Abstract
Objective
Osteoarthritis (OA) is a degenerative joint disease that is more prevalent in women than men, especially later in life. This suggests that sexual dimorphism may be present in the pathogenesis of the disease. The purpose of this review is to discuss evidence of sexual dimorphism in knee OA development and presentation as it is framed by two contrasting paradigms: biomechanics and biology.
Methods
A comprehensive search of databases was conducted including, but not limited to, MEDLINE via Ovid, PubMed, and Google Scholar. Keywords including osteoarthritis, sex differences, and/or sexual dimorphism were searched in combination with knee biomechanics, ACL, joint malalignment, estrogen, chondrocyte signal(l)ing, growth factor and integrin(s).
Results
The biomechanical approach has identified sex differences in joint malalignment, bone shape, gait, and lower limb muscle strength leading to altered load transmission, as well as increased knee laxity in women predisposing them to joint injury. The biological approach has largely focused on the influence of estrogen receptor signaling on the maintenance of joint tissues. Preliminary work identifying sexual dimorphism in chondrocyte signaling pathways involving growth factors and collagen receptors has been reported in addition to more systemic levels of inflammatory cytokines and metabolites.
Conclusion
Understanding the true etiology of OA is crucial for developing effective, individualized treatment in the age of personalised medicine. A shift from a ‘one size fits all’ mentality towards an individualized approach for therapeutic treatment must begin with the acknowledgment of sex differences in the biomechanical and biological factors underlying the onset and development of OA.
Keywords: Knee, Osteoarthritis, Sexual dimorphism, Biomechanics, Cell signaling
Abbreviations: OA, Osteoarthritis; ACL, Anterior Cruciate Ligament; ER, Estrogen Receptor; EGFR, Epidermal Growth Factor Receptor
1. Introduction
Osteoarthritis (OA) is a degenerative disease characterized by progressive deterioration of joint cartilage, narrowing of the joint space, thickening of the subchondral bone, formation of osteophytes, and synovitis.1 OA is the most common form of arthritis and a leading cause of disability in North America.1 Typically, OA develops gradually over time, though it can also develop post-traumatically following joint injury such as an anterior cruciate ligament (ACL) tear.2 Currently, there is no cure for OA and thus treatment is aimed at addressing the symptoms of the disease rather than slowing or stopping the disease itself.1 Therefore, in order to develop an effective treatment that targets the root of the problem, the true etiology of OA must be better understood.
The development of OA is influenced by numerous factors including age, obesity and sex.1 For the purpose of this review, we will focus solely on the influence of sex on OA development. While the prevalence of OA is similar between males and females until around the age of 50, females are twice as likely to develop OA later in life.3 Sex affects other conditions including autoimmune diseases and several types of cancer and has been identified as a major factor influencing pain and drug metabolism.4, 5, 6 Indeed, women are at a higher risk of having adverse reactions to drugs compared to males6 and eight out of ten prescription drugs are withdrawn from the US market due to women's health concerns.7 Despite this evidence, the majority of treatment options for these diseases follow a ‘one size fits all’ approach and the supporting research is conducted in an overwhelmingly male testing population.6,7
Specifically, in terms of knee OA, the majority of research considering sexual dimorphism has been framed by two contrasting paradigms: biomechanics and biology. The biomechanical approach has identified sex differences in joint malalignment, bone shape, gait, and lower limb muscle strength leading to altered load transmission across the joint, as well as increased knee laxity in women predisposing them to joint injury.8,9 The biological approach has documented disparity in sex hormones and the influence of estrogen receptor signaling on the maintenance of joint tissues.10 Furthermore, sexual dimorphism has been identified in systemic levels of inflammatory cytokines and metabolites, in addition to chondrocyte signaling pathways involving growth factor and collagen receptors.11, 12, 13, 14 While the biomechanical paradigm for sexual dimorphism in OA has been extensively reported, differences in the underlying biological mechanisms within the cartilage are not well understood. In this review, sexual dimorphism in the development of knee OA will be discussed in terms biomechanical variances as well as differences in cell signaling mechanisms responsible for the maintenance of cartilage homeostasis.
2. Biomechanical variances
It is well established that women and men differ in terms of frontal plane knee malalignment, also referred to as varus/valgus alignment.15 Varus and valgus alignment are associated with an increased risk of medial or lateral knee OA respectively, and women are more likely than men to have valgus alignment.16 Additionally, bone shape has been shown to affect load distribution across the knee, leading to gait asymmetry and increasing risk of OA development over time.17 STR/Ort mice develop spontaneous knee OA around 18 weeks of age, with males progressing faster and more severely than females.18 Analyses of the tibiae and fibulae of these mice indicate decreased bone retention, less favourable bone shape for load distribution and greater gait asymmetry in males compared to females.17
In addition to joint malalignment and bone shape, sex differences in gait biomechanics and lower limb muscle strength have been investigated. Kinematic data shows that female recreational runners have significantly increased knee extension moment, peak knee adduction moment, patellofemoral loads, and contact pressures compared to males.9 Furthermore, when OA patients are compared, women have greater knee flexion and extension moments during level walking and stair descent compared to men.19 Women walk with a smaller knee internal/external rotation moment compared to men, and only women alter their lower limb biomechanics in association with OA.20 Finally, women have weaker quadriceps muscles compared to men at various stages of OA progression.21 Thus, sexual dimorphism is apparent in gait biomechanics and lower limb muscle strength across the spectrum of healthy and OA patients.
In addition to joint malalignment and gait biomechanics, sexual dimorphism has been documented in ligament laxity and injury, and thus the associated risk of post-traumatic OA. Females have more compliant ligaments and are more than twice as likely to suffer an ACL injury compared to males.22,23 Serum hormone concentrations and knee laxity vary widely throughout the menstrual cycle and between subjects.8,24 Some have reported knee laxity mirroring estrogen levels,8 while others link knee laxity to fluctuations in testosterone and progesterone, in addition to estrogen.24 Most recently, changes to both hip and knee mechanics during the menstrual cycle have been shown to increase risk for ACL injury in women. In this regard, knee valgus moment and anterior laxity increase with estradiol and progesterone at ovulation, and peak vertical ground reaction forces increase during menses.25 An alternate approach to investigating the influence of sex hormones on ligament laxity has been to study hormone supplementation and ligament properties. Using this approach, no association was found between patellar tendon properties and hormone levels during the menstrual cycle in athletes using, or abstaining from, oral contraceptives.26 In contrast, fluctuations in ACL elasticity and serum estradiol concentrations throughout the menstrual cycle were reduced in oral contraceptive users compared to non-users.27 Taken together these studies show sexual dimorphism in ligament laxity, contributing to an increased risk of injury and post-traumatic OA development in females. The exact relationship between systemic estrogen levels and variance in ligament laxity, however, requires further clarification.
In summary, joint malalignment, bone shape, gait and ligament laxity have been identified as biomechanical variances between men and women that contribute to altered load distribution across the knee and increased risk of OA development, especially in women. In contrast, differences in the underlying biological mechanisms within cartilage that may contribute to sexual dimorphism in OA are not well understood. Sex differences in systemic levels of inflammatory cytokines and hormones as well as the influence of estrogen receptor signaling on the maintenance of joint tissues have been investigated. These will now be discussed in addition to sexual dimorphism in chondrocyte signaling pathways involving growth factor and collagen receptors.
3. Biological influences
Chondrocytes govern a complex network of signaling cascades responsible for cartilage homeostasis. When these mechanisms are disrupted, degenerative conditions like OA can develop.1 As OA is a multifactorial condition, it is reasonable to think that there are numerous biological events taking place in the body that would affect joint health in a sex-specific manner. These might include specific chondrocyte signaling pathways associated with systemic hormone, cytokine and/or metabolite activity.
Perhaps the most investigated mechanism thought to contribute to sex differences in OA are sex hormones. Incidence rates of OA in men and women diverge around the age of 50, corresponding to the onset of menopause and the associated decline in systemic estrogen levels in women.28 A review of in vitro and in vivo animal as well as human studies describes the influence of estrogen on joint tissues involving various molecular pathways and cellular levels.10 Though studies report varying effectiveness and some conflicting evidence, there are potential protective effects of estrogen on joint tissues which may explain why its decrease at menopause correlates with a greater propensity for OA in women.10 Studies assessing chronic changes in sex hormones report that oral contraceptive use, high number of pregnancies or postmenopausal hormone replacement therapy all independently increase the risk of knee OA for women.29 In contrast, acute fluctuations in systemic estrogen levels throughout the menstrual cycle have been shown to increase muscle metabolism and reduce collagen degradation protecting joint tissues. While to the contrary, estrogen fluctuations can also lead to more compliant ligaments and joint injury as discussed earlier.30,31 A review of studies investigating the effects of estrogen treatment and/or the modulation of estrogen receptor activity on OA-like cartilage changes reveals conflicting reports.32 About half the studies document a beneficial effect of estrogen on joint cartilage, while the other half report increased degeneration, cell death and worsening cartilage damage with estrogen treatment, especially at higher doses.32 With both chondro-protective and -detrimental effects of estrogen reported, further work is required to elucidate the mechanism(s) through which estrogen acts on joint tissues.
Physiologically, the primary active form of estrogen in the body, estradiol, elicits its effects through estrogen receptors (ERs).33 The two isoforms, ERα and ERβ, have been identified in the articular cartilage of humans and other mammals.34,35 Interestingly, some studies have shown increased protein expression of ERs in females compared to males,36 while others report the opposite.37 Furthermore, a study investigating ER deficient mice saw that female mice carrying a deletion for either ERα or ERβ showed only mild cartilage changes, but when both receptors were simultaneously deleted, there was significantly increased osteophyte formation as well as thinning of the subchondral plate.38 These studies highlight the connection between ER signaling in cartilage and OA development, however there is conflicting evidence of disparate expression patterns in male versus female tissues.
While sex hormones have been extensively studied, there is growing evidence of a role for the extracellular matrix receptor integrin α1β1 and epidermal growth factor receptor (EGFR) within this biological paradigm. Integrin α1β1 is expressed by chondrocytes and is upregulated in both subclinical and end stage OA.39,40 Importantly, studies utilising itga1-null mice have demonstrated that integrin α1β1 plays a protective role against both spontaneous and post-traumatic OA development, with the latter being more robust in females compared to males.12,40 Though not well understood, the molecular mechanisms underlying this chondroprotective effect may involve the interplay of integrin α1β1 and growth factor receptor signaling pathways, including EGFR. EGFR signaling in articular chondrocytes results in the production of reactive oxygen species and catabolic proteinases.11 Integrin α1β1 downregulates the activity of EGFR through T-cell protein tyrosine phosphatase and thus EGFR activity is upregulated in itga1-null mice, possibly contributing to the earlier onset of OA.41 Interestingly, research investigating EGFR signaling in cartilage has revealed further disparity between the sexes. In a model of post-traumatic OA, treatment with the EGFR antagonist erlotinib protected female mice against boney signs of OA but had either no effect or was detrimental to males.12 Other studies investigating sexual dimorphism in EGFR signaling using a variety of EGFR inhibitors and animal models have shown conflicting results.11,42,43 Therefore, integrin α1β1 and EGFR signaling appear to be regulated differently in males and females however the mechanism for this is unknown. Interestingly, emerging evidence supports crosstalk between EGFR and ER, suggesting that ER could be involved in the integrin-EGFR-mediated sex differences in chondrocyte signaling.10 Growth factor receptors such as EGFR can activate ER in the absence of ER ligands, and this crosstalk can occur in the cytoplasm and/or nucleus of cells.17 Therefore, it is possible that changes in EGFR activity, mediated by integrin α1β1, could influence ER expression and thus contribute to the sexual dimorphism of knee OA.
In addition to systemic hormone activity and the associated effects on estrogen receptor signaling cascades, differences in cytokine and metabolite levels may also influence the biological milieu contributing to sex differences in OA. For example, in patients with knee OA, higher baseline interleukin-17A or interleukin-23 levels double the risk of increased bone marrow lesion scores in females, but this is not true of males.13 Additionally, though serum metabolite profiles were not associated with post-traumatic OA development in mice, the itga1-null genotype and erlotinib treatment, were.14 Erlotinib-treated mice revealed six metabolites unique to females and nine unique to males, the latter including a potential biomarker for resistance to EGFR tyrosine kinase inhibitors - glutamine.14 Together these results suggest that sexual dimorphism in serum metabolic profiles and/or cytokine expression, even subclinically, could be indicative of differences in OA pathogenesis between men and women, contributing to disparities in disease presentation later in life.
4. Conclusion
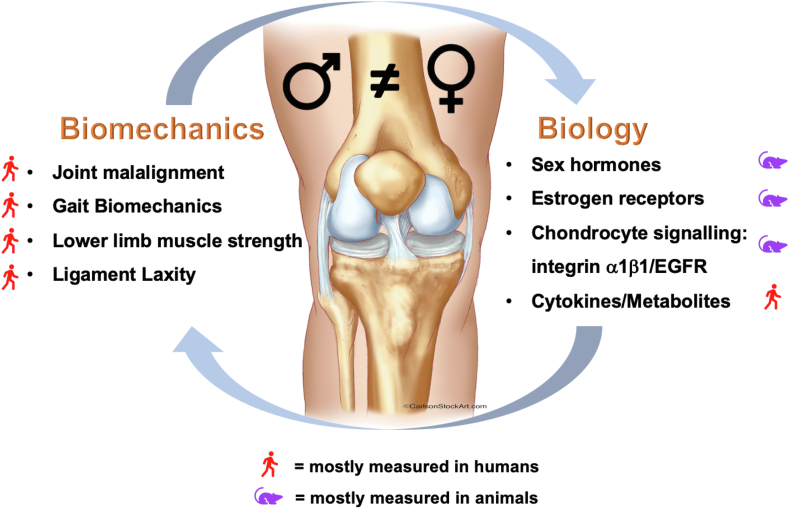
In this review, the biomechanical and biological factors influencing the sexual dimorphism in OA development have been discussed (Fig. 1). Within the biomechanical paradigm, women and men differ in terms of frontal plane alignment, bone shape, lower limb kinematics and muscle strength.15,20 Together these factors alter the magnitude and distribution of loads across joints, increasing wear on articular cartilage. Additionally, fluctuations in ligament laxity driven by sex hormones in women increase the risk of ligamentous injuries, predisposing them to post-traumatic OA.22 In contrast, the biological paradigm is lacking clarity. Variation in sex hormones has been associated with joint health however the exact role of estrogen signaling and ER expression on OA progression remains elusive.10,29 Sex differences in chondrocyte integrin-EGFR-mediated signaling, possibly involving ERs, offer a signaling pathway that can disparately influence OA.12,42,43 Finally, evidence is emerging regarding sex differences in cytokine and metabolomic profiles, potentially contributing to altered predisposition to cartilage damage.13,14
Fig. 1.
Summary of biomechanical and biological factors influencing sexual dimorphism in knee OA. While these factors are often investigated independently of one another, the reality of the synovial joint is that these paradigms coexist and are robustly interconnected. Biomechanical investigations are readily carried out in humans, however biological analyses often require animal models exacerbating the disconnect.
It is clear that sexual dimorphism abounds in numerous parameters that can influence the onset and development of OA. Furthermore, in contrast to the biomechanical paradigm, evidence supporting the biological paradigm is sparse and contradictory. This review highlights the need for further research with particular focus on estrogen-independent biological factors influencing OA and underlines the importance of the inclusion of male and female subjects in basic and clinical research studies of OA. While for the purposes of this review we have separated the biomechanical and biological paradigms, the reality of the synovial joint is that these paradigms coexist and are robustly interconnected. To this end, future collaborative research bridging the biomechanical-biological divide is necessary for the development of effective, personalised therapeutic strategies to treat this debilitating disease.28
In this review we present evidence of sex differences in fundamental biomechanical and biological factors influencing the development of OA (Fig. 1). Moving forwards, it is critical that our testing populations in basic and clinical research more accurately reflect the general population, including both male and female subjects. Thus, it is imperative that researchers recognize sex as a biological variable in human clinical trials as well as basic, subclinical experiments conducted on cells and animals. As sexual dimorphism is wholeheartedly embraced in our investigations into the biomechanical/biological mechanisms underlying OA then our research dollars will be more effective in developing treatments for this debilatative disease.
To conclude, understanding the true etiology of OA is crucial for developing effective, individualized treatment within the context of personalised medicine. This approach is expected to yield better health outcomes, increase the efficacy of treatments, and reduce adverse drug reactions. A shift from a ‘one size fits all’ mentality towards an individualized approach for therapeutic treatment must begin with the acknowledgment of sex differences in the biomechanical and biological factors underlying the onset and development of OA.
Funding/sponsorship
This work was funded by a Canadian Institutes of Health Research Open Operating Grant MOP136800 (AC).
Informed consent
Does not apply.
Institutional ethical committee approval
Does not apply.
Author contribution
Alicia Black: Conceptualization, Methodology, Investigation, Data Curation, Writing – Original Draft, Writing – Review & Editing, Visualization. Andrea Clark: Conceptualization, Methodology, Validation, Writing – Review & Editing, Visualization, Supervision, Project Administration, Funding Acquisition.
Declaration of competing interest
None.
Acknowledgements
None.
References
- 1.Glyn-Jones S., Palmer A.J.R., Agricola R., et al. Osteoarthritis. Lancet. 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 2.Lohmander L., Brandt K., Doherty M., Doherty M., Lohmander S. Oxford University Press; Oxford: 2003. Osteoarthritis. [Google Scholar]
- 3.Arden N., Nevitt M.C. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006;20(1):3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Kim A.M., Tingen C.M., Woodruff T.K. Sex bias in trials and treatment must end. Nature. 2010;465(7299):688–689. doi: 10.1038/465688a. [DOI] [PubMed] [Google Scholar]
- 5.Malfait A.M., Miller R.E. Why we should study osteoarthritis pain in experimental models in both sexes. Osteoarthritis Cartilage. 2020;28(4):397–399. doi: 10.1016/j.joca.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarto G.E. The gender gap: new challenges in women's health. Sex Reproduction Menopause. 2004;2(1):9–14. doi: 10.1016/S0015-0282(03)01141-5. [DOI] [PubMed] [Google Scholar]
- 7.Simon V. Wanted: women in clinical trials. Science. 2005;308(5728):1517. doi: 10.1126/science.1115616. [DOI] [PubMed] [Google Scholar]
- 8.Park S.K., Stefanyshyn D.J., Loitz-Ramage B., Hart D.A., Ronsky J.L. Changing hormone levels during the menstrual cycle affect knee laxity and stiffness in healthy female subjects. Am J Sports Med. 2009;37(3):588–598. doi: 10.1177/0363546508326713. [DOI] [PubMed] [Google Scholar]
- 9.Sinclair J., Selfe J. Sex differences in knee loading in recreational runners. J Biomech. 2015;48(10):2171–2175. doi: 10.1016/j.jbiomech.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Roman-Blas J.A., Castañeda S., Largo R., Herrero-Beaumont G. Osteoarthritis associated with estrogen deficiency. Arthritis Res Ther. 2009;11:24. doi: 10.1186/ar2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X., Zhu J., Liu F., et al. Reduced EGFR signaling enhances cartilage destruction in a mouse osteoarthritis model. Bone Res. 2014;2:14015. doi: 10.1038/boneres.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin S.Y., Pozzi A., Boyd S.K., Clark A.L. Integrin α1β1 protects against signs of post-traumatic osteoarthritis in the female murine knee partially via regulation of epidermal growth factor receptor signaling. Osteoarthritis Cartilage. 2016;24(10):1795–1806. doi: 10.1016/j.joca.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Z., Otahal P., Wang B., et al. Cross-sectional and longitudinal associations between serum inflammatory cytokines and knee bone marrow lesions in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2017;25:499–505. doi: 10.1016/j.joca.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Mickiewicz B., Shin S.Y., Pozzi A., Vogel H.J., Clark A.L. Serum metabolite profiles are altered by erlotinib treatment and the integrin α1-null genotype but not by post-traumatic osteoarthritis. J Proteome Res. 2016;15:815–825. doi: 10.1021/acs.jproteome.5b00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma L., Song J., Dunlop D., et al. Varus and valgus alignment and incident and progressive knee osteoarthritis. Ann Rheum Dis. 2010;69(11):1940–1945. doi: 10.1136/ard.2010.129742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar D., Souza R.B., Subburaj K., et al. Are there sex differences in knee cartilage composition and walking mechanics in healthy and osteoarthritis populations? Clin Orthop Relat Res. 2015;473:2548–2558. doi: 10.1007/s11999-015-4212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Javaheri B., Razi H., Piles M., et al. Sexually dimorphic tibia shape is linked to natural osteoarthritis in STR/Ort mice. Osteoarthritis Cartilage. 2018;26:807–817. doi: 10.1016/j.joca.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Staines K.A., Poulet B., Wentworth B.N., Pitsillides A.A. The STR/ort mouse model of spontaneous osteoarthritis – an update. Osteoarthritis Cartilage. 2017;25:802–808. doi: 10.1016/j.joca.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman K.R., Hughes C., Morrey B.F., Morrey M., An K.N. Gait characteristic of patients with knee osteoarthritis. J Biomech. 2001;34:907–915. doi: 10.1016/s0021-9290(01)00036-7. [DOI] [PubMed] [Google Scholar]
- 20.McKean K.A., Landry S.C., Hubley-Kozey C.L., Dunbar M.J., Stanish W.D., Deluzio K.J. Gender differences exist in osteoarthritic gait. Clin Biomech. 2007;22:400–409. doi: 10.1016/j.clinbiomech.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Logerstedt D.S., Zeni J., Snyder-Mackler L. Sex differences in patients with different stages of knee osteoarthritis. Arch Phys Med Rehabil. 2014;95:2376–2381. doi: 10.1016/j.apmr.2014.07.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanley L.E., Kerr Z.Y., Dompier T.P., Padua D.A. Sex differences in the incidence of anterior cruciate ligament, medial collateral ligament, and meniscal injuries in collegiate and high school sports: 2009-2010 through 2013-2014. Am J Sports Med. 2016;44(6):1565–1572. doi: 10.1177/0363546516630927. [DOI] [PubMed] [Google Scholar]
- 23.Pollard C.D., Braun B., Hamill J. Influence of gender, estrogen and exercise on anterior knee laxity. Clin Biomech. 2006;21(10):1060–1066. doi: 10.1016/j.clinbiomech.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Shultz S.J., Kirk S.E., Johnson M.L., Sander T.C., Perrin D.H. Relationship between sex hormones and anterior knee laxity across the menstrual cycle. Med Sci Sports Exerc. 2004;36(7):1165–1174. doi: 10.1249/01.MSS.0000132270.43579.1A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell D.R., Blackburn J.T., Hackney A.C., Marshall S.W., Beutler A.I., Padua D.A. Jump-landing biomechanics and knee-laxity change across the menstrual cycle in women with anterior cruciate ligament reconstruction. J Athl Train. 2014;49(2):154–162. doi: 10.4085/1062-6050-49.2.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen M., Couppe C., Hansen C.S.E., et al. Impact of oral contraceptive use and menstrual phases on patellar tendon morphology, biochemical composition, and biomechanical properties in female athletes. J Appl Physiol. 2013;114(8):998–1008. doi: 10.1152/japplphysiol.01255.2012. [DOI] [PubMed] [Google Scholar]
- 27.Lee H., Petrofsky J.S., Daher N., Berk L., Laymon M. Differences in anterior cruciate ligament elasticity and force for knee flexion in women: oral contraceptive users versus non-oral contraceptive users. Eur J Appl Physiol. 2014;114(2):285–294. doi: 10.1007/s00421-013-2771-z. [DOI] [PubMed] [Google Scholar]
- 28.Srikanth V.K., Fryer J.L., Zhai G., Winzenberg T.M., Hosmer D., Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13(9):769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Hussain S.M., Wang Y., Giles G.G., Graves S., Wluka A.E., Cicuttini F.M. Female reproductive and hormonal factors and incidence of primary total knee arthroplasty due to osteoarthritis. Arthritis Rheumatol. 2018;70(7):1022–1029. doi: 10.1002/art.40483. [DOI] [PubMed] [Google Scholar]
- 30.Leblanc D.R., Schneider M., Angele P., Vollmer G., Docheva D. The effect of estrogen on tendon and ligament metabolism and function. J Steroid Biochem Mol Biol. 2017;172:106–116. doi: 10.1016/j.jsbmb.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Slauterbeck J.R., Fuzie S.F., Smith M.P., et al. The menstrual cycle, sex hormones, and anterior cruciate ligament injury. J Athl Train. 2002;37(3):275–280. [PMC free article] [PubMed] [Google Scholar]
- 32.Sneikers Y.H., Weinans H., Bierma-Zeinstra S.M., van Leeuwen J.P.T.M., van Osch G.J.V.M. Animal models for osteoarthritis: the effect of ovariectomy and estrogen treatment – a systemic approach. Osteoarthritis Cartilage. 2008;16:533–541. doi: 10.1016/j.joca.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Matthews J., Gustafsson J.A. Estrogen signaling: a subtle balance between ERα and ERβ. Mol Interv. 2003;3(5):281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- 34.Pelletier G. Localization of androgen and oestrogen receptors in rat and primate tissues. Histol Histopathol. 2000;15:1261–1270. doi: 10.14670/HH-15.1261. [DOI] [PubMed] [Google Scholar]
- 35.Claassen H., Hassenpflug J., Schunke M., Sierralta W., Thole H., Kurz B. Immunohistochemical detection of oestrogen receptor alpha in articular chondrocytes from cows, pigs and humans: in situ and in vitro results. Ann Anat. 2001;183:223–227. doi: 10.1016/s0940-9602(01)80221-1. [DOI] [PubMed] [Google Scholar]
- 36.Elbaradie K.B.Y., Wang Y., Boyan B.D., Schwartz Z. Sex-specific response of rat costochondral cartilage growth plate chondrocytes to 17β-estradiol involves differential regulation of plasma membrane associated estrogen receptors. Biochim Biophys Acta Mol Cell Res. 2013;1833:1165–1172. doi: 10.1016/j.bbamcr.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Van der Eerden B.C.J., Van Til N.P., Brinkman A.O., Lowik C.W.G., Wit J.M., Karperein M. Gender differences in expression of androgen receptor in tibial growth plate and metaphyseal bone of the rat. Bone. 2002;30(6):891–896. doi: 10.1016/s8756-3282(02)00723-8. [DOI] [PubMed] [Google Scholar]
- 38.Sniekers Y.H., van Osch G.J.V.M., Ederveen A.G.H., et al. Development of osteoarthritic features in estrogen receptor knockout mice. Osteoarthritis Cartilage. 2009;17(10):1356–1361. doi: 10.1016/j.joca.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Loeser R.F., Carlson C.S., McGee M.P. Expression of beta 1 integrins by cultured articular chondrocytes and in osteoarthritic cartilage. Exp Cell Res. 1995;217(2):248e57. doi: 10.1006/excr.1995.1084. [DOI] [PubMed] [Google Scholar]
- 40.Zemmyo M., Meharra E.J., Kuhn K., Creighton-Achermann L., Lotz M. Accelerated, aging-dependent development of osteoarthritis in alpha1 integrin-deficient mice. Arthritis Rheum. 2003;48(10):2873–2880. doi: 10.1002/art.11246. [DOI] [PubMed] [Google Scholar]
- 41.Chen X., Abair T.D., Ibanez M.R., et al. Integrin α1β1 controls reactive oxygen species synthesis by negatively regulating epidermal growth factor receptor-mediated Rac activation. Mol Cell Biol. 2007;27(9):3313–3326. doi: 10.1128/MCB.01476-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pest M.A., Russell B.A., Zhang Y.W., Jeong J.W., Beier F. Disturbed cartilage and joint homeostasis resulting from a loss of mitogen-inducible gene 6 in a mouse model of joint dysfunction. Arthritis Rheum. 2014;66(10):2816–2827. doi: 10.1002/art.38758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Appleton C.T.G., Usmani S.E., Pest M.A., Pitelka V., Mort J.S., Beier F. Reduction in disease progression by inhibition of transforming growth factor α-CCL2 signaling in experimental posttraumatic osteoarthritis. Arthritis Rheum. 2015;67(10):2691–2701. doi: 10.1002/art.39255. [DOI] [PubMed] [Google Scholar]