Summary
Background
Remdesivir was the first prodrug approved to treat coronavirus disease 2019 (COVID-19) and has the potential to be used during pregnancy. However, it is not known whether remdesivir and its main metabolite, GS-441524 have the potential to cross the blood-placental barrier. We hypothesize that remdesivir and predominant metabolite GS-441524may cross the blood-placental barrier to reach the embryo tissues.
Methods
To test this hypothesis, ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) coupled with multisite microdialysis was used to monitor the levels of remdesivir and the nucleoside analogue GS-441524 in the maternal blood, fetus, placenta, and amniotic fluid of pregnant Sprague-Dawley rats. The transplacental transfer was evaluated using the pharmacokinetic parameters of AUC and mother-to-fetus transfer ratio (AUCfetus/AUCmother).
Findings
Our in-vivo results show that remdesivir is rapidly biotransformed into GS-441524 in the maternal blood, which then readily crossed the placenta with a mother-to-fetus transfer ratio of 0.51 ± 0.18. The Cmax and AUClast values of GS-441524 followed the order: maternal blood > amniotic fluid > fetus > placenta in rats.
Interpretation
While remdesivir does not directly cross into the fetus, however, its main metabolite, GS-441524 readily crosses the placenta and can reside there for at least 4 hours as shown in the pregnant Sprague-Dawley rat model. These findings suggest that careful consideration should be taken for the use of remdesivir in the treatment of COVID-19 in pregnancy.
Funding
Ministry of Science and Technology of Taiwan.
Keywords: Remdesivir, GS-441524, Microdialysis, Blood-placental barrier, Pharmacokinetics
Research in context.
Evidence before this study
Remdesivir became available for pregnant women with severe COVID-19 through the emergency use authorization (EUA) by the United States Food and Drug Administration as of 1 May 2020. Although several clinical trials revealed that remdesivir appears to be safe, well tolerated with a few cases of severe recorded adverse side effects, up to date no study has investigated the transplacental transfer of remdesivir and its main metabolite, GS-441524 in human or other species. The pharmacokinetics parameters of remdesivir and its metabolites in the maternal blood, placenta, fetus, and amniotic fluid are unclear.
Added value of this study
In this study, a novel quantitative method of UHPLC-MS/MS coupled with a microdialysis sampling technique was used to monitor remdesivir and its metabolite GS-441524 in pregnant rats. We report the penetration rate of these anti-viral agents across the rat placenta and showed that GS-441524 may reach the fetus.
Implications of all the available evidence
Our findings provide evidence that remdesivir does not pass through the blood-placental barrier. However, the main active metabolite, GS-441524, remains in the fetus for at least 4 hours in pregnant rats. These findings indicate that informed clinical use of remdesivir needs careful consideration for use in pregnancy.
Alt-text: Unlabelled box
Introduction
Remdesivir (Veklury®, GS-5734), an anti-viral RNA-dependent RNA polymerase, was the first drug approved to treat coronavirus disease 2019 (COVID-19) by the U.S. Food and Drug Administration (FDA). This drug is a nucleotide prodrug and is able to penetrate cell membranes, which is converted to the major metabolite GS-441524 monophosphate.1 GS-441524 monophosphate is then biotransformed to its active triphosphate metabolites, which act to inhibit RNA polymerase replication in viruses, including COVID-19.2 Thus, GS-441524 has the potential to be developed further as an orally available anti-viral drug.3 A double-blind, randomized, placebo-controlled clinical trial of over 1000 patients from 60 countries showed that remdesivir was superior to placebo in shortening the rehabilitation time of hospitalized patients, while lowering the frequency of respiratory tract infections.4
The COVID-19 pandemic affects all members of the population, including pregnant women, who make up approximately 9% of positive COVID-19 cases.5 Pregnant women infected with COVID-19 are up to five times more likely to be admitted to intensive care and require invasive mechanical ventilation.6 It also poses a higher risk of preterm birth (75%).7 and neonatal morbidities.8 However, due to considerations around the safety of remdesivir in pregnant women, including its potential transplacental transfer,9 more than 80% of clinical trials set pregnancy as an exclusion criterion.10 Consequently, there is limited information on the pharmacokinetics of remdesivir during pregnancy.11 Nevertheless, the Compassionate Use Programme (CUP) recently approved clinical trial data involving 86 pregnant women with COVID-19. The study revealed that 93% of pregnant women recovered, with 90% getting discharged soon after receiving remdesivir. Furthermore, remdesivir was well tolerated, with few serious adverse effects.12
Based on this evidence, we hypothesized that the main metabolite of remdesivir crosses the blood-placenta barrier, to reach the fetus. To the best of our knowledge, no study has reported the simultaneous monitoring of remdesivir and its metabolites in the maternal blood, fetus, placenta, and amniotic fluid of pregnant experimental animals. To address this unmet need, a multiple microdialysis sampling systems coupled with UHPLC-MS/MS was developed to assay and monitor remdesivir and GS-441524 deposition in various maternal and embryonic tissues.
Methods
Ethics
The experimental animals were approved by the Institutional Animal Care and Use Committee of the National Yang Ming Chiao Tung University (IACUC no. 1101117), which followed a guidebook for the care and use of laboratory animals (8th edition).
Chemicals and reagents
Remdesivir was provided by Formosa Laboratories Inc. (Taoyuan, Taiwan). GS-441524 was purchased from Selleck Chemicals (Houston, TX, USA). Formic acid, polyethylene glycol 400 (PEG400), and urethane were obtained from Sigma-Aldrich (St. Louis, MO, USA). Critic acid, dextrose, sodium chloride, and sodium citrate were purchased from Merck (Darmstadt, Germany). MS-grade acetonitrile (ACN) was acquired from J.T. Baker Inc. (Phillipsburg, NJ, USA). Triply deionized water (Millipore, Bedford, MA, USA) was used to prepare the stock solutions and mobile phase. Standard stock solutions of remdesivir and GS-441524 were prepared using 10% DMSO in ACN, at a concentration of 1 mg/mL, and stored at -20°C. Calibration curves of remdesivir and GS-441524 were prepared using blank matrices (blood, fetus, placenta, and amniotic fluid) as 5 μL standard stock solutions added into (spike) 45 μL blank matrices, to generate solutions with concentrations of 0.5, 1, 5, 10, 50, and 100 ng/mL and 5, 10, 50, 100, 250, and 500 ng/mL, respectively.
UHPLC-MS/MS conditions
The UHPLC-MS/MS system comprised an LC-MS/MS-8030 mass spectrometer (Shimadzu, Kyoto, Japan) coupled with a positive electrospray ionization (ESI) source. The mass spectrometry conditions were optimized as follows: interface voltage, 3.5 kV; nebulizing gas flow (nitrogen), 3.0 L/min; drying gas flow (nitrogen), 15.0 L/min; collision-induced dissociation gas (argon), 230 kPa; desolvation line temperature, 250°C; and heat block temperature, 400°C. The collision-induced dissociation mode of the multiple reaction monitor (MRM) was selected.
Analytical separation was achieved using a C18 reverse-phase column (100 × 2.1 mm, 2 μm, Purospher STAR, Merck KGaA, Darmstadt, Germany). The mobile phase consisted of 0.2% formic acid (pH=2.45) as the aqueous phase (A) and acetonitrile as the organic solvent (B), with an isocratic ratio of A:B = 40:60 (v/v). The flow rate was 0.3 mL/min, with an injection volume of 20 μL.
Method validation
Analytical validation was achieved using calibration curves and metrics of accuracy and precision, based on the guidance issued by the US FDA in 2018. Stock solutions of remdesivir and GS-441524 (1 mg/mL) were prepared in 10% DMSO and ACN, and frozen at -20°C. Calibration curves were prepared in blank matrices (45 μL) of maternal blood, placenta, fetus, and amniotic fluid dialysis, with stock solutions (5 μL) of concentrations 0.5, 1, 5, 10, 50, and 100 ng/mL (remdesivir) and 5, 10, 50, 100, 250, and 500 ng/mL (GS-441524).
Precision and accuracy were assessed between the lower limit of quantification (LLOQ) and low, medium, and high concentrations within the linear region of the calibration curves for remdesivir and GS-441524. Precision was calculated using the following formula: (% RSD) = [standard deviation/Cobs] × 100%, while accuracy was calculated using the following formula: (% bias) = [(Cobs-Cnom)/Cnom] × 100%, where Cobs is the observed concentration and Cnom is the nominal concentration. Acceptable criteria were ±15%, with an LLOQ of ±20%.
The recovery of the microdialysis probe was defined as the relative extent of remdesivir and GS-441524 recovery in the dialysate. Microdialysis probes were placed in Eppendorf vials containing three different concentrations (low, medium, and high) of remdesivir and GS-441524 anticoagulant citrate dextrose (ACD) solution. The microdialysis recovery (Rdial) was determined by comparing the increment concentration (Cout) with the standard (Cs) of triplicate working solutions, according to the following formula: Rdial (%) = (Cout/Cs) × 100. The free-drug concentrations (Cf) of remdesivir and GS-441524 were calibrated using in vitro probe recovery and the following ratio: Cf = Cm/Rdial, where Cm represents the concentration directly obtained from the microdialysis probe.
Pharmacokinetics of transplacental transfer
Experimental animals
The surgical procedures were approved by the Institutional Animal Care and Use Committee of National Yang Ming Chiao Tung University (IACUC no. 1101117). Pregnant Sprague-Dawley rats at a gestational age of 16 d and weighing 300 ± 10 g were supplied by the Laboratory Animal Center of National Yang Ming Chiao Tung University, Taipei, Taiwan. Animals were provided with food and water ad libitum (Laboratory Rodent Diet 5001, PMI Feeds, Richmond, IN, USA). Rats were excluded from the study if they experienced weight loss, reduced appetite, or other symptoms with poor outcomes, as assessed by a veterinarian. An intravenous (i.v.) dose of remdesivir (30 mg/kg) was administered to the pregnant rats (n=6). Each rat was individually housed.
Multiple microdialysis system
The multiple microdialysis system in the transplacental transfer model was implemented in line with our previous study.13 The microdialysis module system comprised of a microinjection pump (CMA 400; CMA Microdialysis, Stockholm, Sweden) and a microinjection collector (CMA/142; CMA Microdialysis). The microdialysis probes were custom-made in-house. Each probe was constructed using a semi-permeable membrane, with a molecular weight cut-off of 13,000 Da (Spectrum Medical Industries, CA, USA). The lengths of the active membrane for blood and embryo (fetus, placenta, and amniotic fluid) sampling were 1.1 cm and 0.6 cm, respectively.14
Microdialysis probe implantation
Surgical implantation of the microdialysis probes was performed under urethane anesthesia (1 g/kg, intraperitoneal). A maternal blood microdialysis probe was catheterized into the right jugular vein, for blood dialysis sampling. Microdialysis probes were inserted into the placenta and amniotic fluid, for fetal sampling.14 Polyethylene tubing (PE-50) was cannulated in the left femoral vein for remdesivir (30 mg/kg, i.v.). The maternal and embryo probes were perfused with ACD solution (13.6 mM dextrose, 7.5 mM sodium citrate, and 3.5 mM citric acid), at a flow rate of 2.0 μL/min. Following a 1-hour stabilization period, remdesivir (30 mg/kg, 10% DMSO in PEG400) was administered intravenously through the femoral vein cannula.13 Dialysis samples were collected every 20 min for 4 h and stored at -20°C until analysis using UHPLC-MS/MS.
Pharmacokinetic analysis
A WinNolin Standard Edition analytical pipeline (version 5.3, Pharsight Corp., Mountain View, CA, USA) was used to evaluate the pharmacokinetic parameters. The transplacental pharmacokinetic parameters of GS-441524 were analyzed using a non-compartment model. These included the area under the curve until the last sample collection time (AUClast), peak concentration (Cmax), time to reach Cmax (Tmax), half-life (t1/2), and penetration ratio from maternal blood to embryo tissue (AUCembryo tissue/AUCblood). Drug concentration-time curves were drawn using SigmaPlot (version 10.0; Systat Statistics, London, UK).
Statistics
Statistical analyses were performed using SPSS Statistics (version 22.0, IBM Corp., Armonk, NY, USA). Statistical contrasts were determined using one-way analysis of variance and Tukey's post-hoc test. The alpha criterion level was set at 0.05. Data have been expressed as mean ± standard error of the mean.
Role of the funders
The funders of this work had no role in the study design, study design, data collection, data analyses, interpretation, or writing of the report.
Results
Optimization of UHPLC-MS/MS conditions
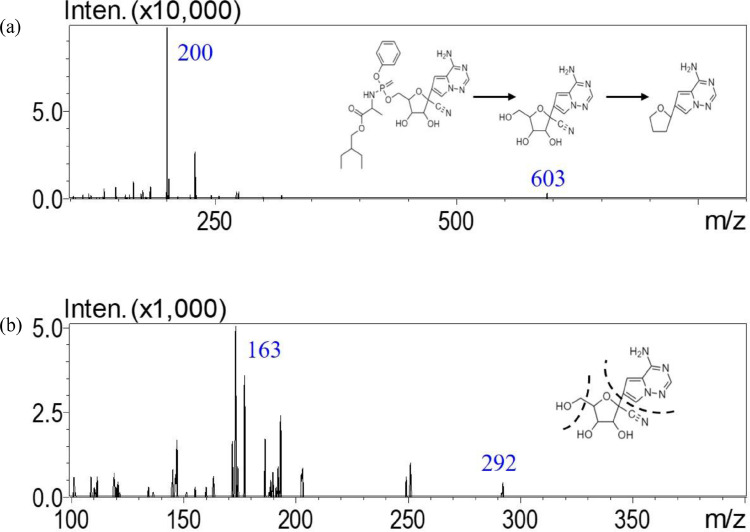
A UHPLC-MS/MS method to analyze remdesivir and GS-441524 in dialysate fluid was established using the positive MRM-ESI mode. This analytical method provided high sensitivity and selectivity for the quantification of remdesivir and GS-441524. After optimization, mass transitions of remdesivir and GS-441524 were observed at 603.2 to 200.1 (m/z) and 292.1 to 163.0 (m/z), respectively. The collision energies were -42 eV and -27 eV for remdesivir and GS-441524, respectively (Figure 1).
Figure 1.
Multiple reaction monitors of product ion mass spectra of (a) remdesivir at m/z 603.2 → 200.1 and (b) GS-441524 at m/z 292.1 → 163.0.
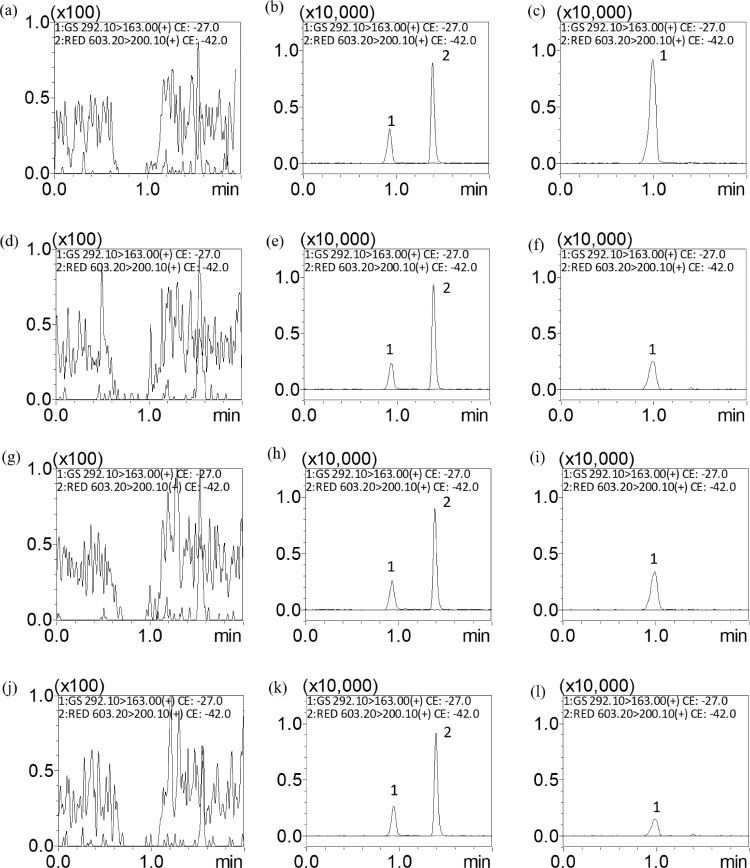
To reach higher intensity and avoid peak tailing, 0.2% formic acid was added to the aqueous phase. Because the chosen composition of the organic phase affected the detection intensity of remdesivir and GS-441524, we carried out an optimization experiment to show that remdesivir and GS-441524 were detected with greater intensity when ACN was used as the organic phase. Representative MRM chromatograms of blank matrices revealed no interference from endogenous eluants during peak elution. The retention times were 0.94 and 1.40 min for GS-441524 and remdesivir, respectively (Figure 2).
Figure 2.
Representative MRM chromatograms of (A) blank blood dialysate, (B) blank blood dialysate spiked with remdesivir (1 ng/mL) and GS-441524 (10 ng/mL), (C) blood dialysate containing GS-441524 (35.05 ng/mL), (D) blank fetus dialysate, (E) fetus dialysate spiked with remdesivir (1 ng/mL) and GS-441524 (10 ng/mL), (F) fetus dialysate containing GS-441524 (10.03 ng/mL), (G) blank amniotic fluid dialysate, (H) blank amniotic fluid dialysate spiked with remdesivir (1 ng/mL) and GS-441524 (10 ng/mL), (I) amniotic fluid dialysate containing GS-441524 (11.10 ng/mL), (J) blank placenta dialysate, (K) placenta dialysate spiked with remdesivir (1 ng/mL) and GS-441524 (10 ng/mL), and (L) placenta dialysate containing GS-441524 (5.26 ng/mL); peak 1 = GS-441524, peak 2 = remdesivir.
Method validation
The UHPLC-MS/MS quantification method showed good linearity (r2) ≥0.995 in the calibration curve range for all dialysate matrices. The LLOQ was determined when the signal-to-noise ratio was 10. The LLOQ was 0.5 ng/mL and 5 ng/mL for remdesivir and GS-441524, respectively. The precision (% RSD) and accuracy (% bias) ranged from -3.33 to 16.30% and -3.53 to 16.20% for remdesivir and GS-441524, respectively. For the LLOQ, all of the values were within ±20%, and those of other concentrations were within ±15%. These results demonstrated that the UHPLC-MS/MS method was reproducible, repeatable, and reliable (Tables 1 and 2).
Table 1.
Method validation for the precision (% RSD) and accuracy (% bias) of the UHPLC-MS/MS method for the determination of remdesivir in the maternal blood, placenta, fetus, and amniotic fluid dialysate of pregnant rats.
| Nominal Concentration (ng/mL) | Intra-day |
(n=5) |
Inter-day |
(n=3) |
||
|---|---|---|---|---|---|---|
| Observed Concentration (ng/mL) | Precision (% RSD) | Accuracy (% bias) | Observed concentration (ng/mL) | Precision (% RSD) | Accuracy (% bias) | |
| Maternal blood | ||||||
| 0.5 | 0.49 ± 0.08 | 16.30 | -1.82 | 0.55 ± 0.06 | 10.40 | 9.33 |
| 1 | 1.08 ± 0.05 | 4.28 | 7.80 | 1.10 ± 0.14 | 12.97 | 9.67 |
| 10 | 11.03 ± 0.56 | 5.06 | 10.28 | 10.03 ± 0.58 | 5.80 | 0.33 |
| 100 | 103.52 ± 4.58 | 4.42 | 3.52 | 100.65 ± 0.85 | 0.84 | 0.65 |
| Placenta | ||||||
| 0.5 | 0.47 ± 0.02 | 4.45 | -6.27 | 0.56 ± 0.08 | 13.32 | 12.67 |
| 1 | 0.99 ± 0.04 | 3.98 | -1.22 | 0.97 ± 0.12 | 12.77 | -3.33 |
| 10 | 10.03 ± 0.42 | 4.15 | 0.25 | 9.75 ± 0.32 | 3.28 | -2.47 |
| 100 | 100.12 ± 2.00 | 1.99 | 0.12 | 99.89 ± 0.70 | 0.70 | -0.11 |
| Fetus | ||||||
| 0.5 | 0.57 ± 0.02 | 3.82 | 14.35 | 0.50 ± 0.06 | 11.62 | -0.67 |
| 1 | 1.06 ± 0.06 | 5.38 | 5.64 | 1.08 ± 0.13 | 11.61 | 7.67 |
| 10 | 9.76 ± 0.23 | 2.33 | -2.38 | 10.16 ± 0.28 | 2.79 | 1.57 |
| 100 | 100.78 ± 2.06 | 2.04 | 0.78 | 99.95 ± 0.16 | 0.16 | -0.05 |
| Amniotic fluid | ||||||
| 0.5 | 0.57 ± 0.04 | 7.20 | 14.02 | 0.50 ± 0.05 | 9.93 | -0.67 |
| 1 | 1.11 ± 0.01 | 1.14 | 10.73 | 1.02 ± 0.09 | 8.71 | 2.00 |
| 10 | 9.80 ± 0.61 | 6.24 | -1.99 | 10.53 ± 0.30 | 2.86 | 5.30 |
| 100 | 100.49 ± 1.03 | 1.02 | 0.49 | 99.98 ± 0.04 | 0.04 | - 0.02 |
Data have been expressed as mean ± SD.
Table 2.
Method validation for the precision (% RSD) and accuracy (% bias) of the UHPLC-MS/MS method for determination of GS-441524 in the maternal blood, placenta, fetus and amniotic fluid dialysate of pregnant rats.
| Nominal concentration (ng/mL) | Intra-day |
(n=5) |
Inter-day |
(n=3) |
||
|---|---|---|---|---|---|---|
| Observed concentration (ng/mL) | Precision (% RSD) | Accuracy (% bias) | Observed concentration (ng/mL) | Precision (% RSD) | Accuracy (% bias) | |
| Maternal blood | ||||||
| 5 | 5.04 ± 0.42 | 8.32 | 0.77 | 4.82 ± 0.13 | 2.76 | -3.53 |
| 10 | 10.10 ± 0.65 | 6.42 | 0.98 | 10.04 ± 0.28 | 2.78 | 0.40 |
| 100 | 101.90 ± 4.39 | 4.30 | 1.90 | 96.76 ± 6.04 | 6.24 | -3.24 |
| 500 | 501.3 ± 26.26 | 5.24 | 0.26 | 499.01 ± 3.50 | 0.70 | -0.20 |
| Placenta | ||||||
| 5 | 5.07 ± 0.69 | 13.60 | 1.33 | 5.39 ± 0.87 | 16.20 | 7.87 |
| 10 | 10.14 ± 0.67 | 6.61 | 1.39 | 10.65 ± 0.75 | 7.00 | 6.47 |
| 100 | 101.80 ± 4.09 | 4.01 | 1.80 | 99.95 ± 3.61 | 3.62 | -0.05 |
| 500 | 504.17 ± 4.70 | 0.93 | 0.83 | 501.25 ± 1.37 | 0.27 | 0.25 |
| Fetus | ||||||
| 5 | 5.76 ± 0.61 | 10.46 | 15.80 | 5.25 ± 0.15 | 2.86 | 5.00 |
| 10 | 10.39 ± 1.13 | 10.92 | 3.89 | 10.67 ± 1.16 | 10.87 | 6.67 |
| 100 | 103.17 ± 6.57 | 6.37 | 3.17 | 100.35 ± 2.23 | 2.22 | 0.35 |
| 500 | 504.01 ± 4.51 | 0.90 | 0.80 | 504.90 ± 7.51 | 1.49 | 0.98 |
| Amniotic fluid | ||||||
| 5 | 5.13 ± 0.27 | 5.17 | 2.57 | 5.29 ± 0.19 | 3.66 | 5.87 |
| 10 | 10.43 ± 0.92 | 8.82 | 4.34 | 10.60 ± 0.57 | 5.38 | 5.97 |
| 100 | 105.0 ± 2.98 | 2.84 | 5.00 | 98.28 ± 9.78 | 9.95 | -1.72 |
| 500 | 500.98 ± 1.49 | 0.30 | 0.20 | 500.45 ± 1.47 | 0.29 | 0.09 |
Data have been expressed as mean ± SD.
The in-vitro recoveries of remdesivir and GS-441524 were assessed using low, medium, and high concentrations of remdesivir (50, 100, and 250 ng/mL) and GS-441524 (100, 250, and 500 ng/mL). The recoveries of remdesivir and GS-441524 were 7.00 ± 0.34% and 54.42 ± 0.62% for blood probes and 3.73 ± 0.19% and 26.58 ± 0.69% for embryonic probes (fetus, placenta, and amniotic fluid). The results suggested that the mean in vitro probe recovery was proportional to the semi-permeable membrane length and independent of the ambient concentration (Table 3). Analytical results were corrected using these recovery values, by dividing by 0.07 for remdesivir in the maternal blood and by 0.26 for GS-441524 in the embryonic tissue.
Table 3.
In vitro microdialysis recovery (%) of remdesivir and GS-441524 using ACD solution as the perfusion solution.
| Concentration (ng/mL) | Recovery (%) in blood probe | Recovery (%) in embryo probe |
|---|---|---|
| Remdesivir | ||
| 50 | 7.12 ± 1.49 | 3.81 ± 0.65 |
| 100 | 7.27 ± 0.80 | 3.54 ± 0.41 |
| 250 | 6.61 ± 0.49 | 3.84 ± 0.80 |
| Average | 7.00 ± 0.34 | 3.73 ± 0.19 |
| GS-441524 | ||
| 100 | 53.95 ± 10.11 | 27.09 ± 1.80 |
| 250 | 55.14 ± 6.82 | 26.87 ± 0.83 |
| 500 | 54.21 ± 0.68 | 25.79 ± 0.11 |
| Average | 54.43 ± 0.62 | 26.58 ± 0.69 |
Data have been expressed as mean ± SEM (n=3).
Blood-placental barrier transfer of remdesivir and GS-441524
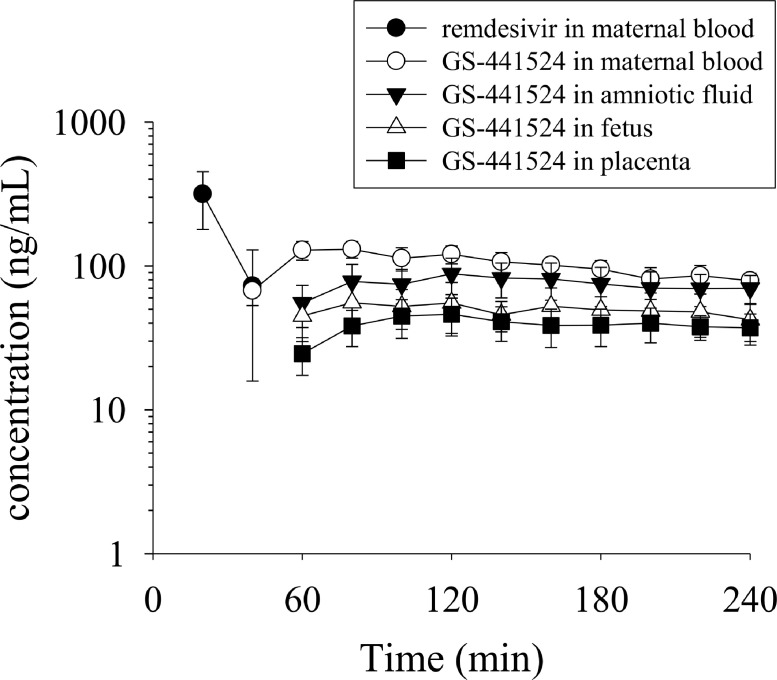
The concentration-time profiles of remdesivir and GS-441524 in the pregnant rats are shown in Figure 3. Remdesivir was only detected in the maternal blood at 40 min after administration (30 mg/kg, i.v.). Owing to the rapid biotransformation of remdesivir to GS-441524, remdesivir was not detectable in subsequent blood and embryo dialysis samples, consistent with a short elimination t1/2 and rapid metabolism. The metabolite GS-441524 first appeared at 40 min in the maternal blood samples, followed by a slow increase to reach a peak concentration (Tmax) of 87 ± 11 min. These results show that remdesivir is rapidly biotransformed to GS-441524, which subsequently achieves sustained levels in the placenta, fetus, and amniotic fluid.
Figure 3.
Concentration-time curves of unbound remdesivir and GS-441524 in the rat maternal blood, amniotic fluid, fetus, and placenta. Data have been expressed as mean ± SEM (n=6).
Owing to biotransformation from remdesivir, the appearance of GS-441524 in the embryo was delayed by approximately 1 h. Furthermore, no remdesivir was detected in the embryo, indicating that GS-441524 was distributed from the maternal blood and appeared in the embryo tissue after a delay of 20 min (Figure 3). The level of GS-441524 slowly declined until the last dialysis collection time-point (4 h), with the t1/2 of GS-441524 ranging from 123 ± 22 (min) to 201 ± 27 (min) in the maternal blood and embryonic tissue.
Based on the concentration-time profiles, the pharmacokinetic parameters of GS-441524 were calculated using a non-compartmental model (Table 4). The maximum concentrations (Cmax) of GS-441524 in the placenta, fetus, and amniotic fluid were 49.02 ± 12.22 ng/mL, 62.43 ± 18.03 ng/mL, and 92.39 ± 21.97 ng/mL, respectively, which were all lesser than the maternal blood concentration (135.97 ± 16.11 ng/mL). The areas under the concentration time curve (AUCs) of GS-441524 in the blood, placenta, fetus, and amniotic fluid were 23.40 ± 2.46, 8.39 ± 2.13, 10.85 ± 3.46, and 16.70 ± 4.48 (min μg/mL), respectively.
Table 4.
Pharmacokinetic parameters of GS-441524 in the rat maternal blood, placenta, fetus, and amniotic fluid, after remdesivir (30 mg/kg, i.v.) administration.
| Parameters | remdesivir (30 mg/kg, i.v.) |
|||
|---|---|---|---|---|
| blood | placenta | fetus | amniotic fluid | |
| Non-compartment model | ||||
| AUClast (min μg/mL) | 23.40 ± 2.46 | 8.39 ± 2.13* | 10.85 ± 3.46 | 16.70 ± 4.48 |
| Cmax (ng/mL) | 135.97 ± 16.11 | 49.02 ± 12.22* | 62.43 ± 18.03* | 92.39 ± 21.97 |
| Tmax (min) | 87 ± 11 | 143 ± 14 | 136 ± 23 | 143 ± 20 |
| t1/2 (min) | 192 ± 14 | 182 ± 31 | 123 ± 22 | 201 ± 27 |
| AUCtissue/AUCblood | - | 0.35 ± 0.09 | 0.46 ± 0.14 | 0.71 ± 0.19 |
AUCtissue/AUCblood represents the maternal blood-to-tissue transfer ratio.
Data have been expressed as mean ± SEM (n=6).
p<0.05, compared with maternal blood within groups, as assessed using ANOVA with Tukey's HSD post-hoc test.
The transfer of GS-441524 between the placenta, fetus, and amniotic fluid was evaluated using the AUCtissue/AUCblood ratio. The mother-to-fetus transfer ratios for the placenta, fetus, and amniotic fluid were 0.35 ± 0.09, 0.46 ± 0.14, and 0.71 ± 0.19, respectively. These results revealed that GS-441524 was transferred across the placenta to the fetus. Moreover, within the fetal tissue, GS-441524 levels were the highest in the amniotic fluid. Interestingly, the AUC ratios of maternal blood to the placenta, fetus, and amniotic fluid were similar, with Cmax ratios of 0.35, 0.46, and 0.71, respectively. A potential explanation is that a high Cmax leads to increased drug exposure in the tissue. In addition, a similar phenomenon of the appearance of GS-441524 was observed in the placenta, amniotic fluid, and fetus, implying that once GS-441524 had passed through the placental barrier, it was rapidly distributed to the fetus and amniotic fluid.
Discussion
In the present study, in vivo microdialysis coupled with a validated UHPLC-MS/MS analytical method.14 was used to investigate the transplacental transfer of remdesivir and its main metabolite, GS-441524, in the maternal blood, fetus, amniotic fluid, and placenta. The methodology used in this study was made compatible with the 3Rs principle (replacement, reduction, and refinement), by reducing the number of animals necessary for longitudinal sampling and measuring the pharmacologically relevant unbound form of the drug in the extracellular compartment. The free fraction of a drug in plasma is an important determinant of the pharmacodynamics15 and pharmacokinetics16 of drug action. This is because only the protein unbound fraction is available for diffusion between the plasma and tissues, where it interacts with pharmacological target proteins, such as ion channels, receptors, and enzymes.17 Furthermore, this technique simultaneously enables the detection of drugs at multiple sampling sites in the same animal. In addition, microdialysis provides clean dialysate samples, which require no further clean-up procedures, and thus, can be directly injected into the UHPLC-MS/MS system. The lack of a clean-up procedure also enables calibration using external standards rather than internal standards. Although microdialysis has advantages over other sample preparation methods, it has some limitations as well. The first is a limited time resolution of approximately 10–20 min to obtain a sufficient volume for analysis,18 owing to which, the initial drug concentration cannot be predicted accurately. Second, in vitro calibration of the microdialysis probe is time consuming.19 Third, for optimal sensitivity, microdialysis must be combined with highly sensitive and relatively expensive analytical instruments, such as UHPLC-MS/MS. Finally, the recovery of the probes is highly dependent on the nature of the semi-permeable membrane, including its length and diameter.
The pharmacokinetic profiles of the parent remdesivir concentrations in the maternal blood declined rapidly after remdesivir administration. This indicates that a high rate of biotransformation and metabolism occurred.20 These results are consistent with the characteristics of remdesivir, which is rapidly metabolized through hydrolysis to form the main metabolite GS-441524. This rapid metabolic conversion results in a shorter elimination t1/2 of remdesivir.21 In addition, owing to the route of i.v. bolus remdesivir administration in this experiment, the Cmax of remdesivir (315.63 ng/mL) was reached during the first sample collection (0–20 min). The most common route of remdesivir administration is intravenous infusion over a period of 30–120 min, with a Tmax of 2 h 16 min.22 Oral administration of remdesivir is generally not recommended because of its high first-pass effect clearance in the gastrointestinal tract, which causes limited absorption and systemic availability.23
GS-441524 exhibited distinctly different pharmacokinetic properties, as compared to remdesivir, in the maternal blood. This main metabolite first appeared during the second sampling time-point, with a peak concentration of 135.97 ng/mL, compared to that of remdesivir, at 315.63 ng/mL, in blood. However, 40 min after remdesivir administration, the concentration of remdesivir was significantly lower than that of GS-441524, and showed a slow elimination phase. These findings are consistent with a previous report, which showed that a large component of remdesivir is transformed into GS-441524,22,24 with a long elimination t1/2 of 21 h.25 The hydrolysis of remdesivir by carboxylesterase produces a more water-soluble monophosphate nucleoside core that tends to remain in the tissue phase.26 The high volume of distribution of GS-441524 (6.36 L/kg) is consistent with this interpretation.27 Upon comparison of differences in pharmacokinetic characteristics in plasma between humans and rats, despite different routes of drug administration, similar remdesivir and GS-441524 pharmacokinetics characteristics were observed. Remdesivir followed a rapid decline28 and the absorption of GS-441524 appeared at the second time-point, with a slow elimination phase.29
Owing to rapid biotransformation, the transplacental transfer phenomenon of remdesivir was not observed in this study. Conversely, only GS-441524 passed through the placenta to the fetus. The Cmax and AUClast values of GS-441524 followed the order: maternal blood > amniotic fluid > fetus > placenta. Interestingly, the AUClast value of amniotic fluid (16.70 ± 4.48 min μg/mL) was roughly 2-fold higher than that of the placenta (8.39 ± 2.13 min μg/mL) and fetus (10.85 ± 3.46 min μg/mL), suggesting that GS-441524 is distributed to the placenta and rapidly reaches the fetus and amniotic fluid. The potential explanation is that GS-441524 does not get easily get metabolized by major CYP family members (such as 1A1, 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, 3A4, or 3A5),30 which implies that GS-441524 may not be metabolized in the liver but remains as GS-441524, to be directly eliminated in the fetal urine; thus, the higher concentrations, higher AUC, and delayed Tmax of GS-441524 in the amniotic fluid might contribute both from the urine of the fetus and placental clearance. As observed in maternal blood, GS-441524 also showed a long residence time in the tissue compartment.
Placental drug transfer is potentially mediated by four main processes: simple diffusion, facilitated diffusion, active transport, and pinocytosis.31 Among these four processes, most of the drug passes through the placenta by means of passive diffusion, governed by Fick's law of diffusion: Q = k A (C1-C2)/d, where Q is the degree of drug diffusion across the placenta, k is the diffusion constant, A is the area of the placental surface, C1 is the maternal blood-free drug concentration, C2 is the fetal free drug concentration, and d is the thickness of the placental tissue.32 From this formulation, a high concentration gradient and the constant k might influence drug transfer from the mother to the fetus. The diffusion constant k is related to physiochemical properties, such as molecular weight, lipid solubility, degree of ionization, and protein binding rate.33 The parent remdesivir reportedly has a high affinity for plasma proteins, with binding as high as 93.6%, whereas only approximately 2% GS-441524 is protein-bound. Furthermore, low molecular weight (<500 Da) and high lipophilicity (high LogP value) are beneficial for rapid placental transfer. The molecular weight and LogP values of remdesivir and GS-441524 are 603 Da (LogP=2.01) and 291 Da (LogP=-1.9), respectively.23 In addition, the calculation of the pKa microstates of remdesivir and GS-441524 revealed that remdesivir possesses acid (10.93) and base (-2.24) microstates, while in comparison, GS-441524 only has a base (3.76) microstate.34 We speculated that acid molecules tend to be highly bound to albumin and have low tissue constituent affinity, leading to low volumes of distribution. In addition, the negative charge of acidic drugs at physiological pH means that remdesivir does not significantly bind to the phospholipid head of the tissue membrane. Thus, the tissue penetration rate of remdesivir is generally considered low.35 Consistent with this notion, remdesivir was not detectable in the spleen or lungs of rodents or monkeys, and did not pass through the blood-brain barrier.36 Moreover, in a cohort study, remdesivir showed a low volume of distribution, ranging between 0.75 and 0.12 L/kg, with a single dose of 10–225 mg in humans.22 Therefore, we speculated that although the higher lipophilicity of remdesivir, as compared to GS-441524, might increase its penetration across the placenta, the ability of the unbound drug to cross the placenta is corrected by the protein binding rate and ionization class,35 which results in a transfer ratio as high as 51% for GS-441524, from mother to fetus. These reports concur with our findings that remdesivir is a prodrug with limited tissue distribution and is rapidly metabolized to the main metabolite (GS-441524) that readily crosses into the embryo. To date, the transmembrane transporters for the metabolite GS-441524 are not fully understood, but one report has indicated that GS-441524 might be a substrate for MDR1, BCRP, CNT3, and ENT1. Additionally, the predominant transporters in the placenta are P-gp, MDR1, BCRP, and OATP in rats, and GS-441524 may pass through the placenta via MDR1 and BCRP transporters.
A multisite transplacental microdialysis approach was successfully developed to investigate the pharmacokinetics of the anti-viral drug remdesivir. This study has shown that remdesivir does not cross the blood-placental barrier, but is instead converted to the main metabolite GS-441524. Limited tissue distribution of remdesivir in the fetus and a long residence time of GS-441524 in the fetal circulation were observed in the present study. Based on the FDA's emergency use authorization during pregnancy, remdesivir should only be prescribed if the potential benefit justifies the risk for the mother and fetus. In conclusion, the findings of this study provide a detailed, temporally dynamic account of the metabolic biotransformation of remdesivir into the main metabolite GS-441524.
Contributors
Ling Yang, I-Hsin Lin, and Lie-Chwen Lin performed the experiments, analyzed the data, and prepared the manuscript. Jeffrey W. Dalley provided intellectual input and edited the paper. Tung-Hu Tsai designed the experiments, edited the paper, and secured funding. Yang and Tsai verified the data. All authors have read and approved the final version of the manuscript.
Declaration of interests
The authors declare no conflicts of interest concerning the materials used in this study or the findings specified in this paper.
Data sharing statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgements
We thank the Chih-Ping Yang Research Group at Formosa Laboratories, Inc., Taoyuan, Taiwan, for supplying us with high-quality remdesivir. This study was supported in part by research grants from the Ministry of Science and Technology of Taiwan (MOST 110-2918-I-239-001, MOST 110-2113-M-A49A-503, and MOST 109-2113-M-010-007) and a graduate student scholarship from the College of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan.
References
- 1.Gordon CJ, Tchesnokov EP, Woolner E, et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem. 2020;295(20):6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheahan TP, Sims AC, Graham RL, et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei D, Hu T, Zhang Y, et al. Potency and pharmacokinetics of GS-441524 derivatives against SARS-CoV-2. Bioorg Med Chem. 2021;46 doi: 10.1016/j.bmc.2021.116364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zambrano LD, Ellington S, Strid P, et al. Update characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status — United States, January 22–October 3, 2020. MMWR Morbidity Mortality Weekly Report. 2020;69(44) doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collin J, Bystrom E, Carnahan A, Ahrne M. Public Health Agency of Sweden's Brief Report: Pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. 2020;99(7):819–822. doi: 10.1111/aogs.13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurol-Urganci I, Jardine JE, Carroll F, et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol. 2021;225(5) doi: 10.1016/j.ajog.2021.05.016. 522 e1- e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jorgensen SCJ, Davis MR, Lapinsky SE. A review of remdesivir for COVID-19 in pregnancy and lactation. J Antimicrob Chemother. 2021 doi: 10.1093/jac/dkab311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louchet M, Sibiude J, Peytavin G, Picone O, Treluyer JM, Mandelbrot L. Placental transfer and safety in pregnancy of medications under investigation to treat coronavirus disease 2019. Am J Obstet Gynecol MFM. 2020;2(3) doi: 10.1016/j.ajogmf.2020.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor MM, Kobeissi L, Kim C, et al. Inclusion of pregnant women in COVID-19 treatment trials: a review and global call to action. The Lancet Global Health. 2021;9(3):e366–e371. doi: 10.1016/S2214-109X(20)30484-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lampejo T. Remdesivir for the treatment of COVID-19 in pregnancy. J Med Virol. 2021;93(7):4114–4119. doi: 10.1002/jmv.26986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burwick RM, Yawetz S, Stephenson KE, et al. Compassionate use of remdesivir in pregnant women with severe Covid-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin IH, Yang L, Hsueh TY, Tsai TH. Blood-placental barrier transfers and pharmacokinetics of unbound morphine in pregnant rats with multiple microdialysis systems. ACS Pharmacol Transl Sci. 2021;4(5):1588–1597. doi: 10.1021/acsptsci.1c00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin IH, Yang L, Dalley JW, Tsai T-H. Trans-placental transfer of nicotine: modulation by organic cation transporters. Biomed Pharmacother. 2022:145. doi: 10.1016/j.biopha.2021.112489. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez D, Schmidt S, Derendorf H. Importance of relating efficacy measures to unbound drug concentrations for anti-infective agents. Clin Microbiol Rev. 2013;26(2):274–288. doi: 10.1128/CMR.00092-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohnert T, Gan LS. Plasma protein binding: from discovery to development. J Pharm Sci. 2013;102(9):2953–2994. doi: 10.1002/jps.23614. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe R, Esaki T, Kawashima H, et al. Predicting fraction unbound in human plasma from chemical structure: improved accuracy in the low value ranges. Mol Pharm. 2018;15(11):5302–5311. doi: 10.1021/acs.molpharmaceut.8b00785. [DOI] [PubMed] [Google Scholar]
- 18.Chefer VI, Thompson AC, Zapata A, Shippenberg TS. Overview of brain microdialysis. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.ns0701s47. [Chapter 7] Unit7 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shippenberg TS, Thompson AC. Overview of microdialysis. Curr Protoc Neurosci. 2001 doi: 10.1002/0471142301.ns0701s00. [Chapter 7] Unit7 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tempestilli M, Caputi P, Avataneo V, et al. Pharmacokinetics of remdesivir and GS-441524 in two critically ill patients who recovered from COVID-19. J Antimicrob Chemother. 2020;75(10):2977–2980. doi: 10.1093/jac/dkaa239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun D. Remdesivir for treatment of COVID-19: combination of pulmonary and IV administration may offer aditional benefit. AAPS J. 2020;22(4):77. doi: 10.1208/s12248-020-00459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humeniuk R, Mathias A, Cao H, et al. Safety, tolerability, and pharmacokinetics of remdesivir, an antiviral for treatment of COVID-19, in healthy subjects. Clin Transl Sci. 2020;13(5):896–906. doi: 10.1111/cts.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dehelean CA, Lazureanu V, Coricovac D, et al. SARS-CoV-2: repurposed drugs and novel therapeutic approaches-insights into chemical structure-biological activity and toxicological screening. J Clin Med. 2020;9(7) doi: 10.3390/jcm9072084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fact sheet for health care providers emergency use authorization (EUA) of VEKLURY® (remdesivir). The U.S. Food and Drug Administration (FDA).
- 25.Du P, Wang G, Yang S, Li P, Liu L. Quantitative HPLC-MS/MS determination of Nuc, the active metabolite of remdesivir, and its pharmacokinetics in rat. Anal Bioanal Chem. 2021;413(23):5811–5820. doi: 10.1007/s00216-021-03561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eastman RT, Roth JS, Brimacombe KR, et al. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci. 2020;6(5):672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sukeishi A, Itohara K, Yonezawa A, et al. Population pharmacokinetic modeling of GS-441524, the active metabolite of remdesivir, in Japanese COVID-19 patients with renal dysfunction. CPT Pharmacometrics Syst Pharmacol. 2021 doi: 10.1002/psp4.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanafin PO, Jermain B, Hickey AJ, et al. A mechanism-based pharmacokinetic model of remdesivir leveraging interspecies scaling to simulate COVID-19 treatment in humans. CPT Pharmacometrics Syst Pharmacol. 2021;10(2):89–99. doi: 10.1002/psp4.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez JC, Moine P, Etting I, Annane D, Larabi IA. Quantification of plasma remdesivir and its metabolite GS-441524 using liquid chromatography coupled to tandem mass spectrometry. Application to a Covid-19 treated patient. Clin Chem Lab Med. 2020;58(9):1461–1468. doi: 10.1515/cclm-2020-0612. [DOI] [PubMed] [Google Scholar]
- 30.Xie J, Wang Z. Can remdesivir and its parent nucleoside GS-441524 be potential oral drugs? An in vitro and in vivo DMPK assessment. Acta Pharm Sin B. 2021;11(6):1607–1616. doi: 10.1016/j.apsb.2021.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peereboom-Stegeman JH, Noordhoek J, Gribnau FW, Russel FG. Mechanisms of drug transfer across the human placenta. Pharm World Sci. 1998;20(4):139–148. doi: 10.1023/a:1008656928861. [DOI] [PubMed] [Google Scholar]
- 32.Griffiths SK, Campbell JP. Placental structure, function and drug transfer. Continuing Educ Anaesthesia Critical Care Pain. 2015;15(2):84–89. [Google Scholar]
- 33.Pacifici GM, Nottoli R. Placental transfer of drugs administered to the mother. Clin Pharmacokinet. 1995;28(3):235–269. doi: 10.2165/00003088-199528030-00005. [DOI] [PubMed] [Google Scholar]
- 34.Deb S, Reeves AA. Simulation of remdesivir disposition and its drug interactions. J Pharm Pharm Sci. 2021;24:277–291. doi: 10.18433/jpps32011. [DOI] [PubMed] [Google Scholar]
- 35.Smith DA, Beaumont K, Maurer TS, Di L. Volume of distribution in drug design. J Med Chem. 2015;58(15):5691–5698. doi: 10.1021/acs.jmedchem.5b00201. [DOI] [PubMed] [Google Scholar]
- 36.Deb S, Reeves AA, Hopefl R, Bejusca R. ADME and pharmacokinetic properties of remdesivir: its drug interaction potential. Pharmaceuticals (Basel) 2021;14(7) doi: 10.3390/ph14070655. [DOI] [PMC free article] [PubMed] [Google Scholar]