Abstract
Background
Knowledge on differences in patients who present with deep vein thrombosis (DVT) and those with pulmonary embolism (PE) is incomplete.
Objective
To determine comorbidities and temporary provoking factors in patients with a first‐time PE or DVT.
Methods
This was a nationwide Swedish registry‐based, retrospective, case‐control study including 298 172 patients with first‐time venous thromboembolism (VTE) and 1 185 079 controls matched for age, sex, and county of residence, free of VTE at the time of matching.
Results
Patients with PE were older than those with DVT (mean age, 69 vs 66 years) and included slightly more women (PE, 53.4% vs DVT, 52.1%). After multivariable adjustment for comorbidities (within 7 years) and temporary provoking factors (within 3 months), heart failure (PE: adjusted odds ratio [aOR], 2.64 [99% confidence interval [CI], 2.55‐2.73]; DVT: aOR, 1.66 [99% CI, 1.60‐1.72]), ischemic heart disease (PE: aOR, 1.51 [99% CI, 1.47‐1.56]; DVT: aOR, 1.01 [99% CI, 0.98‐1.04]), and chronic obstructive pulmonary disease (PE: aOR, 2.51 [99% CI, 2.40‐2.63]; DVT, 1.54 [99% CI, 1.47‐1.62]) were among diseases that showed higher odds ratios in patients with PE than in those with DVT, compared with controls. Comorbidities registered within 6 months were associated with higher aORs than those within 7 years. The highest population attributable risks for PE were for cancer (13.0%) and heart failure (11.7%).
Conclusion
Cardiopulmonary diseases, particularly with recent onset, imply a higher risk for PE, whereas orthopedic surgery and lower‐extremity fractures carry a higher risk of DVT.
Keywords: case‐control studies, comorbidity, deep vein thrombosis, incidence, pulmonary embolism, registries, venous thromboembolism
Essentials.
There are few large‐scale studies on deep vein thrombosis (DVT) and pulmonary embolism (PE).
Patients with DVT or PE were compared with controls in a nationwide study of 1.48 million people.
Diseases in heart and lungs were more common among patients with PE than in patients with DVT.
Orthopedic surgery and fractures were more frequent in patients with DVT than in patients with PE.
1. INTRODUCTION
Venous thromboembolism (VTE), comprising deep vein thrombosis (DVT) and pulmonary embolism (PE), is the third most frequent cardiovascular disease worldwide. 1
Several previous publications have addressed incidence and causes of VTE. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 However, studies exploring differences in risk factors between patients with PE and those with DVT are sparse. The factor V Leiden mutation is more strongly correlated to risk of DVT, 12 while cardiac diseases have been shown to be a stronger risk for PE, which might also be the case for pulmonary conditions such as chronic obstructive pulmonary disease (COPD). 12 , 13 Previous studies on differences are either small, 14 , 15 are exclusively based on old data, 5 , 12 , 16 or only evaluated a limited number of comorbidities. 13 , 17 Given the difference in severity and mortality between PE and DVT, there is a need for a large, comprehensive study focusing on differences between patients with PE and patients with DVT.
The aim of this study was to determine age‐specific incidence rates of PE and DVT as well as the prevalence of comorbidities and temporary provoking factors at the time of a first‐time PE or DVT in comparison with a matched control population.
2. METHODS
2.1. Study population and design
This retrospective case‐control study was based on four linked Swedish nationwide health and administrative registries. Sweden has a publicly financed health care system that provides low‐cost outpatient and hospital care to all citizens. The Swedish Patient Register includes diagnostic and procedural codes of hospital‐based care, including inpatient data with complete national coverage since 1987 and hospital outpatient data since 2001. The Swedish Cause of Death Register includes all deaths of persons registered in Sweden. The Total Population Register has kept a record of all Swedish residents since 1968. 18 The Prescribed Drug Register includes information on all prescribed medications dispensed at any Swedish pharmacy since July 1, 2005. With very few exceptions, patients with PE and DVT in Sweden are cared for in hospital inpatient or specialized hospital‐based outpatient clinics, and not in primary care by general practitioners. Therefore, our register data has close to 100% coverage of all VTE cases in Sweden.
We identified all patients with a registered diagnosis of PE or DVT in the Swedish Patient Register between January 1, 1987, and December 31, 2018. For cases registered before July 1, 2005, a VTE diagnosis was defined as one of the following: (1) a single first inpatient diagnosis of DVT/PE; (2) one first DVT or PE outpatient diagnosis and a subsequent, identical diagnosis within 3 months; or (3) a single first diagnosis of DVT or PE in the Cause of Death Registry. For individuals registered with VTE after July 1, 2005, a VTE diagnosis was defined as one of the following: (1) a single first inpatient diagnosis of DVT or PE and at least one prescription of anticoagulant medication within 6 months after discharge; (2) a single first outpatient diagnosis of DVT or PE and at least one prescription of anticoagulant medication within 3 months; or (3) a single first diagnosis of DVT or PE in the Cause of Death Register. Cases were classified as DVT or PE according to the first registered diagnosis. If a first DVT and PE were registered on the same discharge date, it was regarded as a PE; however, concomitant DVT is not routinely diagnosed in patients with PE in Sweden.
For every case, up to four controls were selected from the Total Population Register, matched by sex, year of birth, and county of residence, who were free of any registered VTE event before the date of VTE for the matching case. However, a control could later be registered with a VTE and then be registered as a case. Thus, the same individual could be registered first as a control and later as a case.
To identify only first‐time VTE events as far as possible, we excluded cases and controls with a registered diagnosis of PE or DVT between January 1, 1980, and December 31, 1986.
The study was approved by the Ethical Review Agency of Sweden (Dnr 2019‐01956). No informed consent was required.
2.2. Definitions
PE and DVT were defined according to the International Classification of Diseases, Revision 8 (ICD‐8: PE, 450; DVT, 451); Revision 9 (ICD‐9: PE, 415B, 416W; DVT, 451 except 451A); or Revision 10 (ICD‐10: PE, I26; DVT, I80 except I80.0). Diagnoses were accepted regardless of whether they were considered a primary or secondary diagnosis or cause of hospitalization or death.
ICD codes as well as surgical and procedural codes used for the definition of comorbidities and temporary provoking factors are listed in Table S1 in the Supplement. Comorbidities were defined as those registered within 7 years before the VTE event; recent comorbidities were defined as those registered within 6 months before the VTE event; and temporary provoking factors were defined as those registered within 3 months before the VTE event; all comorbidities and temporary provoking factors could be registered on the same discharge date as the index VTE.
2.3. Statistical analysis
Categorical variables are presented as number with percentage, and continuous variables are presented as mean and standard deviation, as well as median with first and third quartiles. For comparisons between cases and controls, chi‐square tests were used for dichotomous variables and t tests for continuous variables. All tests were two‐tailed and with a 1% significance level.
Sex‐ and age‐stratified incidence rates were calculated by dividing the VTE cases by individuals at risk (Swedish population) in the same age group for each year, using population data from the statistical database from Statistics Sweden. The total incidence rate was calculated by dividing the total number of first‐time VTE cases by the total person‐years at risk (inhabitants in Sweden each year).
Odds ratios (ORs) with 99% confidence intervals (CIs) were calculated separately for PE and DVT using conditional logistic regression according to case‐control matching. Population attributable risk (PAR) was calculated according to Bruzzi et al. 19 PAR is used to estimate the reduction in percentage of PEs and DVTs in the population if a risk factor is eliminated.
Independent variables included in the multivariable analyses were diagnoses of heart failure, ischemic heart disease, atrial fibrillation (AF); ischemic stroke; hemorrhagic stroke; COPD; cancer; inflammatory bowel disease; depression; psychosis; alcohol abuse; and (within 3 months previous to VTE) gastrointestinal surgery, musculoskeletal surgery, other major surgery, and hospitalization for trauma and lower extremity fracture (see Table S1 for definitions). Separate multivariable analyses were carried out for comorbidities registered within 7 years and within 6 months before the VTE diagnosis. In the multivariable analyses, when each risk factor was analyzed, adjustments were made for all other risk factors. ORs are presented as forest plots and ORs with 99% CIs are presented.
All statistical analyses were performed using SAS version 9 for Windows (SAS Institute Inc., Cary, NC, USA).
3. RESULTS
3.1. Study population
In total, 298 172 patients in Sweden were registered with at least one diagnosis of VTE during the 32‐year study period. Of these, 141 264 had a first diagnosis of PE (including 11 516 individuals with a PE and DVT registered on the same date), and 156 908 with a DVT (Table 1). In total, 220 634 patients were registered in the inpatient register and 76 559 in the outpatient register, whereas 979 patients were first registered with VTE in the Cause of Death Register (Table S2). In 222 110 patients, VTE was registered as a primary diagnosis and as a secondary diagnosis in 76 062 patients. The matched control population comprised 1 185 079 individuals.
TABLE 1.
Baseline characteristics for 298 172 patients with VTE and 1 185 079 matched controls, presented for all VTE as well as for DVT and PE separately
| VTE | DVT | PE | ||||
|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Case | Control | |
| n = 298 172 | n = 1 185 079 | n = 156 908 | n = 623 112 | n = 141 264 | n = 561 967 | |
| Demographic factors | ||||||
| Age, y, mean (SD), median (IQR) | 68 (16.2) | 68 (16.2) | 66 (16.7) | 66 (16.7) | 69 (15.4) | 69 (15.4) |
| 71 (59‐80) | 71 (59‐80) | 70 (57‐79) | 70 (57‐79) | 73 (62‐81) | 73 (62‐81) | |
| Female sex, n (%) | 157 224 (52.7) | 625 117 (52.7) | 81 748 (52.1) | 324 678 (52.1) | 75 496 (53.4) | 300 439 (53.5) |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
|---|---|---|---|---|---|---|
| Comorbidities | ||||||
| Heart failure | 42 865 (14.4) | 72 228 (6.1) | 16 229 (10.3) | 35 854 (5.8) | 26 636 (18.9) | 36 374 (6.5) |
| Ischemic heart disease | 43 595 (14.6) | 106 065 (9.0) | 17 302 (11.0) | 52 964 (8.5) | 26 293 (18.6) | 53 101 (9.4) |
| Atrial fibrillation | 32 371 (10.9) | 69 977 (5.9) | 13 474 (8.6) | 34 976 (5.6) | 18 897 (13.4) | 35 001 (6.2) |
| Ischemic stroke | 21 159 (7.1) | 44 528 (3.8) | 10 302 (6.6) | 22 221 (3.6) | 10 857 (7.7) | 22 307 (4.0) |
| Hemorrhagic stroke | 4363 (1.5) | 7062 (0.6) | 2154 (1.4) | 3584 (0.6) | 2209 (1.6) | 3478 (0.6) |
| Chronic obstructive pulmonary disease | 16 588 (5.6) | 26 117 (2.2) | 6223 (4.0) | 13 148 (2.1) | 10 365 (7.3) | 12 969 (2.3) |
| Cancer | 60 397 (20.3) | 99 373 (8.4) | 30 240 (19.3) | 49 506 (7.9) | 30 157 (21.3) | 49 867 (8.9) |
| Systemic connective tissue disorders | 11 890 (4.0) | 19 371 (1.6) | 5625 (3.6) | 9577 (1.5) | 6265 (4.4) | 9794 (1.7) |
| Inflammatory bowel syndrome | 6422 (2.2) | 11 466 (1.0) | 3400 (2.2) | 6013 (1.0) | 3022 (2.1) | 5453 (1.0) |
| Liver disease | 3008 (1.0) | 4871 (0.4) | 1487 (0.9) | 2471 (0.4) | 1521 (1.1) | 2400 (0.4) |
| Kidney failure | 9751 (3.3) | 13 216 (1.1) | 4786 (3.1) | 6659 (1.1) | 4965 (3.5) | 6557 (1.2) |
| Depression | 9662 (3.2) | 18 895 (1.6) | 4510 (2.9) | 9991 (1.6) | 5152 (3.6) | 8904 (1.6) |
| Psychosis | 6819 (2.3) | 15 803 (1.3) | 3607 (2.3) | 7881 (1.3) | 3212 (2.3) | 7922 (1.4) |
| Alcohol abuse | 5701 (1.9) | 12 820 (1.1) | 3049 (1.9) | 7038 (1.1) | 2652 (1.9) | 5782 (1.0) |
| Temporary provoking factors | ||||||
| Gastrointestinal surgery | 11 930 (4.0) | 5518 (0.5) | 5027 (3.2) | 2871 (0.5) | 6903 (4.9) | 2647 (0.5) |
| Obstetric surgery | 782 (0.3) | 444 (0.0) | 432 (0.3) | 238 (0.0) | 350 (0.2) | 206 (0.0) |
| Surgery of the musculoskeletal system | 26 784 (9.0) | 8532 (0.7) | 15,111 (9.6) | 4193 (0.7) | 11 673 (8.3) | 4339 (0.8) |
| Surgery, other major | 21 442 (7.2) | 11 423 (1.0) | 10 129 (6.5) | 5846 (0.9) | 11 313 (8.0) | 5577 (1.0) |
| Trauma | 12 092 (4.1) | 4286 (0.4) | 6384 (4.1) | 2054 (0.3) | 5708 (4.0) | 2232 (0.4) |
| Lower extremity fracture | 16 051 (5.4) | 4393 (0.4) | 9043 (5.8) | 2165 (0.3) | 7008 (5.0) | 2228 (0.4) |
Comorbidities were defined as a diagnosis registered within 7 years before the VTE event and temporary provoking factors were defined as a diagnosis registered within 3 months previous to the VTE event.
DVT, deep vein thrombosis; IQR, interquartile range (Q1‐Q3); PE, pulmonary embolism; SD, standard deviation; VTE, venous thromboembolism.
3.2. Incidence rates
Age‐ and sex‐specific incidence rates per 100 000 inhabitants for PE and DVT are shown in Figure 1 and Tables S3 and S4. Corresponding figures for any VTE (PE or DVT) are shown in Figure S1 and Table S5. The total annual incidence rates in the population with a first VTE were 105.2 per 100 000 inhabitants, 49.8 per 100 000 for PE, and 55.4 per 100 000 for DVT.
FIGURE 1.
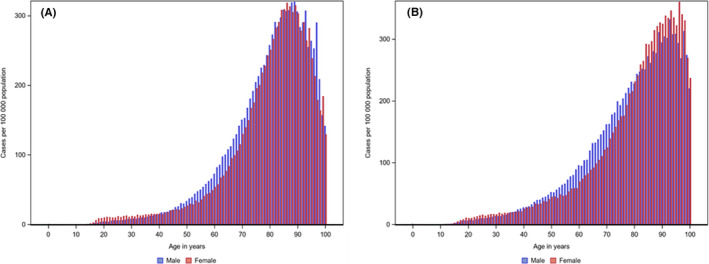
Incidence rate of pulmonary embolism (A) and deep vein thrombosis (B), per 100 000 Swedish inhabitants and age at diagnosis, shown separately for women and men
For PE, the incidence increased from 0.9 to 290 per 100 000 inhabitants from ages 0 to 19 to 80 to 89 years (Figure 1). PE was more common in women aged <40 years than in men of the same age but was more frequent in men than women aged 40 to 79 years.
For DVT, the incidence increased gradually from 1.5 per 100 000 at age 0 to 19 years to 323.6 per 100 000 among those aged ≥90 years (Figure 1). Women aged <40 years and those aged ≥80 years had a slightly higher incidence of DVT than men of the same age.
3.3. Comorbidity and temporary provoking factors
Baseline characteristics are presented separately for patients with DVT and PE in Table 1, with comorbidities registered within 7 years and temporary provoking factors registered within 3 months before the VTE event. On average, patients with PE were 3 years older than patients who had DVT (69 vs 66 years), and the proportion of women was slightly higher among patients with PE (53.4%) than those with DVT (52.1%). The most prevalent comorbidity among all patients was cancer (PE, 21.3%; DVT, 19.3%). Heart failure (PE, 18.9%; DVT, 10.3%) and ischemic heart disease (PE, 18.6%; DVT, 11.0%) were more common in patients with PE than in those with DVT.
Univariable ORs for patients with DVT and PE compared to controls are presented in Table 2, and ORs adjusted for various comorbidities registered within 7 years before the VTE and temporary provoking factors are presented in Figure 2A. Several comorbidities showed a stronger correlation to PE than to DVT in multivariable analysis, including heart failure (PE: adjusted OR [aOR], 2.64 [99% CI, 2.55‐2.73], DVT: aOR,1.66 [99% CI, 1.60‐1.72]), ischemic heart disease (PE: aOR, 1.51 [99% CI, 1.47‐1.56]; DVT; aOR, 1.01 [99% CI, 0.98‐1.04]), AF (PE: aOR, 1.29 [99% CI, 1.25‐1.34], DVT: aOR, 1.10 [99% CI, 1.06‐1.15]), COPD (PE: aOR, 2.51 [99% CI, 2.40‐2.63], DVT: aOR, 1.54 [99% CI, 1.47‐1.62]), and depression (PE: aOR, 1.90 [99% CI, 1.79‐2.01], DVT: aOR, 1.47 [99% CI, 1.39‐1.56]). ORs for PE and DVT were similar for other comorbidities, such as ischemic stroke and inflammatory bowel disease. Temporary provoking factors, such as musculoskeletal surgery (DVT: aOR, 9.81 [99% CI, 9.26‐10.38]; PE: aOR, 7.43 [99% CI, 6.97‐7.92]) and lower‐limb fracture (DVT: aOR, 4.56 [99% CI, 4.19‐4.96]; PE: aOR, 2.56 [99% CI, 2.33‐2.82]) were more common among patients with DVT than those with PE.
TABLE 2.
Unadjusted ORs and PARs for comorbidities within 7 years of the VTE event for 156 908 patients with DVT compared to 623 112 controls and 141 264 patients with PE compared to 561 967 controls
| DVT | PE | |||
|---|---|---|---|---|
| OR case vs control (99% CI) | PAR, % | OR case vs control (99% CI) | PAR, % | |
| Comorbidities | ||||
| Heart failure | 1.98 (1.94‐2.02) | 5.1 | 3.75 (3.69‐3.82) | 13.8 |
| Ischemic heart disease | 1.35 (1.33‐1.38) | 2.9 | 2.30 (2.26‐2.34) | 10.5 |
| Atrial fibrillation | 1.62 (1.59‐1.66) | 3.3 | 2.47 (2.43‐2.52) | 8.0 |
| Ischemic stroke | 1.93 (1.89‐1.98) | 3.2 | 2.05 (2.00‐2.10) | 3.9 |
| Hemorrhagic stroke | 2.40 (2.28‐2.54) | 0.8 | 2.56 (2.42‐2.70) | 1.0 |
| Chronic obstructive pulmonary disease | 1.93 (1.87‐1.99) | 1.9 | 3.43 (3.34‐3.53) | 5.2 |
| Cancer | 2.95 (2.90‐3.00) | 12.7 | 2.97 (2.92‐3.01) | 14.1 |
| Inflammatory bowel disease | 2.29 (2.19‐2.39) | 1.2 | 2.24 (2.14‐2.35) | 1.2 |
| Depression | 1.83 (1.76‐1.90) | 1.3 | 2.38 (2.29‐2.46) | 2.1 |
| Psychosis | 1.87 (1.80‐1.95) | 1.1 | 1.66 (1.59‐1.73) | 0.9 |
| Alcohol abuse | 1.75 (1.68‐1.83) | 0.8 | 1.86 (1.78‐1.95) | 0.9 |
| Temporary provoking factor | ||||
| Gastrointestinal surgery | 7.16 (6.83‐7.50) | 2.8 | 10.82 (10.34‐11.33) | 4.4 |
| Obstetrical surgery | 7.76 (6.59‐9.14) | 0.2 | 7.48 (6.24‐8.96) | 0.2 |
| Surgery of the musculoskeletal system | 15.77 (15.22‐16.35) | 9.0 | 11.67 (11.26‐12.10) | 7.6 |
| Surgery, other major | 7.35 (7.11‐7.60) | 5.6 | 8.76 (8.47‐9.06) | 7.1 |
| Trauma | 13.36 (12.68‐14.06) | 3.8 | 11.18 (10.62‐11.77) | 3.7 |
| Lower‐extremity fracture | 17.89 (17.04‐18.78) | 5.4 | 13.46 (12.82‐14.15) | 4.6 |
CI, confidence interval; DVT, deep vein thrombosis; OR, odds ratio; PAR, population attributable risk; PE, pulmonary embolism; VTE, venous thromboembolism.
FIGURE 2.
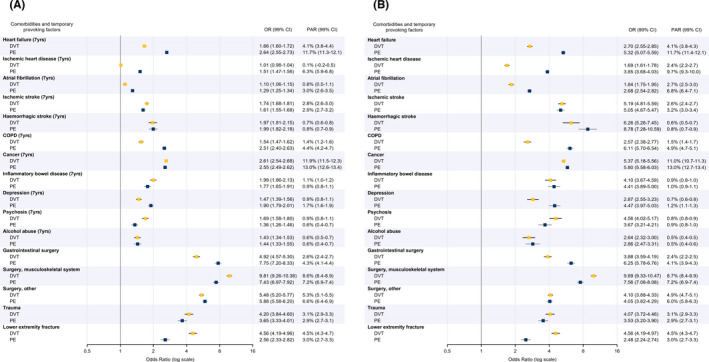
(A) Multivariable ORs for cases of venous thromboembolism compared to controls and PARs for various comorbidities (within ≤7 years) and temporary provoking factors (within ≤3 months). (B) Multivariable ORs for cases of venous thromboembolism compared to controls and PARs for various recent comorbidities (within ≤6 months) and temporary provoking factors (within ≤3 months). Data was divided into patients with PE and those with DVT and adjusted for all other comorbidities and temporary provoking factors. CI, confidence interval; COPD, chronic obstructive pulmonary disease; DVT, deep vein thrombosis; OR, odds ratio; PAR, population attributable risk; PE, pulmonary embolism
The factors with the highest PARs for PE in the multivariable analysis were cancer (13.0%), heart failure (11.7%), ischemic heart disease (6.3%), and musculoskeletal surgery (7.2%). The highest PARs for DVT were found for cancer (11.9%) and musculoskeletal surgery (8.6%; Figure 2A).
We also examined the multivariable aORs and PARs for recently registered comorbidities (first registered within 6 months of the VTE diagnosis). The differences between PE and DVT were consistent, but the aORs were considerably higher in this analysis (Figure 2B).
4. DISCUSSION
In this large retrospective case‐control study based on data from several national registries, we found differences in comorbidities and temporary provoking factors between patients presenting with PE and those presenting with DVT, when compared to matched controls. We also found considerably stronger associations when including only recently diagnosed comorbidities. To the best of our knowledge, this study among a population of nearly 300 000 patients with VTE is the largest such study to date, providing us with the means to study a large variety of associated factors among patients presenting with a clinical diagnosis of PE or DVT.
Previously reported incidence rates vary from 79 to 269 per 100 000 person‐years for VTE, from 39 to 115 per 100 000 person‐years for PE, and from 53 to 162 per 100 000 person‐years for DVT, 9 , 10 , 11 , 20 and these are strongly dependent on age. 9 , 10 , 21 Our reported numbers are in the lower range of previous estimates, which is to be expected because our analysis encompassed all age groups, including children; however, the age‐specific incidence rates are similar to smaller previous studies. 16 , 22 , 23 We also report a sex difference in VTE presentation. Patients with PE were more often women and older than patients with DVT. This is in line with previous studies. 10 , 24
Adjusted relative risk (RR) estimates vary considerably depending on the time frame within which the comorbidities have been registered. Higher RRs have been reported when including more recent comorbidities, as opposed to comorbidities registered over a longer period of time, 13 in line with our results. Previous studies also vary in terms of adjustment for confounding factors, with some studies adjusting for only age and sex 5 or for a limited number of potential risk factors and comorbidities. 16
In this study, the association of cardiac diseases with VTE was greater for PE than for DVT. This confirms previous findings from a large Danish register‐based case‐control study. In that study, patients with a diagnosis of myocardial infarction, heart failure, and AF within 3 months before their VTE had a markedly higher aOR for isolated PE than DVT alone, in comparison with controls. 13 When heart disease was recorded >3 months before the VTE, the aORs were considerably lower, in accordance with our results. 13 Other studies have found RRs for patients with heart failure ranging from 1.1 to 1.35 for DVT and 2.15 to 3.03 for PE, 5 , 16 , 25 and a PAR associated with heart failure of 9.5 (95% CI, 3.3‐15.8) for VTE, 4 similar to our results. Other small studies have also shown a stronger relationship of myocardial infarction with PE compared with DVT, with aOR ranges of DVT 0.9 to 2.4 and PE 1.9 to 8.5, and one study reported a PAR for PE of 6.2%. 5 , 16 , 26 Previous studies with limited sample size have shown a tendency toward higher frequency of AF in patients with PE compared with those with DVT. 16 , 24 , 27 In recent years, most patients with AF are treated with anticoagulants, 28 and it is reasonable to believe that the correlation between AF and VTE is even stronger in patients with AF without anticoagulation.
Nearly 15% of acute exacerbations of COPD have been reported to be caused by PE, which is frequently unrecognized in the clinical setting. 29 We found COPD to have a stronger correlation with PE than with DVT. This is in line with previous, smaller studies. 16 , 30
There are several possible explanations for the higher OR of PE compared to DVT in patients with cardiopulmonary disease. Overlapping symptoms between cardiopulmonary disease and PE could make clinicians more likely to investigate for PE. However, previous autopsy studies have shown the opposite, 31 with many cases of PE either undiagnosed or misinterpreted as being respiratory or cardiovascular disease. 32 Another explanation could be that patients with lower cardiopulmonary capacity owing to other diseases may already be symptomatic, with small pulmonary emboli. A third possibility is that patients with inflammatory processes in the thorax are at higher risk of pulmonary thrombosis, 15 , 33 a theory that has gained attention during the COVID‐19 pandemic. 34 In the case of AF, one proposed mechanism is the development of right atrial thrombi. 27 , 35 A bidirectional risk increase between VTE and AF has been shown. 36
We also report increased OR for ischemic and hemorrhagic stroke, inflammatory bowel disease, and psychiatric diseases. Stroke is a recognized risk factor for VTE with a markedly increased risk especially within the first month of the event. 37 The higher risk of VTE after hemorrhagic stroke, compared with that after ischemic stroke, was reported in a smaller study. 38 The increased risk of VTE in patients with inflammatory bowel disease has been reported previously. 39 , 40 A systematic review and meta‐analysis of psychiatric diseases and VTE showed an association between psychotic diseases and VTE but not with depression and VTE in high‐quality studies. 41 However, our results should be interpreted with caution. Comorbidities typically treated in primary care, such as depression, could potentially be diagnosed and registered in hospital records more often for patients with VTE than for controls owing to admission for VTE.
The relationship between cancer and VTE is well known. In a review article by Timp et al., RRs of VTE varied between 4.1 and 6.7. 42 The adjusted PAR of VTE owing to cancer has been reported to be 11.3% (95% CI, 9.4‐13.2) in a cohort study in the United States among patients with VTE during 1995 to 2008, in line with our results. 43
In our study, recent surgery and trauma displayed the strongest association with VTE. Comparisons with prior studies on the risk of VTE related to surgery are difficult because these risks may vary depending on the type and duration of surgery. We report lower ORs for major surgery than in several prior studies, 3 , 4 , 16 possibly due to our adjustment for factors such as cancer and trauma. Our reported higher OR for DVT than PE in lower‐extremity fracture confirms results from a previous, smaller study. 12 Another previous study including lower‐extremity fracture and musculoskeletal surgery was inconclusive with large CIs. 16
4.1. Strengths and limitations
The two major strengths of this study are the very large study population and the nearly complete national coverage. We specifically only excluded population‐based controls with a prior VTE event at the time of matching, but not thereafter, aiming to avoid bias caused by using a control population that is healthier than the general Swedish population. However, there is still a risk that cases have a larger number of registered diagnoses in the patient registry compared to controls simply because they have been in contact with a hospital for VTE care. This limitation mainly concerns diagnoses that are commonly diagnosed and treated in primary care. Thus, the OR for diagnoses such as depression, COPD, atrial fibrillation, and alcohol abuse might be overestimated.
Other potential limitations of this study include the lack of external validation of the VTE diagnoses. The validity of VTE diagnoses has been questioned. 44 , 45 In this study, we verified the diagnosis of VTE by retrieval of an anticoagulant prescription, a method previously suggested to increase the accuracy of VTE diagnosis. 44 However, this was possible only after July 2005. We have a low reported number of concomitant PE and DVT. This is probably due to underreporting; imaging of the lower‐extremity veins is rarely performed in Sweden following the diagnosis of a PE. Therefore, we cannot study isolated PE compared to PE with DVT. In addition, patients admitted with a VTE could not be distinguished from those acquiring a VTE while in the hospital in our data.
Another limitation is the potential ascertainment bias. Patients with heart failure or COPD more often have shortness of breath than patients without these conditions. This could lead to more frequent chest imaging. The same could be true for patients with recent orthopedic surgery, with leg swelling, and imaging for DVT. However, as mentioned above, the opposite could also be true since clinicians already have a plausible explanation for the symptoms.
The PARs for each factor correlated with VTE provide an estimate of the influence of each risk factor on a population level but should be interpreted with caution. VTE is a multifactorial disease, and both genetic and acquired risk factors interact to cause VTE. 46 It is also important to keep in mind that it is not possible to determine causality using these retrospective observational data.
Another limitation is the lack of information on body weight or body mass index. Obesity is a growing global problem and previous studies have found an increased risk of PE and DVT associated with obesity. 47
Poor socioeconomic status has been found to be associated with an increased risk of both VTE 48 , 49 and associated risk factors. 50 No such information is available in the included registries; hence, we were not able to adjust for this potential confounder.
The generalizability of the results is likely to be high for persons with similar thrombotic risk and socioeconomic situation as the Swedish population. However, the external reliability of the results for other populations is unknown.
5. CONCLUSION
In this study, patients with PE were considerably more likely to have cardiopulmonary disease, in particular of recent onset, while those who had recent surgery of the musculoskeletal system or lower‐extremity fracture were more likely to be diagnosed with DVT.
RELATIONSHIP DISCLOSURE
KGS has received speaker’s honoraria from Pfizer, Bristol‐Meyers Squibb, Bayer and Leo Pharma. JS has received speaker’s honoraria from Bayer. POH, AR, and SJ report no conflicts of interest.
AUTHOR CONTRIBUTIONS
POH conceived the idea for the study. All authors made important contributions to the design of the study, in particular POH, AR, and KGS. KGS wrote the first draft of the manuscript. KGS, POH, and JS provided clinical input at all stages of the project. All authors were involved in the interpretation of data and critical revision of the manuscript and approved the final draft. KGS had final responsibility for the submission of the article. POH, AR, SJ, and KGS provided funds for the project.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Bengt Bengtsson and Nils‐Gunnar Pehrsson, Statistical consultant group, Gothenburg, Sweden, for consultation and performing the statistical analysis.
Glise Sandblad K, Rosengren A, Sörbo J, Jern S, Hansson P‐O. Pulmonary embolism and deep vein thrombosis—comorbidities and temporary provoking factors in a register‐based study of 1.48 million people. Res Pract Thromb Haemost. 2022;6:e12714. doi: 10.1002/rth2.12714
Handling Editor: Dr Neil Zakai
Funding information
This work was supported by grants from the following: the Swedish state under an agreement concerning research and education of doctors [ALFGBG‐716901, ALFGBG‐721351, ALFGBG‐717211, and ALFGBG‐ 720711, the Swedish Heart and Lung Foundation [2018‐0366]; the Swedish Research Council [2018‐02527, VRREG 2019‐00193]. The work was also supported by grants from funds from the Sahlgrenska Academy.
REFERENCES
- 1. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J. 2019;54(3):1901647. [DOI] [PubMed] [Google Scholar]
- 2. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117(1):19‐25. [DOI] [PubMed] [Google Scholar]
- 3. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23 suppl 1):I9‐16. [DOI] [PubMed] [Google Scholar]
- 4. Heit JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study. Arch Intern Med. 2002;162(11):1245‐1248. [DOI] [PubMed] [Google Scholar]
- 5. Ocak G, Vossen CY, Verduijn M, et al. Risk of venous thrombosis in patients with major illnesses: results from the MEGA study. J Thromb Haemost. 2013;11(1):116‐123. [DOI] [PubMed] [Google Scholar]
- 6. Lijfering WM, Rosendaal FR, Cannegieter SC. Risk factors for venous thrombosis ‐ current understanding from an epidemiological point of view. Br J Haematol. 2010;149(6):824‐833. [DOI] [PubMed] [Google Scholar]
- 7. Goldhaber SZ. Risk factors for venous thromboembolism. J Am Coll Cardiol. 2010;56(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 8. Kearon C, Ageno W, Cannegieter SC, et al. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost. 2016;14(7):1480‐1483. [DOI] [PubMed] [Google Scholar]
- 9. Lehnert P, Lange T, Møller CH, Olsen PS, Carlsen J. Acute pulmonary embolism in a National Danish Cohort: increasing incidence and decreasing mortality. Thromb Haemost. 2018;118(3):539‐546. [DOI] [PubMed] [Google Scholar]
- 10. Dentali F, Ageno W, Pomero F, Fenoglio L, Squizzato A, Bonzini M. Time trends and case fatality rate of in‐hospital treated pulmonary embolism during 11 years of observation in Northwestern Italy. Thromb Haemost. 2016;115(2):399‐405. [DOI] [PubMed] [Google Scholar]
- 11. Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34(11):2363‐2371. [DOI] [PubMed] [Google Scholar]
- 12. van Langevelde K, Flinterman LE, van Hylckama VA, Rosendaal FR, Cannegieter SC. Broadening the factor V Leiden paradox: pulmonary embolism and deep‐vein thrombosis as 2 sides of the spectrum. Blood. 2012;120(5):933‐946. [DOI] [PubMed] [Google Scholar]
- 13. Sørensen HT, Horvath‐Puho E, Lash TL, et al. Heart disease may be a risk factor for pulmonary embolism without peripheral deep venous thrombosis. Circulation. 2011;124(13):1435‐1441. [DOI] [PubMed] [Google Scholar]
- 14. Fletcher‐Sanfeliu D, Redón J, García‐Granero Á, et al. “Pulmonary thrombosis in situ”: risk factors, clinic characteristics and long‐term evolution. Blood Coagul Fibrinolysis. 2020;31(7):469‐475. [DOI] [PubMed] [Google Scholar]
- 15. Ten Cate V, Eggebrecht L, Schulz A, et al. Isolated pulmonary embolism is associated with a high risk of arterial thrombotic disease: results from the VTEval study. Chest. 2020;158(1):341‐349. [DOI] [PubMed] [Google Scholar]
- 16. Huerta C, Johansson S, Wallander MA, García Rodríguez LA. Risk factors and short‐term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med. 2007;167(9):935‐943. [DOI] [PubMed] [Google Scholar]
- 17. Van Gent JM, Zander AL, Olson EJ, et al. Pulmonary embolism without deep venous thrombosis: De novo or missed deep venous thrombosis? J Trauma Acute Care Surg. 2014;76(5):1270‐1274. [DOI] [PubMed] [Google Scholar]
- 18. Ludvigsson JF, Almqvist C, Bonamy AK, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125‐136. [DOI] [PubMed] [Google Scholar]
- 19. Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C. Estimating the population attributable risk for multiple risk factors using case‐control data. Am J Epidemiol. 1985;122(5):904‐914. [DOI] [PubMed] [Google Scholar]
- 20. Wendelboe AM, Raskob GE. Global burden of thrombosis. Circ Res. 2016;118(9):1340‐1347. [DOI] [PubMed] [Google Scholar]
- 21. Venous thromboembolism in adult hospitalizations ‐ United States, 2007‐2009. MMWR Morb Mortal Wkly Rep. 2012;61(22):401‐404. [PubMed] [Google Scholar]
- 22. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study. Arch Intern Med. 1998;158(6):585‐593. [DOI] [PubMed] [Google Scholar]
- 23. Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: a population‐based study. J Thromb Haemost. 2007;5(4):692‐699. [DOI] [PubMed] [Google Scholar]
- 24. Palareti G, Antonucci E, Dentali F, et al. Patients with isolated pulmonary embolism in comparison to those with deep venous thrombosis. Differences in characteristics and clinical evolution. Eur J Intern Med. 2019;69:64‐70. [DOI] [PubMed] [Google Scholar]
- 25. Beemath A, Stein PD, Skaf E, Al Sibae MR, Alesh I. Risk of venous thromboembolism in patients hospitalized with heart failure. Am J Cardiol. 2006;98(6):793‐795. [DOI] [PubMed] [Google Scholar]
- 26. Rinde LB, Lind C, Småbrekke B, et al. Impact of incident myocardial infarction on the risk of venous thromboembolism: the Tromsø study. J Thromb Haemost. 2016;14(6):1183‐1191. [DOI] [PubMed] [Google Scholar]
- 27. Enga KF, Rye‐Holmboe I, Hald EM, et al. Atrial fibrillation and future risk of venous thromboembolism: the Tromsø study. J Thromb Haemost. 2015;13(1):10‐16. [DOI] [PubMed] [Google Scholar]
- 28. Forslund T, Komen JJ, Andersen M, et al. 1458Improved stroke prevention in atrial fibrillation after the introduction of NOACs; the Stockholm experience. Eur Heart J. 2018;39(suppl_1):ehy565‐1458. [Google Scholar]
- 29. Lankeit M, Held M. Incidence of venous thromboembolism in COPD: linking inflammation and thrombosis? Eur Respir J. 2016;47(2):369‐373. [DOI] [PubMed] [Google Scholar]
- 30. Schneider C, Bothner U, Jick SS, Meier CR. Chronic obstructive pulmonary disease and the risk of cardiovascular diseases. Eur J Epidemiol. 2010;25(4):253‐260. [DOI] [PubMed] [Google Scholar]
- 31. Pineda LA, Hathwar VS, Grant BJ. Clinical suspicion of fatal pulmonary embolism. Chest. 2001;120(3):791‐795. [DOI] [PubMed] [Google Scholar]
- 32. Ossei PPS, Owusu IK, Owusu‐Asubonteng G, Ankobea‐Kokroe F, Ayibor WG, Niako N. Prevalence of venous thromboembolism in Kumasi: a postmortem‐based study in a tertiary hospital in Ghana. Clin Med Insights Circ Respir Pulm Med. 2020;14:1179548420956364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marongiu F, Mameli A, Grandone E, Barcellona D. Pulmonary thrombosis: a clinical pathological entity distinct from pulmonary embolism? Semin Thromb Hemost. 2019;45(8):778‐783. [DOI] [PubMed] [Google Scholar]
- 34. Cattaneo M, Bertinato EM, Birocchi S, et al. Pulmonary embolism or pulmonary thrombosis in COVID‐19? Is the recommendation to use high‐dose heparin for thromboprophylaxis justified? Thromb Haemost. 2020;120(8):1230‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Waleed KB, Guan X, Li X, et al. Atrial fibrillation is related to lower incidence of deep venous thrombosis in patients with pulmonary embolism. J Thorac Dis. 2018;10(3):1476‐1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lutsey PL, Norby FL, Alonso A, et al. Atrial fibrillation and venous thromboembolism: evidence of bidirectionality in the Atherosclerosis Risk in Communities Study. J Thromb Haemost. 2018;16(4):670‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rinde LB, Småbrekke B, Mathiesen EB, et al. Ischemic stroke and risk of venous thromboembolism in the general population: the Tromsø study. J Am Heart Assoc. 2016;5(11):e004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Skaf E, Stein PD, Beemath A, Sanchez J, Bustamante MA, Olson RE. Venous thromboembolism in patients with ischemic and hemorrhagic stroke. Am J Cardiol. 2005;96(12):1731‐1733. [DOI] [PubMed] [Google Scholar]
- 39. Chung WS, Lin CL, Hsu WH, Kao CH. Inflammatory bowel disease increases the risks of deep vein thrombosis and pulmonary embolism in the hospitalized patients: a nationwide cohort study. Thromb Res. 2015;135(3):492‐496. [DOI] [PubMed] [Google Scholar]
- 40. Cheng K, Faye AS. Venous thromboembolism in inflammatory bowel disease. World J Gastroenterol. 2020;26(12):1231‐1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kowal C, Peyre H, Amad A, et al. Psychotic, mood, and anxiety disorders and venous thromboembolism: a systematic review and meta‐analysis. Psychosom Med. 2020;82(9):838‐849. [DOI] [PubMed] [Google Scholar]
- 42. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer‐associated venous thrombosis. Blood. 2013;122(10):1712‐1723. [DOI] [PubMed] [Google Scholar]
- 43. Heit JA, Ashrani A, Crusan DJ, McBane RD, Petterson TM, Bailey KR. Reasons for the persistent incidence of venous thromboembolism. Thromb Haemost. 2017;117(2):390‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abdul Sultan A, West J, Stephansson O, et al. Defining venous thromboembolism and measuring its incidence using Swedish health registries: a nationwide pregnancy cohort study. BMJ Open. 2015;5(11):e008864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Öhman L, Johansson M, Jansson JH, Lind M, Johansson L. Positive predictive value and misclassification of diagnosis of pulmonary embolism and deep vein thrombosis in Swedish patient registries. Clin Epidemiol. 2018;10:1215‐1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet (London, England). 1999;353(9159):1167‐1173. [DOI] [PubMed] [Google Scholar]
- 47. Glise Sandblad K, Jern S, Åberg M, et al. Obesity in adolescent men increases the risk of venous thromboembolism in adult life. J Intern Med. 2020;287(6):734‐745. [DOI] [PubMed] [Google Scholar]
- 48. Jørgensen H, Horváth‐Puhó E, Laugesen K, Braekkan S, Hansen JB, Sørensen HT. Socioeconomic status and risk of incident venous thromboembolism. J Thromb Haemost. 2021;19(12):3051‐3061. [DOI] [PubMed] [Google Scholar]
- 49. Rosengren A, Fredén M, Hansson PO, Wilhelmsen L, Wedel H, Eriksson H. Psychosocial factors and venous thromboembolism: a long‐term follow‐up study of Swedish men. J Thromb Haemost. 2008;6(4):558‐564. [DOI] [PubMed] [Google Scholar]
- 50. de Mestral C, Stringhini S. Socioeconomic status and cardiovascular disease: an update. Curr Cardiol Rep. 2017;19(11):115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


