Abstract
Intravenous immunoglobulins are an efficacious treatment for chronic inflammatory demyelinating polyradiculoneuropathy. Biomarkers for disease activity are lacking, making the need for ongoing treatment difficult to assess, leading to potential overtreatment and high health-care costs. Our objective was to determine whether intravenous immunoglobulin withdrawal is non-inferior to continuing intravenous immunoglobulin treatment and to determine how often patients are overtreated.
We performed a randomized, double-blind, intravenous immunoglobulin-controlled non-inferiority trial in seven centres in the Netherlands (Trial registration: ISRCTN 13637698; www.isrctn.com/ISRCTN13637698). Adults with clinically stable chronic inflammatory demyelinating polyradiculoneuropathy using intravenous immunoglobulin maintenance treatment for at least 6 months were included. Patients received either intravenous immunoglobulin withdrawal (placebo) as investigational treatment or continuation of intravenous immunoglobulin treatment (control). The primary outcome was the mean change in logit scores from baseline to 24-week follow-up on the patient-reported Inflammatory Rasch–Overall Disability Scale. The non-inferiority margin was predefined as between-group difference in mean change scores of −0.65. Patients who deteriorated could reach a relapse end point according to predefined criteria. Patients with a relapse end point after intravenous immunoglobulin withdrawal entered a restabilization phase. All patients from the withdrawal group who remained stable were included in an open-label extension phase of 52 weeks.
We included 60 patients, of whom 29 were randomized to intravenous immunoglobulin withdrawal and 31 to continuation of treatment. The mean age was 58 years (SD 14.7) and 67% was male. The between-group difference in mean change Inflammatory Rasch–Overall Disability Scale scores was −0.47 (95% CI −1.24 to 0.31), indicating that non-inferiority of intravenous immunoglobulin withdrawal could not be established. In the intravenous immunoglobulin withdrawal group, 41% remained stable for 24 weeks, compared to 58% in the intravenous immunoglobulin continuation group (−17%; 95% CI −39 to 8). Of the intravenous immunoglobulin withdrawal group, 28% remained stable at the end of the extension phase. Of the patients in the restabilization phase, 94% restabilized within 12 weeks.
In conclusion, it remains inconclusive whether intravenous immunoglobulin withdrawal is non-inferior compared to continuing treatment, partly due to larger than expected confidence intervals leading to an underpowered study. Despite these limitations, a considerable proportion of patients could stop treatment and almost all patients who relapsed were restabilized quickly. Unexpectedly, a high proportion of intravenous immunoglobulin-treated patients experienced a relapse end point, emphasizing the need for more objective measures for disease activity in future trials, as the patient-reported outcome measures might not have been able to identify true relapses reliably. Overall, this study suggests that withdrawal attempts are safe and should be performed regularly in clinically stable patients.
Keywords: CIDP, IVIg, overtreatment, withdrawal
Adrichem et al. show that although the majority of CIDP patients require maintenance treatment with IVIg, a considerable proportion are able to stop IVIg treatment without relapsing. Treatment withdrawal is safe and should be attempted regularly in clinically stable CIDP patients.
Introduction
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is a heterogeneous disease with an unpredictable disease course, which can be progressive, relapsing–remitting and monophasic.1 Several studies demonstrated short-term superiority of treatment with intravenous immunoglobulins (IVIg) over placebo in CIDP.2 In patients who improve on treatment, maintenance treatment is often started as most patients run a chronic course.1 However, there is limited evidence on how long maintenance treatment should be given and how IVIg dependency should be assessed. IVIg overtreatment in up to 60% of CIDP patients has been suggested in previous studies, based on lack of clinical deterioration in the patients who were included in placebo arms during a variable period of follow-up.3–6 However, none of these trials were specifically designed to assess IVIg overtreatment.
Clinical evaluation after tapering or stopping IVIg is currently the only way to assess ongoing need for IVIg. Many patients and physicians are reluctant to perform withdrawal trials because of the risk of deterioration.7 In practice, this means that many patients receive IVIg for years without verifying whether ongoing treatment is needed. Preventing IVIg overtreatment would reduce healthcare burden, adverse events and healthcare costs.
The objective of this study was to investigate whether withdrawal of IVIg treatment was non-inferior to continuing IVIg treatment in clinically stable CIDP patients and to determine how often these patients are overtreated with IVIg.
Materials and methods
Study design
We conducted a multicentre randomized, double-blind, non-inferiority trial in clinically stable CIDP patients based on the hypothesis that IVIg withdrawal is non-inferior to continuation of IVIg treatment. IVIg withdrawal was considered as the interventional treatment, while continuation of IVIg treatment was considered as the standard or control treatment. The trial was registered at the ISRCTN registry (ISRCTN13637698). In addition, we performed an open-label prospective follow-up study to provide a better estimate of the risks of IVIg withdrawal by assessing the number of patients that successfully stopped IVIg for an additional period of 52 weeks, and by assessing the rates and time to full restabilization in patients who deteriorated after IVIg withdrawal. The study was approved by the ethical committee of the Amsterdam UMC.
Patients
Patients were included in five university hospitals and two regional hospitals in the Netherlands from April 2014 until November 2018 when the last patient was included. Adult patients were eligible if they had been diagnosed with probable or definite CIDP according to the European Federation of Neurological Societies/Peripheral Nerve Society 2010 criteria, and had stable disease under IVIg treatment for at least 6 months with a treatment interval between 2 and 6 weeks.1 Disease stability was judged by treating physicians; subjective, minor wear-off symptoms were permitted. Patients were excluded if they had experienced deterioration after IVIg withdrawal in the previous 12 months; if there were changes in IVIg dose or interval in the previous 6 months or changes in additional CIDP treatment (e.g. corticosteroids) in the previous 3 months; a prolonged period (>6 weeks) of disability increase following an earlier IVIg withdrawal attempt or a history of CIDP-related respiratory failure. Written informed consent was obtained from all subjects according to the Declaration of Helsinki.
Randomization and masking
After informed consent, patients were randomly allocated to IVIg withdrawal or continuation of IVIg treatment. The randomization procedure was web-based (TENALEA, https://www.aleaclinical.eu/products/), using the non-deterministic minimization method as described by Pocock and Simon.8 The method used duration of prior IVIg treatment (6–12 months versus >12 months) for balancing. We chose the minimization procedure to prevent predictability of upcoming randomizations considering the fact that deblinding took place when a patient reached a study end point. After inclusion, the local investigator provided a prescription for IVIg to the trial pharmacist for a total period of 24 weeks. The randomization code and treatment allocations were provided by TENALEA also to the trial pharmacist. The pharmacist prepared investigational medicinal product (IMP). Blinded infusion bags and closure systems and coated intravenous lines were used to ensure adequate masking. As IVIg/placebo volume ratio changed during tapering, volume and number of infusion bags of IVIg and/or placebo were adjusted to keep these parameters constant during the first three study treatments in order to maintain blinding. An unblinded nurse prepared the IMP for administration at the central pharmacy site and transported IMP to patients’ homes, where a second nurse, blinded for treatment allocation, administered the infusions. Outcome assessors were blinded for treatment allocation. After completion of the final visit of each patient, the treating physician contacted the study team who contacted the trial pharmacist. The trial pharmacy disclosed the allocation of the patients to the treating physician. Deblinding during the trial was only possible in case of reaching a relapse end point requiring change or addition of treatment.
Procedures
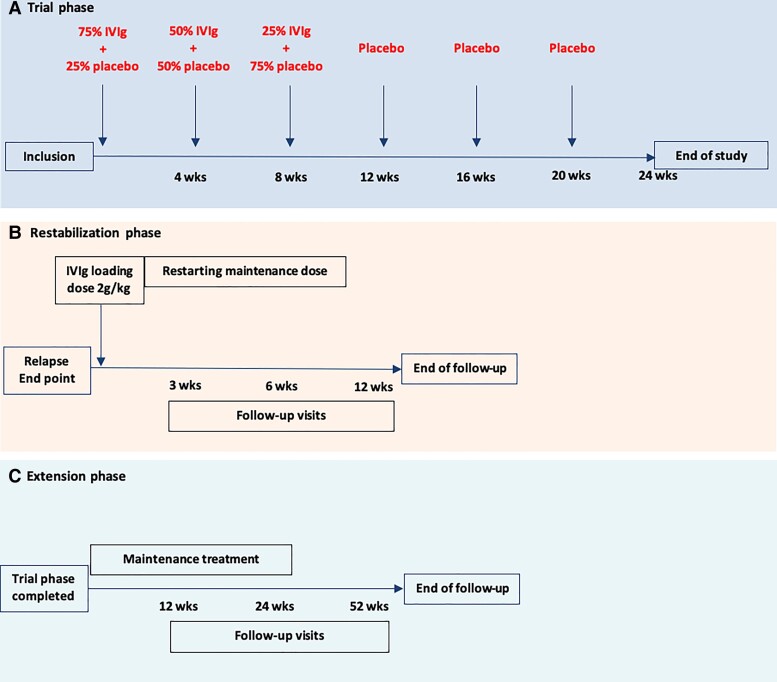
After the baseline visit, patients received an unblinded IVIg treatment at the same dose and interval as prior to the study to measure trough and peak IgG levels. Serum was collected at the same day directly before and after the IVIg treatment. Patients received IMP infusions at the same interval as IVIg prior to the study (Fig. 1A). IVIg withdrawal consisted of an IVIg tapering phase and a placebo-only phase. Tapering consisted of three infusions of 75%, 50% and 25%, respectively, of the patients’ individual pre-study IVIg dose combined with placebo, which was followed by 100% placebo infusions. Placebo consisted of a sodium chloride solution (NaCl 0.9%) in identical volume as the previous IVIg treatment. Patients allocated to continuation of treatment continued the same IVIg treatment (brand, dose and interval) as prior to the study. Follow-up visits were scheduled every 6 weeks. Patients received a phone call in between visits to monitor a possible relapse.
Figure 1.
IVIg withdrawal schedule in a patient on a 4-weekly interval. (A) An example schedule of the investigational treatment in a patient with an IVIg interval of 4 weeks. Follow-up visits were performed every 6 weeks. (B) Restabilization phase consisting of a loading dose and maintenance treatment. Follow-up visits were performed at 3, 6 and 12 weeks. (C) Extension study: maintenance treatment was at the physician’s discretion. Follow-up visits were performed at 12, 24 and 52 weeks.
Patients randomized into the withdrawal group who reached a predefined relapse end point during follow-up received a rescue IVIg loading dose of 2 g/kg followed by maintenance IVIg treatment (Fig. 1B). A maintenance dose equal to the second last dose prior to the first signs of deterioration was advised. For example, a patient who deteriorated after receiving 25% of their baseline IVIg dose received a maintenance dose of 75% of the baseline IVIg dose after their rescue loading dose. We did not restart patients on a lower maintenance dose than 50% of their baseline IVIg dose. Total duration of the restabilization phase was 12 weeks. Visits were scheduled at 3, 6 and 12 weeks after administering the loading dose. There was no fixed restabilization schedule for patients randomized into the IVIg continuation group who reached a relapse end point. The treating physician made the decision if a loading dose, an extra dose or just continuation of treatment was necessary.
Patients from the IVIg withdrawal group who remained stable at 24 weeks were included in an open-label 52-week extension phase to assess potential relapses after the trial phase (Fig. 1C). In the extension phase, follow-up visits were scheduled at 12, 24 and 52 weeks, or earlier if a relapse occurred.
Outcomes
Trial phase
The primary outcome was the mean change score from baseline to final follow-up on the inflammatory Rasch–Overall Disability Scale (iRODS). The iRODS is an interval disability scale based on Rasch methodology (Supplementary Table 1), with the standard unit of measurement expressed in logits.9 The primary end point was reached at 24 weeks after first study treatment or earlier, in case of a relapse. In the original protocol, a relapse end point was defined as a deterioration of >0.65 logits on the iRODS. In addition, deterioration warranting treatment according to the physicians’ or patients’ judgement was regarded as a relapse end point regardless of the iRODS score.
The main secondary outcome was the proportion of patients who did not meet the criteria for a relapse end point and completed the 24-week follow-up. Other secondary outcomes were assessed at 24 weeks, or at a relapse end point if appropriate. These included: muscle strength measured using the Medical Research Council (MRC) sum score; grip strength (Martin-Vigorimeter) of the dominant hand or, in case of asymmetric weakness, the most affected hand; sensory impairment using the INCAT-Sensory Sum Score (INCAT-SS)10; pain using the Pain Intensity Numerical Rating Scale (PI-NRS); fatigue using a 7-item linear modified Rasch-built Fatigue Severity Scale (FSS)11; disability using the generic AMC Linear Disability Score (ALDS)12; patient’s perception of clinical deterioration or improvement on a 5-point Patient Global Impression of Change scale (PGIC), and quality of life using the 36-item Short Form Health Survey (SF-36).13 Additional information on the outcome measures, including ranges of the scales can be found in Supplementary Table 1. The treating physician blinded for treatment allocation performed muscle strength, grip strength and sensory assessments. The other outcome instruments were based on patient self-reports. Assessments were scheduled every 6 weeks during 24 weeks of follow-up after the first IMP treatment, or earlier if a relapse was suspected. At the end of the study, patients were asked to guess to which treatment group they were allocated.
Restabilization phase
Restabilization was assessed using the individual minimally clinically important difference (MCID) on the iRODS and grip strength. Additionally, restabilization was assessed using a 5-point PGIC. Patients and physicians were asked to indicate whether restabilization was reached up to the baseline level before entering the trial at each visit. For iRODS and grip strength, patients were considered restabilized if the score difference between follow-up (before IVIg withdrawal) and baseline was less than the individual MCID on the iRODS and respectively less than 8 kPa on the grip strength, or if score at follow-up was higher than baseline. On the PGIC and physician’s questionnaire, restabilization was defined by scores at follow-up indicating no change or better compared to baseline.
Extension phase
In the extension phase, the proportion of patients stable without the need of treatment 76 weeks after start of treatment withdrawal was assessed. This included the 24-week follow-up of the trial phase and the 52-week follow-up of the extension phase. Stable disease was defined as no change or a change less than the MCID on the iRODS compared to baseline, without restart of treatment.
Protocol changes during study
The study protocol was changed twice during the study. After randomization of the first two patients (both received a single IMP), a paper was published that enabled calculation of the MCID based on a change of at least 1.96 standard error, for each individual iRODS score across the iRODS continuum.14 This MCID is equivalent to a score between 4 and 8 points on the non-linear scale (0–48 points) in clinical practice, depending on the individual baseline iRODS score. The study protocol was amended to define a relapse end point as the individual MCID on iRODS rather than a fixed cut-off of −0.65 logits.
Secondly, an explorative iRODS measurement was added just before the last regular IVIg infusion. This was advised by the Data Safety Monitoring Board to identify possible wear-off symptoms as a reason for the unexpectedly high number of patients in the IVIg group who reached a preliminary outcome.
Statistical analyses
Sample size calculation
Rationale of the non-inferiority margin
The non-inferiority margin was −0.65 logits. This non-inferiority threshold reflects a deterioration to a functional ability level of (0.35–0.65) = −0.30 logit. This means that an ‘average’ patient could still do shopping, but experience minor difficulties to walk one flight of stairs. This non-inferiority margin was considered clinically acceptable given the given the extremely high cost of IVIg, potential (severe) side effects of IVIg, the patient burden of treatment and possibility to restart IVIg if necessary. The non-inferiority margin corresponds with a deterioration of approximately 3 points on the non-linear iRODS score from 0 to 48.
Conceptual background of the sample size calculation
For the sample size calculation, we first defined a clinically acceptable deterioration on the iRODS in an average CIDP patient based on the original iRODS paper.9 The functional ability level of stable patients on the iRODS ranges from −6.95 to 8.11 logits, with a mean logit score of 0.35 (SD 0.84).9 We considered a non-inferiority margin of −0.65 logit (lower confidence interval of the difference in mean change score) as acceptable given the very high costs and patient burden of IVIg overtreatment (for clarification see Supplementary Table 1). The null hypothesis being tested is that withdrawal of IVIg is not non-inferior to the treatment continuation. In other words, withdrawal is worse than continuation of treatment. If the null hypothesis is rejected, withdrawal is not worse than continuation. The null hypothesis is rejected if the lower bound of the 95% CI of the difference between withdrawal and continuation of treatment is higher than −0.65.
When statistically testing the null hypothesis a one-sided 0.025 significance level is used as we are only interested in the lower boundary of the 95% CI.
Sample size calculation
When the sample size in each group is 27, a two-group one-sided 0.025 significance level t-test will have 80% power to reject the null hypothesis that the mean change score of withdrawal of IVIg treatment (µW) is more than 0.65 logit worse than the mean score of continuation of IVIg treatment (µC) (difference µW minus µC < −0.65) in favour of the alternative hypothesis that the mean change score of withdrawal of IVIg treatment is more alike or even better than the mean change score of continuation of IVIg treatment (difference µW minus µC > −0.65). It was assumed that the expected difference in mean change scores is 0 and the common SD is 0.84. Anticipating a 10% attrition rate, 30 (27/0.90) patients per treatment arm (60 patients in total) were included.
Trial phase
The primary outcome was statistically tested for non-inferiority based on the intention-to-treat principle. Additionally, the primary outcome was also analysed on a per-protocol basis. The intention to treat population included all randomized patients, regardless of protocol deviations. The per-protocol population encompassed patients included and treated in accordance with the study protocol. Patients who had been unblinded were excluded from the per-protocol population (Supplementary Table 2).
Baseline assessments were summarized using simple descriptive statistics. The main analysis focused on the between-group difference in the mean change iRODS (logit) scores. Statistical uncertainty of this difference was expressed in a two-sided 95% CI. If the lower limit of the CI crosses the non-inferiority margin of −0.65 logits, non-inferiority of the IVIg withdrawal group cannot be established (for additional explanation, see the previous text in the subsection ‘Sample size calculation’). Inferiority can be established when the upper limit of the CI is below the non-inferiority margin.
Additionally, the non-inferiority of treatment withdrawal was tested using multivariable linear regression with iRODS follow-up scores as the dependent variable, adjusting for both the iRODS baseline scores and the minimization variable (duration of prior IVIg treatment). The linear regression modelling was performed within the context of a non-inferiority design. The coefficient for withdrawal treatment was expressed with its 95% CI.
Regarding the secondary end points, baseline, end point and group change scores were summarized using descriptive statistics. In all secondary outcomes analyses statistical uncertainty was expressed in two-sided 95% CI. As the MRC scores were not normally distributed we expressed the point estimate and CI were analysed using the Hodges–Lehmann approach.15
Unplanned post hoc analysis
We described the number of patients experiencing wear off symptoms at start of trial and with a relapse end point during the trial phase. Furthermore, the between-group difference in the time to relapse end point was analysed by plotting Kaplan–Meier curves and comparing them using the log-rank test.
Restabilization phase
For the restabilization phase, we described the number of patients who restabilized within 12 weeks on the different scales.
Extension phase
For the extension phase, we described the number of patients from the IVIg withdrawal group with stable disease at end of follow up.
An independent Data Safety Monitoring Board performed an interim safety analyses after 10 patients reached a relapse end point and after the inclusion of 30 patients. No interim efficacy analyses were performed. Sample size calculation and statistical analyses were performed in nQuery (v8.5.1) and IBM SPSS Statistics (v25), respectively.
Data availability
The corresponding author (F.E.) has full access to the study protocol and all the data in the study. Data are available upon reasonable request.
Results
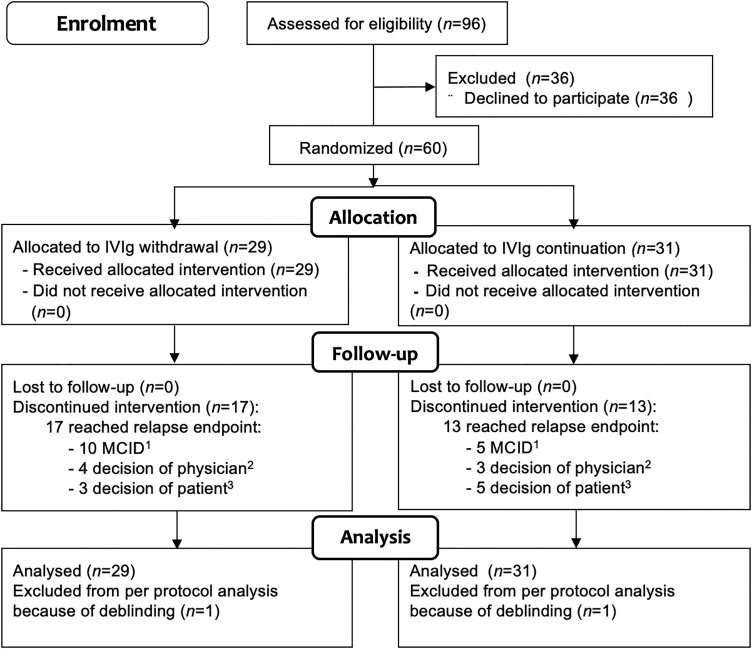
A total of 96 patients were considered eligible (Fig. 2) and 60 patients were included between April 2014 and November 2018. Twenty-nine patients were allocated to the IVIg withdrawal group and 31 to the IVIg continuation group (Fig. 2).
Figure 2.
Enrolment and randomization. (1) MCID on iRODS. (2) Decision of treating physician: relapse, but not captured on the iRODS. (3) Decision of patient: subjective relapse.
Baseline characteristics in both groups were comparable (Table 1). The baseline iRODS logit scores were higher in the IVIg continuation group [mean 4.66 (SD 2.29)] compared to the withdrawal group [3.80 (SD 2.86)]. Previous treatment withdrawal attempts had been performed in 11 of 29 patients (38%) in the IVIg withdrawal group and in 15 of 31 patients (54%) in the IVIg continuation group. Wear-off symptoms were reported in 6 of 29 patients (21%) in the IVIg withdrawal group and 9 of 31 patients (29%) in the IVIg continuation group.
Table 1.
Baseline demographic and clinical characteristics
| IVIg withdrawal group (29) | IVIg continuation group (31) | |
|---|---|---|
| Sex, male | 21 (72%) | 21 (68%) |
| Age [mean ± SD, (range)] | 60.1 (13.54, 21–86) | 57.7 (15.97, 29–81) |
| CIDP | ||
| Typical | 25 (86%) | 22 (71%) |
| Atypical | 4 (14%) | 9 (29%) |
| Asymmetric CIDP | 2 (7%) | 4 (13%) |
| Pure motor/sensory | 2 (7%) | 5 (16%) |
| Disease duration in months (median, range) | 64 (7–586) | 50 (9–299) |
| Wear-off symptoms | 6 (21%) | 9 (29%) |
| MRC sum score (median, range) | 58 (38–60) | 60 (49–60) |
| Grip strength [mean ± SD, (range)] | 84kPa (SD 34.37; 18–145) | 79kPa (SD 28.29; 9–155) |
| Duration of IVIg treatment | ||
| 6–12 months | 15 (52%) | 16 (51%) |
| >12 months | 14 (48%) | 15 (49%) |
| Patients with previous withdrawal attempts | 11 (38%) | 15 (54%) |
| IVIg interval | ||
| 2 weeks | 3 (10%) | 1 (3%) |
| 3 weeks | 16 (55%) | 16 (52%) |
| 4 weeks | 9 (31%) | 9 (29%) |
| 5 weeks | – | 3 (10%) |
| 6 weeks | 1 (3%) | 2 (6%) |
| IVIg dose per infusion (median, range) | 45 g (10–80) | 40 g (10–80) |
| IVIg brand | ||
| Nanogam® | 18 (62%) | 16 (52%) |
| Kiovig® | 10 (35%) | 14 (45%) |
| Privigen® | 1 (3%) | 0 |
| Gamunex® | 0 | 1 (3%) |
| Immunosuppressive treatment besides IVIga | 1 (3%) | 0 (0%) |
| Serum IgG level change after last regular IVIg infusionb (mean ± SD) | 13.19 g/l (SD 7.99) | 12.80 g/l (SD 5.78) |
| iRODS (mean ± SD) logits | 3.80 (SD 2.86) | 4.66 (SD 2.29) |
Daily oral prednisone 5 mg for rheumatic polymyalgia.
Serum was collected before and after IVIg treatment at the day of the treatment.
Trial phase
In the IVIg withdrawal group, 17 of 29 patients (59%) reached a predefined relapse end point compared to 13 of 31 (42%) in the IVIg continuation group. In other words, 12 of 29 (41%) and 18 of 31 patients (58%), respectively, remained stable during the 24-week follow-up in the trial phase (difference −17%; 95% CI −39 to 8).
Of the patients with a relapse end point, 10 of 17 patients (59%) in the IVIg withdrawal group worsened by their individual MCID on the iRODS compared to 5 of 13 (38%) in the IVIg continuation group.
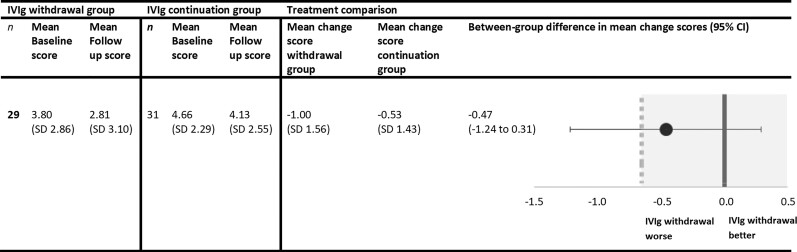
The primary outcome is depicted in Fig. 3. Both groups showed a lower mean logit score at end point compared to baseline. The between-group difference in mean change scores was −0.47 (95% CI: −1.24 to 0.31). The results from the primary outcome were inconclusive. As the lower bound of the CI crosses the non-inferiority margin of −0.65, non-inferiority of IVIg withdrawal could not be demonstrated. Alternatively, we could also not demonstrate that IVIg withdrawal was significantly inferior to IVIg continuation, as the upper bound of the CI was not below the non-inferiority margin. Additional multivariable linear regression also fails to demonstrate non-inferiority of IVIg withdrawal. After adjustment, the coefficient for withdrawal treatment was −0.56, with the lower bound of the CI well below the non-inferiority margin of −0.65 (95% CI: −1.35 to 0.23). See the Supplementary material for further details.
Figure 3.
Primary outcome. Between-group comparisons of the primary outcome expressed in mean change logit scores on the iRODS. The dotted line represents the non-inferiority margin of −0.65. The shaded area marks the non-inferiority zone. †Reported mean changes and differences in mean changes may slightly differ from apparent differences due to rounding.
In total, 28 patients from the IVIg withdrawal group and 30 patients from the IVIg continuation group were included in the per-protocol analysis. Two patients were excluded because of early unblinding (Supplementary Table 2). In the per-protocol population too, non-inferiority in the withdrawal group could not be demonstrated, with the lower bound of the CI of the between-group difference in mean change scores of −0.47 again well below the non-inferiority margin (95% CI −1.27 to 0.33).
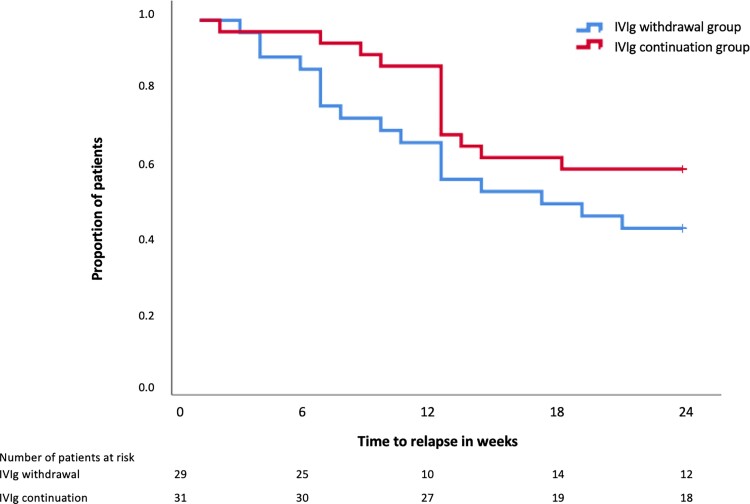
The results of the secondary outcomes are shown in Table 2. In general, variable levels of deterioration were observed in all outcomes in both study arms. However, the deterioration seemed to be more pronounced in the IVIg withdrawal group with regard to grip strength and the PGIC. Fig. 4 shows the proportion of patients that reached a relapse end point on the different time points in both groups. There was no significant difference in time to relapse between both treatment groups.
Table 2.
Secondary outcomes
| IVIg withdrawal group | IVIg continuation group | Treatment comparison | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | End point | Mean changea | n | Baseline | End point | Mean changea | Difference in mean change scores | 95% CI | |
| MRC sum score (median, range) | 29 | 58 (38–60) | 58 (41–60) | 0b (−10 to −3) | 31 | 60 (49−60) | 59 (42−60) | 0b (−7 to −2) | 0c | −1 to 0d |
| Grip strength (mean ± SD) | 29 | 85.3 kPa (34.4) | 73.5 kPa (31.2) | −11.8 kPa (14.2) | 31 | 79.3 kPa (29.2) | 75.8 kPa (28.9) | −3.5 kPa (17.8) | −8.3 | −16.8 to 0.2 |
| INCAT-SS (mean ± SD) | 29 | 5.2 (4.6) | 5.6 (4.2) | 0.4 (2.4) | 31 | 3.8 (3.7) | 3.5 (3.6) | −0.4 (2.3) | 0.8 | −0.4 to 2.0 |
| PI-NRS (mean ± SD) | 28 | 1.6 (1.9) | 2.4 (2.5) | 0.8 (2.4) | 29 | 1.8 (2.4) | 2.3 (2.5) | 0.5 (1.6) | 0.2 | −0.9 to 1.3 |
| FSS score (mean ± SD) | 27 | 35.0 (10.1) | 35.9 (12.1) | 0.9 (6.5) | 29 | 31.9 (12.3) | 32.9 (12.4) | 1.0 (10.8) | −0.1 | −6.3 to 4.0 |
| ALDS (mean ± SD) | 27 | 83.2 (10.3) | 80.6 (12.5) | −2.6 (7.4) | 30 | 87.4 (6.0) | 86.7 (11.7) | −0.7 (5.0) | 1.9 | −1.5 to 5.3 |
| SF-36 (mean ± SD) | 27 | 29 | ||||||||
| Physical component | 42.1 (9.9) | 37.8 (11.2) | −4.4 (9.4) | 45.2 (8.5) | 41.2 (10.5) | −4.0 (8.9) | −0.4 | −5.3 to 4.5 | ||
| Mental component | 50.9 (10.3) | 53.1 (7.9) | 2.1 (9.4) | 49.7 (11.8) | 47.9 (12.4) | −1.8 (13.9) | 3.9 | −2.4 to 10.4 | ||
| PGIC scale, n (%) | 27 | 29 | ||||||||
| Same or better than prior to study | NA | 10 (37%) | NA | NA | 17 (59%) | NA | NA | −44 to 4 | ||
| Worse than prior to the study | NA | 17 (63%) | NA | NA | 12 (41%) | NA | NA | |||
ALDS = AMC Linear Disability Score; FSS = Fatigue Severity Scale; INCAT-SS = INCAT Sensory Sum Score; PI-NRS = Pain Intensity Numeric Rating Scale; SF 36 = Short Form 36.
Reported mean changes and differences in mean changes may slightly differ from apparent differences due to rounding.
Median change scores on the MRC.
The within-group median change score was calculated as the 50th percentile of all individual differences.
Between-group difference on the MRC expressed in median difference in change scores; point estimate and 95% CI were analysed using the Hodges–Lehmann approach.
Figure 4.
Relapse end point during trial phase in both treatment arms.
Post hoc analysis
Post hoc analysis showed that 10 of 15 patients (67%) with wear-off symptoms reached a relapse end point and 5 of 15 (33%) patients remained stable. Four of six patients (67%) with wear-off in the withdrawal group reached a relapse end point, compared to six of nine (67%) in the IVIg continuation group. In Table 3 we show the secondary outcomes in all patients who reached a relapse end point and in patients who reached a relapse end point based on the MCID on the iRODS. Most secondary outcomes showed a trend in which patients with relapse in the IVIg withdrawal group had a larger difference compared to baseline compared to patients from the IVIg continuation group, Supplementary Table 3 shows deterioration on other outcome measures (grip strength, MRC sum score and the PGIC scale) in patients who reached a relapse end point on the different time points.
Table 3.
Post hoc analyses in patients who reached a relapse end point
| IVIg withdrawal group | IVIg continuation group | Treatment comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | Mean change | n | Baseline | Mean change | Difference in mean change scores | 95% CI | ||
| iRODS(mean ± SD) Logits | Relapse end point | 17 | 3.3 (3.1) | −1.9 (1.3) | 13 | 3.9 (2.6) | −1.3 (1.0) | 0.54 | −0.33 to 1.42b |
| MCID end pointa | 10 | 3.4 (2.4) | −2.5 (1.2) | 5 | 4.3 (2.7) | −2.2 (0.8) | −0.3 | −1.6 to 1.01 | |
| MRC sum score (median, range) | Relapse end point | 17 | 58 (38–60) | 0 (−10 to −3)c | 13 | 60 (49−60) | −1 (−7 to −0)c | 0d | −1.0 to 2.0d |
| MCID end point | 10 | 58.5 (52–60) | −2 (−10 to −0) | 5 | 60 (52−60) | 0 (−6−0) | 0d | −4.0 to 2.0d | |
| Grip strength (mean ± SD) | Relapse end point | 17 | 79.5 kPa (38.1) | −16.4 (15.4) | 13 | 77.6 kPa (32.7) | −11.6 (17.9) | −4.9 kPa | −17.8 to 8.1 |
| MCID End point | 9 | 87.8 kPa (42.2) | −19.4 (15.2) | 5 | 82.0 kPa (52.8) | −5.6 (9.0) | −13.8 | −30.1 to 2.4 | |
| INCAT-SS (mean ± SD) | Relapse end point | 17 | 5.7 (5.1) | 0.8 (2.2) | 13 | 4.2 (3.5) | 0.5 (2.9) | 0.3 | −1.7 to 2.2 |
| MCID end point | 10 | 5.7 (4.1) | 1.1 (2.3) | 5 | 2.8 (3.0) | 1.8 (2.2) | −0.7 | −3.4 to 2.4 | |
| PI-NRS (mean ± SD) | Relapse end point | 16 | 1.8 (2.0) | 1.8 (2.7) | 11 | 2.0 (2.5) | 1.1 (1.7) | 0.7 | −1.2 to 2.4 |
| MCID End point | 9 | 1.1 (1.7) | 3.1 (2.6) | 5 | 1.2 (2.7) | 1.4 (2.2) | 1.7 | −1.3 to 4.7 | |
| FSS score (mean ± SD) | Relapse end point | 16 | 31.7 (13.1) | −6.0 (6.5) | 11 | 32.3 (13.0) | −4.4 (8.0) | −1.6 | −7. to 1.4 |
| MCID end point | 9 | 37.0 (7.4) | −6.2 (7.0) | 5 | 33.0 (11.5) | −6.6 (4.6) | 0.4 | −9.6 to 10.3 | |
| ALDS (mean ± SD) | Relapse end point | 15 | 81.4 (11.1) | −3.9 (9.6) | 13 | 85.3.8 (9.2) | −2.6 (18.1) | 1.4 | −5.8 to 8.6 |
| MCID end point | 10 | 82.9 (7.9) | −7.1 (9.1) | 4 | 79.8 (11.1) | −7.4 (11.3) | −0.2 | −12.8 to 12.3 | |
| SF-36 (mean ± SD) | |||||||||
| Physical component | Relapse end point | 15 | 38.8 (10.5) | −7.2 (10.1) | 12 | 42.4 (8.6) | −8.0 (8.4) | 0.8 | −6.7 to 8.9 |
| MCID end point | 9 | 41.2 (9.3) | −10.9 (10.0) | 4 | 41.5 (11.3) | −8.8 (9.8) | −2.1 | −15.3 to 11.0 | |
| Mental component | Relapse end point | 15 | 50.9 (10.1) | 0.3 (8.4) | 12 | 47.2 (13.5) | −4.5 (16.6) | 4.8 | −5.3 to 14.9 |
| MCID end point | 9 | 51.4 (10.2) | −0.5 (5.8) | 4 | 44.7 (18.9) | −3.8 (23.0) | 3.2 | −34.2 to 40.7 | |
ALDS = AMC Linear Disability Score; FSS = Fatigue Severity Scale; INCAT-SS = INCAT Sensory Sum Score; PI-NRS = Pain Intensity Numeric Rating Scale; SF 36 = Short Form 36.
Patients who reached a relapse end point based on the MCID on the iRODS. Change of −1.9 logits and −1.3 logits on the iRODS correspond with −12.4 and −8.8 centile points, respectively. Difference in mean change score of 0.54 logits corresponds with 3.6 centile points.
Point estimate and 95% CI were analysed using an independent sample t-test according superiority analysis.
The within-group median change score was calculated as the 50th percentile of all individual differences.
Between-group difference on the MRC expressed in median difference in change scores; point estimate and 95% CI were analysed using the Hodges–Lehmann approach.
At the end of the study, 52 of 60 patients answered the question to which group they thought they were allocated. In the IVIg withdrawal group, 21 of 25 patients (84%) guessed they were in the IVIg withdrawal group and 4 of 25 (16%) thought that they were in the IVIg continuation group. In the IVIg continuation group, 19 of 27 patients (70%) guessed they were allocated to the IVIg withdrawal group and 8 of 27 (30%) to the IVIg continuation group. All patients who reached a relapse end point (23/23) guessed they were in the placebo group.
In the IVIg withdrawal group, 4 of 12 patients (33%) who remained stable thought they were allocated to the IVIg continuation group. One patient still had side effects and one patient still had wear-off symptoms. Two patients did not give a specific reason. Eight of 12 patients (67%) thought they were allocated to the IVIg withdrawal group. Three patients experienced an increase in CIDP symptoms, one patient had no side effects and one patient had fewer wear-off symptoms. Three patients did not give a specific reason.
Of the patients who completed the 24-week follow up, 9 of 17 (53%) patients in the IVIg group thought they were allocated to IVIg continuation group. Of these nine patients, four patients still had side effects and one patient still experienced wear-off symptoms. The other four patients did not write down a specific reason. Eight of 17 patients (47%) thought they were allocated to the IVIg withdrawal group. One patient noted an increase in CIDP symptoms, three patients had fewer side effects. Four patients did not give a specific reason.
Restabilization
Patients from the IVIg withdrawal group with a relapse end point during the trial phase entered the restabilization protocol. Overall, 16 of 17 (94%) restabilized within the restabilization phase of 12 weeks on iRODS and grip strength (Table 4). At 12 weeks, 14 of 15 (93%) restabilized on the PGIC scale and 15 of 17 (88%) according to the treating physician. After the loading dose, five patients were restarted on a lower IVIg dose, based on the second to last dose on which they were stable during the trial. All five patients relapsed again during the follow-up period.
Table 4.
Number of restabilized patients on different time points
| Week 3 | Week 6 | Week 12 | |
|---|---|---|---|
| iRODS | 15/17 (88%) | 15/17 (88%) | 16/17 (94%) |
| Grip strength | 12/17 (71%) | 14/17(82%) | 16/17 (94%) |
| PGIC scale | 9/15 (60%) | 13/16 (81%) | 14/15 (93%) |
| Restabilization according to physician | 13/16 (81%) | 14/17 (82%) | 15/17 (88%) |
Of the 13 patients who reached a relapse end point in the IVIg continuation group, two patients received a loading dose of IVIg (2 g/kg over 5 days), four patients received an extra IVIg dose and in seven patients their maintenance treatment was continued. During the extension phase, three patients needed a higher maintenance dose or shorter interval and in four patients the maintenance dose was not changed. In two patients the maintenance dose was lowered and in two patients the treatment was successfully stopped.
Extension phase
The 12 patients from the IVIg withdrawal group who remained stable during the trial phase entered the extension phase. Four patients relapsed during the additional 52-week follow-up, two of whom just prior to their final visit. Overall, 8 of 29 (28%) patients from the IVIg withdrawal group remained stable during the trial and extension phase (combined duration 76 weeks).
Discussion
We could not demonstrate non-inferiority of withdrawal of IVIg maintenance treatment compared to continuation of treatment in clinically stable CIDP patients, as our study turned out to be underpowered due to much larger than expected confidence intervals. We chose a non-inferiority design as this reflects best the clinical equipoise in patients who are stable on maintenance IVIg treatment. Efficacy of IVIg in CIDP in patients with active disease has been demonstrated in various trials and we expected that a proportion of patients would need ongoing treatment, as also demonstrated in this study. Our hypothesis was that many patients on chronic treatment do not have active disease requiring further treatment, leading to non-inferiority on disability level on a group level and that in these stable patients, withdrawing IVIg will not lead to deterioration in their daily functioning and permanent disability.
As expected, our findings confirm that many CIDP patients included in this trial required IVIg maintenance treatment. Nevertheless, our study also confirms that a large proportion of patients do not need IVIg maintenance treatment as 41% remained clinically stable at 24 weeks after IVIg withdrawal during the trial. Overall, 28% remained stable during 76 weeks after start of IVIg withdrawal. It might be possible that some of these patients experience a relapse after these 76 weeks as reported by Nobile-Orazio et al.16 However, the majority of relapse end points in both groups were reached within 12 weeks, as illustrated by Fig. 4. Also in other studies, including the larger FORCIDP trial and the ICE trial, a relapse in most patients occurred within the first months after treatment withdrawal.4,6 Together with other studies, this suggests that most IVIg-dependent patients can be identified within 3–6 months after IVIg withdrawal.3,16
If the main objective of an IVIg treatment withdrawal is to determine whether there is any disease activity, stopping treatment directly is probably the fastest way to determine IVIg dependency. Our experience is that many patients feel more comfortable with slower withdrawal rather than directly stopping treatment. This was the main reason to choose a three-step withdrawal schedule, in which the initial maintenance dose was lowered with 25% per infusion. Currently, there is no consensus on the optimal way to attempt IVIg withdrawals. This is the first study that uses the three-step withdrawal schedule, while in previous studies IVIg was often directly stopped. It is not clear whether IVIg-dependent patients deteriorate less severely or restabilize more quickly after tapering compared to directly stopping treatment. In the open-label IVIg dependency test of the PATH study, 11% of patients who stopped IVIg treatment and deteriorated could not be restabilized to their baseline disability score during a 12-week follow-up.17 In this study, all but one patient with a relapse end point in our withdrawal group were restabilized within 12 weeks. More importantly, a vast majority of patients were considered restabilized by 3 weeks on the iRODS. As we expected that not all patients with a relapse end point would reach their MCID on the iRODS, we also used grip strength and restabilization as perceived by the patient as well as the treating physician to assess restabilization. Similar proportions and speed of restabilization was seen when using these alternative scales. All patients were considered restabilized within 24 weeks. Therefore, the three-step withdrawal schedule in combination with a loading dose of IVIg, if a patient relapses, used in this study appears to be a safe method to assess IVIg dependency, but given the small numbers of patients and not universally accepted criteria of restabilization, we cannot confirm that complete restabilization will occur in all patients. In addition, although restabilization was fast in the majority of patients, occasionally restabilization can take more time in some patients which will probably also influence future withdrawal attempts.
There is no commonly accepted threshold of a relapse in IVIg withdrawal or IVIg substitution trials. Generally, in CIDP, disability scales are considered the most appropriate outcome scales for both improvement and deterioration, but some have advocated to include impairment scales to determine deterioration.18 This study was designed to limit deterioration as much as possible to prevent long-term disability. Therefore, we used a broader definition for a relapse end point, in which we allowed the judgement of the treating physicians as well as the patients, which probably also mirrors clinical practice. Not allowing severe relapses might explain why there was no difference on the secondary outcomes between both groups when focusing only on patients who reached a relapse end point. Unexpectedly, however, 42% of the IVIg continuation group also reached a relapse end point. Disease progression despite IVIg treatment is an unlikely cause of the high number of patients with a relapse end point, as all were considered to have stable disease at inclusion and because they were treated with the same IVIg brand, dose and interval during the trial. Fluctuations of symptoms might have contributed to this finding, especially when a longer follow-up period is performed. A minority of patients reported wear-off symptoms at the end of an IVIg cycle prior to the study that might have resulted in patients experiencing deterioration between infusions during the trial. However, wear-off symptoms were not associated with a relapse end point, nor were they captured by deterioration on the iRODS between baseline and the last pre-trial infusion. Interestingly, of the patients who reached a relapse end point, a smaller proportion of patients in the IVIg continuation group reached their MCID on the iRODS compared to the withdrawal group (38% versus 59%). Similarly, the proportion of patients with a relapse end point, whose end point was based on subjective deterioration as perceived by the patient, was higher in the IVIg continuation group (38% versus 18%). These findings emphasize the need to use validated clinical outcome measures, although further studies are needed to determine the clinically relevant differences on these scales. This is also illustrated by the fact that almost half of patients who reached a relapse end point in the IVIg continuation group reached commonly accepted MCID criteria for grip strength and MRC sum score.
Finally, as patients can be reluctant to undergo IVIg withdrawal because of fear of increase of symptoms or reduced functioning, the possibility of being randomized to placebo might have led to a nocebo effect in some of our patients. The nocebo effect refers to the phenomenon that negative expectations of patients have a negative effect on the outcome.19 A clear majority of patients in this study indicated at their final visit that they received placebo, including half of patients from the IVIg continuation group who remained stable during the trial period, both supporting this hypothesis of nocebo effect. This is in line with a recent systematic review that showed a considerable nocebo effect in CIDP trials, especially when using non-deterioration as the primary end point.20 Importantly, a nocebo effect in this study might also have led to a higher proportion of patients with a relapse end point in the withdrawal group and an underestimate of overtreatment. In addition, entering the trial might have been a negative trigger for patients to report worse than they normally would have (observation bias). Some patients might have been reluctant to take part in the trial, as suggested by the 30% of eligible patients that were not willing to participate in the study. This, together with the use of subjective outcome measures, might have skewed the results in both treatment groups towards more frequent deterioration.
This study had several limitations. First of all, the results of our primary outcome were inconclusive. We observed higher than expected standard deviations of the iRODS changes scores in both treatment groups, partly due to the unexpectedly high number of patients in the IVIG continuation group who reached a relapse end point. As this lead to an imprecise estimate, this means that our study turned out to be underpowered to address the primary question. Also, the non-inferiority margin was based on an earlier published average patient logit score of 0.35 on the iRODS and the MCID at this position of the scale.9 In our study, baseline scores in the IVIg withdrawal and continuation group were considerably higher. However, the non-inferiority margin of −0.65 logits remains valid, as the size of the individual MCID based on the expected average score was comparable to the individual MCID based on the (higher) average score found in this study. The non-inferiority margin might also be considered to be large and was based on what we believe is an acceptable clinically relevant deterioration, given the extremely high cost of IVIg, potential (severe) side effects of IVIG and the patient burden of treatment.
Furthermore, a total of 50% of patients reached a relapse end point during the trial, of which only half were captured by the predefined MCID on the iRODS. We believe that patients who experience minor deterioration consider this as clinically important when they are on a stable IVIg dose. For these reasons, the use of the patient-reported disability scale, such as the iRODS, as the only primary outcome measure might also be considered as a limitation in this trial. Combining the iRODS with impairment measurements, such as grip strength, could have made reported health changes more objective.21 On the other hand, a more stringent definition of a relapse end point would probably have led to less willingness of patients to participate in the study. More important, it would limit the external validity of the results, as we believe that the patient’s voice is often leading in the decision to restart IVIg.
The inclusion rate was slow, as over 30% of eligible patients refused to participate in the study. This may have resulted in a selection of patients, limiting external validity. The majority of these patients did not want to taper treatment, although some wanted to stop treatment directly or preferred slower tapering than used in the trial. Additionally, we only included patients who were previously stable. Withdrawal attempts in patients with unstable disease should generally be avoided as it is uncertain whether these patients can be restabilized as well as in our study population.
We did not have any missing data on the primary end points and no patients were lost to follow-up. Two patients were unblinded during the trial. Both patients completed the follow-up without experiencing a relapse end point and were included in the intention-to-treat analysis, but were excluded in the per-protocol analysis. Before deblinding, all patients who deteriorated (both treatment groups) guessed that they received placebo, while similar proportions of patients who remained stable guessed their treatment allocation correctly. For these reasons, we do not have reason to believe that blinding was not maintained in this study.
In conclusion, it remains inconclusive whether IVIg withdrawal is non-inferior compared to continuing treatment, partly due to much larger than expected confidence intervals, leading to an underpowered study. Despite these limitations, we found that a considerable proportion of CIDP patients could stop treatment. This study emphasizes that treatment withdrawal is safe and suggests that attempts should be performed regularly in clinically stable CIDP patients, preferably including objective measurements. In our experience, discussing withdrawal attempts with patients when starting IVIg prevents reluctance in patients when a withdrawal attempt is actually planned in the future. Until we identify biomarkers of disease activity that can identify patients in need of IVIg maintenance treatment, we should probably use at least one objective outcome measure instead of solely relying on patient-reported outcomes, both in trials as well as clinical practice. Alternatively, for future withdrawal studies, other approaches such as a causal interference design might be considered that allow studying the effect of an intervention with adjustment for different confounders without the need for randomization.22
Supplementary Material
Acknowledgements
We thank Janneke Zwiers and Mary Muijs from Sanquin Plasma Products B.V., the research nurses from Penthecilia B.V. and Margit Dros from Mediq Tefa for ensuring the blinding and distribution of the IMP. We also like to thank Michael Lunn, Joke Dijk and Brent Opmeer for participating in the Data Safety Monitoring Board.
Glossary
Abbreviations
- CIDP
chronic inflammatory demyelinating polyradiculoneuropathy
- IMP
investigational medicinal product
- iRODS
inflammatory Rasch-Overall Disability Scale
- IVIg
intravenous immunoglobulins
- MCID
minimally clinically important difference
- MRC
Medical Research Council sum score
- PGIC
patient global impression of change
Funding
The study was funded by a Dutch Governmental grant (ZonMw). Sanquin Plasma Products B.V. provided the placebo, preparation, blinding and distribution of the study treatment. The funders had no role in the trial design, data collection, data analysis, data interpretation, or the writing of the report.
Competing interests
F.E. reports grants from ZonMw (Dutch Governmental Agency), non-financial support from Sanquin Blood Supply, during the conduct of this study; he also reports grants from CSL Behring, Kedrion, Terumo BCT and Takeda Pharmaceutical Company, outside the submitted work. Grants were paid to institution and are used for investigator-initiated studies within INCbase, an international CIDP registry. In addition, he received consultancy/lecturing fee from UCB pharma and CSL Behring, paid to institution, outside the submitted work. I.v.S. reports grants from Dutch Governmental grant (ZonMw/Rational Pharmacotherapy program), non-financial support from Sanquin Plasma Products B.V., during the conduct of the study; other from CSL-Behring, outside the submitted work. P.v.D. reports grants from Takeda, during the conduct of the study; grants from Prinses Beatrix Spierfonds, grants from Sanquin Blood Supply, grants from Grifols, other from Hansa, Annexion, Argenx, CSL and Octapharma, outside the submitted work. I.M. reports personal fees and other from UCB, personal fees from Octapharma, outside the submitted work. K.K. reports grants from Baxalta, other from Sanquin, outside the submitted work. C.F. reports grants from European Union’s Horizon 2020 research and innovation programme Marie Sklodowska-Curie grant for PAIN-Net, Molecule-to-man pain network (grant no. 721841), grants from Prinses Beatrix Spierfonds, grants from Grifols and Lamepro for a trial on IVIg in small fibre neuropathy, other from steering committees/advisory board for studies in small fibre neuropathy of Biogen/Convergence and Vertex, outside the submitted work. L.W. reports grants from Grifols, outside the submitted work. H.S.G. reports grants from Prinses Beatrix Spierfonds, during the conduct of the study; grants and personal fees from Shire/Takeda, outside the submitted work. M.A., I.L., R.d.H., N.V., A.V., L.V., M.D. and N.N. have nothing to disclose.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Van den Bergh PY, Hadden RD, Bouche P, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral nerve society. Eur J Neurol. 2010;17(3):356–363. [DOI] [PubMed] [Google Scholar]
- 2. Eftimov F, Winer JB, Vermeulen M, de Haan R, van Schaik IN. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev 2013;(12):CD001797. [DOI] [PubMed] [Google Scholar]
- 3. van Schaik IN, Bril V, van Geloven N, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2018;17(1):35–46. [DOI] [PubMed] [Google Scholar]
- 4. Hughes RAC, Donofrio P, Bril V, et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): A randomised placebo-controlled trial. Lancet Neurol. 2008;7(2):136–144. [DOI] [PubMed] [Google Scholar]
- 5. Nobile-Orazio E, Cocito D, Jann S, et al. Intravenous immunoglobulin versus intravenous methylprednisolone for chronic inflammatory demyelinating polyradiculoneuropathy: A randomised controlled trial. Lancet Neurol. 2012;11(6):493–502. [DOI] [PubMed] [Google Scholar]
- 6. Hughes R, Dalakas MC, Merkies I, et al. Oral fingolimod for chronic inflammatory demyelinating polyradiculoneuropathy (FORCIDP Trial):A double-blind, multicentre, randomised controlled trial. Lancet Neurol. 2018;17(8):689–698. [DOI] [PubMed] [Google Scholar]
- 7. Gelinas D, Katz J, Nisbet P, England JD. Current practice patterns in CIDP: A cross-sectional survey of neurologists in the United States. J Neurol Sci. 2019;397:84–91. [DOI] [PubMed] [Google Scholar]
- 8. Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 9. van Nes SI, Vanhoutte EK, van Doorn PA, et al. Rasch-built Overall Disability Scale (R-ODS) for immune-mediated peripheral neuropathies. Neurology. 2011;76(4):337–345. [DOI] [PubMed] [Google Scholar]
- 10. Merkies ISJ, Schmitz PIM, van der Meché FGA, Van Doorn PA. Psychometric evaluation of a new sensory scale in immune-mediated polyneuropathies. Neurology. 2000;54(4):943–949. [DOI] [PubMed] [Google Scholar]
- 11. van Nes SI, Vanhoutte EK, Faber CG, Garssen M, van Doorn PA, Merkies IS. Improving fatigue assessment in immune-mediated neuropathies: The modified Rasch-built fatigue severity scale. J Peripher Nerv Syst. 2009;14(4):268–278. [DOI] [PubMed] [Google Scholar]
- 12. Holman R, Weisscher N, Glas CA, et al. The Academic Medical Center Linear Disability Score (ALDS) item bank: Item response theory analysis in a mixed patient population. Health Qual Life Outcomes. 2005;3:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263. [DOI] [PubMed] [Google Scholar]
- 14. Draak TH, Vanhoutte EK, van Nes SI, et al. Changing outcome in inflammatory neuropathies: Rasch-comparative responsiveness. Neurology. 2014;83(23):2124–2132. [DOI] [PubMed] [Google Scholar]
- 15. Hodges JL, Lehmann E. Estimates of location based on rank tests. Ann Math Statist. 1963;34:598–611. [Google Scholar]
- 16. Nobile-Orazio E, Cocito D, Jann S, et al. Frequency and time to relapse after discontinuing 6-month therapy with IVIg or pulsed methylprednisolone in CIDP. J Neurol Neurosurg Psychiatry. 2015;86(7):729–734. [DOI] [PubMed] [Google Scholar]
- 17. Mielke O, Bril V, Cornblath DR, et al. Restabilization treatment after intravenous immunoglobulin withdrawal in chronic inflammatory demyelinating polyneuropathy: Results from the pre-randomization phase of the Polyneuropathy and Treatment with Hizentra study. J Peripher Nerv Syst. 2019;24(1):72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Draak TH, Gorson KC, Vanhoutte EK, et al. Correlation of the patient’s reported outcome Inflammatory-RODS with an objective metric in immune-mediated neuropathies. Eur J Neurol. 2016;23(7):1248–1253. [DOI] [PubMed] [Google Scholar]
- 19. Wojtukiewicz MZ, Politynska B, Skalij P, Tokajuk P, Wojtukiewicz AM, Honn KV. It is not just the drugs that matter: The nocebo effect. Cancer Metastasis Rev. 2019;38(1–2):315–326. [DOI] [PubMed] [Google Scholar]
- 20. Lewis RA, Cornblath DR, Hartung HP, et al. Placebo effect in chronic inflammatory demyelinating polyneuropathy: The PATH study and a systematic review. J Peripher Nerv Syst. 2020;25(3):230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vanhoutte EK, Latov N, Deng C, et al. Vigorimeter grip strength in CIDP: A responsive tool that rapidly measures the effect of IVIG—the ICE study. Eur J Neurol. 2013;20(5):748–755. [DOI] [PubMed] [Google Scholar]
- 22. Keil AP, Edwards JK, Richardson DB, Naimi AI, Cole SR. The parametric g-formula for time-to-event data: Intuition and a worked example. Epidemiology. 2014;25(6):889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The corresponding author (F.E.) has full access to the study protocol and all the data in the study. Data are available upon reasonable request.