Abstract
Therapeutic resistance and metastatic progression are responsible for the majority of cancer mortalities. In particular, the development of resistance is a significant barrier to the efficacy of cancer treatments such as chemotherapy, radiotherapy, targeted therapies, and immunotherapies. Cancer stem cells (CSCs) underlie treatment resistance and metastasis. p38 mitogen-activated protein kinase (p38 MAPK) is downstream of several CSC-specific signaling pathways, and it plays an important role in CSC development and maintenance and contributes to metastasis and chemoresistance. Therefore, the development of therapeutic approaches targeting p38 can sensitize tumors to chemotherapy and prevent metastatic progression.
Subject terms: Cancer stem cells, Metastasis
Introduction
Mitogen-activated protein kinases (MAPKs) allow cells to interpret and respond to a wide variety of signals. This includes DNA damaging genotoxic agents, inflammatory cytokines, and extracellular stimuli such as changes in osmolarity, oxidative stress, and heat shock [1, 2] (Fig. 1A). The p38 MAPKs are serine/threonine-specific protein kinases characterized by a Thr-Gly-Tyr dual phosphorylation motif [3] (Fig. 1A). Four p38 isoforms have been identified (p38α, β, γ, and δ). Recently, these genes have been named as MAPK14 for p38α, MAPK11 for p38β, MAPK12 for p38γ, and MAPK13 for p38δ (Fig. 2A, B). Among them, p38ɑ and β are ubiquitously expressed and share 75% sequence identity with one another at the amino acid level. Whereas p38γ and δ have tissue-specific expression and share 70% homology with each other and 62% and 61% sequence homology with p38α, respectively [4, 5]. The expression of p38γ is limited to the skeletal muscle, whereas p38δ is expressed in the pancreas, kidneys, small intestine, testis, and lungs [4] (Fig. 2A).
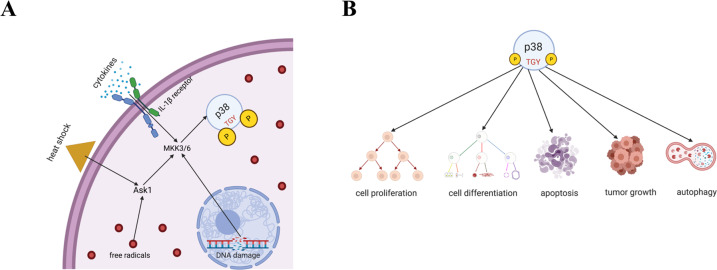
Fig. 1. Functions of p38.
A p38 enables cells to respond to various stimuli, including DNA damaging agents, cytokines, heat shock, and oxidative stress. B In addition, p38 affects cell proliferation, cell differentiation, apoptosis, autophagy, and tumor growth in ways dependent on cell type and the signaling pathways involved.
Fig. 2. Domain structures of p38 isoforms.
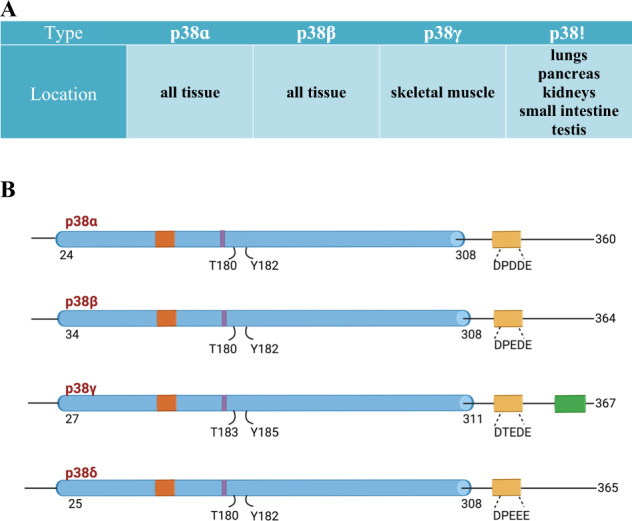
A Expression sites of different p38 isoforms. B p38ɑ and p38β share more sequence homology with each other than with p38γ and p38δ.
All four p38 isoforms serve as nexuses for signal transduction and play crucial roles in many biological processes. This includes cell proliferation, differentiation, glucose and lipid metabolism, secretion, senescence, stress responses, apoptosis, autophagy, and cell migration (Fig. 1B) [3, 6–11]. Depending on the context, p38 proteins can be tumor-suppressive or tumor-promoting [3, 12]. The expression levels of the four different isoforms of p38 vary across different cancer types. For example, primary tumors of breast cancer, lung adenocarcinoma, and glioblastoma multiforme have similar or significantly lower MAPK11 (p38β) and MAPK12 (p38γ) protein expression levels compared to normal tissue (Supplementary Figs. 1 and 2). On the other hand, MAPK13 (p38δ) and MAPK14 (p38α) expression levels are significantly higher in the primary tumor in most cancers (Supplementary Figs. 3 and 4) [13]. Moreover, MAPK14 (p38α) T180/Y182 phosphorylation, which is induced by stress signals, is significantly reduced in primary tumor compared to normal tissue in 2 out of the three cancers tested (Supplementary Fig. 5). There is a similar survival probability between breast cancer patients with low and with high MAPK14 expression, and interestingly, MAPK14 phospho-T180/Y182 seems to have a protective effect (Supplementary Fig. 6) [14, 15]. p38α has many phosphorylation sites that regulate different downstream functions, thus different phosphorylated residues could have different correlation with survival.
p38ɑ and p38β cooperate in heart development, mitotic entry, regulatory T cell induction, and sex determination [16–19]. Similarly, p38γ and p38δ coordinate and regulate tissue regeneration and immune responses [20]. Besides, recent studies have demonstrated that p38 MAPKs regulate the behavior and function of cancer stem cells (CSCs).
CSCs are a subpopulation of tumor cells capable of self-renewal and differentiation that drive tumor initiation, recurrence, progression, and metastasis. CSCs can survive in circulation and have the potential to establish a metastatic tumor at a distant site. CSCs also contribute to the development of chemoresistance, which is responsible for 90% of treatment failures [21]. Various mechanisms contribute to the development of resistance to chemotherapy by CSCs. These cells can express drug-resistance genes such as MDR1, which encodes an energy-dependent exporter, or they can overexpress drug-efflux transporters from the ATP-binding cassette (ABC) family, which can remove chemotherapeutic agents from the cell [21]. CSCs also express aldehyde dehydrogenase 1 (ALDH1), which aids in the protection against alkylating agents such as paclitaxel [22]. In this review, we discuss the mechanisms through which p38 attenuates and augments CSC properties contingent on the cancer type and how p38 can be targeted to overcome chemoresistance and block metastasis.
Role of p38 in CSCs
Enrichment of CSCs by p38
Anywhere between 0.1 and 25% of the total cell population in a solid tumor comprises of CSCs [23, 24]. CSCs can be identified and enriched using various cell surface markers, such as CD44hi/CD24lo for breast cancer [25], CD133 for brain cancer [26], and many others [23, 27]. CSCs divide asymmetrically and generate one daughter cell retaining the stem-cell identity, while the other cell differentiates and is often highly proliferative [23]. Patient-to-patient, the CSC populations are highly variable. This is because CSC properties are influenced by tumor-specific genetic aberrancies, the stage of disease progression, and the types of drugs used to combat tumor growth [28].
Besides the transformation of normal stem cells to cancer stem cells, CSCs can also come from differentiated cancer cells through the activation of the epithelial-to-mesenchymal transition (EMT) [23, 29]. Several studies have shown that high levels of expression of EMT-inducing transcription factors ZEB1/2, SNAI1/2, TWIST1/2, and FOXC2 in cancer cells trigger the expression of stemness factors such as SOX2, BMI1, and OCT4 and enhance the ability to self-renew and to form mammospheres, all of which are characteristics of CSCs [29–32]. In pancreatic cancer cells, mechanical stress upregulates EMT transcription factors, activates p38, and enhances cell migration [33]. Although the transcription factors that promote EMT and enrichment of CSCs are known, these proteins are hard to target pharmacologically relative to kinases like p38.
In human mammary epithelial cells induced to undergo EMT, p38α phosphorylates FOXC2 at serine 367, stabilizes FOXC2, confer stem-cell attributes in vitro, and metastatic competence in vivo [12, 34] (Fig. 3). Additionally, inhibition of p38α blocks stem-cell properties such as sphere-forming potential and the CD44hi/CD24lo stem-cell marker profile (Fig. 3). Besides, depletion of p38α from epithelial cells by shRNA knockdown blocks these cells’ ability to undergo EMT in response to Snail or Twist [34], suggesting that many EMT- and CSC-promoting signals are potentially transmitted through p38.
Fig. 3. Schematic of the p38-FOXC2 signaling axis.

The p38-FOXC2 signaling axis is critical in the formation of CSCs. p38, regulated by many upstream kinases, phosphorylates FOXC2, which then activates ZEB1. Activated FOXC2 and ZEB1 promote the EMT, initiating metastasis and conferring stemness in cancer cells. p38 inhibition reverses the EMT and inhibits stemness in cancer cells.
Overexpression of p38γ in the luminal A breast cancer cell line MCF7 increases CSCs and tumorspheres [35, 36]. Similarly, knockdown of p38γ expression decreases the frequency of the CSC population and blocks tumorsphere formation of breast cancer cell lines MCF7-ErbB2 and BT47, which express HER2 oncogene [36]. In the same way, knockdown of p38γ from triple negative breast cancer (TNBC) cell lines MDA-MB 231 and MDA-MB 468 significantly reduces sphere formation, suggesting that p38γ is also capable of manipulating the CSC population [37]. In addition, silencing of p38γ significantly decreases the key CSC drivers Nanog, OCT3/4, SOX2, and CD44 [37]. Interestingly, p38γ expression alone is sufficient to induce expansion of the CSC population, likely due to its direct stimulation of Nanog expression via c‐Jun‐mediated binding to the activator protein 1 (AP1) site of the Nanog promoter [37]. Nanog then transcriptionally induces the expression of SOX2 and OCT3/4, leading to CSC expansion and inducing TNBC progression [37]. Thus, p38 acts to manipulate CSC properties in multiple breast cancer subtypes.
In addition to serving as an upstream regulator of CSC-inducing transcription factors, p38 also functions as a downstream target of transcription factors through different signaling axes. For example, in response to chemotherapy, the hypoxia-inducible factor HIF1 induces increases in DUSP9 and decreases in DUSP16 expression, leading to activation of the p38 signaling pathway in TNBCs [38]. Activation of p38 then increases the expression of Nanog and KLF4 through phosphorylation and inactivation of ZFP36L1, thus increasing the CSC pool [38]. This complex dual involvement of p38 is critical in regulating CSC maintenance and expansion in breast cancer.
In the head and neck squamous cell carcinoma cell line SCC-131, inhibition of p38 significantly reduces tumor spheroid formation and decreases the expression of the CSC markers SOX2, OCT4, KLF4, c-MYC, and CD44, indicating that p38 plays a key role in the maintenance of stemness properties in this cancer type [39]. Activation of the p38 pathway also enhances the survival of colorectal CSCs under hypoxia and serum-depletion conditions, which are two well-defined capabilities of CSCs [40]. The interaction of neuropilin 1 (NRP-1) and VEGF-A initiates a cascade involving GIPC1, SYX, RhoA/ROCK, and MEK3/6 to activate p38 necessary to enrich epidermal CSCs, as p38 depletion reduces CSC spheroid formation and invasion [41].
KLF family of zinc finger transcription factors KLF4 activates the p38 signaling pathway in osteosarcoma, promoting cancer stemness [42]. Not only does KLF4 overexpression cause a substantial increase in osteosphere formation and dimension, but it also significantly increases the transcription of stem-cell-associated genes, including CD133, ALDH1A1, and ABCG2 [42]. Interestingly, when the expression of p38 is inhibited using siRNA, the KLF4 induced spheres are also reduced, suggesting that the p38 signaling pathway activates the expression of stem-cell transcription factors and downstream transducers of these transcription factors [42]. Downregulation of p38γ using shRNA in breast cancer cells overexpressing ErbB2 decrease the alcohol-induced increase in CSCs, mammosphere formation, and migration and invasion [43]. The cytokine IL-17 activates the p38 signaling pathway, thereby stimulating self-renewal of ovarian CD133+ CSCs, and IL-17-promoted self-renewal is compromised upon p38 inhibition [44]. In hepatocellular carcinoma, long-term tobacco exposure increases IL33 expression in liver tissues. The increased IL33 promotes p38 activation, inducing EMT and increasing the levels of CSC markers CD133, Nanog, and OCT4, and this can be abrogated by p38 inhibition [45].
Role of p38 in tumor-initiating vs. metastatic CSCs
Tumor-initiating CSCs (tiCSCs) and metastatic CSCs (mCSCs) are the two predominant pools of CSCs within tumors. While tiCSCs are responsible for the development of heterogeneous lineages of cancer cells that comprise the primary tumor [46], the mCSCs possess migratory and invasive capabilities, survival capacities in circulation, and metastatic potential [29]. Although mCSCs and tiCSCs share some phenotypic similarities, mCSCs are shown to accumulate new genetic alterations at secondary sites that make them resistant to chemotherapies that are effective against tumor-initiating CSCs [47]. Stemness plays an important role in metastasis, specifically in relation to the expression of various integrins and the formation of circulating tumor cells.
In breast cancer, the expression of integrin αvβ3 induces CSC properties, including tumorsphere formation [48] and the integrin subunits β1 and β3 are used as CSC markers [49]. The β3 is necessary and sufficient for the CSC phenotype in lung, prostate, and breast cancers [48, 50, 51]. Expression of those same integrins, αvβ3, β1, and β3, contributes to lymph node and bone metastases [52–57]. Several studies reported that most circulating tumor cells have CSC-like features [58–62]. Because p38 promotes CSC properties and stemness is implicated in metastasis, p38 might specifically enrich mCSCs as opposed to tiCSCs. Moreover, mCSCs are characterized by CXCR4 expression, which promotes metastasis by activating p38, thus suggesting the involvement of p38 in the production of mCSCs in particular [63].
Activation of the EMT program also induces stemness and p38 signaling [34]. By concomitantly activating the EMT and p38 signaling, cancer cells gain a migratory and mesenchymal phenotype, which is required for metastasis [64, 65]. Inhibition of p38 inhibits FOXC2 and reverts prostate cancer cells from a mesenchymal phenotype with metastatic properties to an epithelial phenotype that is incapable of developing metastatic growth [66]. In addition, p38 inhibition impedes metastasis in breast cancer but not primary tumor growth [34]. The former is mediated by mCSCs, whereas the latter is underpinned by tiCSCs. These findings are strong indicators that p38 proteins play a critical role specifically in mCSCs.
p38 diminishes the CSC population
Although substantial evidence supports a pro-CSC role for p38, some findings argue that p38 can attenuate CSC properties and decrease the CSC population [67–73]. This warrants further characterization of the contributions of each of the four p38 isoforms to CSC properties. The p38/nuclear factor (NF)-kB/Snail signaling pathway is involved in caffeic acid-induced inhibition of CSC properties and migratory capacity of malignant human keratinocyte HaCaT cells [67]. In caffeic acid-treated HaCaT cells, phosphorylation of p38 is increased and NF-kB binding to the Snail promoter is decreased, resulting in the downregulation of Snail, a transcription factor linked to the acquisition of CSC-like characteristics [67]. Caffeic acid-treated HaCaT cells also have attenuated sphere-forming capacity and decreased expression of CD34 and the keratin encoding K5, which are markers of CSCs and skin stem cells, respectively [67]. Thus, through this pathway, p38 acts as an upstream regulator of NF-kB and Snail to reduce CSC properties in skin cancer.
A non-anticoagulant heparan sulfate hexasaccharide sequence, HS06, selectively inhibits CSC self-renewal and induces apoptosis in breast, colorectal, and pancreatic CSCs [68]. HS06 inhibition of CSCs is dependent on early and sustained activation of p38α and β, which inhibits TCF4-mediated signaling and, therefore, CSC self-renewal [68]. When p38 is inhibited using SB203580 in cells treated with HS06, sphere formation is enhanced, and CSC markers (CD44 and CD133) and self-renewal factors (c-MYC and BMI1) are expressed at higher levels than in cells treated with HS06 alone [68]. Glycosaminoglycans (GAGs) are also essential regulators of stemness. Like HS06, G2.2, a sulfated non-saccharide GAG mimetic, induces early and sustained activation of p38 in human colorectal HT29 spheroids [69]. Importantly, pharmacological inhibition of p38 with SB203580 reverses G2.2-mediated inhibition of CSC self-renewal, as evidenced by the increased 3D spheroid formation and expression of CSC and self-renewal markers [69]. These results imply that p38α and β can also inhibit CSC self-renewal under certain circumstances.
Activation of the p38γ and δ isoforms abolishes the CSC properties and tumor-initiating ability of non-small cell lung cancer (NSCLC) cells through ubiquitination and degradation of stemness proteins SOX2, OCT4, Nanog, KLF4, and c-MYC through MK2-mediated phosphorylation of Hsp27, a fundamental component of the proteasomal degradation machinery [70]. The inactivation of p38 induces the upregulation of stemness proteins in NSCLC cells, causing them to acquire CSC properties [70]. WIP1, a p38 phosphatase frequently overexpressed in cancer, promotes stemness-related protein expression and CSC properties by inhibiting p38 activity in NSCLC cells [71].
p38 also regulates ubiquitin-mediated degradation of stemness factors in glioma CSCs, as activation of the p38 pathway leads to reduced epidermal growth factor receptor (EGFR) surface expression via ubiquitin ligase-mediated degradation of EGFR, thereby attenuating the sphere-forming and self-renewal capacities of the CSCs [72]. Upon p38 inhibition, the number of undifferentiated CSCs increases [72]. In contrast to the reported enhancement of CSC properties upon overexpression of p38γ in MCF7 cells [36], in another CSC model resulting from ectopic overexpression of the stem-cell marker Nanog in MCF7 cells, activation of p38 and AMPKα pathways result in inhibition of CSC growth and induction of apoptosis [73]. In addition, in a breast cancer model, activation of p38 from extracellular matrix-dependent compressive forces promoted a differentiated cell phenotype [74]. Although there is limited evidence for a CSC-suppressive role of p38, further study of the involvement of the different p38 isoforms in CSC suppression is warranted.
Mechanisms of p38-mediated chemoresistance
Metastasis is the primary cause of cancer-related mortality, accounting for more than 90% of cancer-related deaths [75]. Although chemotherapy remains the main treatment for many types of cancer, chemoresistance is almost always observed in patients with metastatic cancer. During treatment, some tumor cells transform into CSCs, become chemoresistant, and acquire the ability to become metastatic. Not only do p38 proteins regulate signaling that contributes to CSC maintenance and expansion, but these proteins are also directly linked with the mechanisms that result in chemoresistance.
Drug efflux
ABC transport proteins ABCB1, ABCC1, ABCG2, and MDR1 are expressed within CSCs [76] (Fig. 4). ABC transporters shield cells from harmful toxins and xenobiotics [77]. For example, MDR1 prevents the entry of foreign toxins into the growing fetus and sensitive organs such as the kidneys and brain, and ABCG2 blocks toxins from invading the mammary gland, hematopoietic stem cells, and the blood-brain barrier [77]. However, CSCs use these same ABC transporters to pump chemotherapy agents (e.g., paclitaxel, doxorubicin, vinblastine, etoposide, and colchicine) out of the cells [77]. Inhibition of p38 by BIRB796 can reverse multidrug resistance induced by ABCB1, ABCC1, and ABCG2 by directly inhibiting their ATPase and transport functions [78] (Fig. 4). Moreover, inhibition of p38 by SB202190 reduces MDR1 expression levels in human gastric cancer cells and sensitizes them to the chemotherapeutic agent vincristine, suggesting a role for p38 in drug-efflux-mechanism-driven chemoresistance [79] (Fig. 4).
Fig. 4. Mechanisms of chemoresistance in cancer stem cells.
Upon p38 activation, CSCs are maintained in a non-proliferating, quiescent state for long periods, thus escaping chemotherapies that target rapidly dividing cells. p38 regulates the expression of ALDHs, such as ALDH2, which detoxify the metabolites of chemotherapeutic agents, rendering them ineffective. The WNT/β-catenin signaling pathway, promoted by p38, mediates chemoresistance via the upregulation of ABC transporter pumps such as ABCC1, ABCG2, and MDR1, which remove chemotherapy agents from inside the cell. These ABC transporters are also directly upregulated by p38. Moreover, the Notch and NF-κB signaling axes promote self-renewal and inhibit apoptosis of CSCs even in the presence of chemotherapy drugs. The activity of NF-κB is stimulated by p38, and Notch activates p38. Additionally, p38 phosphorylates BCL-2, promoting apoptosis.
ALDH activity
Cytosolic ALDHs oxidize intracellular aldehydes and convert them into carboxylic acids [80] (Fig. 4). CSCs express high levels of ALDHs, which confer chemoresistance to CSCs by detoxifying the aldehyde intermediates produced in CSCs treated with certain chemotherapy agents and protecting CSCs from the noxious effects of elevated reactive oxygen species levels, which result from oxidative stress generated by chemotherapy drugs [81]. Resistance to paclitaxel and epirubicin is seen in breast tumor samples enriched in CSCs with high levels of ALDH activity [82]. Additionally, when compared to counterparts that do not express these dehydrogenases, ALDH-positive cells from lung cancer cell lines have higher resistance to multiple chemotherapy agents [83]. Detoxification of other chemotherapeutics, such as the alkylating agent cyclophosphamide, also occurs through ALDH activity. Once inside the cell, the pro-drug form of cyclophosphamide is converted to 4-hydroxy cyclophosphamide and then to aldophosphamide [76]. As shown by studies in leukemia cells, aldophosphamide is detoxified by ALDHs specifically in the CSC subpopulation, making this enzyme a primary driver for CSC chemoresistance [76] (Fig. 4). In acute myeloid leukemia, TGF-β1-mediated p38 activation induces ALDH2 expression and chemoresistance [84] (Fig. 4). It is likely that p38 regulates the expression of ALDHs in other cancers, suggesting that p38 inhibition could reduce the expression of ALDHs and sensitize CSCs to chemotherapy.
Quiescence and dormancy of CSCs
Quiescence by cancer cells is reversible, and a quiescent cell may reenter the cell cycle in response to physiological cell stimuli [85]. Dormant tumors consist of CSCs in a non-proliferating state, and these quiescent tumor cells may persist for long periods at metastatic sites [85]. Activation of p38 signaling is dependent on the secretion of BMP7 and promotes dormancy and quiescence in prostate CSC-like cells [86] (Fig. 4). Bone-secreted factors DKK3, vasorin, and neogenin induce dormancy in prostate cancer cells via p38 activation as well [87] (Fig. 4). p38 activation also induces dormancy in squamous carcinoma cells [88]. p38 signaling allows these dormant tumor cells to resist chemotherapy by activation of pro-survival mechanisms driven by upregulation of the protein kinases PERK and BiP, which prevent the activation of Bax, a pro-apoptotic protein [88] (Fig. 4). In addition, whereas a high ratio of ERK to p38 induces tumor growth, a low ratio results in tumor growth arrest [89]. In a head and neck squamous carcinoma cell model, TGF-β2 signaling in the bone marrow activates p38α and β, resulting in a low ERK to p38 signaling ratio and dormancy of malignant disseminated tumor cells [90] (Fig. 4). Activation of p38 can also act through other pathways. For example, high levels of p38 result in CDC42 expression, which induces p21/p27 and represses cyclin D1 expression, thereby causing cell-cycle arrest [11, 91, 92] (Fig. 4).
Because chemotherapeutics target rapidly dividing cells, the slow-cycling, quiescent phenotype of CSCs renders them resistant to conventional therapy. For example, slow-cycling glioma stem cells in a transgenic mouse model survive the alkylating drug temozolomide and eventually cause recurrence [93]. However, when the slow-cycling CSCs are removed from the tumor, the tumor becomes chemosensitive, and the mice survive for a much longer time [93]. Thus, it is possible that p38 inhibition could push CSCs out of their quiescent state, making them more susceptible to chemotherapy-induced death.
Defective DNA repair
Mechanisms such as proofreading, mismatch repair, nucleotide excision repair, and base excision repair fix errors introduced during DNA replication. Because cancer cells rapidly divide, they are often in S phase, which is a vulnerable phase for DNA damage induced by chemotherapeutic agents (including the common analogs of cisplatin, carboplatin, and oxaliplatin) [76]. Due to defective DNA repair pathways, most cancer cells cannot recover from the DNA damage-induced stress and undergo apoptosis. In contrast, CSCs have increased levels of checkpoint kinases and greater DNA repair capacity than a typical cancer cell; consequently, CSCs avoid death usually induced by chemotherapy [94]. Activation of p38ɑ, which is seen in many CSC populations, leads to the activation of the G2 checkpoint in tumors treated with agents such as temozolomide [95]. Activation of the G2 checkpoint increases the fidelity of DNA repair and thus the maintenance of CSCs. p38ɑ also maintains genomic stability and enables CSC survival through activating the ATR-Chk1 signaling axis, promoting DNA replication and repair [96]. Additionally, p38ɑ directly phosphorylates and activates CtIP, which is responsible for DNA double-strand break resection and proper DNA repair [96]. Moreover, activated p38 MAPK signaling increases the expression levels of BRD4 and 53BP1 (key DNA damage response factors that promote nonhomologous end joining repair), thereby repairing damaged DNA and protecting cells from apoptosis [97]. p38 inhibition could be utilized to prevent the activation of cell-cycle checkpoints, particularly the G2 checkpoint, and DNA repair pathways in order to increase the accumulation of DNA damage in CSCs, resulting in induction of apoptosis.
Signaling gone awry
Many signaling pathways involving p38 lead to chemoresistance. The WNT/β-catenin signaling pathway, which is promoted by p38β via phosphorylation of LRP6, is required for normal stem and CSC self-renewal in numerous cell types. This signaling has also been shown to contribute to chemoresistance [77, 98] (Fig. 4). The Notch signaling pathway, which plays an important role in tumor progression and metastasis, is also implicated in CSC maintenance and chemoresistance [99]. Notch proteins, specifically Notch1, stimulate the p38 pathway, which in turn results in enrichment of the CD133+ CSC population [100] (Fig. 4). Moreover, NF-κB, a key regulator of the inflammatory response with activity that is enhanced by p38, contributes to chemoresistance [101] (Fig. 4). On the other hand, phosphorylation of BCL-2 by p38 diminishes the anti-apoptotic potential of BCL-2, thus making CSCs more susceptible to death from chemotherapy [102] (Fig. 4).
p38 plays various roles in other forms of therapy resistance like in immunotherapy. On one hand, in breast cancer clinical samples and mouse models, GS-CSF signaling through p38 activates myeloid cell ARG1 expression, inhibiting antitumor immunity from T cells [103]. This forms an immunosuppressive tumor microenvironment which renders immunotherapy ineffective. On the other hand, some studies show that p38 does not contribute to immunotherapy resistance. For example, a study found that through the HMGCR/p38 signaling pathway, AMPK suppresses tumor progression by downregulating PD-1 in regulatory T cells [104].
In summary, p38 is involved in many chemoresistance mechanisms and pathways. Because p38 activation leads to CSC enrichment and CSCs are linked to chemoresistance, p38 inhibition could be used to overcome chemoresistance. p38 inhibition not only decreases expression of stemness factors and compromises intravasation, distant colonization, and survival of circulating tumor cells, but it would also push metastatic, mesenchymal-like tumor cells into an epithelial state by inducing the mesenchymal-to-epithelial transition, thereby rendering them chemosensitive [34, 105, 106].
p38 combination treatments to overcome chemoresistance
The efficacy of chemotherapy drugs depends on the cancer type and on the stage of cancer. Therefore, it is important to identify the chemotherapy drugs that have increased efficacy in combination with p38 inhibition. In neuroblastoma cells, treatment with etoposide alone activated the p38 pathway, thus increasing the number of neurospheres, which are the CSCs of neuronal origin, and upregulating MDR1, which directly contributes to chemoresistance [2, 79, 107] (Fig. 5). However, pre-treatment and co-treatment with the p38 inhibitor SB203580 dramatically sensitizes neuroblastoma cells to etoposide, strongly reducing the dosage needed to inhibit tumorigenicity and neurosphere formation [107] (Fig. 5). p38 inhibition increases the sensitivity of lymphoma cells to etoposide as well [108].
Fig. 5. p38 inhibition sensitizes tumor cells to standard chemotherapy.

Schematic of the effects of A chemotherapy alone, B pre-treatment with the p38 inhibitor SB203580 followed by chemotherapy, and C co-treatment with the p38 inhibitor SB203580 and chemotherapy. The combination treatments overcome the chemoresistance of tumor cells by inhibiting the formation of CSCs and decreasing cell viability and tumorigenicity. This can lead to lower rates of cancer recurrence and higher survival rates.
Combination of the p38 inhibitor LY479754 and temozolomide heightens the vulnerability of glioma cells to chemotherapy [109]. p38 inhibition also increases sensitivity to cisplatin chemotherapy in head and neck squamous cell carcinoma cells [110]. In gastric cancer cells, the combination of SB203580 with doxorubicin significantly reduces cell viability and increases cell death, as inactivation of the p38 signaling pathway results in an increase in expression of Bax, an apoptotic protein, and a concomitant decrease in the expression of BCL-2, an anti-apoptotic protein [111]. Likewise, p38 inhibition significantly increases colorectal cancer cell sensitivity to 5-FU. Similarly, combination of SB203580 with 5-FU significantly reduces cell viability and increases cell death and cellular caspase activity compared to 5-FU treatment alone and the SB203580 sensitizes cancer cells to 5-FU through an increase in Bax expression [112]. p38 inhibition also increases the sensitivity of colorectal cancer cells to irinotecan [113], which alone results in activation of p38, as demonstrated by increased levels of phosphorylated p38 [113]. p38 activation enhances CSC properties and DNA repair and inhibits autophagy and cell death, hence conferring chemoresistance [113, 114].
A p38 inhibitor/chemotherapy combination also has potential in gynecological cancers. In TNBC lines, the combinations of p38 inhibitors LY2228820, VX-702, or PH-797804 with gemcitabine and with epirubicin reduce TNBC cell proliferation to a greater extent than either of the two chemotherapy drugs individually [115]. This chemosensitization may be due to a loss of CSC features, as demonstrated by a decrease in the levels of the stemness marker FOXC2 and an increase in the epithelial marker E-cadherin resulting from inhibition of p38 activity [115]. In a mouse model of breast cancer, inhibition of p38 cooperates with cisplatin to target tumor cells [116]. Importantly, the breast tumors were smaller and in a less advanced stage at the end of the study period in the case of the combined therapy compared to cisplatin alone [116]. Moreover, in patient-derived xenografts from TNBC and luminal breast tumors, p38ɑ inhibition enhanced the anti-tumoral effect of taxanes alone, leading to greater tumor clearance [96]. Combination of p38ɑ inhibition with taxane-based chemotherapies such as paclitaxel and docetaxel increased DNA damage, missegregation, and aneuploidy in cancer cells, leading to tumor regression and prevention of tumor relapse [96].
There is also evidence from a human clinical trial that the addition of ralimetinib, a p38 inhibitor, to gemcitabine and carboplatin results in a modest improvement in progression-free survival in ovarian cancer patients compared to administration of chemotherapy alone [117]. Furthermore, metformin combined with a p38 inhibitor improves cisplatin sensitivity in cisplatin-resistant ovarian cancer cells [118]. As recently reviewed, p38 inhibition appears to make hybrid epithelial/mesenchymal cells, which possess enhanced stem-like properties and are known to be chemoresistant, sensitive to taxanes and anthracyclines [119]. Although further investigation is needed, there is a plenty of in vitro, in vivo, and clinical trial evidence that inhibition of p38 sensitizes several different cancer types to standard-of-care chemotherapy treatment.
Conclusions and future perspectives
In most cancers, p38 inhibition decreases the expression of stemness factors and compromises intravasation, distant colonization, and survival of circulating tumor cells. p38 inhibitors can also be employed in cancer treatment regimens to circumvent and overcome chemoresistance. Importantly, systemic p38 inhibition has very minimal side effects. Combining p38 inhibitors with standard-of-care chemotherapies will allow treatment with lower doses of these toxic drugs while eliminating residual CSCs and circulating tumor cells.
Further studies of p38 functions are critical because, in certain scenarios, p38 activation may be desired. It is important to understand in what tissues and cancers p38 activation enhances or diminishes the CSC population. The opposing roles of p38 may result from its functions in different signaling axes. As a result, targeting p38 could sometimes produce the opposite of the intended effect, thus posing a barrier to incorporating p38 inhibition as a pharmacological strategy in diseases involving stem cells and CSCs. Despite this limitation, p38 inhibitors have been successfully implemented in cancer treatment regimens to overcome chemoresistance when used in conjunction with various chemotherapeutics.
Further studies in preclinical models should explore whether pre-treatment or co-treatment with p38 inhibitors will effectively increase sensitivity to chemotherapeutic agents. Determination of the correct treatment schedule is important for the successful treatment of cancer patients. Thus, whether p38 signaling should be inhibited before additional treatments are given or if simultaneous targeting is as or more efficacious needs to be determined. We speculate that, in most cases, reprogramming the CSCs before chemotherapy will increase efficacy and prevent the enrichment of CSCs. p38 inhibition may not induce chemosensitivity in certain cancer cells, such as those with extreme mesenchymal phenotypes, which are associated with very high levels of chemoresistance [120–122]. In fact, particular chemotherapy drugs themselves, such as doxorubicin, can activate p38 in certain cell lines, potentially enhancing CSC properties and chemoresistance in turn [111]. Therefore, certain cancers might respond to initial treatment with chemotherapy followed by p38 inhibitor treatment. There is also great promise in combining p38 inhibition with targeted therapy and immunotherapy in CSC-enriched tumors. In summary, using a p38 inhibitor in combination therapy may allow dose reductions of highly toxic chemotherapy drugs and may prevent resistance to targeted and immunotherapy to eliminate CSC-enriched cancers and prevent metastasis.
Supplementary information
Acknowledgements
This research was awarded to SAM and supported by CPRIT (RP160710/RP170172), NIH/NCI (R01CA200970/2R01CA155243), the NSF (PHY-1935762), the Bowes Foundation and in part by the MD Anderson Cancer Center Support Grant CA016672 (MRP to SAM).
Author contributions
Conception and design by SK, PdH and SAM. Writing, reviewing, and revising the manuscript by SK, PdH, SAM. Figures by SK. All authors reviewed the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41388-022-02329-3.
References
- 1.Gui T, Sun Y, Shimokado A, Muragaki Y. The roles of mitogen-activated protein kinase pathways in TGF-beta-induced epithelial-mesenchymal transition. J Signal Transduct. 2012;2012:289243. doi: 10.1155/2012/289243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez-Limon A, Joaquin M, Caballero M, Posas F, de Nadal E. The p38 pathway: from biology to cancer therapy. Int J Mol Sci. 2020;21:1–18. doi: 10.3390/ijms21061913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–44. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uddin S, Ah-Kang J, Ulaszek J, Mahmud D, Wickrema A. Differentiation stage-specific activation of p38 mitogen-activated protein kinase isoforms in primary human erythroid cells. Proc Natl Acad Sci USA. 2004;101:147–52. doi: 10.1073/pnas.0307075101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahu V, Nigam L, Agnihotri V, Gupta A, Shekhar S, Subbarao N, et al. Diagnostic significance of p38 isoforms (p38alpha, p38beta, p38gamma, p38delta) in head and neck squamous cell carcinoma: comparative serum level evaluation and design of novel peptide inhibitor targeting the same. Cancer Res Treat. 2019;51:313–25. doi: 10.4143/crt.2018.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao W, Collins QF, Becker TC, Robidoux J, Lupo EG, Jr., Xiong Y, et al. p38 Mitogen-activated protein kinase plays a stimulatory role in hepatic gluconeogenesis. J Biol Chem. 2005;280:42731–7. doi: 10.1074/jbc.M506223200. [DOI] [PubMed] [Google Scholar]
- 7.Xiong Y, Collins QF, An J, Lupo E, Jr., Liu HY, Liu D, et al. p38 mitogen-activated protein kinase plays an inhibitory role in hepatic lipogenesis. J Biol Chem. 2007;282:4975–82. doi: 10.1074/jbc.M606742200. [DOI] [PubMed] [Google Scholar]
- 8.Keely SJ, Barrett KE. p38 mitogen-activated protein kinase inhibits calcium-dependent chloride secretion in T84 colonic epithelial cells. Am J Physiol Cell Physiol. 2003;284:C339–48. doi: 10.1152/ajpcell.00144.2002. [DOI] [PubMed] [Google Scholar]
- 9.He Y, She H, Zhang T, Xu H, Cheng L, Yepes M, et al. p38 MAPK inhibits autophagy and promotes microglial inflammatory responses by phosphorylating ULK1. J Cell Biol. 2018;217:315–28. doi: 10.1083/jcb.201701049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juo P, Kuo CJ, Reynolds SE, Konz RF, Raingeaud J, Davis RJ, et al. Fas activation of the p38 mitogen-activated protein kinase signalling pathway requires ICE/CED-3 family proteases. Mol Cell Biol. 1997;17:24–35. doi: 10.1128/MCB.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haq R, Brenton JD, Takahashi M, Finan D, Finkielsztein A, Damaraju S, et al. Constitutive p38HOG mitogen-activated protein kinase activation induces permanent cell cycle arrest and senescence. Cancer Res. 2002;62:5076–82. [PubMed] [Google Scholar]
- 12.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–8. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 13.Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi B, et al. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–58. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gyorffy B. Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput Struct Biotechnol J. 2021;19:4101–9. doi: 10.1016/j.csbj.2021.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osz A, Lanczky A, Gyorffy B. Survival analysis in breast cancer using proteomic data from four independent datasets. Sci Rep. 2021;11:16787. doi: 10.1038/s41598-021-96340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.del Barco Barrantes I, Coya JM, Maina F, Arthur JS, Nebreda AR. Genetic analysis of specific and redundant roles for p38alpha and p38beta MAPKs during mouse development. Proc Natl Acad Sci USA. 2011;108:12764–9. doi: 10.1073/pnas.1015013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warr N, Carre GA, Siggers P, Faleato JV, Brixey R, Pope M, et al. Gadd45gamma and Map3k4 interactions regulate mouse testis determination via p38 MAPK-mediated control of Sry expression. Dev Cell. 2012;23:1020–31. doi: 10.1016/j.devcel.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llopis A, Salvador N, Ercilla A, Guaita-Esteruelas S, Barrantes Idel B, Gupta J, et al. The stress-activated protein kinases p38alpha/beta and JNK1/2 cooperate with Chk1 to inhibit mitotic entry upon DNA replication arrest. Cell Cycle. 2012;11:3627–37. doi: 10.4161/cc.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayakawa M, Hayakawa H, Petrova T, Ritprajak P, Sutavani RV, Jimenez-Andrade GY, et al. Loss of functionally redundant p38 isoforms in T cells enhances regulatory T cell induction. J Biol Chem. 2017;292:1762–72. doi: 10.1074/jbc.M116.764548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escos A, Risco A, Alsina-Beauchamp D, Cuenda A. p38gamma and p38delta mitogen activated protein kinases (MAPKs), new stars in the MAPK galaxy. Front Cell Dev Biol. 2016;4:31. doi: 10.3389/fcell.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The different mechanisms of cancer drug resistance: a brief review. Adv Pharm Bull. 2017;7:339–48. doi: 10.15171/apb.2017.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prieto-Vila M, Takahashi RU, Usuba W, Kohama I, Ochiya T. Drug resistance driven by cancer stem cells and their niche. Int J Mol Sci. 2017;18:1–22. doi: 10.3390/ijms18122574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. Int J Biochem Cell Biol. 2012;44:2144–51. doi: 10.1016/j.biocel.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 25.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 27.Lathia J, Liu H, Matei D. The clinical impact of cancer stem cells. Oncologist. 2020;25:123–31. doi: 10.1634/theoncologist.2019-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–3. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pradella D, Naro C, Sette C, Ghigna C. EMT and stemness: flexible processes tuned by alternative splicing in development and cancer progression. Mol Cancer. 2017;16:8. doi: 10.1186/s12943-016-0579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci USA. 2007;104:10069–74. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalli M, Li R, Mills GB, Stylianopoulos T, Zervantonakis IK. Mechanical stress signaling in pancreatic cancer cells triggers p38 MAPK- and JNK-dependent cytoskeleton remodeling and promotes cell migration via Rac1/cdc42/Myosin II. Mol Cancer Res. 2022;20:485–97. doi: 10.1158/1541-7786.MCR-21-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werden SJ, Sphyris N, Sarkar TR, Paranjape AN, LaBaff AM, Taube JH, et al. Phosphorylation of serine 367 of FOXC2 by p38 regulates ZEB1 and breast cancer metastasis, without impacting primary tumor growth. Oncogene. 2016;35:5977–88. doi: 10.1038/onc.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Afify SM, Seno M. Conversion of stem cells to cancer stem cells: undercurrent of cancer initiation. Cancers. 2019;11:1–19. doi: 10.3390/cancers11030345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu M, Wang S, Wang Y, Wu H, Frank JA, Zhang Z, et al. Role of p38γ MAPK in regulation of EMT and cancer stem cells. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3605–17. doi: 10.1016/j.bbadis.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi X, Yin N, Ma S, Lepp A, Tang J, Jing W, et al. p38γ MAPK is a therapeutic target for triple-negative breast cancer by stimulation of cancer stem-like cell expansion. Stem Cells. 2015;33:2738–47. doi: 10.1002/stem.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu H, Tran L, Park Y, Chen I, Lan J, Xie Y, et al. Reciprocal regulation of DUSP9 and DUSP16 expression by HIF1 controls ERK and p38 MAP kinase activity and mediates chemotherapy-induced breast cancer stem cell enrichment. Cancer Res. 2018;78:4191–202. doi: 10.1158/0008-5472.CAN-18-0270. [DOI] [PubMed] [Google Scholar]
- 39.Roy S, Roy S, Kar M, Padhi S, Saha A, Anuja K, et al. Role of p38 MAPK in disease relapse and therapeutic resistance by maintenance of cancer stem cells in head and neck squamous cell carcinoma. J Oral Pathol Med. 2018;47:492–501. doi: 10.1111/jop.12707. [DOI] [PubMed] [Google Scholar]
- 40.Lin SP, Lee YT, Wang JY, Miller SA, Chiou SH, Hung MC, et al. Survival of cancer stem cells under hypoxia and serum depletion via decrease in PP2A activity and activation of p38-MAPKAPK2-Hsp27. PLoS ONE. 2012;7:e49605. doi: 10.1371/journal.pone.0049605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grun D, Adhikary G, Eckert RL. NRP-1 interacts with GIPC1 and α6/β4-integrins to increase YAP1/∆Np63α-dependent epidermal cancer stem cell survival. Oncogene. 2018;37:4711–22. doi: 10.1038/s41388-018-0290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qi X-T, Li Y-L, Zhang Y-Q, Xu T, Lu B, Fang L, et al. KLF4 functions as an oncogene in promoting cancer stem cell-like characteristics in osteosarcoma cells. Acta Pharmacol Sin. 2019;40:546–55. doi: 10.1038/s41401-018-0050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu M, Ren Z, Wang X, Comer A, Frank JA, Ke Z-J, et al. ErbB2 and p38γ MAPK mediate alcohol-induced increase in breast cancer stem cells and metastasis. Mol Cancer. 2016;15:52. doi: 10.1186/s12943-016-0532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang T, Long H, He L, Han X, Lin K, Liang Z, et al. Interleukin-17 produced by tumor microenvironment promotes self-renewal of CD133+ cancer stem-like cells in ovarian cancer. Oncogene. 2015;34:165–76. doi: 10.1038/onc.2013.537. [DOI] [PubMed] [Google Scholar]
- 45.Xie C, Zhu J, Wang X, Chen J, Geng S, Wu J, et al. Tobacco smoke induced hepatic cancer stem cell-like properties through IL-33/p38 pathway. J Exp Clin Cancer Res. 2019;38:39. doi: 10.1186/s13046-019-1052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 47.Li F, Tiede B, Massagué J, Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 2007;17:3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- 48.Desgrosellier JS, Lesperance J, Seguin L, Gozo M, Kato S, Franovic A, et al. Integrin αvβ3 drives slug activation and stemness in the pregnant and neoplastic mammary gland. Dev Cell. 2014;30:295–308. doi: 10.1016/j.devcel.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medema JP. Cancer stem cells: the challenges ahead. Nat Cell Biol. 2013;15:338–44. doi: 10.1038/ncb2717. [DOI] [PubMed] [Google Scholar]
- 50.Lo PK, Kanojia D, Liu X, Singh UP, Berger FG, Wang Q, et al. CD49f and CD61 identify Her2/neu-induced mammary tumor-initiating cells that are potentially derived from luminal progenitors and maintained by the integrin-TGFβ signaling. Oncogene. 2012;31:2614–26. doi: 10.1038/onc.2011.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seguin L, Kato S, Franovic A, Camargo MF, Lesperance J, Elliott KC, et al. An integrin β3-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat Cell Biol. 2014;16:457–68. doi: 10.1038/ncb2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kren A, Baeriswyl V, Lehembre F, Wunderlin C, Strittmatter K, Antoniadis H, et al. Increased tumor cell dissemination and cellular senescence in the absence of beta1-integrin function. EMBO J. 2007;26:2832–42. doi: 10.1038/sj.emboj.7601738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCabe NP, De S, Vasanji A, Brainard J, Byzova TV. Prostate cancer specific integrin alphavbeta3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007;26:6238–43. doi: 10.1038/sj.onc.1210429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takayama S, Ishii S, Ikeda T, Masamura S, Doi M, Kitajima M. The relationship between bone metastasis from human breast cancer and integrin alpha(v)beta3 expression. Anticancer Res. 2005;25:79–83. [PubMed] [Google Scholar]
- 55.Hosotani R, Kawaguchi M, Masui T, Koshiba T, Ida J, Fujimoto K, et al. Expression of integrin alphaVbeta3 in pancreatic carcinoma: relation to MMP-2 activation and lymph node metastasis. Pancreas. 2002;25:e30–5. doi: 10.1097/00006676-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 56.Bakewell SJ, Nestor P, Prasad S, Tomasson MH, Dowland N, Mehrotra M, et al. Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proc Natl Acad Sci USA. 2003;100:14205–10. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Felding-Habermann B. Tumor cell-platelet interaction in metastatic disease. Haemostasis. 2001;31:55–8. [PubMed] [Google Scholar]
- 58.Kasimir-Bauer S, Hoffmann O, Wallwiener D, Kimmig R, Fehm T. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res. 2012;14:R15. doi: 10.1186/bcr3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barrière G, Riouallon A, Renaudie J, Tartary M, Rigaud M. Mesenchymal and stemness circulating tumor cells in early breast cancer diagnosis. BMC Cancer. 2012;12:114. doi: 10.1186/1471-2407-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. 2009;11:R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raimondi C, Gradilone A, Naso G, Vincenzi B, Petracca A, Nicolazzo C, et al. Epithelial-mesenchymal transition and stemness features in circulating tumor cells from breast cancer patients. Breast Cancer Res Treat. 2011;130:449–55. doi: 10.1007/s10549-011-1373-x. [DOI] [PubMed] [Google Scholar]
- 62.Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, et al. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12:5615–21. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 63.Hiratsuka S, Duda DG, Huang Y, Goel S, Sugiyama T, Nagasawa T, et al. C-X-C receptor type 4 promotes metastasis by activating p38 mitogen-activated protein kinase in myeloid differentiation antigen (Gr-1)-positive cells. Proc Natl Acad Sci USA. 2011;108:302–7. doi: 10.1073/pnas.1016917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kucuksayan H, Akca H. The crosstalk between p38 and Akt signaling pathways orchestrates EMT by regulating SATB2 expression in NSCLC cells. Tumour Biol. 2017;39:1010428317706212. doi: 10.1177/1010428317706212. [DOI] [PubMed] [Google Scholar]
- 65.Lin Y, Mallen-St Clair J, Wang G, Luo J, Palma-Diaz F, Lai C, et al. p38 MAPK mediates epithelial-mesenchymal transition by regulating p38IP and Snail in head and neck squamous cell carcinoma. Oral Oncol. 2016;60:81–9. doi: 10.1016/j.oraloncology.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 66.Paranjape AN, Soundararajan R, Werden SJ, Joseph R, Taube JH, Liu H, et al. Inhibition of FOXC2 restores epithelial phenotype and drug sensitivity in prostate cancer cells with stem-cell properties. Oncogene. 2016;35:5963–76. doi: 10.1038/onc.2015.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang Y, Li Y, Wang K, Wang Y, Yin W, Li L. P38/NF-κB/snail pathway is involved in caffeic acid-induced inhibition of cancer stem cells-like properties and migratory capacity in malignant human keratinocyte. PLoS ONE. 2013;8:e58915. doi: 10.1371/journal.pone.0058915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel NJ, Sharon C, Baranwal S, Boothello RS, Desai UR, Patel BB. Heparan sulfate hexasaccharide selectively inhibits cancer stem cells self-renewal by activating p38 MAP kinase. Oncotarget. 2016;7:84608–22. doi: 10.18632/oncotarget.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boothello RS, Patel NJ, Sharon C, Abdelfadiel EI, Morla S, Brophy DF, et al. A unique nonsaccharide mimetic of heparin hexasaccharide inhibits colon cancer stem cells via p38 MAP kinase activation. Mol Cancer Ther. 2019;18:51–61. doi: 10.1158/1535-7163.MCT-18-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fang Y, Wang J, Wang G, Zhou C, Wang P, Zhao S, et al. Inactivation of p38 MAPK contributes to stem cell-like properties of non-small cell lung cancer. Oncotarget. 2017;8:26702–17. doi: 10.18632/oncotarget.15804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deng K, Liu L, Tan X, Zhang Z, Li J, Ou Y, et al. WIP1 promotes cancer stem cell properties by inhibiting p38 MAPK in NSCLC. Signal Transduct Target Ther. 2020;5:36. doi: 10.1038/s41392-020-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soeda A, Lathia J, Williams BJ, Wu Q, Gallagher J, Androutsellis-Theotokis A, et al. The p38 signaling pathway mediates quiescence of glioma stem cells by regulating epidermal growth factor receptor trafficking. Oncotarget. 2017;8:33316–28. doi: 10.18632/oncotarget.16741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang J, Wu W, Yang F, Liu L, Yang Z, Liu L, et al. Marine sponge-derived smenospongine preferentially eliminates breast cancer stem-like cells via p38/AMPKα pathways. Cancer Med. 2018;7:3965–76. doi: 10.1002/cam4.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Munne PM, Martikainen L, Raty I, Bertula K, Nonappa, Ruuska J, et al. Compressive stress-mediated p38 activation required for ERalpha + phenotype in breast cancer. Nat Commun. 2021;12:6967. doi: 10.1038/s41467-021-27220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog. 2013;18:43–73. doi: 10.1615/CritRevOncog.v18.i1-2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas ML, Coyle KM, Sultan M, Vaghar-Kashani A, Marcato P. Chemoresistance in cancer stem cells and strategies to overcome resistance. Chemotherapy. 2014;3:10. [Google Scholar]
- 77.Abdullah LN, Chow EK. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med. 2013;2:3. doi: 10.1186/2001-1326-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He D, Zhao XQ, Chen XG, Fang Y, Singh S, Talele TT, et al. BIRB796, the inhibitor of p38 mitogen-activated protein kinase, enhances the efficacy of chemotherapeutic agents in ABCB1 overexpression cells. PLoS ONE. 2013;8:e54181. doi: 10.1371/journal.pone.0054181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo X, Ma N, Wang J, Song J, Bu X, Cheng Y, et al. Increased p38-MAPK is responsible for chemotherapy resistance in human gastric cancer cells. BMC Cancer. 2008;8:375. doi: 10.1186/1471-2407-8-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ikawa M, Impraim CC, Wang G, Yoshida A. Isolation and characterization of aldehyde dehydrogenase isozymes from usual and atypical human livers. J Biol Chem. 1983;258:6282–7. doi: 10.1016/S0021-9258(18)32405-0. [DOI] [PubMed] [Google Scholar]
- 81.Raha D, Wilson TR, Peng J, Peterson D, Yue P, Evangelista M, et al. The cancer stem cell marker aldehyde dehydrogenase is required to maintain a drug-tolerant tumor cell subpopulation. Cancer Res. 2014;74:3579–90. doi: 10.1158/0008-5472.CAN-13-3456. [DOI] [PubMed] [Google Scholar]
- 82.Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15:4234–41. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- 83.Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–8. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yuan B, El Dana F, Ly S, Yan Y, Ruvolo V, Shpall EJ, et al. Bone marrow stromal cells induce an ALDH+ stem cell-like phenotype and enhance therapy resistance in AML through a TGF-β-p38-ALDH2 pathway. PLoS ONE. 2020;15:e0242809. doi: 10.1371/journal.pone.0242809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen W, Dong J, Haiech J, Kilhoffer MC, Zeniou M. Cancer stem cell quiescence and plasticity as major challenges in cancer therapy. Stem Cells Int. 2016;2016:1740936. doi: 10.1155/2016/1740936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med. 2011;208:2641–55. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu-Lee L-Y, Lee Y-C, Pan J, Lin S-C, Pan T, Yu G, et al. Bone secreted factors induce cellular quiescence in prostate cancer cells. Sci Rep. 2019;9:18635. doi: 10.1038/s41598-019-54566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ranganathan AC, Zhang L, Adam AP, Aguirre-Ghiso JA. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res. 2006;66:1702–11. doi: 10.1158/0008-5472.CAN-05-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK) Cancer Res. 2003;63:1684–95. [PubMed] [Google Scholar]
- 90.Bragado P, Estrada Y, Parikh F, Krause S, Capobianco C, Farina HG, et al. TGF-β2 dictates disseminated tumour cell fate in target organs through TGF-β-RIII and p38α/β signalling. Nat Cell Biol. 2013;15:1351–61. doi: 10.1038/ncb2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Molnár A, Theodoras AM, Zon LI, Kyriakis JM. Cdc42Hs, but not Rac1, inhibits serum-stimulated cell cycle progression at G1/S through a mechanism requiring p38/RK. J Biol Chem. 1997;272:13229–35. doi: 10.1074/jbc.272.20.13229. [DOI] [PubMed] [Google Scholar]
- 92.Takenaka K, Moriguchi T, Nishida E. Activation of the protein kinase p38 in the spindle assembly checkpoint and mitotic arrest. Science. 1998;280:599–602. doi: 10.1126/science.280.5363.599. [DOI] [PubMed] [Google Scholar]
- 93.Chen J, Li Y, Yu T-S, McKay RM, Burns DK, Kernie SG, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–6. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mathews LA, Cabarcas SM, Farrar WL. DNA repair: the culprit for tumor-initiating cell survival? Cancer Metastasis Rev. 2011;30:185–97. doi: 10.1007/s10555-011-9277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hirose Y, Katayama M, Stokoe D, Haas-Kogan DA, Berger MS, Pieper RO. The p38 mitogen-activated protein kinase pathway links the DNA mismatch repair system to the G2 checkpoint and to resistance to chemotherapeutic DNA-methylating agents. Mol Cell Biol. 2003;23:8306–15. doi: 10.1128/MCB.23.22.8306-8315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cánovas B, Igea A, Sartori AA, Gomis RR, Paull TT, Isoda M, et al. Targeting p38α increases DNA damage, chromosome instability, and the anti-tumoral response to taxanes in breast cancer cells. Cancer Cell. 2018;33:1094–110. doi: 10.1016/j.ccell.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 97.Mak VC, Li X, Rao L, Zhou Y, Tsao SW, Cheung LW. p85beta alters response to EGFR inhibitor in ovarian cancer through p38 MAPK-mediated regulation of DNA repair. Neoplasia. 2021;23:718–30. doi: 10.1016/j.neo.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Červenka I, Wolf J, Mašek J, Krejci P, Wilcox WR, Kozubík A, et al. Mitogen-activated protein kinases promote WNT/beta-catenin signaling via phosphorylation of LRP6. Mol Cell Biol. 2011;31:179–89. doi: 10.1128/MCB.00550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer. 2011;11:338–51. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- 100.Kumar D, Kumar S, Gorain M, Tomar D, Patil HS, Radharani NNV, et al. Notch1-MAPK signaling axis regulates CD133(+) cancer stem cell-mediated melanoma growth and angiogenesis. J Investig Dermatol. 2016;136:2462–74. doi: 10.1016/j.jid.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 101.Saha RN, Jana M, Pahan K. MAPK p38 regulates transcriptional activity of NF-kappaB in primary human astrocytes via acetylation of p65. J Immunol. 2007;179:7101–9. doi: 10.4049/jimmunol.179.10.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.De Chiara G, Marcocci ME, Torcia M, Lucibello M, Rosini P, Bonini P, et al. Bcl-2 Phosphorylation by p38 MAPK: identification of target sites and biologic consequences. J Biol Chem. 2006;281:21353–61. doi: 10.1074/jbc.M511052200. [DOI] [PubMed] [Google Scholar]
- 103.Su X, Xu Y, Fox GC, Xiang J, Kwakwa KA, Davis JL, et al. Breast cancer-derived GM-CSF regulates arginase 1 in myeloid cells to promote an immunosuppressive microenvironment. J Clin Investig. 2021;131:1–18. doi: 10.1172/JCI145296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pokhrel RH, Acharya S, Ahn JH, Gu Y, Pandit M, Kim JO, et al. AMPK promotes antitumor immunity by downregulating PD-1 in regulatory T cells via the HMGCR/p38 signaling pathway. Mol Cancer. 2021;20:133. doi: 10.1186/s12943-021-01420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Du B, Shim JS. Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules. 2016;21:1–15. doi: 10.3390/molecules21070965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Antoon JW, Nitzchke AM, Martin EC, Rhodes LV, Nam S, Wadsworth S, et al. Inhibition of p38 mitogen-activated protein kinase alters microRNA expression and reverses epithelial-to-mesenchymal transition. Int J Oncol. 2013;42:1139–50. doi: 10.3892/ijo.2013.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marengo B, De Ciucis CG, Ricciarelli R, Furfaro AL, Colla R, Canepa E, et al. p38MAPK inhibition: a new combined approach to reduce neuroblastoma resistance under etoposide treatment. Cell Death Dis. 2013;4:e589–e. doi: 10.1038/cddis.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kurosu T, Takahashi Y, Fukuda T, Koyama T, Miki T, Miura O. p38 MAP kinase plays a role in G2 checkpoint activation and inhibits apoptosis of human B cell lymphoma cells treated with etoposide. Apoptosis. 2005;10:1111–20. doi: 10.1007/s10495-005-3372-z. [DOI] [PubMed] [Google Scholar]
- 109.Demuth T, Reavie LB, Rennert JL, Nakada M, Nakada S, Hoelzinger DB, et al. MAP-ing glioma invasion: mitogen-activated protein kinase kinase 3 and p38 drive glioma invasion and progression and predict patient survival. Mol Cancer Ther. 2007;6:1212–22. doi: 10.1158/1535-7163.MCT-06-0711. [DOI] [PubMed] [Google Scholar]
- 110.Roy S, Roy S, Kar M, Thakur S, Akhter Y, Kumar A, et al. p38 MAPK pathway and its interaction with TRF2 in cisplatin induced chemotherapeutic response in head and neck cancer. Oncogenesis. 2018;7:53. doi: 10.1038/s41389-018-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tan W, Yu HG, Luo HS. Inhibition of the p38 MAPK pathway sensitizes human gastric cells to doxorubicin treatment in vitro and in vivo. Mol Med Rep. 2014;10:3275–81. doi: 10.3892/mmr.2014.2598. [DOI] [PubMed] [Google Scholar]
- 112.Yang SY, Miah A, Sales KM, Fuller B, Seifalian AM, Winslet M. Inhibition of the p38 MAPK pathway sensitises human colon cancer cells to 5-fluorouracil treatment. Int J Oncol. 2011;38:1695–702. doi: 10.3892/ijo.2011.982. [DOI] [PubMed] [Google Scholar]
- 113.Paillas S, Boissière F, Bibeau F, Denouel A, Mollevi C, Causse A, et al. Targeting the p38 MAPK pathway inhibits irinotecan resistance in colon adenocarcinoma. Cancer Res. 2011;71:1041–9. doi: 10.1158/0008-5472.CAN-10-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Comes F, Matrone A, Lastella P, Nico B, Susca FC, Bagnulo R, et al. A novel cell type-specific role of p38α in the control of autophagy and cell death in colorectal cancer cells. Cell Death Differ. 2007;14:693–702. doi: 10.1038/sj.cdd.4402076. [DOI] [PubMed] [Google Scholar]
- 115.Hollander PD, Shah S, Zhou X, Redwood A, Cai S-R, Sobieski M, et al. Abstract 4669: overcoming therapy resistance in stem cell-rich triple negative breast cancer through p38 MAP kinase inhibition. Cancer Res. 2019;79:4669. [Google Scholar]
- 116.Pereira L, Igea A, Canovas B, Dolado I, Nebreda AR. Inhibition of p38 MAPK sensitizes tumour cells to cisplatin-induced apoptosis mediated by reactive oxygen species and JNK. EMBO Mol Med. 2013;5:1759–74. doi: 10.1002/emmm.201302732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vergote I, Heitz F, Buderath P, Powell M, Sehouli J, Lee CM, et al. A randomized, double-blind, placebo-controlled phase 1b/2 study of ralimetinib, a p38 MAPK inhibitor, plus gemcitabine and carboplatin versus gemcitabine and carboplatin for women with recurrent platinum-sensitive ovarian cancer. Gyneco Oncol. 2020;156:23–31. doi: 10.1016/j.ygyno.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 118.Xie Y, Peng Z, Shi M, Ji M, Guo H, Shi H. Metformin combined with p38 MAPK inhibitor improves cisplatin sensitivity in cisplatin‑resistant ovarian cancer. Mol Med Rep. 2014;10:2346–50. doi: 10.3892/mmr.2014.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jolly MK, Somarelli JA, Sheth M, Biddle A, Tripathi SC, Armstrong AJ, et al. Hybrid epithelial/mesenchymal phenotypes promote metastasis and therapy resistance across carcinomas. Pharmacol Ther. 2019;194:161–84. doi: 10.1016/j.pharmthera.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 120.Hara J, Miyata H, Yamasaki M, Sugimura K, Takahashi T, Kurokawa Y, et al. Mesenchymal phenotype after chemotherapy is associated with chemoresistance and poor clinical outcome in esophageal cancer. Oncol Rep. 2014;31:589–96. doi: 10.3892/or.2013.2876. [DOI] [PubMed] [Google Scholar]
- 121.Loret N, Denys H, Tummers P, Berx G. The role of epithelial-to-mesenchymal plasticity in ovarian cancer progression and therapy resistance. Cancers. 2019;11:1–22. doi: 10.3390/cancers11060838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.El Amrani M, Corfiotti F, Corvaisier M, Vasseur R, Fulbert M, Skrzypczyk C, et al. Gemcitabine-induced epithelial-mesenchymal transition-like changes sustain chemoresistance of pancreatic cancer cells of mesenchymal-like phenotype. Mol Carcinog. 2019;58:1985–97. doi: 10.1002/mc.23090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.