PIKfyve is named after its function and domain structure — phosphoinositide kinase for five position containing a FYVE finger — and was first identified over two decades ago as a lipid kinase that phosphorylates phosphatidylinositol-3-phosphate (PI(3)P), producing PI(3,5)P2 (FIG. 1a). PIKfyve plays a critical role in the endosomal and lysosomal system, regulating membrane homeostasis, endosomal trafficking and autophagy (Toxicol. Appl. Pharmacol. 383, 114771; 2019), and is emerging as a target for cancer, viral infections and neurodegenerative diseases.
Fig. 1 |. PIKfyve as a drug target.
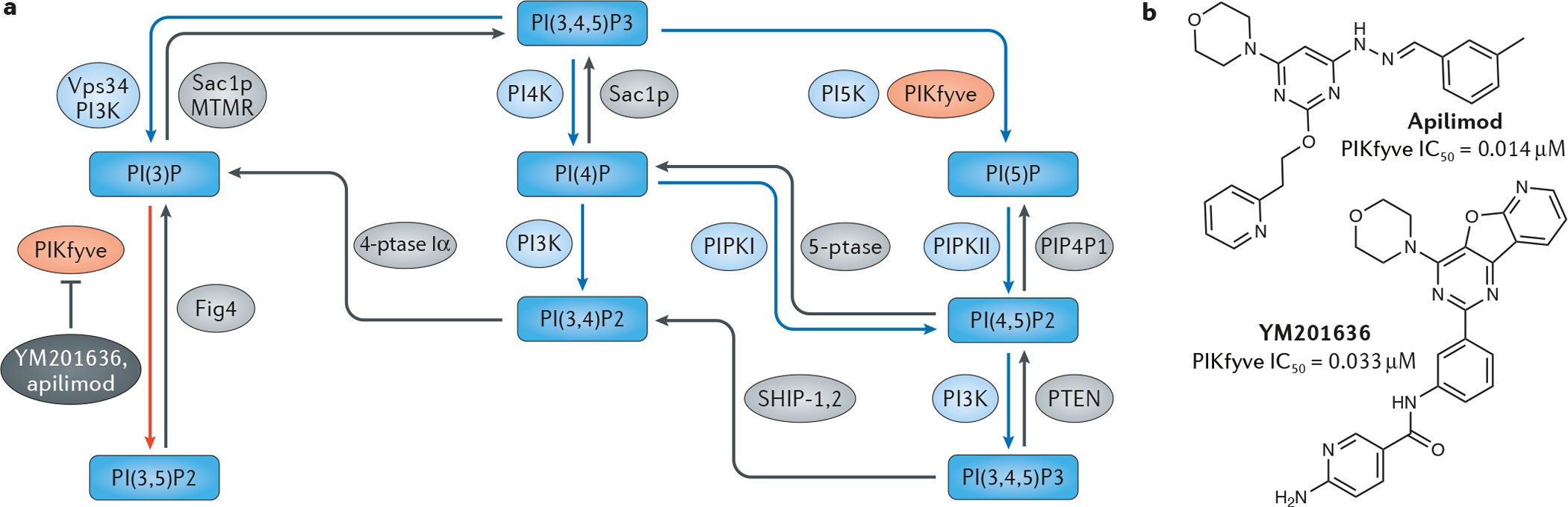
a | The phosphoinositide signalling pathway. Blue squares represent phosphoinositides. Blue arrows indicate the respective kinases, and grey arrows indicate the phosphatases. b | Structures of the PIKfyve inhibitors apilimod and YM201636.
Roles in disease
One of the first studies to indicate the therapeutic relevance of PIKfyve focused on apilimod, a small molecule that was developed to block production of interleukin IL-12 and IL-23 induced by Toll-like receptor (TLR) activation (Chem. Biol. 20, 912–921; 2013). Apilimod was ineffective in clinical trials for autoimmune diseases including Crohn’s disease and rheumatoid arthritis about a decade ago, but it was safe and well tolerated. The molecular targets of apilimod were unknown at the time the trials were started, and this study showed that its effects on TLR-induced signalling were mediated by PIKfyve.
PIKfyve has been implicated in various cancers. For example, apilimod was found to have potent and selective antiproliferative effects in a screen of clinical-stage drugs against B-cell non-Hodgkin lymphoma cell lines (Blood 129, 1768-1778; 2017). Further studies, including shRNA knockdown of PIKfyve, showed that PIKfyve was the target underlying the antiproliferative activity, which was driven by disruption of late endosome/lysosome function.
PIKfyve is also involved in the entry of viruses into host cells, including filoviruses such as Ebola virus and Marburg virus and coronaviruses such as SARS-CoV-2, which causes COVID-19. Pharmacological inhibition of PIKfyve by apilimod was shown to suppress trafficking of Ebola virus to its fusion site on endosomes and subsequently the release of the viral genome into the cytoplasm. Similarly, pharmacological inhibition of PIKfyve by apilimod impaired SARS-CoV-2 entry into various mammalian cell lines and potently suppressed viral replication (Proc. Natl Acad. Sci. USA 117, 20803–20813; 2020).
Finally, PIKfyve also has roles in neurodegenerative diseases. The PIKfyve inhibitor YM201636 was found to improve the survival of motor neurons derived from induced pluripotent stem cells from patients with amyotrophic lateral sclerosis caused by a repeat expansion in C9ORF72, and the role of PIKfyve was confirmed with apilimod (Nat. Med. 24, 313–325; 2018). Furthermore, PIKfyve was identified as a regulator of the subcellular distribution of tau aggregates, and its pharmacological inhibition with YM201636 decreased the lysosomal delivery of tau aggregates, halting a key step in the progression of tauopathies (J. Biol. Chem. 296, 100636; 2021).
Chemical tools
Although PIKfyve is not routinely screened in larger kinome panels, there are a number of high-quality inhibitors available and its crystal structure is known (PDB: 7K2V).
The initial inhibitor identified for PIKfyve was YM201636, a pyrimidine-based kinase inhibitor with good potency against PIKfyve in vitro (IC50 = 0.033 μM) and selectivity over several lipid kinase family members (Toxicol. Appl. Pharmacol. 383, 114771; 2019). As noted above, apilimod (FIG. 1b) has been found to be both a potent PIKfyve inhibitor (IC50 = 0.014 μM) and highly selective across the kinome (Chem. Biol. 20, 912–921, 2013; Blood 129, 1768-1778; 2017). However, although detailed pharmacokinetics of apilimod are largely unknown, literature data suggests that low plasma availability soon after dosing is an issue (Toxicol. Appl. Pharmacol. 383, 114771; 2019). Spurred by the wide-ranging therapeutic potential of PIKfyve inhibition, other compounds are now under development.
Acknowledgements
This article is part of a series from the NIH Common Fund Illuminating the Druggable Genome (IDG) program. The goal of IDG is to catalyse research on understudied proteins from druggable gene families by providing reagents, phenotypes and a mineable database; focusing on GPCRs, kinases and ion channels. For more information, see https://druggablegenome.net/
Footnotes
Competing interests
The authors declare no competing interests.
RELATED LINKS
Pharos (PIKfyve): https://pharos.nih.gov/targets/PIKfyve


