Abstract
Frontotemporal dementia refers to a group of neurodegenerative disorders characterized by behaviour and language alterations and focal brain atrophy. Amyotrophic lateral sclerosis is a rapidly progressing neurodegenerative disease characterized by loss of motor neurons resulting in muscle wasting and paralysis. Frontotemporal dementia and amyotrophic lateral sclerosis are considered to exist on a disease spectrum given substantial overlap of genetic and molecular signatures. The predominant genetic abnormality in both frontotemporal dementia and amyotrophic lateral sclerosis is an expanded hexanucleotide repeat sequence in the C9orf72 gene. In terms of brain pathology, abnormal aggregates of TAR-DNA-binding protein-43 are predominantly present in frontotemporal dementia and amyotrophic lateral sclerosis patients. Currently, sensitive and specific diagnostic and disease surveillance biomarkers are lacking for both diseases. This has impeded the capacity to monitor disease progression during life and the development of targeted drug therapies for the two diseases. The purpose of this review is to examine the status of current biofluid biomarker discovery and development in frontotemporal dementia and amyotrophic lateral sclerosis. The major pathogenic proteins implicated in different frontotemporal dementia and amyotrophic lateral sclerosis molecular subtypes and proteins associated with neurodegeneration and the immune system will be discussed. Furthermore, the use of mass spectrometry-based proteomics as an emerging tool to identify new biomarkers in frontotemporal dementia and amyotrophic lateral sclerosis will be summarized.
Keywords: frontotemporal dementia, amyotrophic lateral sclerosis, biomarkers, neurofilament, proteomics
Frontotemporal dementia and amyotrophic lateral sclerosis are neurodegenerative diseases that lie on the same disease spectrum. Katzeff, Bright et al. explore and evaluate potential biomarkers that could distinguish the two diseases and ultimately improve patient diagnosis and treatment.
Introduction
Frontotemporal dementia refers to a group of neurodegenerative disorders characterized by altered behaviour and language, with a progressive decline in executive function.1 Frontotemporal dementia is the second most common form of younger-onset dementia after Alzheimer’s disease, frequently occurring before 65 years of age.2 Frontotemporal dementia is categorized clinically into various subtypes; the main three include behavioural-variant frontotemporal dementia and two language variants, semantic dementia (also known as semantic variant primary progressive aphasia) and progressive non-fluent aphasia (also known as non-fluent variant primary progressive aphasia). In addition, frontotemporal dementia overlaps with movement disorders having two additional subtypes, progressive supranuclear palsy and corticobasal degeneration. Behavioural-variant frontotemporal dementia is the most common form of frontotemporal dementia presenting with a range of symptoms that include disinhibited behaviour, apathy, increased consumption of sweet foods and alcohol, loss of empathy and emotional processing, and impaired executive function.1,3–5 Semantic dementia is characterized by a loss of semantic knowledge that typically presents as progressive anomia, in the context of fluent expressive speech. In contrast, progressive non-fluent aphasia is characterized by effortful and distorted speech with or without agrammatism in the context of preserved comprehension.4,6,7
Amyotrophic lateral sclerosis, also known as motor neuron disease, is a rapidly progressing neurodegenerative disease characterized by degeneration of motor neurons in the brain and spinal cord, leading to muscle atrophy and paralysis.8,9 Fatality in amyotrophic lateral sclerosis frequently occurs within 3–5 years of diagnosis.10,11 Amyotrophic lateral sclerosis most commonly occurs around 60 years of age, affecting more males than females.10,11 Amyotrophic lateral sclerosis is categorized depending on whether symptoms are predominantly related to upper motor neurons or lower motor neurons. In ∼70% of amyotrophic lateral sclerosis cases, both upper motor neurons and lower motor neurons are affected, while minor subtypes predominantly involve either upper motor neurons or lower motor neurons.9,12,13 Amyotrophic lateral sclerosis often presents with either onset of limb weakness or bulbar symptoms affecting speech or swallowing.
Approximately 15% of frontotemporal dementia cases exhibit motor symptoms and 15–18% of amyotrophic lateral sclerosis cases exhibit frontotemporal dementia symptoms.14 Furthermore, 50% of amyotrophic lateral sclerosis cases have evidence of frontotemporal dementia-like cognitive changes.11 Pathologically, frontotemporal dementia cases are characterized by abnormal aggregates of TDP-43, tau or FET proteins (FET proteins refer to a group of three proteins: fused in sarcoma, EWSR1 and TAF15) while amyotrophic lateral sclerosis cases are pathologically characterized by TDP-43, superoxide dismutase-1 (SOD1) or FET proteins.14–16 Of particular interest, abnormal aggregates of TDP-43 are identified in ∼50% of frontotemporal dementia cases and 95% of amyotrophic lateral sclerosis cases.17 In 2011, TDP-43 aggregates were shown to be associated with the C9orf72 repeat expansion in both frontotemporal dementia and amyotrophic lateral sclerosis.18,19 Together, these studies provide clinical, molecular and genetic evidence supporting the existence of frontotemporal dementia and amyotrophic lateral sclerosis on a disease spectrum. While genetic abnormalities in patients are useful in identifying the underlying molecular pathologies, biomarker development in association with genetic status and clinical assessment is necessary to identify and distinguish molecular signatures in patients who exhibit similar clinical symptoms. Sensitive and specific biomarkers have the potential to assist with targeting specific molecular subtypes for mechanistic treatments, tracking disease progression during life and streamlining patients for clinical trials that are currently lacking for both frontotemporal dementia and amyotrophic lateral sclerosis.
Gene-based biomarkers
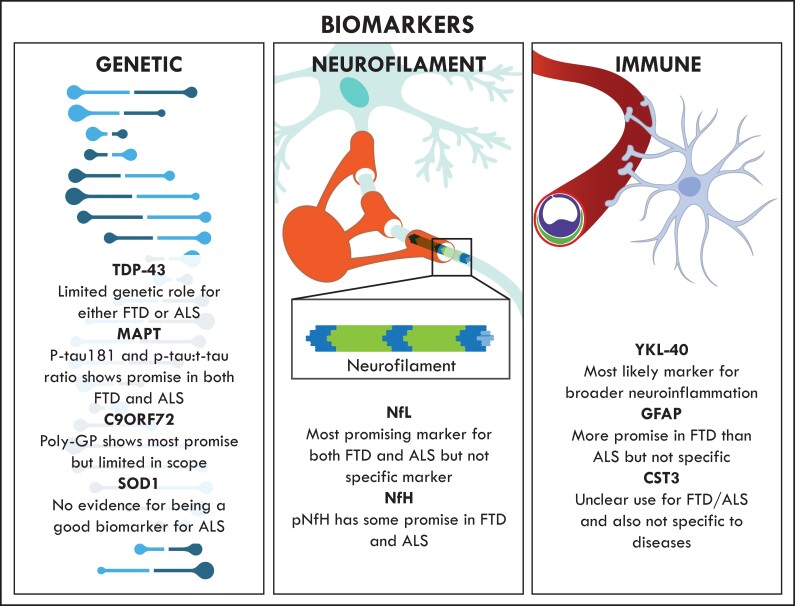
In general, gene-based biomarkers (Fig. 1) have the potential to determine disease susceptibility and assist with discriminating early-stage versus late-stage disease. Genetic mutations in TARDBP, C9orf72, MAPT and SOD1 have been used to classify frontotemporal dementia and amyotrophic lateral sclerosis. While the genetic subtypes of frontotemporal dementia and amyotrophic lateral sclerosis continue to be extensively explored and advances have been made in correlating pathological subtypes with causal genes, the use of gene-based biomarkers, particularly those genes that overlap between frontotemporal dementia and amyotrophic lateral sclerosis, remains to be fully investigated. Although gene-based biomarkers provide promise for biomarker discovery in frontotemporal dementia and amyotrophic lateral sclerosis, it is likely that they will need to be used in combination with other types of biomarker that target additional pathways and mechanisms of disease.
Figure 1.
An overview of current biomarker development in frontotemporal dementia and amyotrophic lateral sclerosis. The main genetic biomarkers evaluated are TDP-43, MAPT, C9ORF72 and SOD1. Neurofilament biomarkers evaluated are NfL and NfH. The main immune-related biomarkers evaluated are YKL-40, GFAP and CST3. All of these markers have limitations in the diagnosis of frontotemporal dementia and amyotrophic lateral sclerosis, and therefore there is an urgent need for new biomarker development for the two diseases.
TARDBP
The TARDBP gene, which encodes TDP-43, accounts for ∼4% of familial amyotrophic lateral sclerosis, 1.5% of sporadic amyotrophic lateral sclerosis patients and ∼2% of behavioural-variant frontotemporal dementia patients.20,21 Pathologically, TDP-43 pathology is present in ∼50% of frontotemporal dementia cases and 95% of amyotrophic lateral sclerosis.17 TDP-43 is an RNA/DNA-binding protein that is widely expressed, particularly in the CNS. While the complete function of TDP-43 is yet to be determined, under physiological conditions TDP-43 is involved in RNA biogenesis and processing.22,23
Use of TDP-43 as a biomarker has been explored in serum, plasma and CSF, predominantly in frontotemporal dementia cases. In behavioural-variant frontotemporal dementia, serum and plasma levels of phosphorylated TDP-43 (pTDP-43) have been used to differentiate between genetic subtypes. While no significant difference pTDP-43 was observed between C9orf72 and GRN-mutation carriers, both groups had higher levels of pTDP-43 compared to other behavioural-variant frontotemporal dementia subtypes and controls in both CSF and plasma samples. Additionally, pTDP-43 levels in plasma correlated positively with CSF; however, there was no correlation in total TDP-43 (tTDP-43) levels within the same samples.24 CSF tTDP-43 failed to differentiate between C9orf72 expansion carriers and non-carriers. CSF tTDP-43 has also been shown to be elevated in amyotrophic lateral sclerosis compared to behavioural-variant frontotemporal dementia patients.25 CSF tTDP-43 was elevated in sporadic amyotrophic lateral sclerosis compared to age-matched controls and other neurodegenerative and inflammatory diseases, including Parkinson’s disease, multiple sclerosis and Guillain–Barré syndrome.26,27
There are, however, two major concerns with the use of TDP-43 as a biomarker. First, there is no consensus as to whether full length TDP-43, pTDP-43 or truncated variants of TDP-43 would be most effective for measurement.28 Second, directly measuring tTDP-43 in a biofluid carries the risk of measuring peripheral rather than brain derived TDP-43. As such, development of methods that can differentiate brain derived TDP-43 from other tissue sources are required to effectively use TDP-43 as a biomarker in behavioural-variant frontotemporal dementia and amyotrophic lateral sclerosis.28,29
MAPT
The tau protein stabilizes microtubules,30,31 and over 50 MAPT mutations have been associated with frontotemporal dementia.32–34 Abnormally phosphorylated tau aggregates, which are the second most common form of pathological protein aggregate in frontotemporal dementia, are mostly associated with behavioural-variant frontotemporal dementia and progressive non-fluent aphasia subtypes.16 The ratio between phosphorylated tau (p-tau181) and total tau (t-tau) has been suggested for differentiation between molecular frontotemporal dementia subtypes. Frontotemporal dementia cases with TDP-43 pathological inclusions have a reduction in the ratio of p-tau181 and t-tau compared to cases with tau pathology.35,36 Additionally, plasma p-tau181 levels are only significantly altered in the MAPT genetic subtype of frontotemporal dementia.37 Tau can also aid in the differentiation between frontotemporal dementia and Alzheimer’s disease, with a higher t-tau:amyloid-β1-42 ratio is indicative of Alzheimer’s disease38 and plasma p-tau181 is elevated in Alzheimer’s disease compared to frontotemporal dementia and controls.39,40 In amyotrophic lateral sclerosis, there is also a significant reduction of both p-tau181 and the p-tau:t-tau ratio compared to frontotemporal dementia with 4-repeat tau inclusions and controls.41 However, the multiple fragments of tau, p-tau, t-tau and non-phosphorylated tau alone are, so far, unable to effectively discriminate between frontotemporal dementia and Alzheimer’s disease, suggesting that tau alone is unlikely to be a disease specific biomarker.42
C9ORF72
The C9orf72 gene consists of 12 exons, of which exons 1a and 1b are alternatively spliced, producing three transcripts and two isoforms.18,43 A G4C2 hexanucleotide repeat expansion is located between exons 1a and 1b, and there are usually 2–20 repeats in healthy individuals. However, in behavioural-variant frontotemporal dementia and amyotrophic lateral sclerosis, hundreds or even thousands of repeats are present; the exact minimum number required for pathology is currently undefined.7 The physiological role of C9ORF72 protein is unclear; however, it may modulate neuronal morphogenesis.44 In addition, C9orf72 has a potential role in neuroinflammation, as demonstrated by C9orf72 knockout rodent studies that exhibit a systemic proinflammatory state, severe autoimmune disease in some strains45,46 and mild neuroinflammation with increased expression of key inflammatory mediators in microglia, and an upregulation of inflammatory genes compared to control cohorts.47 Furthermore, C9orf72 has been identified to be required for normal function of myeloid cells, and altered microglial function is suggested to contribute to neurodegeneration in C9orf72 expansion carriers.47 Therefore, C9ORF72 may have the potential to also be used as a marker of altered immune status in frontotemporal dementia and amyotrophic lateral sclerosis; however, this remains to be investigated. Another aspect of C9orf72 that could contribute towards the development biomarkers for frontotemporal dementia and amyotrophic lateral sclerosis is the study of methylation of G4C2 hexanucleotide repeat. Methylation studies have revealed that large repeats are methylated whereas small or intermediate repeats are not, providing a new avenue for distinguishing repeat sizes.48,49 The same phenomenon was observed in blood, brain and spinal cord tissues of an individual.49 Further investigation into C9ORF72 methylation may help to establish a more accurate cut-off for pathogenic repeat.
Pathologically, C9orf72 is the most common gene implicated in behavioural-variant frontotemporal dementia and amyotrophic lateral sclerosis, affecting ∼40% of familial amyotrophic lateral sclerosis and 25% of familial behavioural-variant frontotemporal dementia.50 A pathological mechanism of C9orf72 gene expansion entails the translation of the expansion into dipeptide repeat proteins.51–53 Formation of dipeptide repeat protein occurs when repeat-associated non-ATG (RAN) translation of the hexanucleotide repeat forms five dipeptide repeat proteins: glycine-alanine (GA), glycine-arginine (GR), proline-alanine (PA), proline-arginine (PR) and glycine-proline (GP).53 Production of poly-PR and poly-GR leads to neurotoxicity via impaired protein translation.54 Poly-GP has attracted attention as a potential biomarker in C9orf72 gene expansion carriers in both behavioural-variant frontotemporal dementia and amyotrophic lateral sclerosis. While asymptomatic mutation carriers have elevated CSF and peripheral blood mononuclear cell poly-GP, these levels were raised further in disease groups.55–57
SOD1
SOD1 is an antioxidant that converts superoxide into molecular oxygen and hydrogen peroxide.58 There are numerous amyotrophic lateral sclerosis-linked SOD1 mutations.59,60 SOD1 protein aggregates are characteristic of amyotrophic lateral sclerosis patients with SOD1 mutations and are the second most common pathological subtype of amyotrophic lateral sclerosis. However, there are no significant changes in CSF SOD1 levels in amyotrophic lateral sclerosis, including sporadic or SOD1 mutation carriers,61 suggesting that SOD1 may not be an effective biomarker for amyotrophic lateral sclerosis. Contrary to this, CSF SOD1 has previously been identified as a robust pharmacodynamic marker in response to antisense oligonucleotide treatment.62 CSF SOD1 has subsequently been used as a pharmacodynamic biomarker in a phase 1–2 ascending dose clinical trial of the SOD1 antisense oligonucleotide Tofersen. In the highest-dosage group, CSF SOD1 levels declined 36% with some evidence of a reduction in disease measures; however, a correlation between treatment and clinical outcomes could not be drawn due to the small study size.63 Therefore, the use of SOD1 as a biomarker may lie primarily in determining the pharmacodynamics of SOD1-lowering therapies as opposed to differentiating between subtypes of amyotrophic lateral sclerosis.
Neurofilament-related biomarkers
Neurofilaments, consisting of neurofilament heavy, medium and light chains (NfH, NfM and NfL; Fig. 1) and α-internexin, are abundantly expressed in neurons and thought to function in axonal growth and maintenance.64 Increased neurofilament levels in CSF or blood have been used as a marker for neurodegeneration so far.65
Neurofilament light chain
NfL has attracted considerable focus as a biomarker of neurodegeneration. NfL has been repeatedly shown to have important prognostic value in frontotemporal dementia. Serum NfL is inversely correlated with survival time.66 Furthermore, presymptomatic cases have shown elevated NfL within serum before disease manifestation.67 Consistent with this, serum NfL was one of the earliest identified altered markers in frontotemporal dementia GRN-mutation carriers.68 Plasma NfL also has prognostic value with plasma NfL elevation occurring in asymptomatic frontotemporal dementia mutation carriers preceding disease onset.69
NfL levels in serum correlate to those in CSF. Importantly, serum and CSF NfL levels correlate with disease severity, functional impairment and brain atrophy.70,71 For diagnosis, CSF NfL combined with p-tau181:t-tau ratio provided 80% sensitivity and 81% specificity in differentiating between frontotemporal dementia cases with tau or TDP-43 pathological inclusions, with NfL levels most significantly elevated in those with TDP-43 pathological inclusions.72 This is consistent with C9orf72 gene expansion carriers found to display higher serum NfL than frontotemporal dementia patients without the mutation.
In amyotrophic lateral sclerosis, NfL has been predominantly studied in CSF and NfL is currently considered the most effective amyotrophic lateral sclerosis biomarker for diagnosis and predicting survival time.73 Elevated CSF NfL is indicative of amyotrophic lateral sclerosis severity and progression.74 Amongst neurodegenerative diseases, frontotemporal dementia and amyotrophic lateral sclerosis exhibit the greatest elevations in CSF NfL.75 Compared to healthy controls, NfL levels are ∼20-fold higher in amyotrophic lateral sclerosis CSF, whereas they are 3-fold higher in frontotemporal dementia CSF.76 In a Swedish cohort study, amyotrophic lateral sclerosis patients exhibited a 709% increase in CSF NfL compared to controls, whereas frontotemporal dementia patients had a 307% increase. In both diseases, elevated NfL inversely correlated to survival time, suggesting that NfL may be a relevant prognostic biomarker.77 NfL has also been studied in amyotrophic lateral sclerosis serum, correlating to disease progression and decreased survival time, but not disease severity.78 Blood and CSF NfL is amongst the earliest markers to change in patients transitioning from presymptomatic to symptomatic.79 Furthermore, NfL could be used to differentiate amyotrophic lateral sclerosis subtypes. For example, plasma NfL levels were significantly higher in bulbar-onset amyotrophic lateral sclerosis patients compared to spinal-onset amyotrophic lateral sclerosis patients.80 Also, NfL could potentially be used as a diagnostic biomarker to differentiate amyotrophic lateral sclerosis from clinically relevant amyotrophic lateral sclerosis mimics.80
However, it is important to note that an elevation in NfL levels is common in other neurodegenerative diseases, including Alzheimer’s disease,81 Parkinson’s disease82 and multiple sclerosis,83 suggesting that NfL is a general marker of neurodegeneration induced axonal damage rather than specific to disease processes in frontotemporal dementia or amyotrophic lateral sclerosis. Nevertheless, NfL is useful as a differentiating biomarker as elevations are greater in frontotemporal dementia and amyotrophic lateral sclerosis compared to atypical parkinsonism and various dementias especially when aged-related concentration cut-offs are considered.84 Overall, NfL is so far the most established biomarker in frontotemporal dementia and amyotrophic lateral sclerosis, although its utility across the spectrum of neurodegenerative diseases indicates that more specific biomarkers are required.
Neurofilament heavy chain
As the NfH chain is phosphorylated, most studies have targeted phosphorylated NfH (pNfH). CSF pNfH has been shown to be elevated in frontotemporal dementia compared to early-onset Alzheimer’s disease.85 In amyotrophic lateral sclerosis, pNfH was elevated in serum, plasma and CSF. In this study, pNfH levels in all biofluids positively correlated with increases in disease progression.86 Both NfL and pNfH are also elevated in amyotrophic lateral sclerosis CSF and serum before symptom onset from nine months to 3.5 years.87 Receiver operating characteristics showed that CSF pNfH could differentiate amyotrophic lateral from amyotrophic lateral mimics.80 While further research is required, pNfH exhibits promise as a biomarker for both frontotemporal dementia and amyotrophic lateral sclerosis.
Neurofilament medium chain
NfM is the least explored neurofilament biomarker so far. A recent study, based on antibody-suspension bead arrays, demonstrated that NfM is elevated in frontotemporal dementia CSF.88 Furthermore, in an earlier study, high levels of NfM were observed in amyotrophic lateral sclerosis plasma.89 Further research is required to establish any potential utility of NfM as a biomarker for frontotemporal dementia and amyotrophic lateral sclerosis.
Immune-related biomarkers
Neuroinflammation is considered a pathological hallmark of neurodegenerative diseases and there has been increasing attention on the potential role of neuroinflammatory and peripheral inflammatory pathways in the pathogenesis of both frontotemporal dementia and amyotrophic lateral sclerosis, as reviewed elsewhere.90,91 As such, various immune-related biomarkers have been explored in both frontotemporal dementia and amyotrophic lateral sclerosis (Fig. 1); however, concerns remain as to the sensitivity and in particular specificity of such markers given their general levels across the spectrum of neurodegenerative diseases.
YKL-40
Chitinase-3-like protein (YKL-40) is a glycoprotein thought to be involved in extracellular matrix remodelling and inflammation.92–94 YKL-40 CSF levels are increased in monogenic and sporadic cases of frontotemporal dementia and amyotrophic lateral sclerosis compared to both asymptomatic mutation carriers and controls.95 These findings have been pathologically validated with elevated YKL-40 prevalent in the amyotrophic lateral sclerosis motor cortex, frontal cortex and spinal cord.96,97 YKL-40 CSF levels are increased in frontotemporal dementia with TDP inclusions compared to controls. Additionally, when combined with p-tau and the p-tau/t-tau ratio, differentiated frontotemporal dementia from non-frontotemporal dementia dementias, including Alzheimer’s disease and dementia with Lewy bodies, with 90% sensitivity and 78% specificity.98 Interestingly, CSF YKL-40 levels are greater in frontotemporal dementia with GRN and MAPT mutations than in C9orf72 expansion carriers.99 However, a recurring finding is that alterations in CSF are not reflected in plasma or serum.95–97,100 Furthermore, YKL-40 is also altered in Alzheimer’s disease, thus YKL-40 alone should be considered a marker of neurodegeneration relating to neuroinflammatory mechanisms rather than a disease specific biomarker.101
GFAP
Glial fibrillary acidic protein (GFAP) is an intermediate filament protein released by astrocytes during astrogliosis.102 GFAP has been considered a potential frontotemporal dementia biomarker with elevated GFAP levels in the CSF103 and plasma of frontotemporal dementia GRN-mutation carriers.104 In serum, GFAP is correlated with cognitive state.105 In amyotrophic lateral sclerosis, GFAP is also elevated in CSF samples.106 However, since GFAP is a measure of astrogliosis, which is common in other neurodegenerative diseases including Alzheimer’s disease and dementia with Lewy bodies, it cannot be used as a specific biomarker for frontotemporal dementia or amyotrophic lateral sclerosis.103
CST3
Cystatin C or cystatin 3 (CST3) is a cysteine protease inhibitor that is abundant in the CSF and implicated in cell signalling, inflammation and neuronal cell death.107–110 CST3 is thought to have a pathological role within the amyotrophic lateral sclerosis brain, being one of only two known proteins in Bunina bodies, an intraneuronal inclusion found only in amyotrophic lateral sclerosis.111,112 However, it is unclear whether CST3 is altered in CSF or serum in amyotrophic lateral sclerosis with different studies reporting contradictory results.113–115 Analysis of CST3 levels in frontotemporal dementia has not been well characterized to date. In one study, CST3 was shown to be decreased in frontotemporal dementia with GRN mutation compared to C9orf72 repeat expansion carriers, suggesting that it may differentiate frontotemporal dementia subtypes.116 However, CST3 levels were also significantly lower in both CSF and serum in Alzheimer’s disease and dementia with Lewy bodies.117 Further research is needed to establish the use of CST3 as a biomarker for amyotrophic lateral sclerosis or frontotemporal dementia.
Emerging biomarkers
An emerging biomarker for amyotrophic lateral sclerosis is T regulatory cells (Tregs). A number of studies has shown a significant and progressive reduction in number of Tregs, and that Tregs are less effective in promoting immune suppression in amyotrophic lateral sclerosis patients.118–121 Tregs levels have been shown to correlate with rate of disease progression and patient survival and are therefore considered a promising therapeutic target for neuroprotection in amyotrophic lateral sclerosis as reviewed in detail elsewhere.122 Aside from presenting as a promising therapeutic target, Tregs measurement in blood is also considered an important pharmacodynamic target of biomarkers across different clinical trials as recently reviewed,123 therefore having the potential to be informative in disease phenotype and clinical stratification.
Another emerging biomarker for amyotrophic lateral sclerosis is the urinary neurotrophin receptor p75 extracellular domain (p75ECD). There is evidence indicating a significant elevation in p75ECD concentrations in the of amyotrophic lateral sclerosis patients, suggesting urinary p75ECD concentration could be a potential biomarker for amyotrophic lateral sclerosis.124,125 Urinary p75ECD concentrations reflected the disease severity and provided additional evidence for an amyotrophic lateral sclerosis diagnosis in patients with clinically suspected amyotrophic lateral sclerosis.125 In a recent meta-analysis, consisting of five case-control studies, urinary p75ECD levels were shown to be significantly higher in patients with amyotrophic lateral sclerosis compared to non-neurological controls.126 The strong association between p75ECD levels and amyotrophic lateral sclerosis supports further investigation of p75ECD as a potential biomarker for amyotrophic lateral sclerosis as a diagnostic biomarker and a progression indicator.126 The practicalities of urinary biomarkers are advantageous given their non-invasive nature and ease of accessibility.
A biomarker strategy that is emerging in the field of neurodegenerative diseases is the polygenic risk score (PRS). PRS is based on a computational algorithm that combines vast measures of genome-wide genetic data to predict an individual’s inherited susceptibility to a disease. It has been widely applied to the analysis of cancer and cardiovascular disease and to a limited degree Alzheimer’s disease.127 It is beginning to be applied to frontotemporal dementia and amyotrophic lateral sclerosis. PRS is particularly useful in the study of these complex diseases, in which the genetic aetiology is multifactorial and heterogeneous. In one study, polygenic risk for frontotemporal dementia was shown to be associated with executive functioning, whereas polygenic risk for amyotrophic lateral sclerosis was associated with verbal-numeric reasoning.128 Future studies that combine PRS with pathway analysis will enable determining more enhanced therapeutic and preventative measures for frontotemporal dementia and amyotrophic lateral sclerosis.
Proteomics biomarker discovery
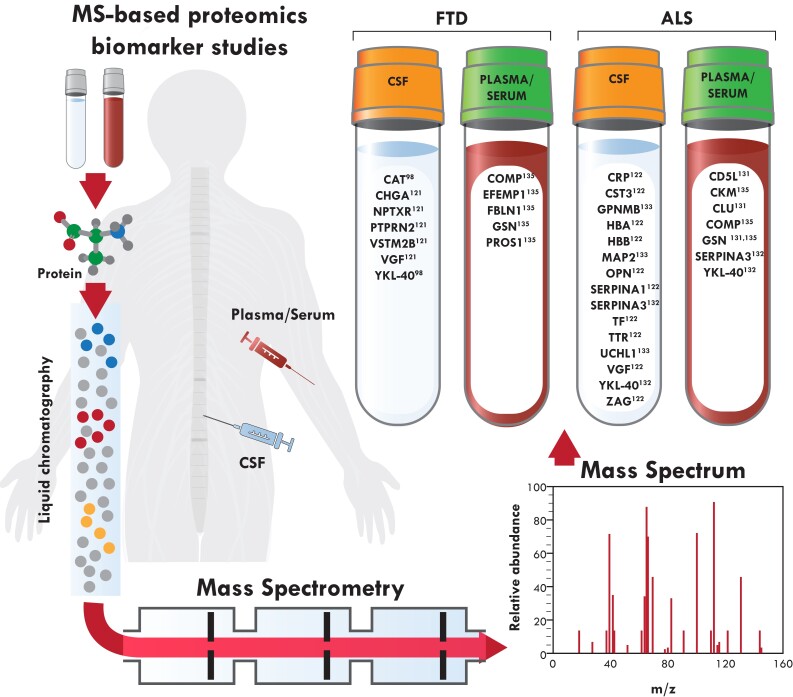
Proteomics allows for unbiased global quantification of alterations in protein levels. Mass spectrometry (MS) is the current gold standard for proteomics analysis.129 MS proteomics is increasingly used in neurodegenerative diseases, particularly for biomarker development in Alzheimer’s disease130 and Parkinson’s disease.131 However, MS proteomics for analysis of frontotemporal dementia and amyotrophic lateral sclerosis biofluids has been hindered by a lack of validation across studies100,132–134 and so far MS proteomics in amyotrophic lateral sclerosis is more comprehensive than in frontotemporal dementia (Fig. 2).
Figure 2.
Mass spectrometry-based proteomics biomarker development in frontotemporal dementia and amyotrophic lateral sclerosis. Patient plasma/serum and CSF samples are processed and analysed by liquid chromatography and mass spectrometry. Proteins identified as potential biomarkers for frontotemporal dementia and amyotrophic lateral sclerosis are listed with their references.
In amyotrophic lateral sclerosis, one of the earliest studies identified CST3 and transthyretin (TTR) as potential biomarkers.113 A meta-analysis of this study and 10 subsequent studies in amyotrophic lateral sclerosis spanning a 10-year period (2005–16) identified 10 proteins (c-reactive protein, CST3, α-globin, β-globin, osteopontin, serpin A1, transferrin, TTR, nerve-growth factor and zinc-alpha-2-glycoprotein) as potential biomarkers in at least two independent studies135 (Fig. 2). For instance, CST3 was identified to be decreased in five studies.113,136–139 However, there were also some inconsistencies in the results across these studies. serpin A1, which was identified in three studies, was increased in amyotrophic lateral sclerosis in two studies137,140 but decreased in the third.141 Additionally, TTR was reported as significantly decreased in amyotrophic lateral sclerosis in three studies113,142 but increased in a fourth study.143 The publications included in this meta-analysis used a variety of different MS technologies, which may explain the inconsistency in the results. However, none of the 10 proteins identified for amyotrophic lateral sclerosis in the meta-analysis were validated in the four MS proteomics studies performed since.134,144–146 Interestingly, TTR and nerve-growth factor ‘inducible’ have also been identified in a subsequent publication as potential biomarkers for frontotemporal dementia.133
So far, only two studies have analysed frontotemporal dementia and amyotrophic lateral sclerosis biofluids side-by-side using MS. The first study used a combination of iTRAQ-based MS, multiple reaction monitoring and single-molecule array to identify and validate eight proteins (chitinase-3-like protein 2, crystallin alpha B, profilin-1, neural proliferation differentiation and control 1, ubiquitin carboxyl-terminal hydrolase L1, neuronal pentraxin receptor, triggering receptor expressed on myeloid cells 2 and transferrin receptor 1) with altered CSF levels in multiple C9orf72 gene expansion cohorts and asymptomatic carriers.147 A more recent study, examining serum from frontotemporal dementia and amyotrophic lateral sclerosis patients identified 23 proteins altered in behavioural-variant frontotemporal dementia and 14 in amyotrophic lateral sclerosis serum. Of these identified proteins, 11 were altered in both diseases and six proteins were validated; cartilage oligomeric matrix protein, EGF containing fibulin extracellular matrix protein-1, FBLN1, gelsolin and protein S in frontotemporal dementia and creatinine kinase M-type, cartilage oligomeric matrix protein and gelsolin in amyotrophic lateral sclerosis.148 Inconsistency in the findings so far highlight the need for more thorough MS proteomics studies, particularly using plasma or serum. A possible explanation for the current inconsistency in results and lack of reproducibility may relate to different MS methodology and batch effects.
Other ‘omics’ approaches are also being used to screen for potential biomarkers and these include lipidomics in frontotemporal dementia149 and amyotrophic lateral sclerosis,150,151 metabolomics in frontotemporal dementia152,153 and amyotrophic lateral sclerosis.154 Most of the research are currently in an exploratory phase and it remains to be seen whether omics approaches can result in identification of biomarkers that are specific to frontotemporal dementia and amyotrophic lateral sclerosis. It is likely that, rather than a single biomarker, a panel of biomarkers that includes a combination of proteins, lipids and metabolites, is needed as an effective biomarker strategy for frontotemporal dementia and amyotrophic lateral sclerosis.
Conclusion
Frontotemporal dementia and amyotrophic lateral sclerosis exist on a disease spectrum with overlapping biochemical and genetic traits. Currently, there are no specific fluid biomarkers for either disease. A number of proteins has been explored as potential fluid biomarkers for frontotemporal dementia and amyotrophic lateral sclerosis, particularly those relating to frontotemporal dementia/amyotrophic lateral sclerosis brain pathology, e.g. TDP-43, tau, C9ORF72 or SOD1. Other proteins, including NfL and immune-related markers YKL-40, GFAP and CST3 have also been explored as potential biomarkers, however, these markers are non-specific indicators of neurodegeneration or astrogliosis across the spectrum of neurodegeneration, thus limiting their utility specifically in frontotemporal dementia and amyotrophic lateral sclerosis.
Earlier research focused heavily on CSF, however, given the difficulty and invasiveness of collecting CSF from patients, there is an increased need for the development of blood-based biomarkers. Recent advances in MS have allowed for a greater detection and measurement of low abundant yet significantly altered proteins in serum and plasma. Consequently, mass spectrometry-based proteomics has come to the forefront as the method of choice for blood-based biomarker discovery.
So far, approaches to overcoming the various hurdles in biomarker development for frontotemporal dementia and amyotrophic lateral sclerosis, including a lack of specificity of biomarkers for frontotemporal dementia and amyotrophic lateral sclerosis given the expression of such markers in other neurodegenerative diseases, matrix issues when testing blood representative of what is expressed in CSF and inconsistencies in replicating results from different research groups, have been limited. Going forward, harnessing the current knowledge of the immune response, including its duration and whether it is a primary or secondary response to neurodegenerative processes, is crucial to identify sensitive and specific biomarkers of disease progression, particularly biofluid biomarkers that are non-invasive, accessible and already established for use in a clinical setting (i.e. blood and urine). An important strategy in the biomarker development will be the integration of genetics and multi-level omics approaches, which may be achieved by harnessing bioinformatics and deep-learning methods that may further integrate imputation of multi-omics and genetic datasets. An advantageous approach would be to collectively and comprehensively analyse all potential biomarkers that have been identified so far across frontotemporal dementia and amyotrophic lateral sclerosis using machine learning and AI technologies to identify common and/or overlapping proteins or pathways or molecular hits that can then be confirmed as informed targets to streamline biomarker discovery.
Acknowledgements
We thank Heidi Cartwright for figure preparation.
Abbreviations
- CST
cystatin
- MS
mass spectrometry
- NfH
neurofilament heavy
- NfL
neurofilament light
- NfM
neurofilament medium
- p75ECD
urinary neurotrophin receptor p75 extracellular domain
- TDP-43
TAR DNA binding protein 43
- YKL-40
chitinase-3-like protein
Funding
This work was supported by funding to ForeFront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neuron disease, from the National Health and Medical Research Council of Australia (NHMRC) program grants (#1132524, #1095127) and partnership project (#1153439). G.M.H. is a NHMRC Leadership Fellow (#1176607), M.C.K. is a NHMRC Practitioner Fellow (#1156093), L.M.I. is a NHMRC Principal Research Fellow (#1136241) and O.P. is a NHMRC Senior Research Fellow (#1103258).
Competing interests
The authors report no competing interests.
References
- 1. Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet. 2015;386(10004):1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lambert MA, Bickel H, Prince M, et al. Estimating the burden of early onset dementia; systematic review of disease prevalence. Eur J Neurol. 2014;21(4):563–569. [DOI] [PubMed] [Google Scholar]
- 3. Olney NT, Spina S, Miller BL. Frontotemporal dementia. Neurol Clin. 2017;35(2):339–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pottier C, Ravenscroft TA, Sanchez-Contreras M, Rademakers R. Genetics of FTLD: Overview and what else we can expect from genetic studies. J Neurochem. 2016;138(Suppl 1):32–53. [DOI] [PubMed] [Google Scholar]
- 5. Schroeter ML, Pawelke S, Bisenius S, et al. A modified reading the mind in the eyes test predicts behavioral variant frontotemporal dementia better than executive function tests. Front Aging Neurosci. 2018;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: A clinical approach. J Neurol. 2018;265(6):1474–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Woollacott IO, Rohrer JD. The clinical spectrum of sporadic and familial forms of frontotemporal dementia. J Neurochem. 2016;138(Suppl 1):6–31. [DOI] [PubMed] [Google Scholar]
- 8. Ciervo Y, Ning K, Jun X, Shaw PJ, Mead RJ. Advances, challenges and future directions for stem cell therapy in amyotrophic lateral sclerosis. Mol Neurodegener. 2017;12(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taylor JP, Brown RH Jr, Cleveland DW. Decoding ALS: From genes to mechanism. Nature. 2016;539(7628):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Talbott EO, Malek AM, Lacomis D. The epidemiology of amyotrophic lateral sclerosis. Handb Clin Neurol. 2016;138:225–38. [DOI] [PubMed] [Google Scholar]
- 11. van Es MA, Hardiman O, Chio A, et al. Amyotrophic lateral sclerosis. Lancet. 2017;390(10107):2084–2098. [DOI] [PubMed] [Google Scholar]
- 12. Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942–55. [DOI] [PubMed] [Google Scholar]
- 13. Turner MR. Motor neuron disease: Biomarker development for an expanding cerebral syndrome. Clin Med (Lond). 2016;16(Suppl 6):s60–s65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lattante S, Ciura S, Rouleau GA, Kabashi E. Defining the genetic connection linking amyotrophic lateral sclerosis (ALS) with frontotemporal dementia (FTD). Trends Genet. 2015;31(5):263–273. [DOI] [PubMed] [Google Scholar]
- 15. Meeter LH, Kaat LD, Rohrer JD, van Swieten JC. Imaging and fluid biomarkers in frontotemporal dementia. Nat Rev Neurol. 2017;13(7):406–419. [DOI] [PubMed] [Google Scholar]
- 16. Tan RH, Ke YD, Ittner LM, Halliday GM. ALS/FTLD: Experimental models and reality. Acta Neuropathol. 2017;133(2):177–196. [DOI] [PubMed] [Google Scholar]
- 17. Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. [DOI] [PubMed] [Google Scholar]
- 18. DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Renton AE, Majounie E, Waite A, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 20 2011;72(2):257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Borroni B, Archetti S, Del Bo R, et al. TARDBP mutations in frontotemporal lobar degeneration: Frequency, clinical features, and disease course. Rejuvenation Res. 2010;13(5):509–517. [DOI] [PubMed] [Google Scholar]
- 21. Mackenzie IR, Rademakers R, Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 2010;9(10):995–1007. [DOI] [PubMed] [Google Scholar]
- 22. Buratti E, Baralle FE. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front Biosci. 2008;13:867–878. [DOI] [PubMed] [Google Scholar]
- 23. Buratti E, Baralle FE. TDP-43: Gumming up neurons through protein-protein and protein-RNA interactions. Trends Biochem Sci. 2012;37(6):237–247. [DOI] [PubMed] [Google Scholar]
- 24. Suarez-Calvet M, Dols-Icardo O, Llado A, et al. Plasma phosphorylated TDP-43 levels are elevated in patients with frontotemporal dementia carrying a C9orf72 repeat expansion or a GRN mutation. J Neurol Neurosurg Psychiatry. 2014;85(6):684–691. [DOI] [PubMed] [Google Scholar]
- 25. Junttila A, Kuvaja M, Hartikainen P, et al. Cerebrospinal fluid TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis patients with and without the C9ORF72 hexanucleotide expansion. Dement Geriatr Cogn Dis Extra. 2016;6(1):142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kasai T, Tokuda T, Ishigami N, et al. Increased TDP-43 protein in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Acta Neuropathol. 2009;117(1):55–62. [DOI] [PubMed] [Google Scholar]
- 27. Majumder V, Gregory JM, Barria MA, Green A, Pal S. TDP-43 as a potential biomarker for amyotrophic lateral sclerosis: A systematic review and meta-analysis. BMC Neurol. 2018;18(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feneberg E, Gray E, Ansorge O, Talbot K, Turner MR. Towards a TDP-43-based biomarker for ALS and FTLD. Mol Neurobiol. 2018;55(10):7789–7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feneberg E, Steinacker P, Lehnert S, et al. Limited role of free TDP-43 as a diagnostic tool in neurodegenerative diseases. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(5-6):351–356. [DOI] [PubMed] [Google Scholar]
- 30. Goedert M. Tau filaments in neurodegenerative diseases. FEBS Lett. 2018;592(14):2383–2391. [DOI] [PubMed] [Google Scholar]
- 31. Hirokawa N. Microtubule organization and dynamics dependent on microtubule-associated proteins. Curr Opin Cell Biol. 1994;6(1):74–81. [DOI] [PubMed] [Google Scholar]
- 32. Poorkaj P, Bird TD, Wijsman E, et al. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43(6):815–825. [DOI] [PubMed] [Google Scholar]
- 33. Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393(6686):702–705. [DOI] [PubMed] [Google Scholar]
- 34. van der Zee J, Van Broeckhoven C. Dementia in 2013: Frontotemporal lobar degeneration—building on breakthroughs. Nat Rev Neurol. 2014;10(2):70–72. [DOI] [PubMed] [Google Scholar]
- 35. Borroni B, Benussi A, Archetti S, et al. Csf p-tau181/tau ratio as biomarker for TDP pathology in frontotemporal dementia. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(1–2):86–91. [DOI] [PubMed] [Google Scholar]
- 36. Hu WT, Watts K, Grossman M, et al. Reduced CSF p-Tau181 to Tau ratio is a biomarker for FTLD-TDP. Neurology. 2013;81(22):1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Foiani MS, Woollacott IO, Heller C, et al. Plasma tau is increased in frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2018;89(8):804–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Irwin DJ, Trojanowski JQ, Grossman M. Cerebrospinal fluid biomarkers for differentiation of frontotemporal lobar degeneration from Alzheimer's disease. Front Aging Neurosci. 2013;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Benussi A, Karikari TK, Ashton N, et al. Diagnostic and prognostic value of serum NfL and p-Tau181 in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry. 2020;91(9):960–967. [DOI] [PubMed] [Google Scholar]
- 41. Grossman M, Elman L, McCluskey L, et al. Phosphorylated tau as a candidate biomarker for amyotrophic lateral sclerosis. JAMA Neurol. 2014;71(4):442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Foiani MS, Cicognola C, Ermann N, et al. Searching for novel cerebrospinal fluid biomarkers of tau pathology in frontotemporal dementia: An elusive quest. J Neurol Neurosurg Psychiatry. 2019;90:740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Burrell JR, Halliday GM, Kril JJ, et al. The frontotemporal dementia-motor neuron disease continuum. Lancet. 2016;388(10047):919–931. [DOI] [PubMed] [Google Scholar]
- 44. Ho WY, Tai YK, Chang JC, et al. The ALS-FTD-linked gene product, C9orf72, regulates neuronal morphogenesis via autophagy. Autophagy. 2019;15(5):827–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Atanasio A, Decman V, White D, et al. C9orf72 ablation causes immune dysregulation characterized by leukocyte expansion, autoantibody production, and glomerulonephropathy in mice. Sci Rep. 2016;6:23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Burberry A, Suzuki N, Wang JY, et al. Loss-of-function mutations in the C9ORF72 mouse ortholog cause fatal autoimmune disease. Sci Transl Med. 2016;8(347):347ra93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. O’Rourke JG, Bogdanik L, Yanez A, et al. C9orf72 is required for proper macrophage and microglial function in mice. Science. 2016;351(6279):1324–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xi Z, van Blitterswijk M, Zhang M, et al. Jump from pre-mutation to pathologic expansion in C9orf72. Am J Hum Genet. 2015;96(6):962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xi Z, Zhang M, Bruni AC, et al. The C9orf72 repeat expansion itself is methylated in ALS and FTLD patients. Acta Neuropathologica. 2015;129(5):715–727. [DOI] [PubMed] [Google Scholar]
- 50. Renton AE, Chio A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci. 2014;17(1):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shi Y, Lin S, Staats KA, et al. Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat Med. 2018;24(3):313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gao FB, Almeida S, Lopez-Gonzalez R. Dysregulated molecular pathways in amyotrophic lateral sclerosis-frontotemporal dementia spectrum disorder. EMBO J. 2017;36(20):2931–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kumar V, Hasan GM, Hassan MI. Unraveling the role of RNA mediated toxicity of C9orf72 repeats in C9-FTD/ALS. Front Neurosci. 2017;11:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kanekura K, Yagi T, Cammack AJ, et al. Poly-dipeptides encoded by the C9ORF72 repeats block global protein translation. Hum Mol Genet. 2016;25(9):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gendron TF, Chew J, Stankowski JN, et al. Poly(GP) proteins are a useful pharmacodynamic marker for C9ORF72-associated amyotrophic lateral sclerosis. Sci Transl Med. 2017;9(383):eaai7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Meeter LHH, Gendron TF, Sias AC, et al. Poly(GP), neurofilament and grey matter deficits in C9orf72 expansion carriers. Ann Clin Transl Neurol. 2018;5(5):583–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lehmer C, Oeckl P, Weishaupt JH, et al. Poly-GP in cerebrospinal fluid links C9orf72-associated dipeptide repeat expression to the asymptomatic phase of ALS/FTD. EMBO Mol Med. 2017;9(7):859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bunton-Stasyshyn RK, Saccon RA, Fratta P, Fisher EM. SOD1 function and its implications for amyotrophic lateral sclerosis pathology: New and renascent themes. Neuroscientist. 2015;21(5):519–29. [DOI] [PubMed] [Google Scholar]
- 59. Rosen DR, Siddique T, Patterson D, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. [DOI] [PubMed] [Google Scholar]
- 60. Saccon RA, Bunton-Stasyshyn RK, Fisher EM, Fratta P. Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain. 2013;136(Pt 8):2342–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zetterstrom P, Andersen PM, Brannstrom T, Marklund SL. Misfolded superoxide dismutase-1 in CSF from amyotrophic lateral sclerosis patients. J Neurochem. 2011;117(1):91–99. [DOI] [PubMed] [Google Scholar]
- 62. Winer L, Srinivasan D, Chun S, et al. SOD1 in cerebral spinal fluid as a pharmacodynamic marker for antisense oligonucleotide therapy. JAMA Neurol. 2013;70(2):201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Miller T, Cudkowicz M, Shaw PJ, et al. Phase 1–2 trial of antisense oligonucleotide Tofersen for SOD1 ALS. N Engl J Med. 2020;383(2):109–119. [DOI] [PubMed] [Google Scholar]
- 64. Yuan A, Rao MV, Veeranna, Nixon RA. Neurofilaments at a glance. J Cell Sci. 2012;125(Pt 14):3257–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577–589. [DOI] [PubMed] [Google Scholar]
- 66. Cajanus A, Katisko K, Kontkanen A, et al. Serum neurofilament light chain in FTLD: Association with C9orf72, clinical phenotype, and prognosis. Ann Clin Transl Neurol. 2020;7(6):903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van der Ende EL, Meeter LH, Poos JM, et al. Serum neurofilament light chain in genetic frontotemporal dementia: A longitudinal, multicentre cohort study. Lancet Neurol. 2019;18(12):1103–1111. [DOI] [PubMed] [Google Scholar]
- 68. Panman JL, Venkatraghavan V, van der Ende EL, et al. Modelling the cascade of biomarker changes in GRN-related frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2021;92:494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rojas JC, Wang P, Staffaroni AM, et al. Plasma neurofilament light for prediction of disease progression in familial frontotemporal lobar degeneration. Neurology. 2021;96:e2296–e2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wilke C, Preische O, Deuschle C, et al. Neurofilament light chain in FTD is elevated not only in cerebrospinal fluid, but also in serum. J Neurol Neurosurg Psychiatry. 2016;87(11):1270–1272. [DOI] [PubMed] [Google Scholar]
- 71. Meeter LHH, Vijverberg EG, Del Campo M, et al. Clinical value of neurofilament and phospho-tau/tau ratio in the frontotemporal dementia spectrum. Neurology. 2018;90(14):e1231–e1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Abu-Rumeileh S, Mometto N, Bartoletti-Stella A, et al. Cerebrospinal fluid biomarkers in patients with frontotemporal dementia spectrum: A single-center study. J Alzheimers Dis. 2018;66(2):551–563. [DOI] [PubMed] [Google Scholar]
- 73. Abu-Rumeileh S, Vacchiano V, Zenesini C, et al. Diagnostic-prognostic value and electrophysiological correlates of CSF biomarkers of neurodegeneration and neuroinflammation in amyotrophic lateral sclerosis. J Neurol. 2020;267:1699–1708. [DOI] [PubMed] [Google Scholar]
- 74. Sun Q, Zhao X, Li S, et al. CSF neurofilament light chain elevation predicts ALS severity and progression. Front Neurol. 2020;11:919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bridel C, van Wieringen WN, Zetterberg H, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: A systematic review and meta-analysis. JAMA Neurol. 2019;76(9):1035–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90:870–881. [DOI] [PubMed] [Google Scholar]
- 77. Skillback T, Mattsson N, Blennow K, Zetterberg H. Cerebrospinal fluid neurofilament light concentration in motor neuron disease and frontotemporal dementia predicts survival. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(5–6):397–403. [DOI] [PubMed] [Google Scholar]
- 78. Brodovitch A, Boucraut J, Delmont E, et al. Combination of serum and CSF neurofilament-light and neuroinflammatory biomarkers to evaluate ALS. Sci Rep. 2021;11(1):703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. van der Ende EL, Bron EE, Poos JM, et al. A data-driven disease progression model of fluid biomarkers in genetic frontotemporal dementia. Brain. 2021;17:e053497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Behzadi A, Pujol-Calderon F, Tjust AE, et al. Neurofilaments can differentiate ALS subgroups and ALS from common diagnostic mimics. Sci Rep. 2021;11(1):22128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat Med. 2019;25(2):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sako W, Murakami N, Izumi Y, Kaji R. Neurofilament light chain level in cerebrospinal fluid can differentiate Parkinson's disease from atypical parkinsonism: Evidence from a meta-analysis. J Neurol Sci. 2015;352(1–2):84–87. [DOI] [PubMed] [Google Scholar]
- 83. Edwards KR, Garten L, Button J, O’Connor J, Kamath V, Frazier C. Neurofilament light chain as an indicator of exacerbation prior to clinical symptoms in multiple sclerosis. Mult Scler Relat Disord. 2019;31:59–61. [DOI] [PubMed] [Google Scholar]
- 84. Ashton NJ, Janelidze S, Al Khleifat A, et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat Commun. 2021;12(1):3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Pijnenburg YA, Janssen JC, Schoonenboom NS, et al. CSF neurofilaments in frontotemporal dementia compared with early onset Alzheimer's disease and controls. Dement Geriatr Cogn Disord. 2007;23(4):225–230. [DOI] [PubMed] [Google Scholar]
- 86. Boylan KB, Glass JD, Crook JE, et al. Phosphorylated neurofilament heavy subunit (pNF-H) in peripheral blood and CSF as a potential prognostic biomarker in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2013;84(4):467–72. [DOI] [PubMed] [Google Scholar]
- 87. Benatar M, Wuu J, Lombardi V, et al. Neurofilaments in pre-symptomatic ALS and the impact of genotype. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(7–8):538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Remnestal J, Oijerstedt L, Ullgren A, et al. Altered levels of CSF proteins in patients with FTD, presymptomatic mutation carriers and non-carriers. Transl Neurodegener. 2020;9(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Haggmark A, Mikus M, Mohsenchian A, et al. Plasma profiling reveals three proteins associated to amyotrophic lateral sclerosis. Ann Clin Transl Neurol. 2014;1(8):544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bright F, Werry EL, Dobson-Stone C, et al. Neuroinflammation in frontotemporal dementia. Nat Rev Neurol. 2019;15(9):540–555. [DOI] [PubMed] [Google Scholar]
- 91. McCauley ME, Baloh RH. Inflammation in ALS/FTD pathogenesis. Acta Neuropathol. 2019;137(5):715–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rathcke CN, Vestergaard H. YKL-40–an emerging biomarker in cardiovascular disease and diabetes. Cardiovasc Diabetol. 2009;8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nordenbaek C, Johansen JS, Junker P, Borregaard N, Sorensen O, Price PA. YKL-40, a matrix protein of specific granules in neutrophils, is elevated in serum of patients with community-acquired pneumonia requiring hospitalization. J Infect Dis. 1999;180(5):1722–1726. [DOI] [PubMed] [Google Scholar]
- 94. Lee CG, Dela Cruz CS, Herzog E, Rosenberg SM, Ahangari F, Elias JA. YKL-40, a chitinase-like protein at the intersection of inflammation and remodeling. Am J Respir Crit Care Med. 2012;185(7):692–694. [DOI] [PubMed] [Google Scholar]
- 95. Oeckl P, Weydt P, Steinacker P, et al. Different neuroinflammatory profile in amyotrophic lateral sclerosis and frontotemporal dementia is linked to the clinical phase. J Neurol Neurosurg Psychiatry. 2019;90:4–10. [DOI] [PubMed] [Google Scholar]
- 96. Vu L, An J, Kovalik T, Gendron T, Petrucelli L, Bowser R. Cross-sectional and longitudinal measures of chitinase proteins in amyotrophic lateral sclerosis and expression of CHI3L1 in activated astrocytes. J Neurol Neurosurg Psychiatry. 2020;91:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Andres-Benito P, Dominguez R, Colomina MJ, Llorens F, Povedano M, Ferrer I. YKL40 in sporadic amyotrophic lateral sclerosis: Cerebrospinal fluid levels as a prognosis marker of disease progression. Aging. 2018;10(9):2367–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Del Campo M, Galimberti D, Elias N, et al. Novel CSF biomarkers to discriminate FTLD and its pathological subtypes. Ann Clin Transl Neurol. 2018;5(10):1163–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Woollacott IOC, Nicholas JM, Heller C, et al. Cerebrospinal fluid YKL-40 and chitotriosidase levels in frontotemporal dementia vary by clinical, genetic and pathological subtype. Dement Geriatr Cogn Disord. 2020;49(1):56–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Teunissen CE, Elias N, Koel-Simmelink MJ, et al. Novel diagnostic cerebrospinal fluid biomarkers for pathologic subtypes of frontotemporal dementia identified by proteomics. Alzheimers Dement (Amst). 2016;2:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Baldacci F, Lista S, Cavedo E, Bonuccelli U, Hampel H. Diagnostic function of the neuroinflammatory biomarker YKL-40 in Alzheimer's disease and other neurodegenerative diseases. Expert Rev Proteomics. 2017;14(4):285–299. [DOI] [PubMed] [Google Scholar]
- 102. Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol. 2011;93(3):421–43. [DOI] [PubMed] [Google Scholar]
- 103. Ishiki A, Kamada M, Kawamura Y, et al. Glial fibrillar acidic protein in the cerebrospinal fluid of Alzheimer's disease, dementia with Lewy bodies, and frontotemporal lobar degeneration. J Neurochem. 2016;136(2):258–261. [DOI] [PubMed] [Google Scholar]
- 104. Heller C, Foiani MS, Moore K, et al. Plasma glial fibrillary acidic protein is raised in progranulin-associated frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2020;91(3):263–270. [DOI] [PubMed] [Google Scholar]
- 105. Benussi A, Ashton NJ, Karikari TK, et al. Serum glial fibrillary acidic protein (GFAP) is a marker of disease severity in frontotemporal lobar degeneration. J Alzheimers Dis. 2020;77(3):1129–1141. [DOI] [PubMed] [Google Scholar]
- 106. Benninger F, Glat MJ, Offen D, Steiner I. Glial fibrillary acidic protein as a marker of astrocytic activation in the cerebrospinal fluid of patients with amyotrophic lateral sclerosis. J Clin Neurosci. 2016;26:75–78. [DOI] [PubMed] [Google Scholar]
- 107. Nycander M, Estrada S, Mort JS, Abrahamson M, Bjork I. Two-step mechanism of inhibition of cathepsin B by cystatin C due to displacement of the proteinase occluding loop. FEBS Lett. 1998;422(1):61–64. [DOI] [PubMed] [Google Scholar]
- 108. George PM, Sheat JM. Cystatin C quantification in CSF. Clin Chem. 1989;35(1):179–180. [PubMed] [Google Scholar]
- 109. Nagai A, Ryu JK, Terashima M, et al. Neuronal cell death induced by cystatin C in vivo and in cultured human CNS neurons is inhibited with cathepsin B. Brain Res. 2005;1066(1–2):120–128. [DOI] [PubMed] [Google Scholar]
- 110. Zi M, Xu Y. Involvement of cystatin C in immunity and apoptosis. Immunol Lett. 2018;196:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Okamoto K, Hirai S, Amari M, Watanabe M, Sakurai A. Bunina bodies in amyotrophic lateral sclerosis immunostained with rabbit anti-cystatin C serum. Neurosci Lett. 1993;162(1–2):125–128. [DOI] [PubMed] [Google Scholar]
- 112. Okamoto K, Mizuno Y, Fujita Y. Bunina bodies in amyotrophic lateral sclerosis. Neuropathology. 2008;28(2):109–115. [DOI] [PubMed] [Google Scholar]
- 113. Ranganathan S, Williams E, Ganchev P, et al. Proteomic profiling of cerebrospinal fluid identifies biomarkers for amyotrophic lateral sclerosis. J Neurochem. 2005;95(5):1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tsuji-Akimoto S, Yabe I, Niino M, Kikuchi S, Sasaki H. Cystatin C in cerebrospinal fluid as a biomarker of ALS. Neurosci Lett. 2009;452(1):52–55. [DOI] [PubMed] [Google Scholar]
- 115. Ren Y, Zhu W, Cui F, et al. Measurement of cystatin C levels in the cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Int J Clin Exp Pathol. 2015;8(5):5419–5426. [PMC free article] [PubMed] [Google Scholar]
- 116. Heywood WE, Hallqvist J, Heslegrave AJ, et al. CSF pro-orexin and amyloid-beta38 expression in Alzheimer's disease and frontotemporal dementia. Neurobiol Aging. 2018;72:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zhong XM, Hou L, Luo XN, et al. Alterations of CSF cystatin C levels and their correlations with CSF Alphabeta40 and Alphabeta42 levels in patients with Alzheimer's disease, dementia with Lewy bodies and the atrophic form of general paresis. PLoS ONE. 2013;8(1):e55328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Beers DR, Henkel JS, Zhao W, et al. Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in mice and correlate with disease progression in patients with amyotrophic lateral sclerosis. Brain. 2011;134(Pt 5):1293–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Beers DR, Zhao W, Wang J, et al. ALS patients’ regulatory T lymphocytes are dysfunctional, and correlate with disease progression rate and severity. JCI Insight. 2017;2(5):e89530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Rentzos M, Evangelopoulos E, Sereti E, et al. Alterations of T cell subsets in ALS: A systemic immune activation? Acta Neurol Scand. 2012;125(4):260–264. [DOI] [PubMed] [Google Scholar]
- 121. Zhao W, Beers DR, Liao B, Henkel JS, Appel SH. Regulatory T lymphocytes from ALS mice suppress microglia and effector T lymphocytes through different cytokine-mediated mechanisms. Neurobiol Dis. 2012;48(3):418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Giovannelli I, Heath P, Shaw PJ, Kirby J. The involvement of regulatory T cells in amyotrophic lateral sclerosis and their therapeutic potential. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21(5–6):435–444. [DOI] [PubMed] [Google Scholar]
- 123. Romano M, Fanelli G, Albany CJ, Giganti G, Lombardi G. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front Immunol. 2019;10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Shepheard SR, Chataway T, Schultz DW, Rush RA, Rogers ML. The extracellular domain of neurotrophin receptor p75 as a candidate biomarker for amyotrophic lateral sclerosis. PLoS ONE. 2014;9(1):e87398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jia R, Shepheard S, Jin J, et al. Urinary extracellular domain of neurotrophin receptor p75 as a biomarker for amyotrophic lateral sclerosis in a Chinese cohort. Sci Rep. 2017;7(1):5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Shi G, Shao S, Zhou J, Huang K, Bi FF. Urinary p75(ECD) levels in patients with amyotrophic lateral sclerosis: A meta-analysis. Amyotroph Lateral Scler Frontotemporal Degener. 2021:1–8. [DOI] [PubMed] [Google Scholar]
- 127. Leonenko G, Baker E, Stevenson-Hoare J, et al. Identifying individuals with high risk of Alzheimer's disease using polygenic risk scores. Nat Commun. 2021;12(1):4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hagenaars SP, Radakovic R, Crockford C, et al. Genetic risk for neurodegenerative disorders, and its overlap with cognitive ability and physical function. PLoS ONE. 2018;13(6):e0198187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hosp F, Mann M. A primer on concepts and applications of proteomics in neuroscience. Neuron. 2017;96(3):558–571. [DOI] [PubMed] [Google Scholar]
- 130. Begcevic I, Brinc D, Brown M, et al. Brain-related proteins as potential CSF biomarkers of Alzheimer's disease: A targeted mass spectrometry approach. J Proteomics. 2018;182:12–20. [DOI] [PubMed] [Google Scholar]
- 131. Lu W, Wan X, Liu B, et al. Specific changes of serum proteins in Parkinson's disease patients. PLoS ONE. 2014;9(4):e95684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Davidsson P, Sjogren M, Andreasen N, et al. Studies of the pathophysiological mechanisms in frontotemporal dementia by proteome analysis of CSF proteins. Brain Res Mol Brain Res. 2002;109(1–2):128–33. [DOI] [PubMed] [Google Scholar]
- 133. Ruetschi U, Zetterberg H, Podust VN, et al. Identification of CSF biomarkers for frontotemporal dementia using SELDI-TOF. Exp Neurol. 2005;196(2):273–281. [DOI] [PubMed] [Google Scholar]
- 134. van der Ende EL, Meeter LH, Stingl C, et al. Novel CSF biomarkers in genetic frontotemporal dementia identified by proteomics. Ann Clin Transl Neurol. 2019;6(4):698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Barschke P, Oeckl P, Steinacker P, Ludolph A, Otto M. Proteomic studies in the discovery of cerebrospinal fluid biomarkers for amyotrophic lateral sclerosis. Expert Rev Proteomics. 2017;14(9):769–777. [DOI] [PubMed] [Google Scholar]
- 136. Pasinetti GM, Ungar LH, Lange DJ, et al. Identification of potential CSF biomarkers in ALS. Neurology. Apr. 2006;66(8):1218–1222. [DOI] [PubMed] [Google Scholar]
- 137. von Neuhoff N, Oumeraci T, Wolf T, et al. Monitoring CSF proteome alterations in amyotrophic lateral sclerosis: Obstacles and perspectives in translating a novel marker panel to the clinic. PLoS ONE. 2012;7(9):e44401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ryberg H, An J, Darko S, et al. Discovery and verification of amyotrophic lateral sclerosis biomarkers by proteomics. Muscle Nerve 2010;42(1):104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Collins MA, An J, Hood BL, Conrads TP, Bowser RP. Label-free LC-MS/MS proteomic analysis of cerebrospinal fluid identifies protein/pathway alterations and candidate biomarkers for amyotrophic lateral sclerosis. J Proteome Res. 2015;14(11):4486–4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Brettschneider J, Mogel H, Lehmensiek V, et al. Proteome analysis of cerebrospinal fluid in amyotrophic lateral sclerosis (ALS). Neurochem Res. 2008;33(11):2358–2363. [DOI] [PubMed] [Google Scholar]
- 141. Chen Y, Liu XH, Wu JJ, et al. Proteomic analysis of cerebrospinal fluid in amyotrophic lateral sclerosis. Exp Ther Med. 2016;11(6):2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ranganathan S, Nicholl GC, Henry S, et al. Comparative proteomic profiling of cerebrospinal fluid between living and post mortem ALS and control subjects. Amyotroph Lateral Scler. 2007;8(6):373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Brettschneider J, Lehmensiek V, Mogel H, et al. Proteome analysis reveals candidate markers of disease progression in amyotrophic lateral sclerosis (ALS). Neurosci Lett. 2010;468(1):23–27. [DOI] [PubMed] [Google Scholar]
- 144. Xu Z, Lee A, Nouwens A, Henderson RD, McCombe PA. Mass spectrometry analysis of plasma from amyotrophic lateral sclerosis and control subjects. Amyotroph Lateral Scler Frontotemporal Degener. 2018;19(5–6):362–376. [DOI] [PubMed] [Google Scholar]
- 145. Bereman MS, Beri J, Enders JR, Nash T. Machine learning reveals protein signatures in CSF and plasma fluids of clinical value for ALS. Sci Rep. 2018;8(1):16334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Oeckl P, Weydt P, Thal DR, Weishaupt JH, Ludolph AC, Otto M. Proteomics in cerebrospinal fluid and spinal cord suggests UCHL1, MAP2 and GPNMB as biomarkers and underpins importance of transcriptional pathways in amyotrophic lateral sclerosis. Acta Neuropathol. 2020;139(1):119–134. [DOI] [PubMed] [Google Scholar]
- 147. Barschke P, Ockl P, Steinacker P, et al. Different CSF protein profiles in amyotrophic lateral sclerosis and frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. J Neurol Neurosurg Psychiatry. 2020;91:503–511. [DOI] [PubMed] [Google Scholar]
- 148. Katzeff JS, Bright F, Lo K, et al. Altered serum protein levels in frontotemporal dementia and amyotrophic lateral sclerosis indicate calcium and immunity dysregulation. Sci Rep. 2020;10(1):13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kim WS, Jary E, Pickford R, et al. Lipidomics analysis of behavioral variant frontotemporal dementia: A scope for biomarker development. Front Neurol. 2018;9:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Sol J, Jove M, Povedano M, et al. Lipidomic traits of plasma and cerebrospinal fluid in amyotrophic lateral sclerosis correlate with disease progression. Brain Commun. 2021;3(3):fcab143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. FernAndez-Eulate G, Ruiz-Sanz JI, Riancho J, et al. A comprehensive serum lipidome profiling of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21(3–4):252–262. [DOI] [PubMed] [Google Scholar]
- 152. Murley AG, Jones PS, Coyle Gilchrist I, et al. Metabolomic changes associated with frontotemporal lobar degeneration syndromes. J Neurol. 2020;267(8):2228–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Santos ALM, Vitório JG, de Paiva MJN, et al. Frontotemporal dementia: Plasma metabolomic signature using gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2020;189:113424. [DOI] [PubMed] [Google Scholar]
- 154. Jia R, Chen Q, Zhou Q, et al. Characteristics of serum metabolites in sporadic amyotrophic lateral sclerosis patients based on gas chromatography-mass spectrometry. Sci Rep. 2021;11(1):20786. [DOI] [PMC free article] [PubMed] [Google Scholar]