: 10.1210/jc.2008-2742 [DOI] [PubMed] [Google Scholar]
- 14.Walker MD, Rundek T, Homma S, et al. Effect of parathyroidectomy on subclinical cardiovascular disease in mild primary hyperparathyroidism. Eur J Endocrinol. 2012;167(2):277-285. doi: 10.1530/EJE-12-0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persson A, Bollerslev J, Rosen T, et al. ; SIPH Study Group . Effect of surgery on cardiac structure and function in mild primary hyperparathyroidism. Clin Endocrinol (Oxf). 2011;74(2):174-180. doi: 10.1111/j.1365-2265.2010.03909.x [DOI] [PubMed] [Google Scholar]
- 16.Lewiecki EM, Miller PD. Skeletal effects of primary hyperparathyroidism: bone mineral density and fracture risk. J Clin Densitom. 2013;16(1):28-32. doi: 10.1016/j.jocd.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 17.Vestergaard P, Mollerup CL, Frøkjaer VG, Christiansen P, Blichert-Toft M, Mosekilde L. Cohort study of risk of fracture before and after surgery for primary hyperparathyroidism. BMJ. 2000;321(7261):598-602. doi: 10.1136/bmj.321.7261.598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson K, Ljunghall S, Krusemo UB, Naessén T, Lindh E, Persson I. The risk of hip fractures in patients with primary hyperparathyroidism: a population-based cohort study with a follow-up of 19 years. J Intern Med. 1993;234(6):585-593. doi: 10.1111/j.1365-2796.1993.tb01017.x [DOI] [PubMed] [Google Scholar]
- 19.Khosla S, Melton LJ III, Wermers RA, Crowson CS, O’Fallon WM, Riggs Bl. Primary hyperparathyroidism and the risk of fracture: a population-based study. J Bone Miner Res. 1999;14(10):1700-1707. doi: 10.1359/jbmr.1999.14.10.1700 [DOI] [PubMed] [Google Scholar]
- 20.Ejlsmark-Svensson H, Rolighed L, Harsløf T, Rejnmark L. Risk of fractures in primary hyperparathyroidism: a systematic review and meta-analysis. Osteoporos Int. 2021;32(6):1053-1060. doi: 10.1007/s00198-021-05822-9 [DOI] [PubMed] [Google Scholar]
- 21.Heide-Jørgensen U, Adelborg K, Kahlert J, Sørensen HT, Pedersen L. Sampling strategies for selecting general population comparison cohorts. Clin Epidemiol. 2018;10:1325-1337. doi: 10.2147/CLEP.S164456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Axelsson KF, Jacobsson R, Lund D, Lorentzon M. Effectiveness of a minimal resource fracture liaison service. Osteoporos Int. 2016;27(11):3165-3175. doi: 10.1007/s00198-016-3643-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Socialstyrelsen. Statistik om hjärtinfarkter 2019. 2020. Accessed December 8, 2021. https://sdb.socialstyrelsen.se/if_hji/val.aspx
- 24.Alexander RT, Hemmelgarn BR, Wiebe N, et al. ; Alberta Kidney Disease Network . Kidney stones and kidney function loss: a cohort study. BMJ. 2012;345:e5287. doi: 10.1136/bmj.e5287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 26.Albertsson-Wikland K, Mårtensson A, Sävendahl L, et al. Mortality is not increased in recombinant human growth hormone-treated patients when adjusting for birth characteristics. J Clin Endocrinol Metab. 2016;101(5):2149-2159. doi: 10.1210/jc.2015-3951 [DOI] [PubMed] [Google Scholar]
- 27.Breslow NE, Day NE. Statistical methods in cancer research: volume II—the design and analysis of cohort studies. IARC Sci Publ. 1987;(82):1-406. [PubMed] [Google Scholar]
- 28.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 29.Vestergaard P, Mosekilde L. Fractures in patients with primary hyperparathyroidism: nationwide follow-up study of 1201 patients. World J Surg. 2003;27(3):343-349. doi: 10.1007/s00268-002-6589-9 [DOI] [PubMed] [Google Scholar]
- 30.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359(9319):1761-1767. doi: 10.1016/S0140-6736(02)08657-9 [DOI] [PubMed] [Google Scholar]
- 31.Hansen S, Hauge EM, Rasmussen L, Jensen JE, Brixen K. Parathyroidectomy improves bone geometry and microarchitecture in female patients with primary hyperparathyroidism: a one-year prospective controlled study using high-resolution peripheral quantitative computed tomography. J Bone Miner Res. 2012;27(5):1150-1158. doi: 10.1002/jbmr.1540 [DOI] [PubMed] [Google Scholar]
- 32.Anagnostis P, Vaitsi K, Veneti S, et al. Efficacy of parathyroidectomy compared with active surveillance in patients with mild asymptomatic primary hyperparathyroidism: a systematic review and meta-analysis of randomized-controlled studies. J Endocrinol Invest. 2021;44(6):1127-1137. doi: 10.1007/s40618-020-01447-7 [DOI] [PubMed] [Google Scholar]
- 33.Yeh MW, Zhou H, Adams AL, et al. The relationship of parathyroidectomy and bisphosphonates with fracture risk in primary hyperparathyroidism: an observational study. Ann Intern Med. 2016;164(11):715-723. doi: 10.7326/M15-1232 [DOI] [PubMed] [Google Scholar]
- 34.Seib CD, Meng T, Suh I, et al. Risk of fracture among older adults with primary hyperparathyroidism receiving parathyroidectomy vs nonoperative management. JAMA Intern Med. 2022;182(1):10-18. doi: 10.1001/jamainternmed.2021.6437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hedbäck G, Odén A. Increased risk of death from primary hyperparathyroidism—an update. Eur J Clin Invest. 1998;28(4):271-276. doi: 10.1046/j.1365-2362.1998.00289.x [DOI] [PubMed] [Google Scholar]
- 36.Palmér M, Adami HO, Bergström R, Akerström G, Ljunghall S. Mortality after surgery for primary hyperparathyroidism: a follow-up of 441 patients operated on from 1956 to 1979. Surgery. 1987;102(1):1-7. [PubMed] [Google Scholar]
- 37.Nilsson IL, Yin L, Lundgren E, Rastad J, Ekbom A. Clinical presentation of primary hyperparathyroidism in Europe—nationwide cohort analysis on mortality from nonmalignant causes. J Bone Miner Res. 2002;17(suppl 2):N68-N74. [PubMed] [Google Scholar]
- 38.Vestergaard P, Mollerup CL, Frøkjaer VG, Christiansen P, Blichert-Toft M, Mosekilde L. Cardiovascular events before and after surgery for primary hyperparathyroidism. World J Surg. 2003;27(2):216-222. doi: 10.1007/s00268-002-6541-z [DOI] [PubMed] [Google Scholar]
- 39.Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2018;9(1):3-19. doi: 10.1002/jcsm.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guirguis-Blake JM, Michael YL, Perdue LA, Coppola EL, Beil TL. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(16):1705-1716. doi: 10.1001/jama.2017.21962 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Definitions
eTable 2. Other Fracture Outcomes
eTable 3. Cardiovascular Outcomes
eTable 4. Overall Death and Cardiovascular Deaths
eTable 5. Clinical Outcomes per Sex
eTable 6. Clinical Outcomes Within One Year
eFigure. Cumulative Incidence Function in PHPT Patients vs. Controls
eTable 7. Subhazard Ratios for PHPT Patients vs Population Controls, With Consideration of Competing Risk of Death
eTable 8. Outcomes for PTX Patients After Surgery Using the Same PTX Patients Before Surgery as Controls


