Abstract
An understanding of the ethical underpinnings of human subjects research that involves some risk to participants without anticipated direct clinical benefit—such as the kidney biopsy procedure as part of the Kidney Precision Medicine Project (KPMP)—requires a critical examination of risks as well as a diverse set of countervailing potential benefits to participants. This kind of deliberation has been foundational to the development and conduct of the KPMP. Herein, we use illustrative features of this research paradigm to develop a more comprehensive conceptualization of the types of benefits that may be important to research participants, including respecting pluralistic values, supporting the opportunity to act altruistically, and enhancing benefits to a participant’s community. This approach may serve as a model to help researchers, ethicists, and regulators to identify opportunities to better respect and support participants in future research that entails some risk to these participants as well as to improve the quality of research for people with kidney disease.
Keywords: Research ethics, human subjects, The Kidney Precision Medicine Project, kidney biopsy, community engagement
The Kidney Precision Medicine Project (KPMP) is a multicenter prospective cohort study, with an objective to develop a more granular depiction of kidney diseases by generating a comprehensive repository of kidney biopsies from adults with a spectrum of types and severities of acute kidney injury and chronic kidney disease.1 This endeavor necessitates the collection of image-guided percutaneous kidney biopsies from participants who would otherwise be unlikely to undergo this procedure for clinical indications. Although kidney biopsy is considered to be a relatively safe procedure, it is nonetheless invasive and associated with a risk of adverse outcomes ranging from mild discomfort to, rarely, significant injury.2 In the early 1990s, a panel of experts convened by the National Institutes of Diabetes and Digestive and Kidney Diseases highlighted some of the potential ethical dimensions of future research involving kidney biopsies, including unique needs for protocol design and informed consent.3 As a paradigmatic example of the type of research anticipated by this panel, careful clinical and ethical evaluation of the risks to study participants has guided the KPMP since its inception.4,5 The intentional approach that resulted from this deliberation may offer researchers, ethicists, and regulators guidance on identifying and weighing risks, benefits, and values in human subjects research to both respect participants and advance the science of care for people with kidney disease.
Risk in research with human subjects in the US
In the US, regulatory approaches to ensuring ethical research practices have developed, in large part, in response to exploitation of research participants in the name of advancing scientific knowledge and the greater good.6–8 Guided by ethical principles described in the Belmont Report—including respect for persons, beneficence, and justice—the Code of Federal Regulations (45 CFR 46) directs appropriate conduct of research with human subjects.9, 10 This set of regulations, known as the Common Rule, requires that research involving human subjects that is funded by federal agencies (including the National Institutes of Health) be reviewed by an Institutional Review Board (IRB) and outlines the processes and criteria by which these IRBs determine the boundaries of permissible research. Specifically, regarding considerations of risk to participants, the Common Rule requires that:
“Risks to subjects are reasonable in relation to anticipated benefits, if any, to subjects, and the importance of the knowledge that may reasonably be expected to result from research.”9
Operationalizing these regulations involves determining what constitutes reasonable risk and benefit in clinical research, and who is to make these assessments. Some research involves “minimal risk,” that is, no more risk than would be expected in the course of day-to-day life or usual medical care.10, 11 Other types of research, such as therapeutic trials for cancer, may pose greater risks but offer some appreciable clinical benefits to participants that may outweigh those risks. However, for research that involves more than minimal risk to participants—such as the risks associated with kidney biopsy in the KPMP—without the expectation of direct clinical benefit, ethical considerations framing appropriate research practices are especially complex.
Kidney disease has a profound impact on human health, with chronic kidney disease impacting an estimated 500 million people world-wide.12 Developing a mechanistic understanding of kidney injury may allow clinicians to better serve the affected population. This goal appeals to the Common Rule’s requirement that research offer the opportunity to generate important knowledge. Minimizing risk to participants has also been of primary concern in developing the KPMP protocols.1, 4 Nevertheless, the invasive nature of kidney biopsy means that risk to participants in the KPMP cannot be entirely abrogated. As such, it is important to critically examine any countervailing benefits anticipated by participants, while acknowledging that these benefits may extend beyond the straightforward advancement of their own health.
Minimizing risk to participants in the KPMP
Minimizing risk is ethically obligatory and has shaped the KPMP recruitment and biopsy processes. Similar to other complex clinical research projects, participation in the KPMP may include multiple types of risk, such as loss of confidentiality and future identification by genetic analyses. We focus here on the risks of the kidney biopsy procedure, which received dedicated attention in study development. The most common adverse events that people may experience are pain at the biopsy site (30–50%) and/or a small amount of bleeding. However, more concerning complications of the procedure are possible, including substantial bleeding requiring a blood transfusion (1–8%)13–15 surgical intervention (0.2%),15–17 or exceedingly rarely, loss of the biopsied kidney (0.01%) or death (0.02%).16 A systematic review of the existing literature informed a standardized KPMP biopsy protocol intended to limit risk,1 such as avoiding large-bore needles,19 instituting a procedural checklist,16, 20 and close post-procedure monitoring and follow-up. All biopsies are performed by certified and experienced nephrologists and radiologists and a maximum of 5 needle passes with a 16-gauge needle are allowed with a goal of obtaining 3 biopsy cores.1 Potential participants who were thought to have a relatively high risk of adverse outcomes from biopsy—such as those with a solitary kidney, uncontrolled hypertension, severe anemia, and/or requiring continuous anti-coagulation—were excluded from enrollment (Table S1). Further opportunities to minimize risks were solicited from an independent institutional review board, data safety and monitoring board, and external expert panel.1 These procedures reflect the current state of knowledge about the risks of kidney biopsy; however, the KPMP is also prospectively collecting data on procedural risks among early KPMP participants and using this information to refine the consent process throughout the course of the study. Adverse events are actively monitored by an internal safety and adjudication committee, an external data and safety monitoring committee, and an external panel of content experts.1
Although these strategies reduce the risk involved in the kidney biopsy procedure, risk cannot be entirely eliminated. Moreover, these efforts focus on the acute risks associated with performing a kidney biopsy, but may not reflect potential, albeit rare, secondary or long-term harms from complications of the procedure. Although the vast majority of these complications are limited in scope, the most severe adverse events related to kidney biopsy may result in need for invasive interventions and/or hospitalization. These acute events may also be associated with chronic complications, such as a decrease in kidney function or immunologic response to blood products that could hinder future kidney transplant options. Further, care for complex adverse events may entail significant financial burden.21, 22 Acknowledging the possibility of these broader and/or unanticipated harms, the KPMP study design includes a unique provision of “no-fault” harm insurance to all participants to cover medical costs resulting from participation in the study, including those related to any hospitalization and/or additional procedures resulting from complications of biopsy.3 This feature exemplifies a more holistic approach to anticipating and addressing the potential harms that can arise through research participation.
Participant-defined benefits of research engagement
The need for a participant-centered understanding of benefit to research participants
For the majority of participants in the KPMP, a kidney biopsy would not have been recommended in the course of routine clinical care. Precision medicine promises to support targeted treatments based on biopsy results in the future, but the majority of KPMP participants are not expected to benefit directly from these advances. Although there is always a possibility that the results of a biopsy could yield unexpected and clinically actionable insights, this is unlikely for many participants with either transient acute kidney injury or scarring changes resulting from advanced chronic kidney disease. This point is uniquely salient for a sub-group of KPMP participants with Type 1 diabetes but without evidence of diabetic kidney disease. From a scientific perspective, biopsies from these individuals may help us to better understand protective mechanisms and to identify therapies to prevent diabetic kidney disease for others. However, because the members of this sub-group of the KPMP participants have no known kidney disease, they are especially unlikely to experience direct clinical benefit from undergoing biopsy (notwithstanding a small possibility of identifying occult diabetic nephropathy23). Given the equivocal clinical value of kidney biopsy for most participants in the KPMP, the justification for assuming the risk of biopsy and participating in the KPMP must rely on values other than direct medical benefit.
Central to a deeper analysis of benefit in human subjects research is an appreciation that what constitutes benefit to a participant must be framed by their own values and goals, rather than being narrowly defined by clinical outcomes or researchers’ assumptions. A person-centered approach to health care, in which the focus of care shifts from treating a disease to supporting a person’s goals, has gained traction in clinical practice.24 A person may choose a particular therapeutic course for a variety of reasons beyond clinical efficacy, such as concerns about cost, impact on lifestyle or reproductive options, and/or familial harmony. Person-centered decision-making is especially important when the likelihood of risks and benefits is uncertain.25 With the advent of a range of clinical diagnostic tools informing understanding of kidney disease (e.g., anti-phospholipase A2 receptor (PLA2R) antibodies and whole genome sequencing), appropriate clinical indications for kidney biopsy are increasingly complex and value-sensitive. Indeed, if the goal of the KPMP to enhance the diagnostic and prognostic value of kidney biopsy is achieved, the balance of clinical benefits versus risk may shift for both patients and research participants.
Within a research context, respect for persons similarly requires that decisions about participation in research be framed by participants’ own values and personal assessment of risk and benefit. Thus, a broader understanding of what these participants value in research is needed to clarify the ethical justifications for research that involves risk to participants and also to identify opportunities to optimize the potential benefits for participants in future studies.
Research participants may experience multiple types of benefits
One potential benefit of participating in the KPMP is the receipt of detailed information from a kidney biopsy about the underlying physiology of one’s disease process. This knowledge may be considered a benefit in itself for participants, even if it does not overtly change monitoring or treatment plans. Existing work suggests that simply knowing more about one’s health condition can be motivating and empowering and may guide a person’s approach to self-care.5, 26, 27 To facilitate this potential benefit of participating in the KPMP, biopsy results (as well as fundoscopic exams, laboratory findings, and other information gathered as part of the KPMP procedures that may have clinical relevance) are routinely communicated to participants (if this is their preference) by site investigators. These data are also provided to participants’ clinicians for placement in their health records.
However, the anticipation of direct personal benefit, even if intangible, may not capture the reasons that many participants choose to engage in medical research. For some participants, being granted the opportunity to “help researchers make discoveries” and “help people with kidney problems in the future”—as stated in the KPMP consent form—may itself be sufficiently compelling. Altruistic behavior may contribute to a participant’s sense of wellbeing and personal integrity regardless of any specific health benefit.28 To best respect a participant’s wish to act altruistically, it is important to ensure that research is designed to maximize the beneficial societal impact. Development of a broad and meaningful contribution to knowledge that is inclusive of diversity in present and future populations has guided planning and conduct of the KPMP.
The Common Rule is framed around consideration of benefits and risks as they accrue to an individual participant. This strict delineation between benefits and risks to participants versus benefits to society also reflects a conceptualization of a research participant’s identity as wholly independent and distinct from their membership within a community. However, in reality, people do not exist in isolation. They exist as members of various and overlapping communities, including not only friends and families, but groups whose membership is defined by an array of social, demographic, geographic, political, religious, ideological, and identity characteristics. The assumption that a person’s individual interests can be isolated from their community risks distorting the reality of what it is to be human and may give insufficient weight to the relationships that are typically important parts of one’s identity. It is common for people to take on personal burdens and risks for their family members or friends. In medical practice, clinicians acknowledge and accommodate the reality of this social embeddedness when they facilitate patients’ choosing care options shaped by considerations of financial burden on family members29 or they respect people’s decision to accept the health risks of engaging in living organ donation.30 People may assume these types of risks for family members or others within their community, not merely because they expect to accrue personal benefit in return, but to fulfill obligations entailed by their relationships.31 Motivation to participate in research may similarly derive from close ties and identification with a community of people with kidney disease and/or with the community of people benefiting from clinical research more broadly.32 For research participants whose sense of self is closely intertwined with others in their community, any gain in scientific knowledge that could serve their community may indeed be perceived to constitute direct benefit to these participants themselves.
Balancing protection of human subjects with support for participant autonomy
Concerns about expansive interpretations of participant benefits
Recognition that values differ among patients and between patients and their clinicians has underlined the importance of supporting people in making clinical decisions that reflect their own values rather than adhering to rigid assumptions about relative risk and benefit. As in clinical practice, respect for persons requires that individual research participants be allowed to make their own determinations about the relative weight of risks and benefits. Unilaterally disallowing participation based on the determination by a research team or the scientific community that risk is too great echoes a paternalistic stance that may limit opportunities for people who would otherwise wish to engage in research that they see as personally beneficial or otherwise valuable. Intentional processes of informed consent that strive to present risks and benefits in a way that truly facilitates understanding and decision-making supports respect for autonomy.
However, respect for participants’ autonomy does not mean that any and all research should be considered permissible as long as participants are willing. In light of a history of inexcusable exploitation of research participants in the US, researchers and IRBs tend to maintain a high bar for accepting risk to participants. This concern aligns with a guiding principle in clinical medicine of non-maleficence, or avoiding harm. While we seek to optimize participants’ autonomy regarding decisions about research participation, it remains true that potential participants may be at risk of exploitation due to a variety of factors, including incomplete understanding of risk and benefits of a study, the influence of pre-existing personal and institutional care relationships with investigators, and therapeutic misconception.33 Ultimately, research ethics are framed by multiple ethical principles, including both autonomy and non-maleficence. Respecting these principles means striking a balance in which participants are allowed to make autonomous, informed choices about whether and how to participate in research as long as the degree of risk remains within the guardrails established by regulations and IRBs and is accompanied by some potential benefit, even if this benefit is not strictly clinical (Figure 1).
Figure 1. Strategies to minimize clinical risks of kidney biopsy and optimize a range of benefits for participants in the Kidney Precision Medicine Project.
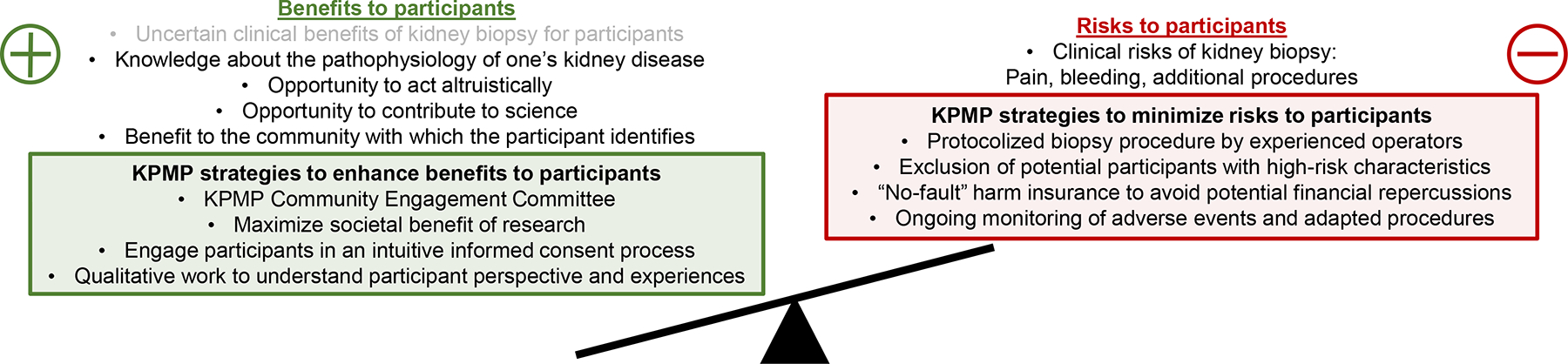
Research ethics as codified in the US Common Rule require that risks to research participants—such as risks associated with kidney biopsy as part of the Kidney Precision Medicine Project (KPMP)--are exceeded by potential benefits. Traditional clinical benefits of kidney biopsy are often equivocal for participants in KPMP, but appreciation of a range of participant-defined benefits reveals opportunities to enhance these benefits in the design and conduct of the KPMP.
Study design and implementation as an ethical dialogue: Contribution of research participants and the KPMP Community Engagement Committee
The manner in which multiple ethical principles guiding research practice are weighed is not solely at the discretion of researchers or even the research community. US research regulatory structures, including the Common Rule, and IRBs serve as the voice of society-at-large, seeking to protect those members of the public who may be vulnerable to unintended exploitation. However, these institutions are best positioned to assess potential benefits to participants in relatively objective and broadly agreed-upon terms, and less equipped to appreciate a broader spectrum of participant-defined benefits.
One potential avenue to enhance the identification and incorporation of the broad array of potential benefits from participation in research is the robust inclusion of members of the communities from which participants are recruited in research planning and design. This stakeholder perspective contributes to a more comprehensive understanding of participant benefits, which can then be integrated into research design from the ground up. The Community Engagement Committee (CEC) of the KPMP is a panel of clinicians, investigators, and—most importantly—a national contingent of people with kidney disease, including those living in the communities from which participants are recruited. This group has been intimately involved from early in the conceptualization and development of the KPMP and throughout the research process.3 For example, the KPMP informed consent form was developed in partnership with the CEC, and the group continues to inform the IRB application and revision process.4, 34 The CEC is well-positioned and empowered to identify and maximize potential benefits to participants and to ground the goals of the project in the needs and priorities of diverse communities.4 The CEC has also been engaged throughout the course of research to give feedback on ongoing questions such as appropriate approaches to disseminating genetic findings and other unique results deriving from deep molecular phenotyping in the KPMP. Future empirical qualitative work investigating the perspectives and preferences of KPMP participants may identify additional opportunities to bolster the value of research for these individuals.
Conclusion.
The US Common Rule regulating research with human subjects requires that any risk to a participant is reasonable in relation to potential benefits to that participant and the value of knowledge gained. Evaluating the ethical foundations of research that involves risk without explicit clinical benefits—such as kidney biopsy in the KPMP—requires efforts to minimize risk as well as to better appreciate the broad array of benefits that participants may enjoy. This includes respecting pluralistic participant values, supporting their opportunity to act altruistically, and appreciating benefits to a participant’s community. This more comprehensive understanding of how participants may view the benefits of taking part in research could inform future research endeavors to better support the participants on which this work relies and to better serve the broader community of people with kidney disease and other medical conditions.
Supplementary Material
Financial Disclosure:
CRB reports research funding and/or stipends from the University of Washington Department of Medicine, National Institute for Diabetes and Digestive and Kidney Diseases (NIDDK), Veterans Affairs Health Services Research & Development, and The American Society of Nephrology. SER reports research funding from NIDDK, Bayer Healthcare, AstraZeneca and Scientific Advisory Board membership for Reata, Relypsa, and Bayer. KRT is supported by four NIDDK/National Institutes of Health (NIH) grants, one National Institute for Advancing Translational Sciences/NIH grant, one National Institute on Minority Health and Health Disparities/NIH grant, and a Center for Disease Control contract all from the US government, as well as a research grants from Goldfinch Bio, Bayer, and Travere. KRT has received consulting fees for diabetes and CKD from Eli Lilly, Boehringer Ingelheim, AstraZeneca, Gilead, Goldfinch Bio, Novo Nordisk and Bayer. The remaining authors declare that they have no relevant financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Catherine R. Butler, Division of Nephrology, Department of Medicine and the Kidney Research Institute, University of Washington; Seattle-Denver Health Services Research and Development Center of Innovation, Veterans Affairs Puget Sound Health Care System, Seattle, WA.
Paul S. Appelbaum, Department of Psychiatry, Columbia University Vagelos College of Physicians & Surgeons; New York State Psychiatric Institute, New York, NY.
Heather Ascani, Division of Nephrology, Department of Internal Medicine, University of Michigan, Ann Arbor, MI.
Mark Aulisio, Department of Bioethics, School of Medicine, Case Western Reserve University, Center for Biomedical Ethics, MetroHealth System, Cleveland, OH.
Catherine E. Campbell, Kidney Precision Medicine Project Patient Partner, American Association of Kidney Patients, Sigma Theta Tau International Honor Society, Case Management Society of America, AARP Volunteer Nursing Leadership Board.
Ian H. de Boer, Division of Nephrology, Department of Medicine and the Kidney Research Institute, University of Washington, Seattle, WA.
Ashveena L. Dighe, Division of Nephrology, Department of Medicine and the Kidney Research Institute, University of Washington, Seattle, WA.
Daniel E. Hall, Departments of Surgery, Anesthesiology and Perioperative Medicine University of Pittsburgh, Pittsburgh, PA; Wolff Center at UPMC; Center for Health Equity Research and Promotion and Geriatric Research Education and Clinical Center, VA Pittsburgh Healthcare System.
Jonathan Himmelfarb, Division of Nephrology, Department of Medicine and the Kidney Research Institute, University of Washington, Seattle, WA.
Richard Knight, KPMP Patient Partner, USA; American Association of Kidney Patients.
Karla Mehl, Division of Nephrology, Columbia University Irving Medical Center, New York, New York..
Raghavan Murugan, The Center for Critical Care Nephrology, Department of Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania.
Sylvia E. Rosas, Kidney and Hypertension Unit, Joslin Diabetes Center, Harvard Medical School, Boston, MA..
John R. Sedor, Department of Nephrology and Hypertension, Glickman Urological and Kidney and Lerner Research Institutes, Cleveland Clinic Foundation; Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH.
John F. O’Toole, Department of Nephrology and Hypertension, Glickman Urological and Kidney and Lerner Research Institutes, Cleveland Clinic Foundation; Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH.
Katherine R. Tuttle, Division of Nephrology, Department of Medicine and Kidney Research Institute, University of Washington, Seattle, Washington.
Sushrut S. Waikar, Section of Nephrology, Boston University School of Medicine and Boston Medical Center; Renal Division, Brigham & Women’s Hospital, Boston, Massachusetts.
Michael Freeman, Division of Pediatric Nephrology and Hypertension, Department of Pediatrics and Humanities, Penn State College of Medicine, Hershey, PA.
References.
- 1.de Boer IH, Alpers CE, Azeloglu EU, et al. Rationale and design of the Kidney Precision Medicine Project. Kidney International. 2021;99(3): 498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poggio ED, McClelland RL, Blank KN, et al. Systematic review and meta-analysis of native kidney biopsy complications. Clin J Am Soc Nephrol. 2020;15(11): 1595–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glassock RJ, Hirschman GH, Striker GE. Workshop on the use of renal biopsy in research on diabetic nephropathy: a summary report. Am J Kidney Disease. 1991;18(5):589–92 [DOI] [PubMed] [Google Scholar]
- 4.Tuttle KR, Knight R, Appelbaum PS, et al. Integrating Patient Priorities with Science by Community Engagement in the Kidney Precision Medicine Project. Clin J Am Soc Nephrol. 2021;16(4):660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown KD, Campbell C, Roberts GV. Precision medicine in kidney disease: the patient’s view. Nature reviews. Nephrology 2020;16(11): 625–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothman DJ. Were Tuskegee & Willowbrook ‘studies in nature’? Hastings Cent Rep. 1982;12(2): 5–7. [PubMed] [Google Scholar]
- 7.Beecher HK. Ethics and clinical research. N Engl J Med. 1966;274(24): 1354–1360. [DOI] [PubMed] [Google Scholar]
- 8.De Castro LD. Exploitation in the use of human subjects for medical experimentation: a re-examination of basic issues. Bioethics. 1995;9(3–4): 259–268. [DOI] [PubMed] [Google Scholar]
- 9.The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Bethesda, MD: Department of Health, Education and Welfare. The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research; 1979. [PubMed] [Google Scholar]
- 10.Protection of Human Subjects. 45 CFR §46 (2009).
- 11.Resnik DB. Eliminating the daily life risks standard from the definition of minimal risk. Journal of Medical Ethics. 2005;31(1): 35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glassock RJ, Warnock DG, Delanaye P. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat Rev Nephrol. 2017;13(2): 104–114. [DOI] [PubMed] [Google Scholar]
- 13.Poggio ED, McClelland RL, Blank KN, et al. Systematic Review and Meta-Analysis of Native Kidney Biopsy Complications. Clin J Am Soc Nephrol. 2020;15(11): 1595–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moledina DG, Luciano RL, Kukova L, et al. Kidney Biopsy-Related Complications in Hospitalized Patients with Acute Kidney Disease. Clinical journal of the American Society of Nephrology : CJASN. 2018;13(11): 1633–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palsson R, Short SAP, Kibbelaar ZA, et al. Bleeding Complications After Percutaneous Native Kidney Biopsy: Results From the Boston Kidney Biopsy Cohort. Kidney Int Rep. 2020;5(4): 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corapi KM, Chen JL, Balk EM, Gordon CE. Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. American journal of kidney diseases. 2012;60(1): 62–73. [DOI] [PubMed] [Google Scholar]
- 17.Luciano RL, Moeckel GW. Update on the Native Kidney Biopsy: Core Curriculum 2019. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2019;73(3): 404–415. [DOI] [PubMed] [Google Scholar]
- 18.Tøndel C, Vikse BE, Bostad L, Svarstad E. Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988–2010. Clin J Am Soc Nephrol. 2012;7(10): 1591–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui S, Heller HT, Waikar SS, McMahon GM. Needle Size and the Risk of Kidney Biopsy Bleeding Complications. Kidney Int Rep. 2016;1(4): 324–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360(5): 491–499. [DOI] [PubMed] [Google Scholar]
- 21.Golestaneh L, Alvarez PJ, Reaven NL, et al. All-cause costs increase exponentially with increased chronic kidney disease stage. Am J Manag Care. 2017;23(10 Suppl): S163–s172. [PubMed] [Google Scholar]
- 22.Stubbs JR. Wrapping our arms around the cost of transfusion therapy. Transfusion. 2014;54(2): 259–262. [DOI] [PubMed] [Google Scholar]
- 23.Klessens CQ, Woutman TD, Veraar KA, et al. An autopsy study suggests that diabetic nephropathy is underdiagnosed. Kidney Int. 2016;90(1): 149–156. [DOI] [PubMed] [Google Scholar]
- 24.Tinetti ME, Fried T. The end of the disease era. Am J Med. 2004;116(3): 179–185. [DOI] [PubMed] [Google Scholar]
- 25.Whitney SN, McGuire AL, McCullough LB. A typology of shared decision making, informed consent, and simple consent. Ann Intern Med. 2004;140(1): 54–59. [DOI] [PubMed] [Google Scholar]
- 26.Umeukeje EM, Young BA, Fullerton SM, et al. You Are Just Now Telling Us About This? African American Perspectives of Testing for Genetic Susceptibility to Kidney Disease. Journal of the American Society of Nephrology : JASN. 2019;30(4): 526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West KM, Blacksher E, Cavanaugh KL, et al. At the Research-Clinical Interface: Returning Individual Genetic Results to Research Participants. Clin J Am Soc Nephrol. 2020;15(8): 1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahana E, Bhatta T, Lovegreen LD, Kahana B, Midlarsky E. Altruism, helping, and volunteering: pathways to well-being in late life. J Aging Health. 2013;25(1): 159–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osamor PE, Grady C. Autonomy and couples’ joint decision-making in healthcare. BMC medical ethics. 2018;19(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freeman MA, Wightman AG. Did parents have it right all along? Parents, risk, and living kidney donation: Revisiting the arguments for and against parental living donation of kidneys. Pediatric transplantation. 2018;22(3): e13153. [DOI] [PubMed] [Google Scholar]
- 31.Lindemann H Why families matter. Pediatrics. 2014;134 Suppl 2: S97–103. [DOI] [PubMed] [Google Scholar]
- 32.Cox SM, McDonald M. Ethics is for human subjects too: participant perspectives on responsibility in health research. Soc Sci Med. 2013;98: 224–231. [DOI] [PubMed] [Google Scholar]
- 33.Appelbaum PS, Lidz CW, Grisso T. Therapeutic misconception in clinical research: frequency and risk factors. IRB: Ethics & Human Research. 2004;26(2): 1–8. [PubMed] [Google Scholar]
- 34.Kimmel PL, Jefferson N, Norton JM, Star RA. How Community Engagement Is Enhancing NIDDK Research. Clin J Am Soc Nephrol. 2019;14(5): 768–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


