Abstract
The claustrum is the most densely interconnected region in the human brain. Despite the accumulating data from clinical and experimental studies, the functional role of the claustrum remains unknown. Here, we systematically review claustrum lesion studies and discuss their functional implications. Claustral lesions are associated with an array of signs and symptoms, including changes in cognitive, perceptual and motor abilities; electrical activity; mental state; and sleep. The wide range of symptoms observed following claustral lesions do not provide compelling evidence to support prominent current theories of claustrum function such as multisensory integration or salience computation. Conversely, the lesions studies support the hypothesis that the claustrum regulates cortical excitability. We argue that the claustrum is connected to, or part of, multiple brain networks that perform both fundamental and higher cognitive functions. As a multifunctional node in numerous networks, this may explain the manifold effects of claustrum damage on brain and behaviour.
Keywords: claustrum, lesion, perception, salience, sleep, pain
The claustrum is the most densely interconnected region in the human brain. By reviewing the literature on human lesions of the claustrum and linking it to the recent explosion in animal research, Atilgan et al. argue that the claustrum contributes to perception, pain, sleep and salience via its integral roles in different networks.
Introduction
The claustrum is a sheet-like bilateral brain region tucked beneath the insular cortex (Fig. 1). Extensive anatomical work across different species, including humans, monkeys, rodents, reptiles, rabbits, and cats, indicates widespread connectivity between the claustrum and the neocortex,1–15 and highlights the need for a more thorough investigation of the claustrum’s circuitry. Strong reciprocal connectivity with most neocortical areas is perhaps the most noteworthy feature of the claustrum,2,7,9,10,14–16 which has the highest connectivity in the human brain by regional volume.17 Recent work has shown corticoclaustral and claustrocortical connections evoke feedforward inhibition in mice,6,18 similar to previous results in cats.19–22 While the anatomy, physiology, and putative functions of the claustrum have been reviewed elsewhere,4,16,23–27 this review seeks to untangle and shed new light on the functional role of the claustrum.
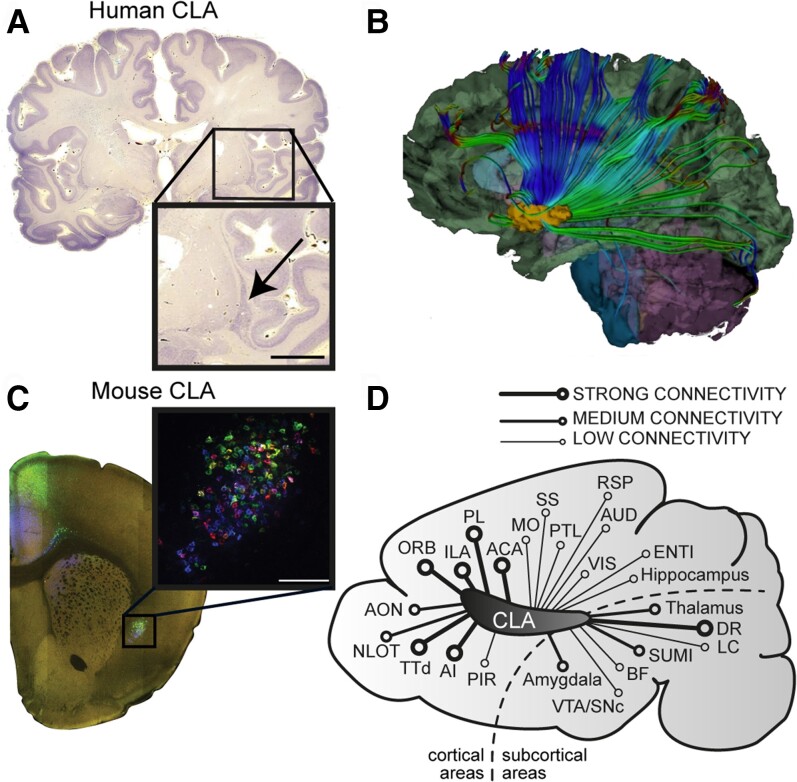
Figure 1.
The location and connectivity of the claustrum. (A) Nissl stained human coronal brain section with inset showing the location of the claustrum. (B) White matter tractography image showing outgoing connections from the claustrum reprinted from Torgerson et al.17 with permission from John Wiley and Sons ©2015. (C) Mouse coronal brain section with inset showing the claustrum labelled by multi-colour retrograde tracers. (D) Schematic illustration of a sagittal mouse brain section showing the relative connectivity strength between the claustrum and other brain regions. Connectivity strength was assessed based on the data provided in Zingg et al.15 and Wang et al.12 Most but not all connections are reciprocal (see reviews Mathur26 and Jackson et al.25). ACA = anterior cingulate area; AI = anterior insular cortex; Aud = auditory cortex; AON = anterior olfactory nucleus; BF = basal forebrain; CLA = claustrum; DR = dorsal raphe; ENTI = entorhinal cortex; ILA = infralimbic area; LC = locus coeruleus; MO = motor cortex; LOT = the nucleus of the lateral olfactory tract; ORB = orbital area; PL = prelimbic area; PTL = parietal cortex; PIR = piriform area; RSP = retrosplenial cortex; SUMI = supramammillary; SS = somatosensory cortex; TTd = dorsal taenia tecta; VTA/SNc = ventral tegmental area/substantia nigra; VIS = visual cortex.
Understanding the anatomy and connectivity of a brain region can provide important insights into its function. Attempts to find classic structure-function relationships are challenging in highly connected brain regions where many functions are possible due to multiple co-existing pathways. In the case of the claustrum, such widespread connectivity has led to a plethora of hypotheses regarding its function. The claustrum has been implicated in processes including the amplification of cortical oscillations,28,29 attentional allocation,2,4,26,30 consciousness,16,31 cognition,6 spatial navigation,32 the transfer of information across sensory modalities,33 sexual function,34 and aesthetic judgement.35
In addition to investigating its anatomy and connectivity, examining the consequences of damage to a brain region may provide complementary insights into its function. The lesion approach, including both acute and chronic interventions, has been immensely powerful in localizing language, episodic memory, and even aspects of executive function, in both human and animal studies.36,37 While imperfect, loss-of-function approaches can enable subsequent neural activity recordings to focus on stimuli or behaviours implicated by the lost functions. Moreover, lesion studies can help reveal a region’s functional connectivity. However, the claustrum’s contorted shape and bilateral location deep in the brain renders this approach difficult, though some studies have attempted to lesion the claustrum in animals.38–40
While the claustrum’s anatomy renders lesion studies challenging, examples of claustrum lesions exist within the literature. To our knowledge no review has comprehensively assessed clinical studies of human claustrum lesions. In this review, we present a meta-analysis of all studies to date that describe cases of claustrum lesions in human patients. We show that claustrum lesions lead to a wide range of signs and symptoms. Most commonly, patients experienced impairments in different levels of cognitive, perceptual, and motor abilities, as well as seizures and disturbances to mental state and sleep. Using these findings, in conjunction with animal studies, we consolidate evidence concerning the claustrum’s role in sensory perception, sleep, pain, and salience, and facilitate a critical re-evaluation of existing hypotheses of the claustrum’s function. Our results do not support prominent hypothesized functions such as multisensory integration and salience processing, but may instead provide evidence for a role in regulating cortical excitability. We hypothesize that the claustrum is connected to, or is a part of, many different networks, and propose that the brain-wide connectivity of the claustrum may have provided a useful scaffold onto which many functional roles could subsequently be built. This may explain the apparent role of the claustrum in fundamental functions such as sleep, as well as in more complex functions such as attention and salience. We argue that the claustrum is likely a multifunctional area, or perhaps possesses a global function to regulate neural activity dynamics across the cerebral mantle. Under this schema, damage to the claustrum would disrupt diverse neurological and behavioural processes.
Diverse findings following lesion or stimulation of the human claustrum
Literature search
The potential functions of the claustrum have not been comprehensively analysed by examining human lesion studies because of the relative scarcity of claustral lesions (the only reported work focused specifically on delusional states).27 To tackle this, we examined claustrum lesion cases by searching the following terms on PubMed and Scopus: ‘claustrum AND (lesion OR contusion OR injury OR trauma)’ (n = 103). Studies were then screened for cases in which reported lesions included the claustrum according to neuroimaging. Thirty-eight individual cases (Supplementary Tables 1 and 2) and 14 cohort studies (Supplementary Table 3) were included in this review. Details of these studies can be found in the Supplementary material.
Specific claustrum lesions
Due to the claustrum’s nestled location, vascularization, and morphology, isolated claustrum lesions are rare, and predominantly occur in the spectrum of autoimmune encephalitis. Unfortunately, available clinical imaging techniques lack sufficient resolution to conclusively resolve the claustrum from the external capsule. Studies that reported lesions to the claustrum and external capsule (Fig. 2B), the external capsule only (Fig. 2C), and the claustrum only (Fig. 2D) reveal strikingly similar imaging results. Given this diversity in the reporting of lesions, it is difficult to distinguish truly claustrum specific lesions from those which may impact both the claustrum and adjacent white matter. Disambiguating what caused the lesion can help. For example, autoimmune encephalitis might be caused by anti-neuronal surface antibodies that have a predilection to epitopes on the claustrum, such as voltage-gated potassium channels antibodies and anti-glutamic acid decarboxylase antibody.41 On the other hand, pathologies that affect white matter such as vasogenic oedema and demyelination might have a predilection for external and extreme capsules. Thus, the diagnosis offers an important clue to claustrum involvement, despite the imaging in these cases being identical. Nonetheless, the lack of isolated claustrum lesions means lesion studies are inherently problematic. It is therefore difficult to attribute a particular functional role to the claustrum based on lesions alone.
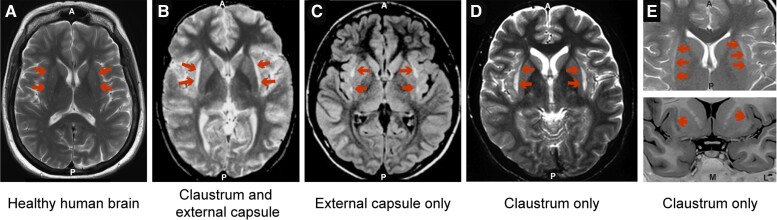
Figure 2.
Similar lesions have been described as affecting either the claustrum, the external capsule, or both. (A) Representative T2-weighted image of a healthy human brain. (B) T2-weighted image showing a lesion reported as affecting the claustrum and external capsule. Reprinted from Sperner et al.45 with permission from Springer Nature ©1996. (C) Fluid-attenuated inversion recovery (FLAIR) image showing a lesion reported as affecting the external capsule. Reprinted from Mumoli et al.43 with permission from Springer Nature ©2014. (D) T2-weighted image showing a lesion reported as affecting the claustrum. Reprinted from Ishii et al.42 with permission from American Society of Neuroradiology ©2011. (E) T2-weighted image (top) and T1-weighted image (bottom) showing a lesion reported as affecting the claustrum. Reprinted from Silva et al.44 with permission from Springer Nature ©2018.
Despite this difficulty, seven case studies have been identified which report relatively exclusive lesions in the claustrum with or without involvement of the external capsule (Supplementary Table 1). Four of these patients presented with status epilepticus and were found to have bilateral lesions affecting the claustrum and external capsule. The four patients also had EEG abnormalities along with a variety of other symptoms including seizures, motor impairment, cognitive impairment, psychotic symptoms, and tremor.42–45 Another patient with abnormalities in the right claustrum and bilateral external capsule experienced non-epileptiform EEG abnormalities, along with motor and cognitive impairment.46 A 55-year-old patient with an isolated left claustrum lesion following an ischaemic stroke experienced unilateral paraesthesia of the face, tongue, and head—similar to a lacunar syndrome—along with polymodal gustatory, auditory, vestibular, and visual disturbances.47 Recently, a patient admitted to hospital with Covid-19 showed bilateral lesions to the claustrum and external capsule. He presented with aggression, cognitive impairment, disorientation, and stupor. Upon follow-up, the claustrum lesions remained despite a complete neuropsychiatric recovery.48 For further details of these cases, see Supplementary Table 1.
While the underlying cause of the claustrum damage was not always clear, the various authors suspected a range of aetiologies including viral encephalitis, ischaemic stroke, and seizure induced damage. Despite the similar and isolated brain abnormalities, the patients presented with a wide and inconsistent array of signs and symptoms. The effects reported in these diverse cases are difficult to interpret. They do not offer strong support to any single hypothesis of the claustrum’s function. Conversely, these results suggest that the claustrum may instead have multiple roles, or an, as yet, unidentified global role that impacts multiple behavioural domains. However, further evidence is required to draw any conclusions.
Non-specific claustrum lesions
Beyond these studies, many others have reported lesions to the claustrum in conjunction with other brain regions (Supplementary Table 2). Other areas commonly included the external capsule (14/38) and insula (12/38), between which the claustrum is interposed, and the hippocampus (9/38). In particular, presumed viral and non-viral encephalitis may cause relatively claustrum-specific changes on MRI neuroimaging, sometimes in conjunction with hippocampal damage. Like the studies with isolated claustrum lesions, these less specific lesion studies also reported a wide variety of symptoms (Table 1). The most common signs and symptoms in individual cases were nonspecific cognitive impairment (19/38), seizures (18/38), motor impairment (17/38), visual disturbances (9/38), non-seizure EEG abnormalities (8/38), loss of consciousness (8/38), speech problems (8/38), auditory disturbances (6/38), sleep disturbances (6/38), delusions (5/38), tremor (5/38), and hallucinations (4/38). We separated these observations into the following four categories: disruption in cognitive, perceptual and motor abilities; changes in electrical activity; changes in mental state; and sleep disturbance.
Table 1.
Lesioned area, symptoms and seizures reported in case studies with claustrum lesions
| Hemispheric distribution | Bilateral | 22 |
| Unilateral (Right/Left/Undefined) | 16 (9/5/2) | |
|---|---|---|
| Extent of lesion | Claustrum only | 7 |
| External capsule | 14 | |
| Insula | 12 | |
| Hippocampus | 9 | |
| Other cortices | 17 | |
| Other subcortices | 22 | |
| Signs and symptoms | Cognitive, perceptual and motor abilities | |
| Cognitive impairment | 19 (50%) | |
| Motor disturbance | 17 (45%) | |
| Visual disturbances | 9 (24%) | |
| Speech disturbances | 8 (21%) | |
| Auditory disturbances | 6 (16%) | |
| Tremor | 5 (13%) | |
| Paraesthesia | 5 (13%) | |
| Electrical activity disturbance | ||
| Seizures | 18 (47%) | |
| Non-seizure EEG abnormalities | 8 (21%) | |
| Mental state | ||
| Loss of consciousness | 8 (21%) | |
| Hallucinations | 4 (11%) | |
| Delusions (Cotard delusion) | 5 (2) (13%) | |
| Sleep disturbances | 6 (16%) | |
| Seizure type (patients may fit >1 severities) | 1) Partial | 7 (18%) |
| 2) Generalized | 15 (39%) | |
| 3) Status epilepticus | 13 (34%) | |
| 4) Refractory status epilepticus | 10 (26%) |
Claustral lesions impair cognitive, perceptual and motor abilities
Patients with claustrum lesions were frequently reported to have perceptual and cognitive impairments. In many cases, the lesions impacted patients’ ability to perceive sensory input in various ways. For example, a 12-year-old experienced temporary loss of vision and hearing.45 Interestingly, another young child experienced permanent bilateral blindness due to damage to the left calcarine cortex and right claustrum, although the most parsimonious explanation for this finding is undetected damage to the right optic radiation.49 In some cases, lower perceptual sensitivity,50 and tinnitus51 were reported. Somatosensory deficits have also been reported.47,52 Cases presumed to be driven by encephalitis often presented with deficits in episodic memory,46,53,54 working memory, and reasoning or with disorientation in time.52 Claustrum damage has even been described as part of neurodegenerative syndromes such as corticobasal degeneration, where it may be accompanied by a disruption in visual attention functions including spatial orientation, optic ataxia,55 and cognitive decline in Lewy body dementia.56 Left claustrum damage could lead to aphasia when accompanied by left putamen damage.57 Despite the varied pathologies, lesion sites, and inconsistent observations across studies, each patient experienced disturbance to some aspect of their cognitive and perceptual abilities. Claustrum lesions led to a wide variety of sensory deficits, suggesting the claustrum may play a functional role in one or more aspects of sensory processing. Notably, no evidence for specific deficits in multisensory integration was reported.
Claustrum lesions result in an increased incidence of electrical disturbances
Patients with claustral lesions often experienced disturbances to the electrical activity of the brain, involving generalized seizures (15/38) and status epilepticus (5 min of constant seizures without any return to consciousness in between; 13/38). Generalized seizures were more common when the claustrum was damaged bilaterally (10/15 studies). Several studies45,51 reported that patients with claustral lesions developed recurrent generalized seizures, with interictal slowing (3–4 Hz) on EEG. Patients who also had claustrum-associated focal seizures reported experiencing delusions and altered mental state (discussed below), which was coupled with pathological EEG slow-wave activity.58 Unfortunately, the majority of the case studies reporting generalized seizures do not report EEG recordings. The studies that did include EEG recordings typically reported generalized slow-wave activity present even in the awake state. A few of these also described generalized spikes or sharp waves.45,59
Strikingly, several patients with claustrum lesions exhibited a form of severe epilepsy (10/38) known as refractory status epilepticus. Refractory status epilepticus is a subset of these cases that fail to respond to the two frontline pharmacological treatments for seizures (benzodiazepines and one anticonvulsant).60 Studies examining new-onset refractory status epilepticus reported lesions in the claustrum as a common pathology, concomitant with slow-wave activity, spikes and sharp waves, and periodic lateralized epileptiform discharges in EEG recordings.61–63 Taken together, these studies suggest that claustrum dysfunction increases global synchronization of the electrical activity of the brain, and potentially leads to seizures.64 This is consistent with a putative role for the claustrum in regulating cortical excitability.
Claustral lesions disturb mental state
Several studies noted that medicated and unmedicated patients with claustrum lesions experienced altered mental states, which can be defined as general changes in brain function characterized by confusion, disorientation and disordered perceptions.65 These included non-seizure loss of consciousness, confusion, stupor, delusions, hallucinations, as well as other mild fluctuations in mental state. Loss of consciousness, defined here as an interruption in one’s awareness of the self and the environment, was reported in 8 of 38 case studies. Similarly, a study of combat veterans with penetrating traumatic brain injuries reported that patients with lesions to the claustrum showed an increased duration but not frequency of loss of consciousness relative to patients with lesions in other locations.66 Recent lesion mapping has uncovered a distributed circuit associated with loss of consciousness, which peaks in the bilateral claustrum.67 These findings suggest that the claustrum may be necessary to support some aspects of maintaining one’s level of consciousness. An alternative explanation is that these processes generate ‘selfhood’, the mental representation of a distinct first-person capable of agency, a role which has been associated with the nearby anterior insula68 but might be extended to parts of the claustrum.
Altered mental states were reported across many claustrum lesion case studies. While some patients manifested broadly altered mental states including anhedonia, delirium, or confusion,61,63 some had narrower neuropsychiatric features such as delusions with paranoid or religious content,58,69 visual hallucinations, and abulia.70 One patient with claustral dysfunction attributed to COVID-19 exhibited ‘delirious behavior’.48 Even more specific features included delusions of jealousy.71 The high frequency of visual and auditory hallucinations, confusion, and delusional states has previously been identified and discussed at length by Patru and Reser.27 They argue persuasively that the claustrum is involved in the process of generating and filtering delusional beliefs (described below).
Intriguingly, claustrum dysfunction may also be linked to Cotard delusion. Patients experiencing Cotard delusion may believe that they are dead, do not exist, are decaying, or have lost internal organs.72 Intriguingly, two patients described above with claustrum lesions experienced symptoms consistent with Cotard delusion.69,73 As only a few hundred instances of Cotard delusions are known worldwide, the finding that one confirmed case of Cotard delusion and another patient with highly overlapping symptoms arose from pathology involving the claustrum is highly suggestive of an association and perhaps a disconnection with ‘self’. Although most reported cases of Cotard delusion do not include claustrum lesions74–76 the claustrum was the only area of overlap between the two cases described. These examples of altered mental states following claustrum lesions suggest that the claustrum may be required to modulate complex cognitive representations such as beliefs and desires.
Claustral lesions disturb sleep
Sleep disturbances were commonly observed in patients with claustrum lesions, usually with other symptoms present. Some studies reported hypersomnia along with altered mental state63,70 while others reported restlessness along with motor disturbance.56 It is unclear whether these sleep disturbances occurred as a direct result of the claustrum lesions themselves, patients’ medications, or damage to other brain structures. Additionally, these studies did not assess the polysomnographic features of the sleep disturbances or provide a systematic investigation of sleep. No details were reported about whether patients had difficulty initiating and/or maintaining sleep. Recently, a genome-wide analysis suggested a link between insomnia and a disruption in claustrum-hypothalamus-striatum circuitry,77 pointing to the role of these areas in sleep initiation. Another mechanism by which circuit-level abnormalities might affect a patient’s sleep-wake cycle is through a disturbance in waking resting state functional connectivity in the default mode network.78 Although evidence points to an important functional role of the claustrum in sleep control, lesion studies are too limited to provide conclusive evidence.
Claustral lesions can result in painful sensations
Multiple case studies included patients who reported painful sensations.61,79–82 These sensations varied in character and severity. In the 1950s, two separate studies described patients with unilateral lesions to the claustrum, among other regions, who experienced hyperalgesia to hot and/or cold in the contralateral upper limb, together with hyperaesthesia, depression, and suicidal ideation.80,82 Pain with hypoaesthesia has also been reported.81 Other cases have described dynamic changes, such as painful cramping beginning in the contralateral hand, with Jacksonian march to the whole of the left side,79 or discomfort with spreading paraesthesia,47 suggesting that pain could relate to heightened excitability rather than neural loss.
Despite these positive findings, caution must be exercised as many painful sensations might more parsimoniously be attributed to causes other than claustral lesions (e.g. myalgia to fever in Choi et al.61). Claustrum stimulation has also induced pain, with a participant reporting painful sensations ‘like a knife jabbing’ and of ‘burning’ during claustrum stimulation.83 This is noteworthy as brain stimulation rarely produces painful sensations, although when it does it has often been reported following stimulation of a region surrounding the posterior insula and claustrum.84 While these are isolated examples, numerous studies have hinted at a connection between the claustrum and pain. However, this possibility has received comparatively less attention than other brain areas.
The lesion studies were inconsistent in their reporting of the neurological examination, and most reported just whether sensation was intact. The lack of any specific mention of pain may reflect an incomplete report, rather than constituting evidence that pain was unaffected. However, it does seem clear that there is no obvious neuropathic pain syndrome associated specifically with claustrum lesions, perhaps contrasting with the more distinct sensory syndromes associated with insula damage.85 Future studies should specifically test pain in such focal cases, ideally using quantitative methods.
Human claustrum stimulation
Like lesion studies, brain stimulation studies can provide strong evidence linking a given region to specific functional roles. The usefulness of stimulation studies is, however, limited by the possibility that observed effects result from excitation or inhibition of brain regions downstream of the region being stimulated.86 This caveat is particularly noteworthy when considering the claustrum given its widespread connectivity throughout the brain. Additionally, due to the small volume of the claustrum, stimulation will almost certainly result in depolarization of neurons in nearby tissue.
A recent study by Bickel & Parvizi83 obtained claustral stimulation data from five patients with pharmacologically resistant epilepsy, whose implanted electrodes partially overlapped with the claustrum. Four of the five patients experienced changes in somatosensation including ‘a strange skin sensation from the face running down to the leg’; ‘a sense of pressure in the mouth’; ‘sensation around the left knee’; ‘hot flashes’; and ‘acid-like sensation in the throat’. However, none of them reported changes in their awareness of the situation and their surroundings. In 2014, Koubeissi et al.31 reported that a patient with intractable epilepsy suffered a reversible disruption of consciousness after an electrode near the claustrum was simulated. However, a few caveats apply to this finding. First, the electrode was located in the extreme capsule, near, but not within the claustrum. Second, the patient had previously undergone an ipsilateral temporal lobectomy which may have functional implications. Third, the current applied by Koubeissi et al.31 was greater in both magnitude and duration than in the Bickel and Parvizi83 study. This may have caused widespread blanket depolarization of claustrocortical neurons, producing cortical inhibition,6 that subsequently disrupted normal information processes.
The signs and symptoms of claustrum dysfunction caused by claustrum lesions and claustrum stimulation vary based on the pathologies and lesion/stimulation sites. Each patient experienced some aspect of disturbance in cognitive function, perceptual abilities, mental state, electrical activity, or sleep. This great diversity of signs and symptoms suggests a structure with a global role affecting diverse cognitive processes. Remarkably, and in stark contrast to this conclusion, patients with well-delineated surgical removal of unilateral claustra for low-grade cerebral glioma had no observed long-term sensorimotor or cognitive impairment.87 The functional role of the unilateral claustrum might be efficiently compensated by neural plasticity of the intact claustrum, explaining why these patients did not suffer any permanent cognitive-perceptual impairments. These studies suggest that due to its involvement in multiple functionally compensated dynamic neural networks the claustrum is involved in numerous neural processes including both high-level cognitive processes as well as more fundamental processes. Taken together, these lesion and stimulation studies highlight the need to critically re-evaluate existing theories of the claustrum’s function.
Re-evaluating the function of the claustrum
The results described above present the most comprehensive survey of claustrum lesions to date. Paradoxically, the most consistent feature of these claustrum lesion studies is their inconsistency. The diverse and overlapping array of clinical pathologies reported in these lesion and stimulation studies suggests that the claustrum may have a far-reaching role. The task of attempting to draw functional conclusions from this rich and varied data is further complicated by limited and inconsistent investigational practices across human studies.
In the sections below we will look more deeply into certain functional hypotheses in light of the lesion studies and propose avenues for future exploration. We will briefly evaluate the claustrum’s involvement in neural correlates of sensory perception and salience as two prominent theories of the claustrum’s function. We then review the claustrum’s involvement in sleep and pain based on recent physiological studies.
Sensory processing
Lesion studies detailing sensory and perceptual abnormalities are suggestive of a functional role for the claustrum in sensory processing. These observations are consistent with anatomical and physiological findings in basic research. Connectivity studies have shown strong claustrocortical projections to sensory cortices and minimal corticoclaustral input from primary sensory areas.12,15,88 Physiological studies have revealed somatotopic responses, retinotopic responses, and direct responses to auditory stimuli within the claustrum.89–91 This anatomical connectivity, when considered in conjunction with single neuron recordings, led to the hypothesis that the claustrum is well positioned to play a key role in multisensory integration.16,28 However, individual claustrum neurons have been shown to respond predominantly to one sensory modality92 without any preference for the properties of the stimulus (i.e. no differences between vocalizations versus environmental sounds93).
These contrary findings might be partially resolved by data showing that claustrum neurons responded to changes in the sensory input93 and responded to the novelty of stimuli pairs.94 This hints that the claustrum does not encode the physical properties of the sensory stimuli but may instead shape sensory perception. This is supported by an early animal study showing that a claustrum lesion impacted the way primary visual cortex encoded a visual feature,95 and by more recent theories that the claustrum may reduce noise in sensory cortices.2,25
The link to sensory processing is suggested by several lesion patients experiencing hallucinations and other delusions (as reviewed in-depth by Patru and Reser27). In that review, the authors argue that the claustrum is poised to act as a common centre for delusional states. They theorize how the claustrum may be involved in common models of delusion such as the glutamate/dopamine model, Κ-opioid receptor model, and models based on deficits in attention, sensory-gating, salience processing, and prediction. It is, however, important to note that these claustrum-related mechanisms of delusion may be directly related to the sensory abnormalities described in the lesion section.
As reported above, claustrum lesions can lead to sensory effects in multiple sensory domains, suggesting that the claustrum may be involved in the modulation of sensory processing. While it is possible that the claustrum plays a role in generating sensory perception, physiological evidence more strongly suggests a nuanced role in shaping the transition from raw sensory input to perceived output as reviewed elsewhere in detail.26
Salience
Recent studies performed in animals have also suggested a role for the claustrum in salience processing. Salience is the property of a stimulus that makes it noticeable. For example, a sudden loud sound carries perceptual salience, whereas the smell of food has motivational salience for a hungry organism. Put another way, salience can be seen as a process of attributing importance to information, which in turn controls information flow in the brain.
Findings from recent connectivity and physiology studies in animal models support the idea that the claustrum may be part of the ‘salience network.’6,96 The salience network is an operationally-defined group of brain regions including the anterior cingulate cortex, insula, amygdala, hypothalamus, ventral striatum, thalamus, and specific brainstem nuclei.97–99 Many of these regions are closely connected with the claustrum (Fig. 3A and B). Moreover, the claustrum also receives strong input from regions linked to aversive and rewarding salience through its connections with the amygdala and dopaminergic system, respectively.100,101 Recent physiological evidence from rodent models also aligns with these connectivity studies. Firstly, claustrum neurons have been reported to respond to salient sensory stimuli.23,93 Secondly, inhibition of the claustrum decreased performance on a forced-choice task only in the presence of a distractor2 and regulated the behavioural sensitization to rewarding salient stimuli.102 Finally, in a classical conditioning paradigm, claustrum responses most closely correlated with the perceptual salience (which was produced by novelty and unexpectedness) of conditioned and unconditioned stimulus associations.94 Based on these and other studies, researchers have proposed a putative role for the claustrum in salience processing.
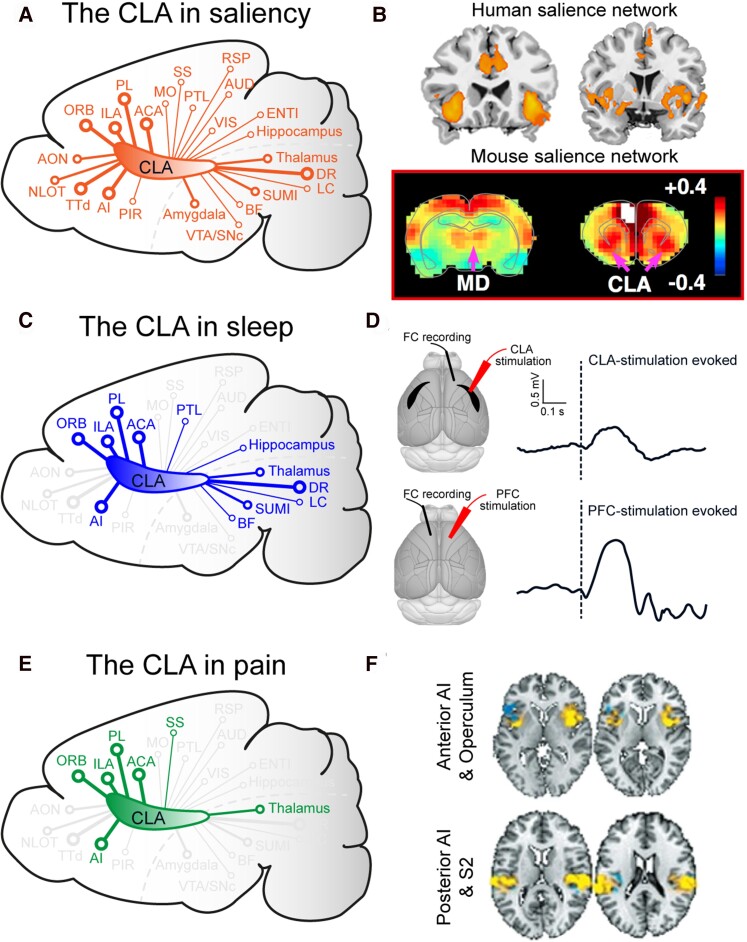
Figure 3.
The claustrum is connected with or part of multiple brain networks. (A) Schematic illustration of a sagittal mouse brain section highlighting the connections between the claustrum (CLA) and regions important in salience. (B) Representative functional MRI images showing the human and rodent salience networks adapted from Seeley et al.98 and Smith et al.,96 respectively (© Society for Neuroscience). (C) Schematic illustration of a sagittal mouse brain section highlighting the connections between the claustrum and regions important in sleep. (D) Claustrum and prefrontal cortex (PFC) stimulation both evoke slow-wave like activity. Reprinted from Narikiyo et al.7 and Vyazovskiy et al.,122 respectively, with permission from Springer Nature ©2020. (E) Schematic illustration of a sagittal mouse brain section highlighting the connections between the claustrum and regions important in pain processing. (F) Brain activity to physical pain using multi-study data and machine learning classification tools. Images kindly provided by Tor Wager. FC = frontal cortex; MD = mediodorsal thalamus.
While much evidence suggests a link, it remains unclear how the claustrum contributes to salience processing. One hypothesis suggests that the salience network is involved with switching between the default mode network and the central executive network.97,99 This switch allows the brain to deploy the cognitive resources necessary to engage with salient stimuli. Functional MRI studies have suggested that the dorsal anterior insula is linked with this network switch.97 However, it is possible that this process may also involve the claustrum. Furthermore, given the connectivity between the claustrum and default mode network, and the inhibitory effect of claustrum stimulation on the cortex, the claustrum is well placed to inhibit the default mode network in response to salient stimuli.6
The claustrum is suggested to contribute to salience processing by acting as a coincidence detector. Paired recordings within the claustrum have revealed dense electrical and chemical synapses between interneurons and a relative paucity of excitatory-excitatory connections.18 Stimulation of corticoclaustral projections reliably evokes feed-forward inhibition in claustrocortical projection neurons.18 This may explain the bimodal activity characteristic of claustrum neuron responses to cortical activation in the presence of feedforward inhibition. As such, strong input within a short temporal window is required to induce suprathreshold responses in the claustrum.103 By responding to coincident input from sensory, limbic, executive104 and neuromodulatory areas, this coincidence detection architecture could allow the claustrum to signal the salience of stimuli activating multiple neural circuits simultaneously.29
On the other hand, the lesion studies discussed above do not support a role for the claustrum in salience. A common sign for the loss of salience in one’s mental representation of the environment is hemispatial neglect.105 Hemispatial neglect is a common neuropsychological syndrome, in which patients fail to detect stimuli located contralaterally to a focal hemispheric lesion, even in the absence of primary sensory or motor deficits.106 None of unilateral claustrum lesion studies reported spatial hemispatial neglect. Furthermore, claustrocortical projections do not align well with the salience network based on cortico-striatal-thalamic loops. The strong ipsilateral claustrocortical projections do not match with the strong interhemispheric projections between cortical and subcortical areas posited as crucial due to hypotheses about the salience network.107 In addition, corticoclaustral projections provide the dominant input to the claustrum (84% of claustrum input originates in the cortex15), but salience processing requires substantial non-cortical involvement. While supporting evidence continues to accumulate, questions remain as to whether the claustrum has a role in salience processing.
Sleep
Many of the lesion studies described above include reports of sleep disturbances. While the studies included limited details of these disruptions, recent developments from animal research support the hypothesis that the claustrum may be involved in sleep (for a more general review of sleep, see Brown et al.,108 Liu and Dan,109 Saper and Fuller110 and Vyazovskiy and Harris111).
The broad anatomical connectivity of the claustrum makes it well placed to play a role in brain-wide processes such as sleep. Sleep is regulated by numerous brain regions, many of which are strongly connected to the claustrum, including those that are highlighted in Fig. 3C. Of particular relevance to sleep are the enriched serotonin receptors in the claustrum112–115 and extensive unidirectional inputs from serotonergic raphe neurons,7,15 which are involved in sleep-wake control.116 Additionally, the claustrum’s abundant reciprocal connections with the prefrontal cortex, insula, and cingulate cortex15—where slow waves can originate117–119—make the claustrum a promising candidate for regulating slow waves. These anatomical connections and physiological findings appear to support a role for the claustrum in sleep-related phenomena. Indeed, the claustrum shows increased expression of markers of neural activity following REM sleep hypersomnia, particularly in claustral neurons connected to frontal cortices such as anterior cingulate cortex.120
A recent study by Narikiyo et al.7 causally linked the claustrum to slow-wave activity. The authors found that stimulation of the claustrum during slow-wave sleep induced a slow wave-like down-state in the cortex followed by a synchronized transition to an up-state (Fig. 3D). In keeping with this, claustrocortical neurons were more active in slow-wave sleep, firing maximally just before and after the up-state of slow waves. Finally, slow-wave activity was reduced by genetic ablation of the claustrum. While this provides a compelling link between the claustrum and sleep, it is important to note that stimulation of the frontal cortex and thalamus121,122 as well as peripheral and transcranial magnetic stimulation123 have both been reported to elicit similar slow-wave activity (Fig. 3D). As such, further research is required to establish a unique role for the claustrum in slow-wave sleep.
Another recent study by Norimoto et al.115 linked the claustrum to other aspects of sleep. The authors identified a reptilian homologue of the claustrum that was important in the generation of sharp-wave ripples. These physiological events are composed of a slow, high amplitude potential (sharp wave) followed by a burst of rapid oscillatory activity (ripple) and are implicated in memory consolidation.124 Increasing and decreasing serotonin levels in the claustrum led to upregulation and downregulation of spontaneous sharp-wave ripples. They did, however, note that lesions of the claustrum had no effect on the alternation between REM and non-REM sleep. The authors conclude that while the claustrum underlies the generation of sharp-wave ripples during slow-wave sleep, it is not involved in the generation of the sleep rhythm itself.
These studies have carefully dissected several global and specific aspects of sleep and found that while the claustrum was involved with various aspects of sleep in different species, it did not control sleep globally. These results strongly implicate the claustrum as part of a network that subserves some of the neurophysiological correlates of sleep. While the link between the claustrum and sleep remains preliminary, the available evidence from animal studies in combination with the data from human studies presented above might lead to fruitful directions for future research.
Pain
The lesion studies outlined above hint at the intriguing possibility that the claustrum may play a role in pain perception. Pain is a multifaceted sensation, involving both sensory and affective dimensions. Acutely painful sensations activate, in a flexibly accessible manner, a bilateral and widely distributed network of brain regions. These include a core set of regions, such as the thalamus, insula, brainstem, secondary somatosensory, mid- and anterior-cingulate, and prefrontal cortices (Fig. 3E).125–127 Pain-related activity in the claustrum, which is closely connected with many of these brain regions, may be obscured by activity in the nearby insula, falsely attributed to the claustrum when genuinely within the insula, or, if in both, attributed to one or the other.
Figure 3F illustrates the classification results for physical pain within a region of the posterior insula.126 It is clear that the extent of activity from the various studies that have contributed to this map might overlap with the adjacent claustrum. Going forward, opportunities with ultra-high field imaging, such as 7 T MRI, with improved signal-to-noise, spatial resolution, and contrast effects are already illustrating for various studies, including pain, the potential it offers for better spatial discrimination between adjacent brain regions or nuclei.128,129 It will be important for future studies to better delineate the precise roles of the claustrum and divisions of the insula, as well as their interactions, in pain.
Recent advances in rodent and human imaging now also enable further isolation of claustral and insular activity.130,131 Notably, the insula has been identified as a potential deep brain stimulation target for treatment of pain132,133; it is possible that electrodes inserted for this purpose also have contacts within the claustrum or affect it via downstream activation. Despite the challenges of isolating claustrum activity during MRI, several studies of human brain activity during various modalities of experimentally induced pain report increased activity in the claustrum, among many other regions.134–144
As well as acute pain, claustrum activity has been reported in a few studies looking at chronic pain.145,146 Furthermore, studies examining the placebo effect, catastrophizing, and empathy have also reported activity within the claustrum among other regions.147–152 The claustrum has further been reported as active in studies investigating acupuncture, migraine, oxygen deprivation, and invasive motor cortex stimulation in patients with chronic pain.153–157 Drawing strong conclusions from these studies is challenging as the authors rarely include any discussion of the claustrum outside of data tables.
In addition to human and animal imaging studies, non-imaging evidence from animal studies also supports a link between the claustrum and pain. The claustrum expresses proteins implicated in pain-related signalling, including kappa, delta, and mu opioid receptors,158–162 and shows increased neural activity following experimentally-induced pain in various species.163–167 One early study assessed the correlation between seizure-induced damage to the brain and changes in rats’ pain threshold. Intriguingly, of all the structures within the diencephalon and telencephalon, damage to the claustrum explained the largest percentage of variance in the pain threshold.168 Cautious interpretation is warranted as the pain threshold test in a rat will be confounded with movement and/or motivation deficits.
The claustrum may also play a role in the sensation of itch, a sensation that provokes the desire to scratch. While pain and itch are distinct sensations, the two are closely related.169–172 Functional neuroimaging studies have noted differences in baseline claustrum activity in patients with chronic pruritus (itching) and healthy volunteers.173,174 Multiple studies have found increased claustrum activity in response to experimentally induced itch,174–176 and this activity correlates with itch intensity.176 The claustrum is also rich in K-opioid receptors which have been linked to itch reduction.158,177 Moreover, claustrum deactivation is positively correlated with itch-reduction following application of butorphanol, a drug with Κ-opioid receptor agonist activity.178
Although the claustrum is not commonly reported as active in studies of pain and itch, its potential involvement merits further investigation. The present lack of discussion, possible reporting bias, and inconsistent activation may be due to the dominance of other structures very near the claustrum that have well defined roles in pain and itch. Despite these challenges, and in light of ultra-high field MRI imaging opportunities and animal imaging techniques, further investigation of a connection between the claustrum and pain is warranted—not least because of its accessibility as a target for deep brain stimulation.
Discussion
Claustrum lesions resulted in numerous symptoms including disturbances in cognitive function, perceptual and motor abilities, mental state, electrical activity, sleep, and pain. Similarly, animal studies have reported roles for the claustrum in numerous functions as outlined above. At first, it seems difficult to reconcile these diverse findings and potential functions. Rather than performing a single function, the claustrum may instead play multiple intersecting roles that underlie the diverse range of disruptions observed following claustrum lesions.
We hypothesise that the widespread connectivity substrate provided by the claustrum may have initially developed for evolutionarily ancient and fundamental functions. Later, it became a useful framework or scaffold upon which more advanced cognitive abilities could be built as they developed over evolutionary timescales. In considering this possibility, it is perhaps helpful to look across the evolutionary history of the claustrum. Putative claustra have been described in all extant mammals, but the claustrum is likely far older. A claustrum homologue was described in birds by Puelles179 and more recently a reptilian claustrum was described by Norimoto et al.115 By comparing more distant branches of the evolutionary tree, we may perhaps extrapolate backwards to the original function of the claustrum. Sleep, one of the most ancient neural processes, has been linked to the claustrum in species separated by hundreds of millions of years of evolution. Norimoto et al.115 found that the reptilian homologue of the claustrum was involved in the generation of sharp-wave ripples during slow-wave sleep. Similarly, Narikiyo et al.7 proposed that the claustrum was involved with the generation of slow-wave sleep in mice, and Jansen et al.77 linked the claustrum to insomnia in humans. While these studies connecting the claustrum with sleep are preliminary (see above), it is clear that the early claustrum was involved in some facet of sleep.
How can this evolutionarily ancient structure involved in the generation of sleep, one of the most ancient of neural processes, also be linked to more complex cognitive processes like salience or sensory processing? While the extensive connectivity of the claustrum may have evolved to facilitate sleep, it may have gained other uses as the brain increased in complexity. With the expansion of the cortex in mammalian lineages, the scaffold of the claustrum linking these diversifying cortices may have taken on new functions to support new behavioural needs. These new functions, by-products of the claustrum’s exceptional connectivity, may have superseded the original function. These functions may even exist as discrete circuits contained within the claustrum complex.
Among the diverse consequences of claustrum lesions, we observed a strikingly high incidence of seizures. Combined with evidence from animal studies linking the claustrum to seizures, these studies are redolent of a role for the claustrum in regulating cortical excitability.39,40,64 Further research will be required to understand if this increased risk of seizures is specific to the claustrum, or whether similar results would be obtained from any such large disruption of brain connectivity.
Conclusion
Recent work on the claustrum is unravelling the multifaceted nature of its role in the brain. We sought to answer the classic question ‘What can’t you do without the claustrum?’ Unfortunately, lesions to the human claustrum are rarely specific due to its anatomy and bilateral location. Such lesions inevitably impact neighbouring areas, resulting in heterogeneous effects ranging from impaired cognitive, perceptual, and motor abilities, to altered electrical activity in the brain, and profound disturbances to mental states and sleep. These diverse findings suggest a far-reaching function for the claustrum.
While recent anatomical findings exclude the claustrum from some functions such as low-level sensory processing (given the lack of inputs from sensory thalamic nuclei and sparse inputs from primary sensory cortices) and direct generation and/or modification of motor output (given lack of connectivity with striatum), research has not yet yielded a conclusive function for the claustrum. It remains possible that the claustrum underlies many discrete functions and/or possesses a, so far, unidentified global function that ties together these disparate findings. The lesion studies described above do not lend conclusive support to any one theory of the claustrum’s function. They do not provide strong evidence for prominent theories such as multisensory integration and salience processing while lending support to other theories such as the regulation of cortical excitability. Further, the lesion studies in the context of current research have led us to hypothesize a role for the claustrum in regulating cortical excitability. While the function—or functions—of the claustrum is not yet known, it is clear that further work is required to understand this enigmatic brain structure.
One possibility is to continue work on the claustrum across species, which has already usefully spanned reptiles, rodents, rabbits, cats, monkeys, and humans. This cross-species approach may be especially important considering that the role of this highly connected brain area could have changed throughout evolution. That the claustrum has been conserved across these diverse lineages over hundreds of millions of years argues for a fundamentally important function.
In neuroscience, we are often tempted to conclude that a brain region or cell type is responsible for a specific function, but this does not always generate understanding. The claustrum is a brain structure where this strategy is particularly unhelpful, urging us to re-think our approach both methodologically and conceptually.
Supplementary Material
Acknowledgements
We would like to thank Anna Hoerder-Suabedissen, Armin Lak, Simon Butt, and Richard Burman for discussions and Philip Schwarz for translation assistance.
Funding
This project has received funding from the Wellcome Trust to A.M.P., the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 852765) to A.M.P., Sir Henry Wellcome Postdoctoral Fellowship to H.A., Natural Sciences and Engineering Research Council of Canada (NSERC) and Clarendon Fund graduate scholarships to D.K.O., Medical Research Council (MR/S01134X/1) to V.V., Medical Research Council Clinician Scientist Fellowship (MR/P00878/X) to S.G.M. and Leverhulme grant (RPG-2018-310) to S.G.M.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Atlan G, Terem A, Peretz-Rivlin N, Groysman M, Citri A. Mapping synaptic cortico-claustral connectivity in the mouse. J Comp Neurol. 2017;525(6):1381–1402. [DOI] [PubMed] [Google Scholar]
- 2. Atlan G, Terem A, Peretz-Rivlin N, et al. The claustrum supports resilience to distraction. Curr Biol. 2018;28(17):2752–2762.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carman JB, Cowan WM, Powell TP. The cortical projection upon the claustrum. J Neurol Neurosurg Psychiatry. 1964;27(1):46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goll Y, Atlan G, Citri A. Attention: the claustrum. Trends Neurosci. 2015;38(8):486–495. [DOI] [PubMed] [Google Scholar]
- 5. Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212(2):149–179. [DOI] [PubMed] [Google Scholar]
- 6. Jackson J, Karnani MM, Zemelman BV, Burdakov D, Lee AK. Inhibitory Control of Prefrontal Cortex by the Claustrum. Neuron. 2018;99(5):1029–1039.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Narikiyo K, Mizuguchi R, Ajima A, et al. The claustrum coordinates cortical slow-wave activity. Nat Neurosci. 2020;23(6):741–753. [DOI] [PubMed] [Google Scholar]
- 8. Sadowski M, Moryś J, Jakubowska-Sadowska K, Narkiewicz O. Rat’s claustrum shows two main cortico-related zones. Brain Res. 1997;756(1-2):147–152. [DOI] [PubMed] [Google Scholar]
- 9. Sherk H. The claustrum and the cerebral cortex. In: Jones EG, Peters A, eds. Sensory-motor areas and aspects of cortical connectivity. Springer; 1986:467–499. [Google Scholar]
- 10. Tanné-Gariépy J, Boussaoud D, Rouiller EM. Projections of the claustrum to the primary motor, premotor, and prefrontal cortices in the macaque monkey. J Comp Neurol. 2002;454(2):140–157. [DOI] [PubMed] [Google Scholar]
- 11. Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. [DOI] [PubMed] [Google Scholar]
- 12. Wang Q, Ng L, Harris JA, et al. Organization of the connections between claustrum and cortex in the mouse. J Comp Neurol. 2017;525(6):1317–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. White MG, Mathur BN. Frontal cortical control of posterior sensory and association cortices through the claustrum. Brain Struct Funct. 2018;223(6):2999–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zingg B, Hintiryan H, Gou L, et al. Neural networks of the mouse neocortex. Cell. 2014;156(5):1096–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zingg B, Dong HW, Tao HW, Zhang LI. Input-output organization of the mouse claustrum. J Comp Neurol. 2018;526(15):2428–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crick FC, Koch C. What is the function of the claustrum? Philos Trans R Soc Lond B Biol Sci. 2005;360(1458):1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Torgerson CM, Irimia A, Goh SYM, Van Horn JD. The DTI connectivity of the human claustrum: Claustrum connectivity. Hum Brain Mapp. 2015;36(3):827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim J, Matney CJ, Roth RH, Brown SP. Synaptic organization of the neuronal circuits of the claustrum. J Neurosci. 2016;36(3):773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cortimiglia R, Crescimanno G, Salerno MT, Amato G. The role of the claustrum in the bilateral control of frontal oculomotor neurons in the cat. Exp Brain Res. 1991;84(3):471–477. [DOI] [PubMed] [Google Scholar]
- 20. Salerno MT, Cortimiglia R, Crescimanno G, Amato G, Infantellina F. Effects of claustrum stimulation on spontaneous bioelectrical activity of motor cortex neurons in the cat. Exp Neurol. 1984;86(2):227–239. [DOI] [PubMed] [Google Scholar]
- 21. Salerno MT, Cortimiglia R, Crescimanno G, Amato G. Effect of claustrum activation on the spontaneous unitary activity of frontal eye field neurons in the cat. Neurosci Lett. 1989;98(3):299–304. [DOI] [PubMed] [Google Scholar]
- 22. Tsumoto T, Suda K. Effects of stimulation of the dorsocaudal claustrum on activities of striate cortex neurons in the cat. Brain Res. 1982;240(2):345–349. [DOI] [PubMed] [Google Scholar]
- 23. Brown SP, Mathur BN, Olsen SR, Luppi PH, Bickford ME, Citri A. New breakthroughs in understanding the role of functional interactions between the neocortex and the claustrum. J Neurosci. 2017;37(45):10877–10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dillingham CM, Jankowski MM, Chandra R, Frost BE, O’Mara SM. The claustrum: Considerations regarding its anatomy, functions and a programme for research. Brain Neurosci Adv. 2017;1:2398212817718962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jackson J, Smith JB, Lee AK. The Anatomy and physiology of claustrum-cortex interactions. Annu Rev Neurosci. 2020;43(1):231–247. [DOI] [PubMed] [Google Scholar]
- 26. Mathur BN. The claustrum in review. Front Syst Neurosci. 2014;8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patru MC, Reser DH. A New perspective on delusional states—evidence for claustrum involvement. Front Psychiatry. 2015;6:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smythies J, Edelstein L, Ramachandran V. Hypotheses relating to the function of the claustrum. Front Integr Neurosci. 2012;6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smythies J, Edelstein L, Ramachandran V. Hypotheses relating to the function of the claustrum II: does the claustrum use frequency codes? Front Integr Neurosci. 2014;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fodoulian L, Gschwend O, Huber C, et al. The claustrum-medial prefrontal cortex network controls attentional set-shifting. bioRxiv. [Preprint]. doi:10.1101/2020.10.14.339259 [Google Scholar]
- 31. Koubeissi MZ, Bartolomei F, Beltagy A, Picard F. Electrical stimulation of a small brain area reversibly disrupts consciousness. Epilepsy Behav. 2014;37:32–35. [DOI] [PubMed] [Google Scholar]
- 32. Jankowski MM, O’Mara SM. Dynamics of place, boundary and object encoding in rat anterior claustrum. Front Behav Neurosci. 2015;9:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hadjikhani N, Roland PE. Cross-modal transfer of information between the tactile and the visual representations in the human brain: A positron emission tomographic study. J Neurosci. 1998;18(3):1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Redouté J, Stoléru S, Grégoire MC, et al. Brain processing of visual sexual stimuli in human males. Hum Brain Mapp. 2000;11(3):162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ishizu T, Zeki S. The brain’s specialized systems for aesthetic and perceptual judgment. Eur J Neurosci. 2013;37(9):1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adolphs R. Human lesion studies in the 21st century. Neuron. 2016;90(6):1151–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wolff SB, Ölveczky BP. The promise and perils of causal circuit manipulations. Curr Opin Neurobiol. 2018;49:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grasby K, Talk A. The anterior claustrum and spatial reversal learning in rats. Brain Res. 2013;1499:43–52. [DOI] [PubMed] [Google Scholar]
- 39. Kudo T, Wada JA. Effect of unilateral claustral lesion on intermittent light stimulation-induced convulsive response in D, L-allylglycine treated cats. Electroencephalogr Clin Neurophysiol. 1995;95(1):63–68. [DOI] [PubMed] [Google Scholar]
- 40. Mohapel P, Hannesson DK, Armitage LL, Gillespie GW, Corcoran ME. Claustral Lesions Delay Amygdaloid Kindling in the Rat. Epilepsia. 2000;41(9):1095–1101. [DOI] [PubMed] [Google Scholar]
- 41. Malter MP, Helmstaedter C, Urbach H, Vincent A, Bien CG. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol. 2010;67(4):470–478. [DOI] [PubMed] [Google Scholar]
- 42. Ishii K, Tsuji H, Tamaoka A. Mumps virus encephalitis with symmetric claustrum lesions. Am J Neuroradiol. 2011;32(7):E139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mumoli L, Labate A, Palamara G, Sturniolo M, Gambardella A. Reversible symmetrical external capsule hyperintensity as an early finding of autoimmune encephalitis. Neurol Sci. 2014;35(7):1147–1149. [DOI] [PubMed] [Google Scholar]
- 44. Silva G, Jacob S, Melo C, Alves D, Costa D. Claustrum sign in a child with refractory status epilepticus after febrile illness: why does it happen? Acta Neurol Belg. 2018;118(2):303–305. [DOI] [PubMed] [Google Scholar]
- 45. Sperner J, Sander B, Lau S, Krude H, Scheffner D. Severe transitory encephalopathy with reversible lesions of the claustrum. Pediatr Radiol. 1996;26(11):769–771. [DOI] [PubMed] [Google Scholar]
- 46. Hwang KJ, Park KC, Yoon SS, Ahn TB. Unusual lesion in the bilateral external capsule following status epilepticus: a case report. J Epilepsy Res. 2014;4(2):88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maximov GK, Hinova-Palova DV, Iliev AA, et al. Ischemic stroke of the left claustrum in a 55-year-old female: a case report. Claustrum. 2018;3(1):1528135. [Google Scholar]
- 48. Zuhorn F, Omaimen H, Ruprecht B, et al. Parainfectious encephalitis in COVID-19: ‘The Claustrum Sign’. J Neurol. 2020;268(6):2031–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Albayrak BS, Gorgulu A. Persistent bilateral amaurosis in a child caused by damage to the calcarine cortex and the claustrum in contralateral hemispheres after a closed head injury. J Trauma. 2008;64(6):E81–E82. [PubMed] [Google Scholar]
- 50. Randerath J, Finkel L, Shigaki C, et al. Does it fit?—Impaired affordance perception after stroke. Neuropsychologia. 2018;108:92–102. [DOI] [PubMed] [Google Scholar]
- 51. Matsuzono K, Kurata T, Deguchi S, et al. Two unique cases with anti-GluR antibody-positive encephalitis. Clin Med Insights Case Rep. 2013;6:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chessa E, Piga M, Floris A, Mathieu A, Cauli A. Severe neuropsychiatric systemic lupus erythematosus successfully treated with rituximab: an alternative to standard of care. Open Access Rheumatol. 2017;9:167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hiraga A, Watanabe O, Kamitsukasa I, Kuwabara S. Voltage-gated Potassium Channel Antibody-associated Encephalitis with Claustrum Lesions. Intern Med. 2014;53(19):2263–2264. [DOI] [PubMed] [Google Scholar]
- 54. Shintaku M, Kaneda D, Tada K, Katano H, Sata T. Human herpes virus 6 encephalomyelitis after bone marrow transplantation: Report of an autopsy case. Neuropathology. 2010;30(1):50–55. [DOI] [PubMed] [Google Scholar]
- 55. Jellinger KA, Grazer A, Petrovic K, et al. Four-repeat tauopathy clinically presenting as posterior cortical atrophy: atypical corticobasal degeneration? Acta Neuropathol. 2011;121(2):267–277. [DOI] [PubMed] [Google Scholar]
- 56. Yoshimura N, Yoshimura I, Asada M, et al. Juvenile Parkinson’s disease with widespread Lewy bodies in the brain. Acta Neuropathol. 1988;77(2):213–218. [DOI] [PubMed] [Google Scholar]
- 57. Seghier ML, Bagdasaryan J, Jung DE, Price CJ. The importance of premotor cortex for supporting speech production after left capsular-putaminal damage. J Neurosci. 2014;34(43):14338–14348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Turkalj I, Stojanovic S, Petrovic K, Njagulj V, Mikov I, Spanovic M. Psychosis following stab brain injury by a billiard stick. Hippokratia. 2012;16(3):275–277. [PMC free article] [PubMed] [Google Scholar]
- 59. Lapenta L, Frisullo G, Vollono C, et al. Super-refractory status epilepticus: Report of a case and review of the literature. Clin EEG Neurosci. 2015;46(4):335–339. [DOI] [PubMed] [Google Scholar]
- 60. Singh SP, Agarwal S, Faulkner M. Refractory status epilepticus. Ann Indian Acad Neurol. 2014;17(5):32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Choi JY, Kim EJ, Moon SY, Kim TJ, Huh K. Prognostic significance of subsequent extra-temporal involvement in cryptogenic new onset refractory status epilepticus (NORSE) initially diagnosed with limbic encephalitis. Epilepsy Res. 2019;158:106215. [DOI] [PubMed] [Google Scholar]
- 62. Meletti S, Giovannini G, d’Orsi G, et al. New-onset refractory status epilepticus with claustrum damage: Definition of the clinical and neuroimaging features. Front Neurol. 2017;8:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Meletti S, Slonkova J, Mareckova I, et al. Claustrum damage and refractory status epilepticus following febrile illness. Neurology. 2015;85(14):1224–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kurada L, Bayat A, Joshi S, Koubeissi MZ. The Claustrum in Relation to Seizures and Electrical Stimulation. Front Neuroanat. 2019;13:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hodgson E, Mailman RB, Chambers JE, eds. Dictionary of toxicology. 3rd ed. Elsevier; 2015. [Google Scholar]
- 66. Chau A, Salazar AM, Krueger F, Cristofori I, Grafman J. The effect of claustrum lesions on human consciousness and recovery of function. Conscious Cogn. 2015;36:256–264. [DOI] [PubMed] [Google Scholar]
- 67. Snider SB, Hsu J, Darby RR, et al. Cortical lesions causing loss of consciousness are anticorrelated with the dorsal brainstem. Hum Brain Mapp. 2020;41(6):1520–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sleigh J, Warnaby C, Tracey I. General anaesthesia as fragmentation of selfhood: insights from electroencephalography and neuroimaging. Br J Anaesth. 2018;121(1):233–240. [DOI] [PubMed] [Google Scholar]
- 69. McMurtray A, Tseng B, Diaz N, Chung J, Mehta B, Saito E. Acute psychosis associated with subcortical stroke: Comparison between basal ganglia and mid-brain lesions. Case Rep Neurol Med. 2014; 2014:428425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shintani S, Tsuruoka S, Shiigai T. Serial positron emission tomography (PET) in gliomatosis cerebri treated with radiotherapy: a case report. J Neurol Sci. 2000;173(1):25–31. [DOI] [PubMed] [Google Scholar]
- 71. Chakraborty S, Singi SR, Pradhan G, Anantha Subramanya H. Neuro-cysticercosis presenting with single delusion: A rare psychiatric manifestation. Int J Appl Basic Med Res. 2014;4(2):131–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Grover S, Aneja J, Mahajan S, Varma S. Cotard’s syndrome: Two case reports and a brief review of literature. J Neurosci Rural Pract. 2014;5(Suppl 1):S59–S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McKay R, Cipolotti L. Attributional style in a case of Cotard delusion. Conscious Cogn. 2007;16(2):349–359. [DOI] [PubMed] [Google Scholar]
- 74. Bott N, Keller C, Kuppuswamy M, Spelber D, Zeier J. Cotard delusion in the context of schizophrenia: A case report and review of the literature. Front Psychol. 2016;7:1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Debruyne H, Portzky M, Van den Eynde F, Audenaert K. Cotard’s syndrome: A review. Curr Psychiatry Rep. 2009;11(3):197–202. [DOI] [PubMed] [Google Scholar]
- 76. Kudlur SNC, George S, Jaimon M. An overview of the neurological correlates of Cotard syndrome. Eur J Psychiatry. 2007;21:99–116. [Google Scholar]
- 77. Jansen PR, Watanabe K, Stringer S, et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet. 2019;51(3):394–403. [DOI] [PubMed] [Google Scholar]
- 78. Lunsford-Avery JR, Damme KSF, Engelhard MM, Kollins SH, Mittal VA. Sleep/wake regularity associated with default mode network structure among healthy adolescents and young adults. Sci Rep. 2020;10(1):509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Serrano-Castro PJ, Quiroga-Subirana P, Payán-Ortiz M, Fernandez-Perez J. The expanding spectrum of febrile infection-related epilepsy syndrome (FIRES). Seizure. 2013;22(2):153–155. [DOI] [PubMed] [Google Scholar]
- 80. Obrador S, Dierssen G, Ceballos R. Consideraciones clinicas, neurológicas y anatómicas sobre el llamado dolor talámico. Acta Neurol Latinoam. 1957;3:58–77. [Google Scholar]
- 81. Barcikowska M. [Lesions of the insula and operculum: a syndrome]. Neurol Neurochir Pol. 1979;13(2):205–209. [PubMed] [Google Scholar]
- 82. Biemond A. The Conduction of pain above the level of the thalamus opticus. AMA Arch Neurol Psychiatry. 1956;75(3):231–244. [DOI] [PubMed] [Google Scholar]
- 83. Bickel S, Parvizi J. Electrical stimulation of the human claustrum. Epilepsy Behav. 2019;97:296–303. [DOI] [PubMed] [Google Scholar]
- 84. Mazzola L, Isnard J, Peyron R, Mauguière F. Stimulation of the human cortex and the experience of pain: Wilder Penfield’s observations revisited. Brain. 2012;135(2):631–640. [DOI] [PubMed] [Google Scholar]
- 85. Starr CJ, Sawaki L, Wittenberg GF, et al. Roles of the insular cortex in the modulation of pain: Insights from brain lesions. J Neurosci. 2009;29(9):2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bota M, Sporns O, Swanson LW. Architecture of the cerebral cortical association connectome underlying cognition. Proc Natl Acad Sci U S A. 2015;112(16):E2093–E2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Duffau H, Mandonnet E, Gatignol P, Capelle L. Functional compensation of the claustrum: lessons from low-grade glioma surgery. J Neurooncol. 2007;81(3):327–329. [DOI] [PubMed] [Google Scholar]
- 88. Carey RG, Neal TL. Reciprocal connections between the claustrum and visual thalamus in the tree shrew (Tupaia glis). Brain Res. 1986;386(1–2):155–168. [DOI] [PubMed] [Google Scholar]
- 89. Clarey JC, Irvine DRF. Auditory response properties of neurons in the claustrum and putamen of the cat. Exp Brain Res. 1986;61(2):432–437. [DOI] [PubMed] [Google Scholar]
- 90. LeVay S, Sherk H. The visual claustrum of the cat. I. Structure and connections. J Neurosci. 1981;1(9):956–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Olson CR, Graybiel AM. Sensory maps in the claustrum of the cat. Nature. 1980;288(5790):479–481. [DOI] [PubMed] [Google Scholar]
- 92. Remedios R, Logothetis NK, Kayser C. Unimodal responses prevail within the multisensory claustrum. J Neurosci. 2010;30(39):12902–12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Remedios R, Logothetis NK, Kayser C. A role of the claustrum in auditory scene analysis by reflecting sensory change. Front Syst Neurosci. 2014;8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Reus-García MM, Sánchez-Campusano R, Ledderose J, et al. The claustrum is involved in cognitive processes related to the classical conditioning of eyelid responses in behaving rabbits. Cereb Cortex. 2021;31(1):281–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sherk H, LeVay S. Contribution of the cortico-claustral loop to receptive field properties in area 17 of the cat. J Neurosci. 1983;3(11):2121–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Smith JB, Watson GDR, Liang Z, Liu Y, Zhang N, Alloway KD. A role for the claustrum in salience processing? Front Neuroanat. 2019;13:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16(1):55–61. [DOI] [PubMed] [Google Scholar]
- 100. Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411(6835):305–309. [DOI] [PubMed] [Google Scholar]
- 101. Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28(3):309–369. [DOI] [PubMed] [Google Scholar]
- 102. Terem A, Gonzales BJ, Peretz-Rivlin N, et al. Claustral neurons projecting to frontal cortex mediate contextual association of reward. Curr Biol. 2020;30(18):3522–3532.e6. [DOI] [PubMed] [Google Scholar]
- 103. Chia Z, Augustine GJ, Silberberg G. Synaptic connectivity between the cortex and claustrum is organized into functional modules. Curr Biol. 2020;30(14):2777–2790.e4. [DOI] [PubMed] [Google Scholar]
- 104. Ettlinger G, Wilson WA. Cross-modal performance: behavioural processes, phylogenetic considerations and neural mechanisms. Behav Brain Res. 1990;40(3):169–192. [DOI] [PubMed] [Google Scholar]
- 105. Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354(1387):1325–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Robertson IH, Halligan PW. Spatial neglect: a clinical handbook for diagnosis and treatment. Psychology Press/Taylor & Francis; 1999:ix, 161. [Google Scholar]
- 107. Peters SK, Dunlop K, Downar J. Cortico-striatal-thalamic loop circuits of the salience network: A central pathway in psychiatric disease and treatment. Front Syst Neurosci. 2016;10:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92(3):1087–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Liu D, Dan Y. A motor theory of sleep-wake control: Arousal-action circuit. Annu Rev Neurosci. 2019;42(1):27–46. [DOI] [PubMed] [Google Scholar]
- 110. Saper CB, Fuller PM. Wake-sleep circuitry: an overview. Curr Opin Neurobiol. 2017;44:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Vyazovskiy VV, Harris KD. Sleep and the single neuron: the role of global slow oscillations in individual cell rest. Nat Rev Neurosci. 2013;14(6):443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gehlert D, Gackenheimer S, Wong D, Robertson D. Localization of 5-HT3 receptors in the rat brain using [3H]LY278584. Brain Res. 1991;553(1):149–154. [DOI] [PubMed] [Google Scholar]
- 113. Koyama Y, Kondo M, Shimada S. Building a 5-HT3A receptor expression map in the mouse brain. Sci Rep. 2017;7:42884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Morales M, Battenberg E, Bloom FE. Distribution of neurons expressing immunoreactivity for the 5HT3 receptor subtype in the rat brain and spinal cord. J Comp Neurol. 1998;402(3):385–401. [PubMed] [Google Scholar]
- 115. Norimoto H, Fenk LA, Li HH, et al. A claustrum in reptiles and its role in slow-wave sleep. Nature. 2020;578(7795):413–418. [DOI] [PubMed] [Google Scholar]
- 116. Oikonomou G, Altermatt M, Zhang RW, et al. The serotonergic raphe promote sleep in zebrafish and mice. Neuron. 2019;103(4):686–701.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24(31):6862–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F, Tononi G. Source modeling sleep slow waves. Proc Natl Acad Sci U S A. 2009;106(5):1608–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Nir Y, Staba RJ, Andrillon T, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70(1):153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Renouard L, Billwiller F, Ogawa K, et al. The supramammillary nucleus and the claustrum activate the cortex during REM sleep. Sci Adv. 2015;1(3):e1400177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Logothetis NK, Augath M, Murayama Y, et al. The effects of electrical microstimulation on cortical signal propagation. Nat Neurosci. 2010;13(10):1283–1291. [DOI] [PubMed] [Google Scholar]
- 122. Vyazovskiy VV, Faraguna U, Cirelli C, Tononi G. Triggering slow waves during NREM sleep in the rat by intracortical electrical stimulation: effects of sleep/wake history and background activity. J Neurophysiol. 2009;101(4):1921–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Riedner BA, Hulse BK, Murphy MJ, Ferrarelli F, Tononi G. Temporal dynamics of cortical sources underlying spontaneous and peripherally evoked slow waves. Prog Brain Res. 2011;193:201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Joo HR, Frank LM. The hippocampal sharp wave–ripple in memory retrieval for immediate use and consolidation. Nat Rev Neurosci. 2018;19(12):744–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–391. [DOI] [PubMed] [Google Scholar]
- 126. Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368(15):1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Xu A, Larsen B, Baller EB, et al. Convergent neural representations of experimentally-induced acute pain in healthy volunteers: A large-scale fMRI meta-analysis. Neurosci Biobehav Rev. 2020;112:300–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Faull OK, Jenkinson M, Ezra M, Pattinson KT. Conditioned respiratory threat in the subdivisions of the human periaqueductal gray. Elife. 2016;5:e12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Schulz E, Stankewitz A, Winkler AM, Irving S, Witkovský V, Tracey I. Ultra-high-field imaging reveals increased whole brain connectivity underpins cognitive strategies that attenuate pain. Elife. 2020;9:e55028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Krimmel SR, Qadir H, Hesselgrave N, et al. Resting state functional connectivity of the rat claustrum. Front Neuroanat. 2019;13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Krimmel SR, White MG, Panicker MH, Barrett FS, Mathur BN, Seminowicz DA. Resting state functional connectivity and cognitive task-related activation of the human claustrum. Neuroimage. 2019;196:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I. The dorsal posterior insula subserves a fundamental role in human pain. Nat Neurosci. 2015;18(4):499–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Galhardoni R, da Silva VA, García-Larrea L, et al. Insular and anterior cingulate cortex deep stimulation for central neuropathic pain. Neurology. 2019;92(18):e2165–e2175. [DOI] [PubMed] [Google Scholar]
- 134. Coghill R, Talbot J, Evans A, et al. Distributed processing of pain and vibration by the human brain. J Neurosci. 1994;14(7):4095–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. De Groote S, De Jaeger M, Van Schuerbeek P, et al. Functional magnetic resonance imaging: cerebral function alterations in subthreshold and suprathreshold spinal cord stimulation. J Pain Res. 2018;11:2517–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Duerden EG, Albanese MC. Localization of pain-related brain activation: A meta-analysis of neuroimaging data. Hum Brain Mapp. 2013;34(1):109–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Gard T, Holzel BK, Sack AT, et al. Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb Cortex. 2012;22(11):2692–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lu CL, Wu YT, Yeh TC, et al. Neuronal correlates of gastric pain induced by fundus distension: a 3T-fMRI study. Neurogastroenterol Motil. 2004;16(5):575–587. [DOI] [PubMed] [Google Scholar]
- 139. Martin L, Borckardt JJ, Reeves ST, et al. A pilot functional MRI study of the effects of prefrontal rTMS on pain perception. Pain Med. 2013;14(7):999–1009. [DOI] [PubMed] [Google Scholar]
- 140. Niddam DM, Yeh TC, Wu YT, et al. Event-related functional MRI study on central representation of acute muscle pain induced by electrical stimulation. Neuroimage. 2002;17(3):1437–1450. [DOI] [PubMed] [Google Scholar]
- 141. Ochsner KN, Ludlow DH, Knierim K, et al. Neural correlates of individual differences in pain-related fear and anxiety. Pain. 2006;120(1–2):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Scheef L, Jankowski J, Daamen M, et al. An fMRI study on the acute effects of exercise on pain processing in trained athletes. Pain. 2012;153(8):1702–1714. [DOI] [PubMed] [Google Scholar]
- 143. Sevel LS, O’Shea AM, Letzen JE, Craggs JG, Price DD, Robinson ME. Effective connectivity predicts future placebo analgesic response: A dynamic causal modeling study of pain processing in healthy controls. Neuroimage. 2015;110:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Xu X, Fukuyama H, Yazawa S, et al. Functional localization of pain perception in the human brain studied by PET. Neuroreport. 1997;8(2):555–559. [DOI] [PubMed] [Google Scholar]
- 145. Niddam DM, Lee SH, Su YT, Chan RC. Brain structural changes in patients with chronic myofascial pain. Eur J Pain. 2017;21(1):148–158. [DOI] [PubMed] [Google Scholar]
- 146. Smallwood RF, Laird AR, Ramage AE, et al. Structural brain anomalies and chronic pain: A quantitative meta-analysis of gray matter volume. J Pain. 2013;14(7):663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Amanzio M, Benedetti F, Porro CA, Palermo S, Cauda F. Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum Brain Mapp. 2013:34(3):738–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ding R, Ren J, Li S, Zhu X, Zhang K, Luo W. Domain-general and domain-preferential neural correlates underlying empathy towards physical pain, emotional situation and emotional faces: An ALE meta-analysis. Neuropsychologia. 2020;137:107286. [DOI] [PubMed] [Google Scholar]
- 149. Gracely RH. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127(4):835–843. [DOI] [PubMed] [Google Scholar]
- 150. Jauniaux J, Khatibi A, Rainville P, Jackson PL. A meta-analysis of neuroimaging studies on pain empathy: investigating the role of visual information and observers’ perspective. Soc Cogn Affect Neurosci. 2019;14(8):789–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kong J, Gollub RL, Polich G, et al. A functional magnetic resonance imaging study on the neural mechanisms of hyperalgesic nocebo effect. J Neurosci. 2008;28(49):13354–13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Palermo S, Benedetti F, Costa T, Amanzio M. Pain anticipation: An activation likelihood estimation meta-analysis of brain imaging studies: Pain anticipation an activation likelihood estimation meta-analysis. Hum Brain Mapp. 2015;36(5):1648–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Biella G, Sotgiu ML, Pellegata G, Paulesu E, Castiglioni I, Fazio F. Acupuncture produces central activations in pain regions. Neuroimage. 2001;14(1):60–66. [DOI] [PubMed] [Google Scholar]
- 154. Jia Z, Yu S. Grey matter alterations in migraine: A systematic review and meta-analysis. Neuroimage Clin. 2017;14:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Liotti M, Brannan S, Egan G, et al. Brain responses associated with consciousness of breathlessness (air hunger). Proc Natl Acad Sci U S A. 2001;98(4):2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Volkers R, Giesen E, Heiden M, et al. Invasive motor cortex stimulation influences intracerebral structures in patients with neuropathic pain: An activation likelihood estimation meta-analysis of imaging data. Neuromodulation. 2020;23(4):436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Yeo S, Rosen B, Bosch P, van den Noort M, Lim S. Gender differences in the neural response to acupuncture: Clinical implications. Acupunct Med. 2016;34(5):364–372. [DOI] [PubMed] [Google Scholar]
- 158. Chen C, Willhouse AH, Huang P, et al. Characterization of a knockin mouse line expressing a fusion protein of kappa opioid receptor conjugated with tdTomato: 3-Dimensional brain imaging via CLARITY. eNeuro. 2020;7(4):ENEURO.0028-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Florin S, Meunier JC, Costentin J. Autoradiographic localization of [3H]nociceptin binding sites in the rat brain. Brain Res. 2000;880(1–2):11–16. [DOI] [PubMed] [Google Scholar]
- 160. Mansour A, Fox CA, Burke S, et al. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350(3):412–438. [DOI] [PubMed] [Google Scholar]
- 161. Mika J, Obara I, Przewlocka B. The role of nociceptin and dynorphin in chronic pain: Implications of neuro–glial interaction. Neuropeptides. 2011;45(4):247–261. [DOI] [PubMed] [Google Scholar]
- 162. Tempel A, Zukin RS. Neuroanatomical patterns of the mu, delta, and kappa opioid receptors of rat brain as determined by quantitative in vitro autoradiography. Proc Natl Acad Sci U S A. 1987;84(12):4308–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Jastreboff P, Sikora M, Frydrychowski A, Słoniewski P. Claustral single cell reactions to tooth pulp stimulation in cats. Acta Neurobiol Exp (Wars). 1983;43(4-5):291–298. [PubMed] [Google Scholar]
- 164. Lei LG, Zhang YQ, Zhao ZQ. Pain-related aversion and Fos expression in the central nervous system in rats. Neuroreport. 2004;15(1):67–71. [DOI] [PubMed] [Google Scholar]
- 165. Sinniger V, Porcher C, Mouchet P, Juhem A, Bonaz B. c-fos and CRF receptor gene transcription in the brain of acetic acid-induced somato-visceral pain in rats. Pain. 2004;110(3):738–750. [DOI] [PubMed] [Google Scholar]
- 166. Słoniewski P, Moryś J, Pilgrim C. Stimulation of glucose utilization in the rat claustrum by pain. Folia Neuropathol. 1995;33(3):163–168. [PubMed] [Google Scholar]
- 167. Tan LL, Pelzer P, Heinl C, et al. A pathway from midcingulate cortex to posterior insula gates nociceptive hypersensitivity. Nat Neurosci. 2017;20(11):1591–1601. [DOI] [PubMed] [Google Scholar]
- 168. Persinger MA, Peredery O, Bureau YR, Cook LL. Emergent properties following brain injury: the claustrum as a major component of a pathway that influences nociceptive thresholds to foot shock in rats. Percept Mot Skills. 1997;85(2):387–398. [DOI] [PubMed] [Google Scholar]
- 169. Hachisuka J, Chiang MC, Ross SE.. Itch and neuropathic itch. Pain. 2018;159(3):603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Liu T, Ji RR. New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms? Pflugers Arch. 2013;465(12):1671–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Schmelz M. Itch and pain. Neurosci Biobehav Rev. 2010;34(2):171–176. [DOI] [PubMed] [Google Scholar]
- 172. Ständer S, Schmelz M. Chronic itch and pain-Similarities and differences. Eur J Pain. 2006;10(5):473–473. [DOI] [PubMed] [Google Scholar]
- 173. Cohen OS, Chapman J, Lee H, et al. Pruritus in familial Creutzfeldt–Jakob disease: a common symptom associated with central nervous system pathology. J Neurol. 2011;258(1):89–95. [DOI] [PubMed] [Google Scholar]
- 174. Papoiu ADP, Emerson NM, Patel TS, et al. Voxel-based morphometry and arterial spin labeling fMRI reveal neuropathic and neuroplastic features of brain processing of itch in end-stage renal disease. J Neurophysiol. 2014;112(7):1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Herde L, Forster C, Strupf M, Handwerker HO. Itch Induced by a Novel Method Leads to Limbic Deactivations— A Functional MRI Study. J Neurophysiol. 2007;98(4):2347–2356. [DOI] [PubMed] [Google Scholar]
- 176. Papoiu ADP, Coghill RC, Kraft RA, Wang H, Yosipovitch G. A tale of two itches. Common features and notable differences in brain activation evoked by cowhage and histamine induced itch. Neuroimage. 2012;59(4):3611–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Nguyen E, Lim G, Ding H, Hachisuka J, Ko MC, Ross SE. Morphine acts on spinal dynorphin neurons to cause itch through disinhibition. Sci Transl Med. 2021;13(579):eabc3774. [DOI] [PubMed] [Google Scholar]
- 178. Papoiu ADP, Kraft RA, Coghill RC, Yosipovitch G. Butorphanol suppression of histamine itch is mediated by nucleus accumbens and septal nuclei: A pharmacological fMRI study. J Invest Dermatol. 2015;135(2):560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Edelstein L, Smythies J, Ramachandran V. The claustrum—Structural, functional and clinical neuroscience. Academic Press; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.