Abstract
Background
In December 2019, novel coronavirus pneumonia was detected in Wuhan, Hubei Province, China, and as the epidemic spread, such cases emerged worldwide. Recently, the World Health Organization (WHO) named a new mutant Omicron (B.1.1.529), which disrupts the binding of most antibodies to the S protein and has a greater ability to break through the vaccine, posing a serious risk to population safety. Positive pregnant women give birth to positive newborns despite appropriate isolation measures taken by medical staff, suggesting that we may have vertical transmission of the novel coronavirus. This article analyzes and studies the possible vertical transmission path of the new coronavirus in the perinatal period of pregnant women and the antibody-dependent enhancement (ADE), and puts forward effective preventive measures for positive pregnant women to provide further reference for clinical work.
Methods
We searched multiple databases, including PubMed, CNKI, Google Scholar, WHO COVID-19 database, and CDC database. Search terms included COVID-19, SARS-CoV-2, vertical transmission, Omicron, Vaginal, Breast Feeding, Vaccine, Neonatal, Severe acute respiratory syndrome coronavirus, Pregnancy, and Semen.
Selection criteria
The following criteria were also met: (1) positive maternal novel coronavirus nucleic acid test; (2) reporting of neonatal outcome; (3) language in Chinese or English; (4) study date or location indicated; (5) no suspected or confirmed duplicated reports.
Results
There is evidence of vertical transmission, and the risk of possible vertical transmission is 5.7% (75/1314). The article listed four possible vertical transmission routes, namely placental transmission, vaginal upstream transmission, breastfeeding transmission and monocyte, and macrophage transmission route, with placental transmission being the most probable. Meanwhile, SARS-CoV-2 may also enter the placenta to infect the fetus through antibody-dependent enhanced substitution mechanism. We recommend three methods for early surveillance of vertical transmission, namely nucleic acid testing, antibody screening, and antigen testing, and analyze their advantages and disadvantages. Finally, the article provides recommendations in four areas: labor management, neonatal management, nosocomial infection prevention and control, and vaccination. As well as suggesting effective preventive measures for positive pregnant women and analyzing the advantages and disadvantages of vaccination, it is recommended that pregnant women should be vaccinated promptly, but considering that the vaccine may cause fever, it is recommended to consider vaccination cautiously in the first trimester of pregnancy.
Conclusion
The article concludes that vertical transmission is possible, with placental transmission being the most likely, and that the risk of possible vertical transmission is 5.7% (75/1314). Good personal protection, patient isolation, ward disinfection, and vaccination are the best means of interrupting SARS-CoV-2.
Keywords: SARS-CoV-2, Mother-to-child transmission, Mechanism, Prevention
Introduction
Novel coronavirus pneumonia refers to pneumonia caused by SARS-CoV-2. In December 2019, novel coronavirus pneumonia was detected in Wuhan, Hubei Province, China, and as the epidemic spread, such cases emerged worldwide. On March 7, 2020, the World Health Organization declared novel coronavirus a global pandemic [1].
According to the World Health Organization (WHO), as of 6 January, 2022, a cumulative total of 234,533,539 cases of novel coronavirus pneumonia and 4,796,222 deaths have been confirmed worldwide [2]. The powerful spread of the epidemic has been accompanied by widespread mutation of the novel coronavirus, with 195 countries reporting cases of the Alpha variant, while 145 countries have reported cases of the Beta variant, 99 countries have reported cases of the Gamma variant, and 192 countries have reported cases of the Delta variant. According to WHO, there are currently 11 variant types alone of greatest concern, and these variant viruses are causing public health events of concern [2, 3]. Recently, a new variant of SARS-CoV-2 was reported in South Africa. On November 26, 2021, WHO named this mutant—Omicron (B.1.1.529) [4]. Omicron has a large number of mutations widely distributed across multiple proteins, but the focus is on mutations in the S-protein receptor binding domain (RBD), which mutations affect both infectivity and vaccine blocking ability. Omicron is 13 times more infectious than the original SARS-CoV-2 and 2.8 times more infectious than Delta [5]. Omicron disrupts the binding of most antibodies to S proteins and has a greater vaccine breakthrough ability. The current COVID-19 vaccine is somewhat less effective against the mutant virus, but the vaccine still has a preventive effect that can control the transmission and infection of Omicron [6]. Due to its frequent mutations and strong transmission capacity, COVID-19 has the ability to spread rapidly worldwide. It is recommended to strengthen existing sanitary and public health measures to curb its spread.
Experimental data showed that mothers with COVID-19 were more likely to have more severe complications, higher rates of preterm birth, and even 22 times higher mortality than undiagnosed mothers [7]. Moreover, most of the positive pregnant women showed mild or moderate symptoms, most commonly fever, cough, smell disturbance, taste disturbance, muscle pain, fatigue, sore throat, chills, headache, and loss of appetite [8]. Although the risk of SARS-CoV-2 infection in pregnant women is consistent with those who are not pregnant, they have more stress and anxiety and are more likely to suffer from post-traumatic stress disorder, so the role of novel coronaviruses in mothers and infants should be emphasized, studied, and explored [9, 10]. Whether the novel coronavirus produces vertical transmission and threatens fetal health has become a focus of attention in the field of perinatal medicine. Now, we analyze and discuss the possible pathways of vertical transmission of novel coronavirus in the perinatal period, as well as the early detection and interruption measures for maternal and fetal health, based on published research data and clinical data.
Vertical transmission of new coronavirus may occur
The literature shows that a total of 205 infants were born to COVID-19-positive pregnant women in 33 studies, of which 6.3% (13/205) tested positive at birth. China reported 167 positive maternal deaths, of which 4.2% (7/167) of infants were positive at birth [11]. Mirbeyk et al. reported in a systematic review that COVID-19-positive pregnant women conceived 302 newborns and a total of 219 newborns underwent nasopharyngeal specimen collection, of which 5% (11/219) tested positive for nucleic acid [12]. Chi et al. reported nucleic acid test results for SARS-CoV-2 in 128 newborns, of which 3.9% (5/128) of the neonates tested positive for nucleic acid [13]. Villar et al. reported nucleic acid test results in 416 neonates whose mothers were COVID-19 positive, of which 12.9% (54/416) tested positive for nucleic acid [7]. The presence of positive children suggests the need for further research into the possibility of vertical transmission.
Dong et al. reported an infected pregnant woman who delivered by cesarean section in a negative pressure ward and whose newborn was isolated immediately after delivery and whose newborn blood sample showed high levels of IgM 2 h after delivery and continued to be elevated [14]. IgM does not cross the placental barrier and is not detectable until at least 3 days after infection [15]. In contrast, high IgM after 2 h of birth indicates that intrauterine infection may have occurred and that the newborn is producing antibodies of its own.
Huiting et al. reported the delivery of a COVID-19 positive pregnant woman, respectively. The newborn was born without contacting the mother and was immediately isolated for observation, and was tested positive for nucleic acid after 36 h, 16 h, and 3 days, respectively [16–18]. A positive result was still detected in an immediately isolated neonate, suggesting the possibility of intrauterine infection.
A systematic review listed 655 nasopharyngeal swabs of newborns, 19 of which were positive for SARS-CoV-2 by RT-PCR, 4 placenta and 1 cord blood specimens were positive, but all nasopharyngeal swabs were negative, and 3 newborns had abnormally elevated IgM antibodies against SARS-CoV-2 in their sera. These findings suggest that SARS-CoV-2 may invade the placenta and even break the placental barrier to infect the fetus [19].
We included 15 articles to calculate the risk of vertical transmission and showed that 75 of the 1314 children born to positive pregnant women were positive, suggesting a possible risk of vertical transmission of 5.7% (75/1314) (Table 1).
Table 1.
Background characteristics of the selected studies
| Authors | Study location | Study design | Number of newborn | Number of positive newborns | |
|---|---|---|---|---|---|
| 1 | Villar et al. [7] | Multinational study | Case report | 706 | 54 |
| 2 | Chen et al. [45] | China | Retrospective cohort | 9 | 0 |
| 3 | Dong et al. [14] | China | Case report | 1 | 0 |
| 4 | Ferrazzi et al. [18] | Italy | Retrospective cohort | 3 | 42 |
| 5 | Liu et al. [33] | China | Case report | 3 | 0 |
| 6 | Lu et al. [93] | China | Case report | 1 | 0 |
| 7 | Di et al. [94] | China | Retrospective cohort | 9 | 0 |
| 8 | Jinhua et al. [95] | China | Retrospective cohort | 17 | 0 |
| 9 | Chao et al. [96] | China | Case report | 1 | 0 |
| 10 | Siyin et al. [97] | China | Case report | 1 | 0 |
| 11 | Huiting et al. [11] | China | Retrospective cohort | 4 | 1 |
| 12 | Kirtsman et al. [31] | Canada | Case report | 3 | 3 |
| 13 | Lopian et al. [39] | Israel | Retrospective cohort | 21 | 1 |
| 14 | Fenizia et al. [35] | Italy | Prospective study | 56 | 0 |
| 15 | González et al. [62] | Spain | Retrospective cohort | 14 | 440 |
Possible routes and mechanisms of the mother-to-child transmission of SARS-CoV-2
Vertical transmission refers to the way of the virus is transmitted from the parental host to the offspring, mainly through the placenta or the birth canal, but also can be seen perinatal breastfeeding or intimate contact infection, etc. [20]. There are four possible routes of mother-to-child transmission of SARS-CoV-2: placental transmission, vaginal ascending transmission, and breastfeeding transmission. Next, I will further explain its transmission mechanism from the following aspects.
Placental transmission
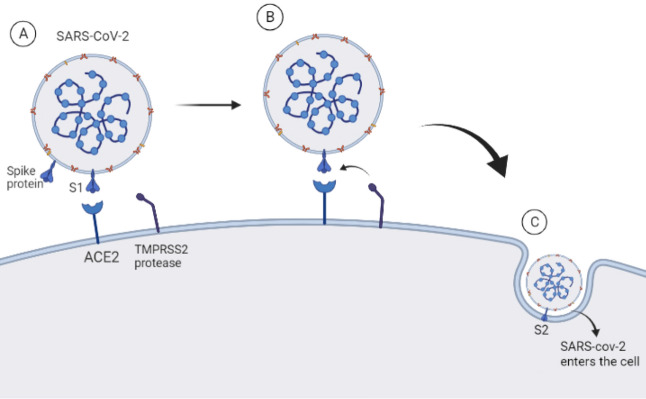
The placenta acts as a barrier and SARS-CoV-2 are found in the placental trophoblast [21]. Angiotensin-converting enzyme 2 (ACE2) binds to the virus and enters the placenta with the aid of transmembrane serine protease 2 (TMPRSS2), leading to mother-to-child transmission [22]. When SARS-CoV-2 invades target cells, the stinger protein (S) binds to ACE2, a receptor on the surface of the target cells. Meanwhile, cellular proteases such as TMPRSS2, Recombinant Cathepsin B (CTSB), and Recombinant Cathepsin L (CTSL) cleave the S protein, thus allowing the S protein to bind to ACE2 into the target cells [23–26]. Therefore, serine protease blockers inhibit serine protease activity, resulting in a lower rate of SARS-CoV-2 infection, and blockers have been used in Japan for the treatment of COVID-19 pneumonia [24].
In addition, ACE2 expression was found in many cells, and ACE2 was expressed in the intestine, kidney, testis, gallbladder, heart, lung, and placenta. Within the placental villi, ACE2 was expressed mainly in syncytial trophoblasts, cytotrophoblasts, endothelial cells, decidual cells, and vascular smooth muscle cells of primary secondary villi [23–27]. The extent of ACE2 and TMPRSS2 expression in the placenta varies with the period of gestation. In early gestation, ACE2 and TMPRSS2 levels are high, and ACE2 is predominantly found in the primary and secondary decidual areas; expression levels gradually decrease with increasing gestational duration. In late gestation, ACE2 and TMPRSS2 were not even expressed [27, 28].
A study reported the miscarriage of an SARS-CoV-2-positive pregnant woman at 13 weeks of gestation, and the replication and synthesis of the viral genome was detected in the placenta, lung, and kidney tissues of both fetuses [29]. Hosier et al. reported that COVID-19 was diagnosed in one pregnant woman at 22 weeks of gestation, and PCR testing of the placenta and fetus revealed a positive placenta and umbilical cord [30]. Kirtsman et al. reported a pregnant woman who tested positive for SARS-CoV-2 before delivery and a newborn who tested positive for SARS-CoV-2 on the day of birth, day 2, and day 7 on nasopharyngeal swabs and placenta [31].
These studies suggest that novel coronaviruses may infect the fetus through the placenta at all stages of pregnancy. Moreover, the gradual decrease in the expression of ACE2 and TMPRSS2 suggests that maternal infection with SARS-CoV-2 in early pregnancy is more likely to lead to placental infection, while infection with SARS-CoV-2 in late pregnancy is less likely to result in vertical transmission, and the maternal and neonatal risks are lower [32], However, it is still necessary to take precautions to avoid infection with the virus to prevent critical situations of placental inflammation, fibrin deposition, or even infarction, which can affect maternal and neonatal health [29] (Fig. 1).
Fig. 1.
SARS-CoV-2 invasion pathway into the placenta. A Stinger protein subunit S1 binds to target cell surface receptor ACE2. B Cellular protease TMPRSS2 cleaves S protein and activates stinger protein subunit S2. C Stinger protein subunit S2 promotes entry of novel coronavirus into cells
Vaginal ascending transmission
Several studies have shown that vaginal secretions of SARS-CoV-2-positive pregnant women tested negative and that maternal vaginal secretions tested negative even when intrauterine infection occurred in the newborn [14, 33, 34]. Fenizia et al. reported vaginal swabs from 45 SARS-CoV-2-positive pregnant women with no detectable SARS-CoV-2 and also tested 34 rectal swabs and found 27% of the samples to be positive; nevertheless, all neonates tested negative [35]. Takmaz et al. reported that none of the vaginal swabs of 38 SARS-CoV-2-positive patients had detectable SARS-CoV-2 [36]. A systematic review showed that all 13 neonates delivered vaginally tested negative for SARS-CoV-2 nucleic acid [37]. Belinda et al. reported an SARS-COV-2-positive pregnant woman who did not undergo mother-infant separation after a vaginal delivery and the infant lived with its parents, both of whom were notably SARS-COV-2 positive. They followed strict viral precautions, washing their hands and wearing surgical masks. The newborns were breastfed throughout. The newborns were tested for COVID-19 24 h after delivery and the results were negative [38]. However, there were still cases of positive pregnant women who were born after vaginal delivery and 2 newborns tested positive for SARS-CoV-2 nucleic acid after day 1 and day 3, respectively [18]. Lopian et al. reported 21 newborns born to 21 SARS-CoV-2 positive patients and 1 newborn tested positive 24 h after birth. However, infection with SARS-CoV-2 is most likely not affected by the mode of delivery and may be related to other routes of transmission [39]. In general, vaginal upstream transmission is very unlikely and pregnant women can choose vaginal delivery.
Some researchers have discussed whether SARS-CoV-2 can appear in sperm and thus be transmitted to women through sexual intercourse [40]. Pan et al. reported 34 men who were infected with SARS-CoV-2 and then recovered, and after 31 days, no SARS-CoV-2 was detected in semen samples [41]. Pavone et al. reported 36 patients with COVID-19-positive [42]. Guo et al. reported 23 patients with COVID-19 with a median time from diagnosis to semen sample production of 32 days and tested their semen specimens during hospitalization and all specimens were negative [43]. Jin et al. reported the semen test results of 38 patients with COVID-19, of which 6 patients had positive test results [44]. Although cases of SARS-CoV-2 in male semen have been reported, most studies suggest that the risk of having SARS-CoV-2 in semen is extremely low. Therefore, the likelihood of sexual intercourse transmission of SARS-CoV-2 is extremely low.
Breastfeeding transmission
Hikmet et al. stated in their study that the expression of ACE2 in the breast is not clear, which tentatively suggests that SARS-CoV-2 is not transmitted to breast milk through the breast [23]. Chen et al. reported nine laboratory-confirmed SARS-CoV-2-positive pregnant women. Amniotic fluid, umbilical cord blood, breast milk samples, and neonatal pharyngeal swabs collected from six of these patients were sampled, and no SARS-CoV-2 was detected [45]. These studies suggest that the likelihood of SARS-CoV-2 transmission to breast milk is extremely low. The Center for Disease Control and Prevention and the American College of Obstetricians and Gynecologists state that breast milk is an important source of nutrition for infants and helps boost their immunity, and that mothers infected with SARS-CoV-2 can breastfeed [46, 47]. And the patient can also breastfeed after the vaccination [9].
Monocyte and macrophage transmission pathways
SARS-CoV-2 can infect monocytes and macrophages through the ADE pathway and also monocytes and macrophages through the ACE2 pathway. MERS-CoV and SARS-CoV can spread the infection further by infecting macrophages. SARS-CoV-2 is highly homologous to MERS-CoV and SARS-CoV [48]. A similar pathway may exist to infect monocytes and macrophages. The S protein of SARS-CoV-2 binds to ACE2 of monocytes and macrophages, allowing SARS-CoV-2 to enter and infect cells [49], and these infected cells migrate into the tissues, allowing the virus to spread [50]. A study found that SARS-CoV-2 entered the macrophage population via ACE2 in the subepithelial and splenic marginal zones of lymph nodes by immunostaining in COVID-19 patients [51]. Jafarzadeh et al. reported that a pregnant woman infected with SARS-CoV-2 presented with miscarriage at 13 weeks of gestation, and SARS-CoV-2 was detected in the placenta with a high degree of inflammation, and the presence of a large number of macrophages was observed, which may have been infected with SARS-CoV-2 and transported to the placenta and then infected with capillary cells, allowing vertical transmission to occur [29]. These studies suggest that SARS-CoV-2 is capable of infecting monocytes and macrophages, and perhaps also infecting the fetus through infected macrophages during pregnancy, with vertical transmission occurring. More evidence is still needed to show that SARS-CoV-2 can infect macrophages and spread the virus to the placenta.
Possible antibody-dependent enhancement (ADE) alternative mechanisms for vertical mother-to-child transmission of SARS-CoV-2
SARS-CoV-2 has been shown to enter cells via the ACE2 pathway, but other entry pathways may still exist. The antibody-dependent enhancement alternative mechanism is present in many coronaviruses, such as feline infectious peritonitis (FIP) virus, SARS-CoV, and Middle East respiratory syndrome coronavirus (MERS-CoV) [48]. SARS-CoV-2 is approximately 79% homologous to SARS-CoV and 50% homologous to MERS-CoV [48, 52]. Therefore, to study the transmission mechanism of SARS-CoV-2, it is important to consider whether ADE will occur.
ADE may be an alternative mechanism: the complex formed by binding of SARS-CoV-2 to antibodies may bind to Fcγ receptors on the surface of B cells or antigen-presenting cells and mediate entry into immune cells, which in turn invade systemic organs [53]. If this hypothesis is confirmed, then instead of preventing the entry of the virus, neutralizing antibodies would promote cellular infection [53]. Another hypothesis is that under the stimulation of a low-level neutralizing antibody (NAb), SARS-CoV-2 binds to the antibody and the complex formed can enter the cell via ACE2 receptor and then release SARS-CoV-2 [48]. This hypothesis suggests that the virus may enter the placenta and even infect the fetus through low levels of neutralizing antibodies. One study showed that IgG antibodies were more responsive to SARS-CoV-2 nuclear capsid protein, with slower viral clearance and more severe infection. In contrast, patients with a weaker antibody response to the nuclear capsid protein cleared the virus earlier, suggesting that we may have an ADE mechanism [48]. Although various hypotheses exist, there is no clear evidence of an ADE mechanism for SARS-CoV-2, and more research is still needed to seek evidence [54].
SARS-CoV-2 early monitoring of mother-to-child transmission
Nucleic acid testing
According to the COVID-19 Treatment Protocol (Trial Version 8, Revised), positive real-time reverse transcription-polymerase chain reaction (RT-PCR) tests and positive tests for SARS-CoV-2-specific IgG antibodies and IgM antibodies in people who have not been vaccinated against COVID-19 are the current gold standard for diagnosing people with SARS-CoV-2 [56]. SARS-CoV-2 nucleic acid can be detected in specimens such as nasal, oropharyngeal swabs, sputum or other lower respiratory secretions, blood, stool, and urine by RT- PCR, NGS, etc. [55].
Antibody test
Compared with the IgG and IgM antibody assay for SARS-CoV-2, RT-PCR is accurate, but the detection cycle is long and complicated, requiring professional personnel and laboratories to operate, which is not conducive to rapid diagnosis. The rapid, simple, sensitive, and accurate antibody assay is more suitable for clinical diagnosis [57].
Serum, plasma, and venous whole blood specimens can all be tested for antibodies. Three-five milliliters of whole blood is recommended for adults to ensure that a sufficient amount of serum can be isolated. Whole blood specimens can be collected directly using ethylenediaminetetraacetic acid (EDTA) anticoagulation tubes [58].
The body produces IgG and IgM as a specific immune response to viral invasion. IgG can cross the placental barrier to the fetus, but IgM molecules are too large to cross the placental barrier [15]. IgM is produced early and can be detected in the patient's blood 3–6 days after infection. Therefore, it can be used as an early detection indicator. IgG is produced late and can only be detected in the patient's blood 8 days after infection [15]. If the newborn tests positive for IgM, intrauterine infection may be suspected. If the test is positive for IgG and negative for IgM, the intrauterine infection is not considered to have occurred [56]. If IgG declines rapidly and IgM is negative, the initial diagnosis is false positive and no intrauterine infection has occurred [59]. Notably, in their study, Xia Chengjing et al. pointed out that false positives may occur in IgG and IgM antibody assays due to insufficient specificity in the selection of labeled antigen sites, and therefore suggested using fusion fragments of SARS-CoV-2 S and N proteins as antigens to improve the specificity of the assay [60].
Antigen detection
The growing number of COVID-19 infections in the population and the increasing number of specimens to be tested have overwhelmed laboratories, leaving them short of resources and delaying the delivery of test results [61]. Although RT-PCR is accurate, the test cycle is long and complex, requiring professionals and laboratories to operate, which is not conducive to rapid diagnosis. Therefore, there is an urgent need for alternative technologies, such as antigen detection tests, which can detect the presence of viruses in respiratory samples in a matter of minutes [62]. Antigen tests are currently available for evaluation, but mostly for the adult population and very few specifically for the pediatric population [61–65], and some researchers have speculated that antigen testing accuracy in children may be lower than in adults [65], so SARS-CoV-2 antigen testing must be evaluated specifically in children. González et al. evaluated the COVID-19 Ag Rapid Test Device (Abbott Rapid Diagnostics Jena GmbH, Jena, Germany), which was specifically designed for the pediatric population. The sensitivity and specificity of the rapid antigen test were 77.78% [95% confidence interval (CI) 51.92–92.63] and 100% (95% CI 98.88–100), respectively. The diagnostic accuracy was 99.09% (95% CI 97.53–99.71) with a kappa coefficient of 0.87 (P < 0.001). The minimum performance requirement for using an antigen test as a SARS-CoV-2 diagnostic is a sensitivity higher than 80%, and test results close to this value but with good specificity, negative and positive predictive values might be considered for use [62]. There are very few studies on antigen detection in the pediatric population and more research data are needed.
SARS-CoV-2 interruption of mother-to-child transmission
SARS-CoV-2 is widely prevalent and extremely transmissible, adaptive, and variable. Due to the decline of maternal immunity in the perinatal period and the underdeveloped immune system of newborns, the risk of SARS-CoV-2 infection is high for this reason. To effectively interrupt the mother-to-child transmission route of SARS-CoV-2, targeted prevention should be carried out in four areas: labor management, neonatal management, nosocomial infection prevention and control, and vaccination.
Maternity ward management
Studies have shown that vaginal ascending transmission is less likely, and for this reason, vaginal delivery is also less likely to infect the newborn. For women with mild symptoms and no contraindications, vaginal delivery can be chosen, but the cesarean section is generally recommended [66, 67]. Of course, the possibility of vaginal ascending transmission cannot be completely excluded. Pregnant women who have been diagnosed with COVID-19 should deliver in a designated hospital, and the isolation ward should be managed by a multidisciplinary team of members, including obstetricians, neonatologists, infectious diseases, respiratory medicine, critical care disciplines and anesthesiologists, nurses, etc. [68]. Pregnant women should deliver in a negative pressure operating room, and pregnant women and physicians should wear tertiary protection (N95 mask, face shield, goggles, gloves, gown, shoe covers, operating cap) [69]. Although delayed cord ligation is beneficial for preterm and term infants, the possibility of mother-to-child transmission is currently unknown, and for this reason, delayed cord ligation is not recommended to reduce the risk of mother-to-child transmission [70].
Neonatal management
Since the potential for mother-to-child transmission is unclear, and the greatest risk for the neonatal route of infection is droplet transmission [56]. For this reason, good neonatal isolation is an important measure to avoid infection. For pregnant women who have been diagnosed with COVID-19 during delivery, postpartum newborns should be promptly sent to a negative pressure isolation room or a single room with a high-efficiency particulate air filter (HEPA) for isolation [69]. And strengthen the implementation of contact isolation, droplet isolation, and air isolation measures [71]. During this period, nasopharyngeal, oropharyngeal swabs or lower respiratory tract aspirates, and alveolar lavage fluid as well as serum should be collected regularly to test for viral nucleic acids in newborns [72]. It is also recommended to stop newborn chaperones and visits and to feed the newborn with formula. If donor breast milk is used, it should be fed after pasteurization [72].
Prevention and control of nosocomial infections
The room of the child should be disinfected preferentially by hydrogen peroxide fogging or vaporization, or by spraying with chlorine-containing agents [72]. The medical waste generated by the child should be placed in a double-layer infectious medical waste bag, and, disinfected with a chlorine-containing preparation for more than 10 min, and then marked [73]. After 7 days of use, the newborn warming box should be disassembled and cleaned and disinfected with chlorine-containing disinfectant, and some contaminated areas should be cleaned and disinfected with stain, and then disinfected and dechlorinated with water [74].
Vaccination
Inactivated vaccines, mRNA vaccines, etc. have been developed. [75]. Studies have indicated that vaccination of pregnant women does not affect pregnancy, delivery, and neonatal complications [76], but local and systemic adverse events may occur after vaccination, with local adverse events including injection site pain, fatigue, headache, and muscle pain. Systemic adverse events include fatigue, headache, muscle pain, chills, fever, and nausea [77, 78]. Differences in the probability of adverse events between the first and second vaccination with Pfizer-BioNTech and Moderna have been reported. For example, the incidence of chills [3% (254/9052) vs 26% (1747/6638)], fever [3% (256/9052) vs 25% (1648/6638)] and nausea [5% (492/9052) vs 20% (1356/6638)]. The incidence of chills [6% (442/7930) vs 49% (2755/5635)], fever [6% (453/7930) vs 46% (2594/5635)], and nausea [8% (638/7930) vs 34% (1909/5635)] after the first and second doses of Moderna, respectively. A higher incidence of adverse events can be found after the second dose of vaccine [78]. In early gestation, maternal fever is likely to cause fetal neural tube defects and may also trigger cardiovascular defects, oral clefts, isolated congenital ear defects, cataracts, hypospadias, renal anomalies, possible anorectal malformations, and general congenital malformations [79], and vaccination may trigger fever, but the likelihood of long-term fever is low [80].
The World Health Organization believes that the benefits of vaccination outweigh the risks and recommends vaccination for pregnant women [81]. The Centers for Disease Control and Prevention (CDC) recommends that pregnant women be vaccinated against SARS-CoV-2, concluding that the mRNA vaccine does not pose a safety issue for pregnant women and infants [82]. The current findings suggest that the vaccine is safe for pregnant women, but considering that fever can increase the risk of congenital malformations, it is recommended that vaccination in the first trimester of pregnancy be considered with caution [79, 83].
It has been shown that the New Crown Pneumonia mRNA vaccine is immunogenic in pregnant and lactating women and induces an immune response to SARS-CoV-2 variants, and that vaccination elicits a higher antibody response than infection [84]. After vaccination, serum antibody and breast milk antibody IgM, IgG, and IgA levels increased in pregnant and lactating women, but the main serum antibody and breast milk antibody and breast milk antibody were IgG [84, 85]. And the more the number of inoculations, the higher the IgG level [85].
Researchers have found higher antibody levels in the cord blood of vaccinated pregnant women than in unvaccinated infected pregnant women [84], suggesting antibody transfer to the newborn through the placenta. There is also a study that suggests that the timing of vaccination may affect the degree of antibody transfer, suggesting that we should perhaps vaccinate as soon as possible to strengthen fetal immunity [85].
As SARS-CoV-2 continues to mutate, one begins to wonder about the possibility of reinfection. Sengpiel et al. reported a pregnant woman who tested positive at gestational week 10 + 2. Neutralizing antibody (NAB) and SARS-CoV-2 antibody tests were negative 2 months after the first infection, which may explain why reinfection occurred. Newborns tested SARS-CoV-2 2 h after birth, indicating that intrauterine infection did not occur [86]. There are very few cases of reinfection during pregnancy after vaccination, and it is unclear how reinfection affects vertical transmission, and more data are needed to study this. However, as SARS-CoV-2 continues to mutate, the efficacy of existing vaccines has decreased to varying degrees and immune escape may occur. The authors speculated that previous infections or vaccinations do not fully ensure protection of pregnant women and newborns from infection [5, 86–89].
The novel coronavirus S protein contributes to virus recognition and entry into host cells [22], and there is an 86% probability of mutations in the gene sequence of the S protein [90], which may enhance the affinity of the virus to the cellular receptor ACE2, leading to increased infectivity and even to viral immune escape [91]. Vaccines now developed have different degrees of reduced effectiveness against different mutant strains [91], and vaccine immune escape may occur if mutant strains continue to drift through the population [92]. Therefore, the gene sequence of SARS-CoV-2 should be monitored in a timely manner and a novel vaccine should be developed to reduce or stop the SARS-CoV-2 pandemic.
Conclusions
The current mechanism of vertical transmission relies mainly on placental transmission, with a possible risk of vertical transmission of 5.7% (75/1314), and further clinical studies are still needed. When diagnosing mother-to-child transmission, attention should be paid to the problem of false positives in newborns and follow-up for 3–6 months to dynamically monitor the changes in IgG and IgM. As a special population, to effectively prevent mother-to-child SARS-CoV-2 infection, it is recommended to conduct comparative vaccination studies along with good hospital infection surveillance and isolation and decontamination of the ward. Vaccination studies in pregnant women have shown that vaccines are safe and reliable, can boost their immunity, and are less likely to be harmful to the fetus, but it is recommended that vaccination be considered carefully in the first trimester of pregnancy. The available literature still lacks dynamic and continuous follow-up of the infection process in positive mothers, and further information needs to be accumulated.
Acknowledgements
I thank Wenbin Dong for the article topic selection and modification and guidance.
Abbreviations
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus
- WHO
World Health Organization
- CDC
The Centers for Disease Control and Prevention
- RBD
Receptor-binding domain
- ACE2
Angiotensin-converting enzyme 2
- TMPRSS2
Transmembrane serine protease 2
- CTSB
Recombinant Cathepsin B
- CTSL
Recombinant Cathepsin L
- FIP
Feline infectious peritonitis
- MERS-CoV
Middle East respiratory syndrome coronavirus
- ADE
Antibody-dependent enhancement
- NAb
Neutralizing antibody
- RT-PCR
Real-time reverse transcription-polymerase chain reaction
- EDTA
Ethylenediaminetetraacetic acid
- CI
Confidence interval
- HEPA
High efficiency particulate air filter
Funding
No funding was secured for this study.
Declarations
Conflicts of interest
There are no conflicts of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferrer-Oliveras R, Mendoza M, Capote S, et al. Immunological and physiopathological approach of COVID-19 in pregnancy. Arch Gynecol Obstet. 2021;304(1):39–57. doi: 10.1007/s00404-021-06061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weekly epidemiological update on COVID-19—6 January 2022 [EB/OL]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---6-january-2022
- 3.Weekly epidemiological update on COVID-19—5 October 2021 [EB/OL]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---5-october-2021
- 4.Kannan S, Shaik Syed Ali P, Sheeza A. Omicron (B.1.1.529)—variant of concern—molecular profile and epidemiology: a mini review. Eur Rev Med Pharmacol Sci. 2021;25(24):8019–8022. doi: 10.26355/eurrev_202112_27653. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Wang R, Gilby NB, et al. Omicron (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. ArXiv. 2021 doi: 10.1021/acs.jcim.1c01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He X, Hong W, Pan X, et al. SARS-CoV-2 Omicron variant: characteristics and prevention. MedComm. 2021;2(4):838–845. doi: 10.1002/mco2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021 doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Toro F, et al. Impact of COVID-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:36–46. doi: 10.1016/j.cmi.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luxi N, Giovanazzi A, Capuano A, et al. COVID-19 vaccination in pregnancy, paediatrics, immunocompromised patients, and persons with history of allergy or prior SARS-CoV-2 infection: overview of current recommendations and pre- and post-marketing evidence for vaccine efficacy and safety. Drug Saf. 2021;44(12):1247–1269. doi: 10.1007/s40264-021-01131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akgor U, Fadıloglu E, Soyak B, et al. Anxiety, depression and concerns of pregnant women during the COVID-19 pandemic. Arch Gynecol Obstet. 2021;304(1):125–130. doi: 10.1007/s00404-020-05944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bwire GM, et al. Possible vertical transmission and antibodies against SARS-CoV-2 among infants born to mothers with COVID-19: A living systematic review. J Med Virol. 2021;93:1361–1369. doi: 10.1002/jmv.26622. [DOI] [PubMed] [Google Scholar]
- 12.Mirbeyk M, Saghazadeh A, Rezaei N. A systematic review of pregnant women with COVID-19 and their neonates. Arch Gynecol Obstet. 2021;304(1):5–38. doi: 10.1007/s00404-021-06049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi J, Gong W, Gao Q. Clinical characteristics and outcomes of pregnant women with COVID-19 and the risk of vertical transmission: a systematic review. Arch Gynecol Obstet. 2021;303(2):337–345. doi: 10.1007/s00404-020-05889-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong L, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323:1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xi C, Guili W. a review of pregnancy outcomes of novel coronavirus infection during pregnancy. Acta Laser Biol Sin. 2020;29:302–308. [Google Scholar]
- 16.Huiting Z, et al. Clinical analysis of perinatal COVID-19 in eight pregnant women. J Chongqing Med Univ. 2020;45:916–921. [Google Scholar]
- 17.Alzamora MC, et al. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020;37:861. doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrazzi E, et al. Vaginal delivery in SARS-CoV-2-infected pregnant women in Northern Italy: a retrospective analysis. BJOG Int J Obste Gynaecol. 2020;127:1116–1121. doi: 10.1111/1471-0528.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettirosso E, et al. COVID-19 and pregnancy: a review of clinical characteristics, obstetric outcomes and vertical transmission. Aust N Z J Obstet Gynaecol. 2020;60:640–659. doi: 10.1111/ajo.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan XZL, Xiaokui PYHMG. Medical microbiology. 9. Beijing: People’s Medical Publishing House; 2018. [Google Scholar]
- 21.Mulvey JJ, et al. Analysis of complement deposition and viral RNA in placentas of COVID-19 patients. Ann Diagn Pathol. 2020;46:151530. doi: 10.1016/j.anndiagpath.2020.151530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komine-Aizawa S, Takada K, Hayakawa S. Placental barrier against COVID-19. Placenta. 2020;99:45–49. doi: 10.1016/j.placenta.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hikmet F, et al. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020;16:e9610. doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann M, et al. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv. 2020 doi: 10.1101/2020.01.31.929042. [DOI] [Google Scholar]
- 25.Hartenian E, et al. The molecular virology of coronaviruses. J Biol Chem. 2020;295:12910–12934. doi: 10.1074/jbc.REV120.013930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan PM, Caplice N. COVID-19 and relative angiotensin-converting enzyme 2 deficiency: role in disease severity and therapeutic response. Open Heart. 2020 doi: 10.1136/openhrt-2020-001302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jing Y, et al. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol Hum Reprod. 2020;26:367–373. doi: 10.1093/molehr/gaaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bloise E, et al. Expression of severe acute respiratory syndrome coronavirus 2 cell entry genes, angiotensin-converting enzyme 2 and transmembrane protease serine 2, in the placenta across gestation and at the maternal-fetal interface in pregnancies complicated by preterm birth or preeclampsia. Am J Obstet Gynecol. 2021;224:298.e1–298.e8. doi: 10.1016/j.ajog.2020.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valdespino-Vázquez MY, et al. Fetal and placental infection with SARS-CoV-2 in early pregnancy. J Med Virol. 2021;93:4480–4487. doi: 10.1002/jmv.26965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosier H, Farhadian SF, Morotti RA, et al. SARS–CoV-2 infection of the placenta. J Clin Investig. 2020 doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirtsman M, et al. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ. 2020;192:E647–E650. doi: 10.1503/cmaj.200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiwen X, Sicong P, Huaping Z. Assessment of the risk of maternal vertical transmission of novel coronavirus pneumonia from mother to child and the safety of breastfeeding and mother-infant interaction. Chin J Neonatol. 2021 doi: 10.21037/tp.2020.02.06. [DOI] [Google Scholar]
- 33.Liu W, Wang Q, Zhang Q et al (2020) Coronavirus disease 2019 (COVID-19) during pregnancy: a case series. Preprints 2020, 2020020373
- 34.Qiu L, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813–817. doi: 10.1093/cid/ciaa375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fornari F. Vertical transmission of Covid-19-a systematic review. J Pediatr Perinatol Child Health. 2020;4:7–13. doi: 10.26502/jppch.74050034. [DOI] [Google Scholar]
- 36.Takmaz O, Kaya E, Erdi B, et al. Severe acute respiratory syndrome coronavirus (SARS-CoV-2) is not detected in the vagina: a prospective study. PLoS ONE. 2021;16(9):e0253072. doi: 10.1371/journal.pone.0253072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fornari F. Vertical transmission of Covid-19-a systematic review. J Pediatr Perinatol Child Health. 2020;4(2):7–13. doi: 10.26502/jppch.74050034. [DOI] [Google Scholar]
- 38.Lowe B, Bopp B. COVID-19 vaginal delivery—a case report. Aust N Z J Obstet Gynaecol. 2020;60(3):465–466. doi: 10.1111/ajo.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopian M, Kashani-Ligumsky L, Czeiger S, et al. Safety of vaginal delivery in women infected with COVID-19. Pediatr Neonatol. 2021;62(1):90–96. doi: 10.1016/j.pedneo.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perry MJ, Arrington S, Neumann LM, et al. It is currently unknown whether SARS-CoV-2 is viable in semen or whether COVID-19 damages spermatozoa. Andrology. 2021;9(1):30–32. doi: 10.1111/andr.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan F, Xiao X, Guo J, et al. No evidence of severe acute respiratory syndrome–coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113(6):1135–1139. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavone C, Giammanco GM, Cascino AP, et al. Assessment of SARS-CoV-2 RNA shedding in semen of 36 males with symptomatic, asymptomatic, and convalescent infection during the first and second wave of COVID-19 pandemic in Italy. Asian J Androl. 2022;24(2):135–138. doi: 10.4103/aja2021103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo L, Zhao S, Li W, et al. Absence of SARS-CoV-2 in semen of a COVID-19 patient cohort. Andrology. 2021;9(1):42–47. doi: 10.1111/andr.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li D, Jin M, Bao P, et al. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open. 2020;3(5):e208292. doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen H, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ACGO. Coronavirus (COVID-19), pregnancy, and breastfeeding: a message for patients [EB/OL]. https://www.acog.org/womens-health/faqs/coronavirus-covid-19-pregnancy-and-breastfeeding#
- 47.CDC. Care for breastfeeding people [EB/OL]. https://www.cdc.gov/coronavirus/2019-ncov/hcp/care-for-breastfeeding-people.html
- 48.Karthik K, et al. Role of antibody-dependent enhancement (ADE) in the virulence of SARS-CoV-2 and its mitigation strategies for the development of vaccines and immunotherapies to counter COVID-19. Hum Vaccin Immunother. 2020;16:3055–3060. doi: 10.1080/21645515.2020.1796425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang Y, et al. Highlight of immune pathogenic response and hematopathologic effect in SARS-CoV, MERS-CoV, and SARS-Cov-2 infection. Front Immunol. 2020;11:1022. doi: 10.3389/fimmu.2020.01022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jafarzadeh A, et al. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: lessons from SARS and MERS, and potential therapeutic interventions. Life Sci. 2020;257:118102. doi: 10.1016/j.lfs.2020.118102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaath H, et al. Single-cell transcriptome analysis highlights a role for neutrophils and inflammatory macrophages in the pathogenesis of severe COVID-19. Cells. 2020 doi: 10.3390/cells9112374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tetro JA. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. 2020;22:72–73. doi: 10.1016/j.micinf.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ulrich H, Pillat MM, Tárnok A. Dengue fever, COVID-19 (SARS-CoV-2), and antibody-dependent enhancement (ADE): a perspective. Cytometry A. 2020;97:662–667. doi: 10.1002/cyto.a.24047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halstead SB, Katzelnick L. COVID-19 vaccines: should we fear ADE? J Infect Dis. 2020;222:1946–1950. doi: 10.1093/infdis/jiaa518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yinghui J, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (Full version) New Med. 2020;30:35–64. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Health Commission New coronavirus pneumonia diagnosis and treatment plan (Trial Version 8) Chin Med J. 2021 doi: 10.1097/CM9.0000000000000866. [DOI] [Google Scholar]
- 57.Xiaozheng L, et al. The diagnostic value of detection of IgG and IgM antibodies to the novel coronavirus. Med Diet Health. 2021;19:146–148. [Google Scholar]
- 58.Ying-Chun XU, Ji-Hong HU. Expert consensus on clinical application of new coronavirus nucleic acid and antibody detection. Int J Lab Med. 2020;41:1665–1669. [Google Scholar]
- 59.Chen W, Yihua Z, Huixia Y. How to obtain evidence of vertical transmission of coronavirus according to serological test of specific IgM and IgG antibodies. Chin J Perinat Med. 2020;23:217–219. [Google Scholar]
- 60.Chengjing X, et al. Analysis of a case of systemic lupus erythematosus leading to false positives of new coronavirus IgM and IgG antibodies in serum. Lab Med Clin. 2021;18:573–575. [Google Scholar]
- 61.Freire-Paspuel B, Morales-Jadan D, Zambrano-Mila M, et al. Analytical sensitivity and clinical performance of “COVID-19 RT-PCR Real TM FAST (CY5) (ATGen, Uruguay) and “ECUGEN SARS-CoV-2 RT-qPCR” (UDLA-STARNEWCORP, Ecuador)”: high quality-low cost local SARS-CoV-2 tests for South America. PLoS Negl Trop Dis. 2022;16(4):e0010082. doi: 10.1371/journal.pntd.0010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.González-Donapetry P, García-Clemente P, Bloise I, et al. Think of the children: evaluation of SARS-CoV-2 rapid antigen test in pediatric population. Pediatr Infect Dis J. 2021;40(5):385–388. doi: 10.1097/INF.0000000000003101. [DOI] [PubMed] [Google Scholar]
- 63.Gonçalves CCA, Barroso SPC, Herlinger AL, et al. COVID-19 diagnosis by RT-qPCR in alternative specimens. Mem Inst Oswaldo Cruz. 2021;116:e210085. doi: 10.1590/0074-02760210085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernandes Q, Inchakalody VP, Merhi M, et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann Med. 2022;54(1):524–540. doi: 10.1080/07853890.2022.2031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Albert E, Torres I, Bueno F, et al. Field evaluation of a rapid antigen test (Panbio™ COVID-19 Ag Rapid Test Device) for COVID-19 diagnosis in primary healthcare centres. Clin Microbiol Infect. 2021;27(3):472.e7–472.e10. doi: 10.1016/j.cmi.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuyan M. Management strategy of pregnant women during the outbreak of 2019 coronavirus disease. J Shandong Univ. 2020;58:38–43. [Google Scholar]
- 67.Yuheng X, Tian L. Integration of systematic prevention measures for neonatal 2019-nCoV. J Chongqing Med Univ. 2020;45:1066–1069. [Google Scholar]
- 68.Fang L, et al. Response plan in the neonatal intensive care unit during epidemic of SARS-CoV-2 infection (2nd edition) Chin J Contemp Pediatr. 2020;22:205–210. doi: 10.7499/j.issn.1008-8830.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vardhelli V, et al. Perinatal COVID-19: review of current evidence and practical approach towards prevention and management. Eur J Pediatr. 2021;180:1009–1031. doi: 10.1007/s00431-020-03866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amatya S, et al. Management of newborns exposed to mothers with confirmed or suspected COVID-19. J Perinatol. 2020;40:987–996. doi: 10.1038/s41372-020-0695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yanqin Y, et al. Recommendations for graded prevention and control for children during the outbreak of corona virus disease 2019. Chin J Child Health Care. 2020;28:237–241. [Google Scholar]
- 72.Laishuan W, et al. Perinatal and neonatal management plan for prevention and control of SARS-CoV-2 infection (2nd edition) Chin J Contemp Pediatr. 2020;22:195–198. doi: 10.7499/j.issn.1008-8830.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuhong L, et al. Prevention and control strategies of new coronavirus infection in newborns. Shandong Med J. 2020;60:110–113. [Google Scholar]
- 74.Xiajuan Y, et al. Nursing advice on prevention and control of neonatal novel coronavirus infection. Chin J Nurs. 2020;55:496–498. [Google Scholar]
- 75.Xiaoyan Q, et al. New coronavirus vaccine research and development progress and its potential adverse reactions. Chin J Clin Pharm. 2021;30:64–72. [Google Scholar]
- 76.Wainstock T, Yoles I, Sergienko R, et al. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine. 2021;39(41):6037–6040. doi: 10.1016/j.vaccine.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blakeway H, Prasad S, Kalafat E, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226(2):236.e1–236.e14. doi: 10.1016/j.ajog.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pratama NR, Wafa IA, Budi DS, et al. Covid-19 vaccination in pregnancy: a systematic review. Medrxiv. 2021 doi: 10.1101/2021.07.04.21259985. [DOI] [Google Scholar]
- 79.Graham JM., Jr Update on the gestational effects of maternal hyperthermia. Birth Defects Res. 2020;112(12):943–952. doi: 10.1002/bdr2.1696. [DOI] [PubMed] [Google Scholar]
- 80.Goldshtein I, Nevo D, Steinberg DM, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326(8):728–735. doi: 10.1001/jama.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.World Health Organization. Is it safe for pregnant women, those planning to become pregnant, and breastfeeding mothers to receive COVID-19 vaccines? [EB/OL]. https://www.who.int/zh/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-(covid-19)-vaccines-safety
- 82.Centers for Disease Control and Prevention (CDC). COVID-19 vaccines while pregnant or breastfeeding [EB/OL]. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html
- 83.COVID-19 vaccination in pregnancy and breastfeeding [EB/OL]. https://www.epicentro.iss.it/en/vaccines/covid-19-pregnancy-breastfeeding
- 84.Collier A-RY, et al. Immunogenicity of COVID-19 mRNA vaccines in pregnant and lactating women. JAMA. 2021 doi: 10.1001/jama.2021.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gray KJ, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sengpiel V, Carlsson Y, Liljeqvist J, et al. Confirmed reinfection with SARS-CoV-2 during a pregnancy: a case report. Clin Case Rep. 2022;10(2):e05400. doi: 10.1002/ccr3.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Araf Y, Akter F, Tang YD, et al. Omicron variant of SARS-CoV-2: genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol. 2022;94(5):1825–1832. doi: 10.1002/jmv.27588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Farrukh L, Mumtaz A, Sana MK. How strong is the evidence that it is possible to get SARS-CoV-2 twice? A systematic review. Rev Med Virol. 2021;31(5):1–12. doi: 10.1002/rmv.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu C, Ginn HM, Dejnirattisai W, et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184(16):4220–4236.e13. doi: 10.1016/j.cell.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao X, et al. Evolution and variation of SARS-CoV-2. Chem Life. 2021;41:215–222. [Google Scholar]
- 91.Yenan F, et al. Global research progress of novel coronavirus variant strains. Chin J Virol. 2021;37(03):695–711. [Google Scholar]
- 92.Yenan F, et al. Analysis of the global early spread of the new coronavirus variant strain VOC 202012/01 and the evolutionary characteristics of the spike protein. Chin J Virol. 2021;37:267–273. [Google Scholar]
- 93.Lu D, Sang L, Du S, et al. Asymptomatic COVID-19 infection in late pregnancy indicated no vertical transmission. J Med Virol. 2020;92(9):1660–1664. doi: 10.1002/jmv.25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lei D, et al. Clinical analysis of nine cases of combined novel coronavirus pneumonia in pregnancy. Chin J Perinat Med. 2020 doi: 10.1016/j.ajog.2020.04.014. [DOI] [Google Scholar]
- 95.Tian J, et al. Clinical analysis of 29 cases of pregnancy combined with novel coronavirus pneumonia. Chin J Infect Dis. 2020;38(10):621–625. [Google Scholar]
- 96.Tong C, et al. A case of preterm delivery after novel coronavirus infection in mid-pregnancy. Chin J Perinat Med. 2020;23(05):321–323. [Google Scholar]
- 97.Zhuang S, et al. A case of perinatal novel coronavirus infection. Chin J Perinat Med. 2020 doi: 10.1002/uog.22006. [DOI] [Google Scholar]