Abstract
Background
Tripterygium glycosides (TG) is widely used in the treatment of diabetic kidney disease (DKD) in China. To systematically assess and synthesize the available evidence, we present an overview of systematic reviews (SRs) and meta-analyses (MAs) on the topic of TG interventions for DKD.
Methods
SRs/MAs on TG interventions for DKD were comprehensively searched in seven databases. Methodological quality, risk of bias, reporting quality, and quality of evidence were assessed using the Assessment of Multiple Systematic Reviews 2 (AMSTAR-2), the Risk of Bias in Systematic (ROBIS) scale, the list of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), as well as the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system.
Results
This overview includes 13 SRs/MAs that use quantitative calculations to comprehensively assess various outcomes in TG interventions for DKD. The methodological quality, reporting quality, and risk of bias of SRs/MAs, and the quality of evidence for outcome indicators are unsatisfactory. Limitations of the included SRs/MAs consist in the lack of essential procedures such as protocol registration, screening of duplicate study, provision of the list of excluded studies, and assessment of publication bias. Besides, the reliance on small samples for quantitative synthesis of effect sizes also constitutes an important limitation.
Conclusion
TG may be a potential complementary treatment modality to DKD therapy. However, this conclusion must be treated with caution as the quality of the evidence provided by SRs/MAs is generally low.
Keywords: tripterygium glycosides, diabetic kidney disease, systematic reviews, meta-analyses, overview
Introduction
Diabetes mellitus (DM) is a thorny medical issue,1 and diabetic kidney disease (DKD) is a serious renal microvascular disease of type 1 or type 2 DM, which occurs in about 25–40% of DM patients.2,3 Clinically characterized by a progressive decline in renal function and persistent proteinuria, DKD has become the leading cause of the end-stage renal disease (ESRD) in China.4 Patients with clinical DKD5,6 will experience pathological changes such as dilated glomerular thylakoid matrix, thickened glomerular basement membrane (GBM), and formation of glomerular nodular sclerosis. The mechanisms underlying the pathogenesis of DKD have not been fully understood, and the treatment currently recommended for DKD is to control blood pressure and blood sugar.7 Although the clinical application of routine drugs such as renin-angiotensin-aldosterone system (RAAS) inhibitors8 can slow down the progression of DKD, it has always been a challenge to effectively reduce proteinuria in patients with severe proteinuria.9,10
Recent studies have shown that inflammation is an important pathogenesis of DKD.9 Excessive activation of the inflammatory response is often observed in patients with diabetes-related renal dysfunction or chronic renal insufficiency,11 and inflammation which is independent of traditional risk factors is considered an important cause of persistent microproteinuria in DKD patients.12 A review also concluded that the renal infiltration of immune cells is of great significance to the development of DKD.13 Therefore, kidney disease researchers are also facing the important task of choosing new drugs for DKD patients from the anti-inflammatory and immunosuppressive perspective.
Tripterygium wilfordii Hook f. is an ancient Chinese botanical medicine, first recorded in one of the four classics of traditional Chinese medicine - The Divine Farmer’s Materia Medica. There are more than 400 natural metabolites, including triptolide and celastrol, isolated and characterized from Tripterygium wilfordii Hook f.,14 and the fat-soluble substance tripterygium glycosides (TG, State Drug Administration Z33020778, Z43020138) extracted from its root nucleus has immunosuppressive and anti-inflammatory effects.15,16 As early as 1977, Chinese scholars firstly reported the effect of TG on reducing glomerulonephritis proteinuria. Since then, TG has been used for clinical therapy of kidney diseases.17 A randomized controlled trial (RCT) showed that TG combination therapy significantly alleviated the inflammatory state, reduced proteinuria levels, and improved renal function in patients with chronic glomerulonephritis.18 With anti-inflammatory function and excellent ability to prevent the rupture of the glomerular membrane induced by oxidation, TG can prevent the progression of DKD and proteinuria.19–21 Several investigators have published systematic reviews (SRs) and meta-analyses (MAs) in this area, but the evidence has not been scientifically evaluated. Therefore, we presented an overview of these SRs/MAs to tap and evaluate the available evidence, aiming to systematically and comprehensively determine the clinical effectiveness and safety of using TG for DKD treatment.
Materials and Methods
The study methods outlined in this overview follow the guidelines set out in the Cochrane handbook and several high-quality overviews.22,23 This overview protocol has been registered with the INPLASY website. (Registration number: INPLASY202230065).
Development of Inclusion and Exclusion Criteria
Literature Inclusion Criteria
Type of Research
SRs/MAs were based on RCTs in the treatment of DKD with TG, and the language involved is limited to English and Chinese.
Types of Participants
The participants are patients diagnosed with DKD based on international24 or national standards,25 regardless of race, age, gender, time of onset, and source of cases.
Type of Intervention
To ensure that the results are more convincing, two types of treatment schemes were included: (1) The control group received conventional treatment (CT) or placebo, and the intervention group received TG treatment on the basis of the CT or placebo. (2) The control group received CT, and the intervention group was treated with TG. The CT included not only regulations in diet, exercise, hypoglycemia, blood pressure, lipid, anticoagulation, and diuresis, etc., but also other routine drugs such as angiotensin-converting enzyme inhibitors (ACEI)/ angiotensin receptor blockers (ARB) (benazepril, telmisartan, valsartan, irbesartan, losartan, candesartan, and fosinopril) and huangkui capsules,26 alprostadil injection, and simvastatin.
Types of Outcomes
The following are the outcome measures: Total clinical effective rate, 24 h-urine total protein (24-UTP), serum albumin (Alb), serum creatinine (SCr), blood urea nitrogen (BUN), urinary β2-microglobulin adverse events, albumin-excretion rate, urinary albumin excretion rates (UAER), endogenous creatinine clearance rate, glomerular filtration rate (eGFR), inflammatory factors, and adverse reaction.
Exclusion Criteria
(1) Animal studies; (2) Network SRs/MAs, research protocols, narrative reviews, overviews, dissertation, and conference abstracts.
Search Strategy
With the search date set to be October 31, 2021, we searched a total of 7 literature databases, including PubMed, Cochrane Library, Embase, Wanfang Database, VIP, CNKI, and Chinese Biological Medicine (CBM). The literature search in this study used MeSH terms or keywords combined with free text words. Our key search terms included tripterygium glycosides, diabetic kidney disease, systematic reviews, and meta-analyses. We also reviewed the references of all the retrieved literatures to avoid missing topic-related SRs/MAs. The specific search strategy is tuned according to different databases. The PubMed search strategy was shown in Table 1, and search strategies for other databases were described in “Supplementary Table S1: Search strategy for all databases”. On March 4, 2022, we updated the related database search using the same search strategy.
Table 1.
Search Strategy for the PubMed Database
| Query | Search Term |
|---|---|
| #1 | “Tripterygium Glycosides” OR “Tripterygium wilfordii polyglycosides” |
| #2 | Diabetic Nephropathies[Mesh] |
| #3 | “Nephropathies, Diabetic” OR “Nephropathy, Diabetic” OR “Diabetic Nephropathy” OR “Diabetic Kidney Disease” OR “Diabetic Kidney Diseases” OR “Kidney Disease, Diabetic” OR “Kidney Diseases, Diabetic”OR “Diabetic Glomerulosclerosis” OR “Glomerulosclerosis, Diabetic” OR “Intracapillary Glomerulosclerosis” OR “Nodular Glomerulosclerosis” OR “Glomerulosclerosis, Nodular” OR “Kimmelstiel-Wilson Syndrome” OR “Kimmelstiel Wilson Syndrome” OR “Syndrome, Kimmelstiel-Wilson” OR “Kimmelstiel-Wilson Disease” OR “Kimmelstiel Wilson Disease” |
| #4 | #2 OR #3 |
| #5 | Meta-Analysis as Topic [Mesh] |
| #6 | “Systematic review” OR “meta-analysis” OR “meta analysis” OR “meta-analyses” OR “Review, Systematic” OR “Systematic reviews” |
| #7 | #5 OR #6 |
| #8 | #1 AND #4 AND #7 |
Eligibility Assessment and Data Extraction
The literature screening (PL-L and CD-D) and information extraction (RC-L and HS-S) were performed independently by two researchers. Endnote X9 was used to filter the relevant articles and remove duplicates. Then the titles and abstracts of the documents were reviewed to search for the documents that may meet the conditions, and the full text was read. Finally, the following information was extracted from each eligible SR/MA by means of standardized tables: First author, country, year of publication, quality assessment tools included in RCTs, treatment measures for treatment groups and control groups, and main conclusion.
SRs/MAs Quality Assessment
Two researchers (P-D and HS-S) independently assessed the methodological and evidential quality of the included SRs/MAs.
Assessment of Methodological Quality
The methodological quality of the included SRs/MAs was assessed using the Assessment System for Evaluating Methodological Quality 2 (AMSTAR-2).27 Seven (2, 4, 7, 9, 11, 13, and 15) of the 16 items in the tool were critical areas.
Assessment of Risk of Bias
The Risk of Bias in Systematic Review (ROBIS)28 scale was used in this overview to evaluate the risk of bias of the inclusion of SRs/MAs, and the evaluation was carried out in three stages.
Assessment of Reporting Quality
The quality of each SR/MA report of the included SRs/MAs was evaluated by the list of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)29 which consists of 27 items focusing on the reporting methods and results that are incorporated into SRs/MAs.
Assessment of Quality of Evidence
The quality of evidence for each SR/MA outcome was evaluated by The Grading of Recommendations Assessment, Development, and Evaluation (GRADE),30 and the degradation of evidence quality resulted from five aspects, namely, limitations, inconsistencies, indirectness, imprecision, and publication bias. Evidence with less than one degrading factor (including one) was rated as high quality, while evidence with two degrading factors was rated as moderate quality, three degrading factors as low quality, and more than three degrading factors as very low quality.
Results
Results on Literature Search and Screening
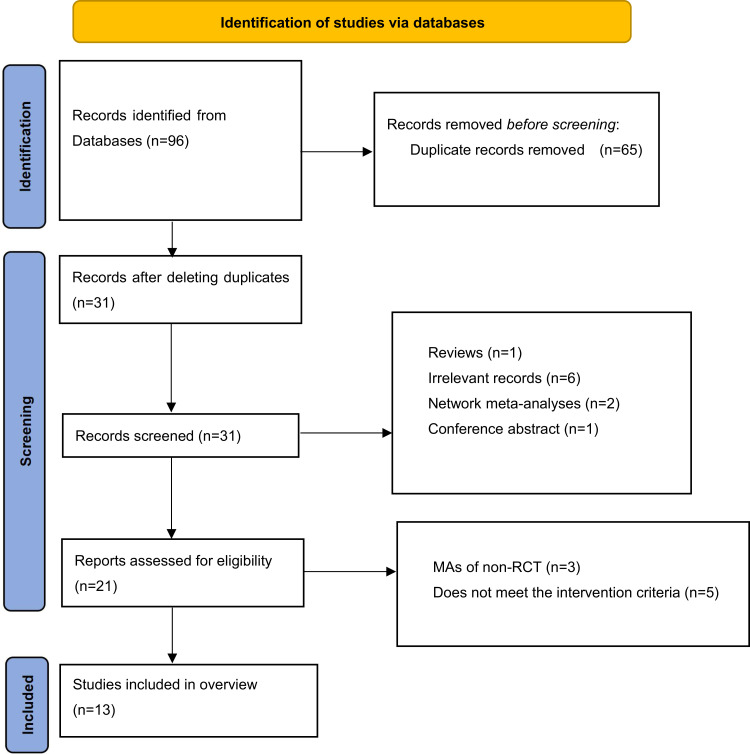
A total of 96 publications were retrieved from seven electronic databases. Twenty publications were retrieved for full-text evaluation after removing duplicates through title/abstract screening. Finally, 13 SRs/MAs31–43 were included in the study based on the assessment made according to the previously established inclusion and exclusion criteria (Figure 1).
Figure 1.
Literature screening flowchart.
Description of Included SRs/MAs
Thirteen SRs/MAs published from 2015 to 2022 were included, and all the included papers were MAs. These literatures were all from China, of which 731–37,43 were in English and 637–42 were in Chinese. The number of RCTs included in the SRs/MAs ranged from 9 to 33, and the total number of subjects included in each SR/MA ranged from 584 to 2764. In 12 SRs/MAs,31–41,43 the intervention adopted for the treatment group was TG combined with CT, and the control group was treated with CT. In 1 SR/MA,42 the intervention group received TG, and the control group received RAAS inhibitor. In terms of quality assessment scales, 12 literatures used the Cochrane risk of bias standard,31–41,43 and only one literature used Jadad.42 The details of the SRs/MAs included were shown in Table 2.
Table 2.
Characteristics of the Included SRs/MAs
| Author, Year (Country) | Trials (Subjects) | Intervention Group | Control Group | Quality Assessment | Main Results |
|---|---|---|---|---|---|
| JinYing Fang, 202031 | 9 (851) | TG + CT | CT | Cochrane criteria | ACEI/ARB plus TG produce greater reductions in 24h-UTP and SCr levels in patients with DKD than ACEI/ARB alone, and better effects might be achieved after long-term administration. There was no difference in side effects between ACEI/ARB plus TG and ACEI/ARB alone. |
| Huabin Guo, 202132 | 26 (1824) | TG + CT | CT | Cochrane criteria | Compared with the control group, TG has a significant effect in reducing 24h-UTP, increasing serum albumin and total efficacy. In terms of adverse reactions, TG significantly reduced the white blood cells of DKD patients. |
| Yizhen Li, 202133 | 31 (2764) | TG + CT | CT | Cochrane criteria | Symptomatic treatments combined with TG can significantly lower 24h-UTP and SCr in DKD patients than the basic treatment without TG. In terms of adverse side effects, patients with DKD who took TG for 3 months and 6 months showed serious adverse reactions. |
| Wanchun Ye, 201834 | 12 (829) | TG + CT | CT | Cochrane criteria | The combination of TG and valsartan in the treatment of DKD can not only increase Alb but also decrease 24h-UTP, urinary albumin excretion rate and urine β2-microglobulin level, and the incidence of adverse reactions of TG combined with valsartan is higher than that of valsartan monotherapy. |
| Xue Wu, 202135 | 23 (1810) | TG + CT | CT | Cochrane criteria | TG combined with ARB significantly improved the 24h-UTP, UAER, SCr and Alb statistically, and slight side effects of the combined treatment were observed, mainly manifested as abnormal liver function. |
| Ying Wang, 202036 | 18 (1160) | TG + CT | CT | Cochrane criteria | The combined treatment of TG and ARB shows that DKD has a good effect in significantly reducing 24h-UTP and improving Alb, but the risk of adverse events is higher. |
| Huan Chen, 202037 | 13 (1141) | TG + CT | CT | Cochrane criteria | The addition of TG on the basis of CT can effectively reduce the increased CRP, IL-6, TNF-α due to DKD compared with the CT group; however, this can increase the incidence of adverse reactions such as leukopenia and abnormal liver function. |
| Jing Huang, 201538 | 13 (1119) | TG + CT | CT | Cochrane criteria | The TG group was better than the control group in terms of reducing the 24h-UTP, increasing the Alb, and improving the overall effective rate and efficacy, but it was shown that the risk of adverse reactions in the combined treatment group was higher than that in the control group. |
| Kui Liu, 201939 | 16 (1482) | TG + CT | CT | Cochrane criteria | The TG group can significantly improve the clinical efficacy, and significantly reduce the 24h-UTP and SCr levels, but the incidence of adverse reactions in the combined treatment group is greater than that in the CT group. |
| Xinhua Liang, 201640 | 10 (584) | TG + CT | CT | Cochrane criteria | The TG group can significantly increase the Alb, SCr and 24h-UTP of DKD patients. |
| Mengjiu Zhang, 202041 | 16 (973) | TG + CT | CT | Cochrane criteria | The 24h-UTP, SCr and BUN values of the treatment group were significantly lower than those of the control group. There was no significant difference in alanine aminotransferase and white blood cell count between the treatment group and the control group, and the incidence of adverse reactions in the test group was higher than that in the control group. |
| Guoshuang Zhu, 201942 | 14 (826) | TG | CT | Jadad | Compared with ACEI/ARB, TG significantly reduced UP, BUN and SCr levels, and there was no statistical difference in adverse reactions. |
| Dandan Xie 202243 | 33 (2034) | TG + CT | CT | Cochrane criteria | Based on our findings, TG combined with western medicine for DKD may be a safe and effective treatment; the optimal duration of treatment may be 3 to 6 months. |
Abbreviations: TG, tripterygium glycosides; CT, conventional treatment; 24-UTP, 24 h-urine total protein; SCr, serum creatinine; Alb, serum albumin; BUN, blood urea nitrogen; UAER, urinary albumin excretion rates; eGFR, glomerular filtration rate; CRP, c-reactive protein; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers.
Results on SRs/MAs Quality Assessment
Methodological Quality Assessment
The evaluation details of the included SRs/MAs on the AMSTAR-2 were shown in Table 3. Regarding the methodological quality of the included SRs/MAs, all were considered to be of very low quality because more than one key item was missing from the SRs/MAs included in the quality assessment. Methodological quality limitations include the following items: Item 2 (Only 3 SRs/MAs have registered the protocol), Item 7 (No list of exclusion was provided by the SRs/MAs), Item 10 (None reported funding of RCTs included in SRs/MAs), and Item 13 (When interpreting the evaluation results, only 5 SRs/MAs considered the risk of bias in the main study).
Table 3.
Result of the AMSTAR-2 Assessments
| Author, Year (Country) | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Q15 | Q16 | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JinYing Fang, 202031 | Y | PY | Y | Y | Y | Y | N | Y | Y | N | Y | Y | N | Y | Y | Y | VL |
| Huabin Guo, 202132 | Y | PY | Y | Y | Y | Y | N | Y | Y | N | Y | Y | Y | N | Y | Y | VL |
| Yizhen Li, 202133 | Y | PY | Y | Y | Y | Y | N | Y | Y | N | Y | N | N | Y | Y | Y | VL |
| Wanchun Ye, 201834 | Y | PY | Y | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | Y | VL |
| Xue Wu, 202135 | Y | PY | Y | PY | Y | Y | N | Y | Y | N | Y | Y | N | Y | N | Y | VL |
| Ying Wang, 202036 | Y | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | N | Y | Y | Y | VL |
| Huan Chen, 202037 | Y | PY | Y | PY | Y | Y | N | Y | Y | N | Y | Y | Y | Y | Y | N | VL |
| Jing Huang, 201538 | Y | PY | Y | Y | Y | Y | N | Y | Y | N | Y | Y | Y | N | Y | N | VL |
| Kui Liu, 201939 | Y | PY | Y | PY | Y | Y | N | Y | Y | N | Y | Y | N | N | N | N | VL |
| Xinhua Liang, 201640 | Y | PY | Y | Y | Y | Y | N | Y | Y | N | Y | Y | Y | Y | N | N | VL |
| Mengjiu Zhang, 202041 | Y | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | N | N | Y | N | VL |
| Guoshuang Zhu, 201942 | Y | PY | Y | PY | Y | Y | N | Y | Y | N | Y | N | N | Y | Y | N | VL |
| Dandan Xie 202243 | Y | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | N | Y | Y | Y | VL |
Notes: Y, yes; PY, partial yes; N, no; VL, very low; L, low; H, high; Q2, Q4, Q7, Q9, Q11, Q13, and Q15 are key areas.
Risk of Bias of the Included SRs/MAs
Regarding the results of the ROBIS assessment, both Phase 1 and Domain 1 rated SRs/MAs as having low risk of bias. Nine of the SRs/MAs were rated as low risk in Domain 2, eight SRs/MAs were rated as low risk in Domain 3, while only four SRs/MAs were rated as low risk in Domain 4. In Phase 3, and the 5 SRs/MAs had a low risk of bias. The results of the ROBIS scale assessment were presented in Table 4.
Table 4.
Results of the ROBIS Assessments
| Author, Year (Country) | Phase 1 | Phase 2 | Phase 3 | |||
|---|---|---|---|---|---|---|
| Assessing Relevance | Domain 1: Study Eligibility Criteria | Domain 2: Identification and Selection of Studies | Domain 3: Collection and Study Appraisal | Domain 4: Synthesis and Findings | Risk of Bias in the Review | |
| JinYing Fang, 202031 | √ | √ | √ | √ | × | × |
| Huabin Guo, 202132 | √ | √ | √ | × | √ | √ |
| Yizhen Li, 202133 | √ | √ | √ | √ | × | × |
| Wanchun Ye, 201834 | √ | √ | √ | √ | √ | √ |
| Xue Wu, 202135 | √ | √ | × | × | × | × |
| Ying Wang, 202036 | √ | √ | √ | √ | × | × |
| Huan Chen, 202037 | √ | √ | × | √ | √ | √ |
| Jing Huang, 201538 | √ | √ | √ | √ | √ | √ |
| Kui Liu, 201939 | √ | √ | × | √ | × | × |
| Xinhua Liang, 201640 | √ | √ | √ | × | × | √ |
| Mengjiu Zhang, 202041 | √ | √ | √ | × | × | × |
| Guoshuang Zhu, 201942 | √ | √ | × | × | × | × |
| Dandan Xie 202243 | √ | √ | √ | √ | × | × |
Note: √, low risk; ×, high risk.
Report Quality of the Included SRs/MAs
The results of the PRISMA assessment were shown in Table 5. Twenty-four of the 27 items had a “yes OR partially yes” response rate of over 70%, indicating the inclusion of relatively complete reporting of SRs/MAs. Nevertheless, there are reporting deficiencies for some items. The reports of Item 5 (Protocol and registration) and Item 8 (Search) were incomplete (with “yes OR partially yes” response rate less than 50%).
Table 5.
Results of the PRISMA Checklist
| Section/Topic | Items | JinYing Fang, 202031 | Huabin Guo, 202132 | Yizhen Li, 202133 | Wanchun Ye, 201834 | Xue Wu, 202135 | Ying Wang, 202036 | Huan Chen, 202037 | Jing Huang, 201538 | Kui Liu, 201939 | Xinhua Liang, 201640 | Mengjiu Zhang, 202041 | Guoshuang Zhu, 201942 | Dandan Xie 202243 | Number of Yes and Partially Yes (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Q1.Title | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13(100%) |
| Abstract | Q2. Structured summary | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | Y | PY | Y | 13(100%) |
| Introduction | Q3. Rationale | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13(100%) |
| Q4. Objectives | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13(100%) | |
| Methods | Q5. Protocol and registration | N | N | N | N | N | Y | N | N | N | N | Y | N | Y | 3(23%) |
| Q6. Eligibility criteria | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13(100%) | |
| Q7. Information sources | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13(100%) | |
| Q8. Search | Y | N | Y | N | N | N | N | Y | N | N | N | N | Y | 4(30.8%) | |
| Q9. Study selection | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13(100%) | |
| Q10. Data collection process | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13(100%) | |
| Q11. Data items | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13(100%) | |
| Q12. Risk of bias in individual studies | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13(100%) | |
| Q13. Summary measures | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13(100%) | |
| Q14. Synthesis of results | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13(100%) | |
| Q15. Risk of bias across studies | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | 12(92.3%) | |
| Q16. Additional analyses | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | 12(92.3%) | |
| Results | Q17. Study selection | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13(100%) |
| Q18. Study characteristics | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13(100%) | |
| Q19. Risk of bias within studies | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | 12(92.3%) | |
| Q20. Results of individual studies | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13(100%) | |
| Q21. Synthesis of results | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13(100%) | |
| Q22. Risk of bias across studies | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | 12(92.3%) | |
| Q23. Additional analysis | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | 12(92.3%) | |
| Discussion | Q24. Summary of evidence | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | PY | Y | PY | 13(100%) |
| Q25. Limitations | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13(100%) | |
| Q26. Conclusions | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 13(100%) | |
| Funding | Q27. Funding | Y | Y | Y | Y | Y | Y | N | Y | N | Y | Y | Y | Y | 11(84.6%) |
Notes: Y, yes; PY, partially yes; N, no. PRISMA checklist adapted from: Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi:10.1136/bmj.b2535.29 Creative Commons Attribution Non-commercial License.
Evidence Quality of the Included SRs/MAs
Results of the Evidence Quality
The 13 SRs/MAs included 73 effect sizes related to the effectiveness of TG for DKD. Of all the effect sizes, 3 were rated as high quality, 13 moderate quality, 30 low quality and 27 very low quality in terms of the quality of evidence. Risk of bias (n = 56) was the most common degrading factor, followed by publication bias (n = 55), inconsistency (n = 27), imprecision (n = 12), and indirectness (n = 0). GRADE specific assessment details were shown in Table 6.
Table 6.
Results of Evidence Quality
| Author, Year (Country) | Outcomes | Studies (Participants) | Limitations | Inconsistency | Indirectness | Imprecision | Publication Bias | Effect Size (95% CI) | P-value | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| JinYing Fang, 202031 | 24h-UTP | 9 (851) | Serious① | Serious② | No | No | No | WMD=−0.34(−0.38,-0.30) | P<0.00001 | Low |
| SCr | 8 (781) | Serious① | No | No | No | No | WMD=−9.87(−13.76, −5.97) | P<0.00001 | Moderate | |
| Huabin Guo, 202132 | 24h-UTP | 24 (847) | No | Serious② | No | No | No | WMD=−0.84(−1.09, −0.59) | P<0.00001 | Moderate |
| Alb | 15 (976) | No | No | No | No | Serious④ | WMD=2.88(1.87, 3.90) | P<0.00001 | Moderate | |
| SCr | 23 (1628) | No | Serious② | No | No | No | WMD=−4.77(−4.78, −1.75) | P=0.002 | Moderate | |
| BUN | 9 (774) | No | Serious② | No | Serious③ | Serious④ | WMD=−0.37(−0.79, 0.04) | P=0.08 | Very low | |
| CCr | 7 (476) | No | No | No | No | Serious④ | SMD=0.42(0.24, 0.60) | P<0.00001 | Moderate | |
| Total clinical efficacy | 5 (571) | No | No | No | No | Serious④ | OR=4.08, 95% CI (2.37, 7.04) | P<0.00001 | Moderate | |
| Adverse reactions | ALT | 10 (539) | Serious① | No | No | Serious③ | Serious④ | WMD=1.18(−0.68, 3.04) | P=0.21 | Very low |
| WBC | 8 (533) | No | No | No | No | Serious④ | WMD=−0.26(−0.38, −0.14) | P<0.00001 | Moderate | |
| Yizhen Li, 202133 | 24h-UTP(3 months) | 14 (1120) | Serious① | Serious② | No | No | Serious④ | WMD=0.30(–0.35, –0.25) | P<0.00001 | Very low |
| 24h-UTP(6 months) | 7 (708) | Serious① | Serious② | No | No | Serious④ | WMD=−0.91(−1.27, −0.56) | P<0.00001 | Very low | |
| SCr(3 months) | 12 (1002) | Serious① | Serious② | No | No | Serious④ | WMD=−12.63(−21.96, −3.31) | P=0.008 | Very low | |
| SCr(6 months) | 8 (780) | Serious① | Serious② | No | No | Serious④ | WMD=−2.85(−5.03, −0.68) | P=0.01 | Very low | |
| Adverse reactions | Adverse reactions (3 months) events | 13 (1148) | Serious① | No | No | No | No | WMD=2.02(1.35, 3.00) | P=0.006 | Moderate |
| Adverse reactions (6 months) events | 11 (1032) | Serious① | No | No | No | No | WMD=3.49(1.96, 6.22) | P<0.00001 | Moderate | |
| Wanchun Ye, 201834 | Total clinical efficacy | 6 (494) | No | No | No | No | No | RR=1.35 (1.22, 1.50) | P<0.00001 | High |
| 24h-UTP | 12 (829) | No | Serious② | No | No | No | WMD=−0.97(−1.19, -0.76) | P<0.00001 | Moderate | |
| SCr | 9 (532) | No | Serious② | No | Serious③ | No | WMD=−0.26(−7.52, 7.00) | P=0.94 | Low | |
| Alb | 8 (417) | No | No | No | No | No | WMD=3.87(3.12, 4.62) | P<0.00001 | High | |
| UAER | 2 (278) | Serious① | Serious② | No | No | Serious⑤ | WMD=−145.53(−227.95, −63.11) | P<0.00001 | Very low | |
| Urinary β2-microglobulin | 2 (278) | Serious① | No | No | No | Serious⑤ | WMD=−11.86(−13.02, −10.69) | P<0.00001 | Low | |
| BUN | 2 (120) | Serious① | No | No | Serious③ | No | WMD=0.25 (−0.23, 0.74) | P=0.31 | Low | |
| Endogenous creatinine clearance rate | 3 (177) | No | No | No | Serious③ | No | WMD=−0.43(−3.48, 2.62) | P=0.78 | Moderate | |
| Adverse reactions | Adverse reaction rate | 8 (448) | No | No | No | No | No | RR=3.41 (1.34, 8.66) | P=0.01 | High |
| Xue Wu, 202135 | 24h-UTP | 18 (1339) | Serious① | Serious② | No | No | Serious④ | SMD=−1.46(−1.84, −1.09) | P<0.00001 | Very Low |
| UAER | 5 (500) | Serious① | Serious② | No | No | Serious④ | SMD=−6.9(−9.65, −4.14) | P<0.00001 | Very Low | |
| SCr | 18 (1397) | Serious① | Serious② | No | No | Serious④ | WMD=−7.65(−12.99, −2.31) | P<0.00001 | Very Low | |
| BUN | 6 (487) | Serious① | No | No | Serious③ | Serious④ | WMD=−0.06(−0.25, 0.13) | P=0.51 | Very Low | |
| Alb | 12 (737) | Serious① | No | No | No | Serious④ | WMD=5.7(4.44, 6.96) | P<0.00001 | Low | |
| HbA1c | 6 (447) | Serious① | No | No | Serious③ | Serious④ | WMD=−0.08(−0.22, 0.06) | P=0.24 | Very Low | |
| Adverse reactions | ALT | 6 (270) | Serious① | No | No | Serious③ | Serious④ | WMD=1.08(0.04, 2.12) | P=0.04 | Very Low |
| Ying Wang, 202036 | 24h-UTP | 17 (1033) | Serious① | Serious② | No | No | Serious④ | WMD=−0.95(−1.17, −0.74) | P<0.00001 | Very Low |
| SCr | NR | Serious① | Serious② | No | Serious③ | Serious④ | NR | P>0.05 | Very Low | |
| eGFR | NR | Serious① | Serious② | No | Serious③ | Serious④ | NR | P>0.05 | Very Low | |
| Alb | 13 (1148) | Serious① | No | No | No | Serious④ | WMD=2.53(1.44, 3.62) | P<0.00001 | Low | |
| Adverse reactions | Adverse reaction rate | 15 (951) | Serious① | No | No | No | No | RR=2.22(1.32, 3.73) | P=0.03 | Moderate |
| Huan Chen, 202037 | CRP | 9 (589) | Serious① | No | No | No | Serious④ | WMD=1. 89(1. 64, 2. 15) | P<0.00001 | Low |
| IL-6 | 6 (557) | Serious① | Serious② | No | No | Serious④ | WMD=3. 79(2. 51, 5. 07) | P<0.00001 | Very Low | |
| TNF-α | 8 (709) | Serious① | No | No | No | Serious④ | WMD=0. 40(0. 31, 0. 49) | P<0.00001 | Low | |
| Adverse reactions | Leukopenia rate | 6 (575) | Serious① | No | No | No | Serious④ | OR=4. 33(1. 08, 17. 47) | P=0.04 | Low |
| Abnormal liver function rate | 6 (448) | Serious① | No | No | No | Serious④ | OR=3. 73(1. 12, 12. 35) | P=0.03 | Low | |
| Adverse gastrointestinal reaction rate | 6 (526) | Serious① | No | No | Serious③ | Serious④ | OR=0(−0. 04, 0. 04) | P=0.94 | Very Low | |
| Jing Huang, 201538 | Total clinical efficacy | 9 (617) | Serious① | No | No | No | Serious④ | OR=4. 32(2. 77, 6. 46) | P<0.00001 | Low |
| 24h-UTP | 12 (1033) | Serious① | Serious② | No | No | No | WMD=−0. 84(−1. 02, −0. 66) | P<0.00001 | Low | |
| Alb | 9 (1033) | Serious① | No | No | No | Serious④ | SMD=0. 98(0. 83, 1. 13) | P<0.00001 | Low | |
| SCr | 10 (787) | Serious① | No | No | Serious③ | Serious④ | WMD=1.35(−0.05, 2.74) | P=0.06 | Very Low | |
| Adverse reactions | Adverse reaction rate | 7 (306) | Serious① | No | No | No | Serious④ | RD=0.07(0.03, 0.12) | P=0.0008 | Low |
| Kui Liu, 201939 | Total clinical efficacy | 10 (787) | Serious① | No | No | No | Serious④ | OR=6.10(3.93, 9.48) | P<0.00001 | Low |
| 24h-UTP | 16 (1475) | Serious① | Serious② | No | No | Serious④ | SMD=−1.27(−2.04, −0.51) | P=0.001 | Very Low | |
| SCr | 10 (787) | Serious① | Serious② | No | No | Serious④ | WMD=−7.49(−13.83, −1.15) | P=0.02 | Very Low | |
| Adverse reactions | Adverse reaction rate | 7 (617) | Serious① | No | No | No | Serious④ | RD=0.07(0.03, 0.11) | P=0.0002 | Low |
| Xinhua Liang, 201640 | Alb | 8 (425) | No | Serious② | No | No | Serious④ | WMD=3.52(0.80, 6.25) | P=0.01 | Low |
| SCr | 7 (446) | No | Serious② | No | No | Serious④ | WMD=−15.25(−23.84, −6.66) | P=0.0005 | Low | |
| 24h-UTP | 6 (275) | No | Serious② | No | No | Serious④ | WMD=−0.66(−0.89, −0.42) | P<0.00001 | Low | |
| BUN | 5 (343) | No | No | No | Serious③ | Serious④ | WMD=−0.05(−0.36, 0.27) | P=0.77 | Low | |
| Mengjiu Zhang, 202041 | 24h-UTP | 16 (1015) | Serious① | No | No | No | Serious④ | SMD=−0.78(−1.03,-0.54) | P<0.00001 | Low |
| Alb | 13 (767) | Serious① | No | No | No | Serious④ | SMD=0.61(0.34,0.87) | P<0.00001 | Low | |
| SCr | 15 (950) | Serious① | Serious② | No | No | Serious④ | SMD=−0.44(−0.85,-0.03) | P<0.00001 | Very Low | |
| BUN | 7 (543) | Serious① | No | No | No | Serious④ | WMD=−0.30(−0.59, −0.01) | P=0.04 | Low | |
| Adverse reactions | ALT | 14 (825) | Serious① | Serious② | No | No | Serious④ | SMD=0.26(−0.06, 0.59) | P=0.04 | Very Low |
| WBC | 10 (614) | Serious① | No | No | Serious③ | Serious④ | WMD=−0.29(−0.72, 0.14) | P=0.18 | Very Low | |
| Adverse reaction rate | 13 (830) | Serious① | No | No | No | Serious④ | RR=1.97(1.22, 3.19) | P=0.006 | Low | |
| Guoshuang Zhu, 201942 | Total clinical efficacy | 4 (254) | Serious① | No | No | No | Serious④⑤ | OR=3.91(1.91,8.01) | P=0.0002 | Low |
| 24h-UTP | 9 (498) | Serious① | No | No | No | Serious④ | WMD=−0.53(−0.77, 0.29) | P<0.00001 | Low | |
| BUN | 3 (220) | Serious① | Serious② | No | No | Serious④ | WMD=−2.2(−2.79, −1.61) | P<0.00001 | Very Low | |
| SCr | 4 (196) | Serious① | No | No | Serious③ | Serious④ | WMD=−5.79(−11.04, 0.11) | P=0.05 | Very Low | |
| Adverse reactions | Adverse reaction rate | 9 (610) | Serious① | No | No | Serious③ | Serious④ | OR=1.01(0.56,1.82) | P=0.46 | Very Low |
| Dandan Xie 202243 | UAER | 9 (694) | Serious① | No | No | No | Serious④ | SMD=−2.55 (−4.70, −0.40) | P=0.02 | Low |
| 24h-UTP | 27 (1842) | Serious① | No | No | No | No | WMD= −0.79 (−1.22, −0.36) | P = 0.0003 | Moderate | |
| SCr | 25 (1698) | Serious① | Serious② | No | No | No | WMD=−8.23 (−14.48, −1.99) | P = 0.01 | Low | |
| Alb | 17 (1174) | Serious① | Serious② | No | No | No | WMD= 4.70 (3.27, 6.13) | P<0.00001 | Low | |
| Adverse reaction rate | 20 (1351) | Serious① | No | No | No | Serious④ | RR=2.55 (1.57, 4.13) | P<0.00001 | Low |
Notes: ①The included studies had a large bias in methodology such as randomization, allocation concealment, and blinding. ②The confidence interval overlapped less or the I2 value of the combined results was larger. ③The sample size from the included studies did not meet the optimal sample size or the 95% confidence interval crossed the invalid line. ④The funnel chart was asymmetry. ⑤Fewer studies were included, and their results were all positive, which may result in a large publication bias.
Abbreviations: CT, conventional treatment; 24-UTP, 24 h-urine total protein; SCr, serum creatinine; Alb, serum albumin; BUN, blood urea nitrogen; UAER, urinary albumin excretion rates; eGFR, glomerular filtration rate; CRP, c-reactive protein, IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; WBC, white blood cell.
Summary of Results of the Included Studies
The result indicators extracted from the included studies were listed in Table 6.
Efficacy of TG for DKD
24h-UTP
12 SRs/MAs31–36,38–43 reported on 24h-UTP, 11 of which reported meta-analyses showing that the effect of TG combination therapy was more significant than that of CT alone, and one SR/MA42 showed that TG was more effective than RAAS inhibitors.
SCr
A total of 12 SRs/MAs31–36,38–43 reported on SCr, of which 11 studies compared TG combined with CT and CT alone, and it was shown that the results of combined treatment were more significant than CT alone. One SR/MA37 reported that TG had no statistically significant improvement in SCr compared with RAAS inhibitors.
Alb
A total of 8 SRs/MAs32,34–36,38,40,41,43 reported on Alb, all of which were TG combined with CT versus CT alone, and the results show that combined treatment can significantly improve Alb.
BUN
A total of 6 SRs/MAs32,34,35,40–42 reported on BUN, and 5 of them were about TG combined with CT versus CT alone, but only one SR/MA41 showed that combined treatment can reduce BUN in patients with DKD. In addition, one SR/MA42 reported that TG can significantly reduce BUN compared with RAAS inhibitors.
Total Clinical Effective Rate
A total of 5 SRs/MAs32,34,38,39,42 reported the total clinical effective rate, of which 4 SRs/MAs32,34,38,39 reported that TG combined with CT was higher than CT alone. One SR/MA42 showed that TG was more clinically effective than RAAS inhibitors.
Other Clinical Effect Sizes
There are 3 SRs/MAs34,35,43 reporting on UAER, and the results show that there was no significant difference in UAER between TG combined with CT and CT alone. Two SRs/MAs reported that TG combination therapy can significantly improve CCr32 and Urinary β2-microglobulin in patients34 with DKD. In addition, there was one SR/MA35 reporting that TG combination therapy could not only reduce the inflammatory state of patients with DKD but also decrease CRP, IL6 and TNF-α.
Adverse Reactions
There were 10 SRs/MAs32–39,41–43 that reported on adverse reactions. The results of 9 SRs/MAs showed that the incidence of adverse reactions in TG combination therapy was significantly increased, and these adverse reactions included abnormal liver function, gastrointestinal reactions, menstrual disorders in women of childbearing age, and decreased white blood cell counts. In addition, one of the SR/MAs showed that there was no significant difference in the incidence of adverse reactions between TG and RAAS inhibitors.
Discussion
DKD is one of the main microvascular diseases in diabetes and an important cause of death in DM patients.44 The treatment of TG for DKD has also attracted increasing attention. This overview summarizes the available evidence to comprehensively assess the efficacy of TG for the treatment of DKD.
Summary of the Main Findings
This overview incorporated 13 SRs/MAs on TG for DKD. These publications were based on the RCT and were published from 2015 to 2021. Ten (11/13, 84.6%) SRs/MAs were published in the last five years, indicating that TG has received increasing attention as an important intervention modality for DKD.
Based on the results of the AMSTAR-2 evaluation in this overview, the methodological quality of all SRs/MAs was very low. The absence of Item 2 (protocol registration, 3/13, 23.1%), Item 7 (list of exclusions, 0/13, 0%), Item 10 (inclusion of research funding information, 0/13, 0%), and Item 13 (risk of bias interpretation, 5/13, 38.4%) was the main reason for the low methodological quality of SRs/MAs. Only 2 SRs/MAs contained initial research protocol registrations which could ensure the standardization of the research process, and the lack of protocol registration will increase the risk of bias and impact the rigor and credibility of the final SRs/MAs results.45 None of the SR/MA provided a complete list of exclusions for each study, which may affect the reliability of the results and the assessment of publication bias. The provision of a list of exclusion researches was a stronger demonstration of the rigor of the literature screening process. In addition, no SR/MA reporting included the RCT’s funding resources, which may increase bias in clinical trials as the results of corporate-funded studies may be biased in favor of the funder. The authors of the 7 SRs/MAs did not consider the risk of bias of including RCTs when interpreting or discussing the study results, which may affect the authenticity of the final results. The ROBIS scale was used to assess the risk of bias of the included SRs/MAs. Among them, incomplete literature search, inaccurate assessment of the risk of bias, and insufficient explanation of the risk of bias in the discussion are the main factors leading to the high risk of bias, which may affect the reliability of the final results. Consistent with the results of the AMSTAR-2 assessment, the lack of study protocol registration was a significant reason for the low quality of the reporting of SRs/MAs included. In addition to this, the lack of a complete search strategy was an important reason for the low quality of the reports since the reproducibility of SRs/MAs can be diminished by providing only search terms.
Based on the GRADE evidence quality assessment, 3 of the 73 effect sizes were rated as high quality, 13 moderate quality, 30 low quality, and 27 very low quality. The risk of bias was the most common degrading factor, followed by the publication bias, inconsistency, imprecision, and indirectness. The risk of bias was mainly reflected in the fact that most of the original RCTs for TG treatment of DKD did not clearly describe the random sequence generation method, the allocation concealment method, or the blinding method. Through further analysis, it was found that the outcome indicators included in the SRs/MAs were either incomplete or had risk of publication bias. Besides, the lack of original researches included in the relevant indicators was also an important reason for missing publication bias assessment. Among the included SRs/MAs, it is worth noting that although almost all SRs/MAs have shown that adding TG to CT was an effective method for the treatment of DKD, the conclusions of SRs/MAs may be different from the real results due to the low methodological quality of the included studies.
Studies have shown that TG can inhibit the expression of hypoxia-inducible factor 1-a, endothelin-1, and vascular endothelial growth factor, thereby reducing inflammation, decreasing thylakoid cell number, suppressing thylakoid matrix proliferation, and delaying glomerulosclerosis.46,47 TG also has immunosuppressive effects48 by interfering with nuclear factor-kB and toll-like receptor signaling pathways and reducing the production of tumor necrosis factor-a, interleukin-5 and immunoglobulin E. In the GRADE evidence evaluation, high- and medium-quality evidence seems to prove that adding TG to the basic treatment can not only significantly reduce 24h-UTP, SCr, CCr, and Urinary β2-microglobulin in patients with DKD, but also significantly increase the total effective rate and Alb. However, due to the low overall methodological quality of the included studies, caution should be exercised when recommending TG as a supplementary intervention for DKD. In addition, the adverse reactions of TG treatment should also be paid attention to. These adverse reactions mainly include abnormal liver function and decreased WBC count. Medical guidance recommends that the TG treatment duration should not exceed 3 consecutive months29 so as to maintain the effectiveness and safety of TG use. Therefore, special attention should be paid to the appropriate dosage and treatment duration in the specific implementation, depending on the pathological symptoms of individual patients. In the absence of high-quality evidence of TG-related adverse reactions, the principles of traditional Chinese medicine theory such as “no overtreatment” and “formula and medicine combination” can be applied. In practice, safe botanical drugs or botanical extracts with leukocyte proliferative effects are added.
Implications for Future Research
This paper provides a comprehensive assessment of all the aspects of the included SRs/MAs using AMSTAR-2, PRISMA, ROBIS, and GRADE, and it is found that the methodological and evidential quality is unsatisfactory, which implies that there is considerable room for improvement in the process of conducting SRs/MAs. When selecting topics for SRs/MAs, investigators should register or publish study protocols in advance to minimize the risk of bias and ensure the standardization of SRs/MAs. In terms of literature search and screening, gray literature should be checked, a complete search formula should be provided, and a list of excluded literatures should be provided with explanations to ensure transparency and avoid publication bias. For literatures with a high risk of bias, patients, care providers, and outcome evaluators should be blinded whenever possible to minimize the risk of bias. A well-designed, rigorously-executed, and complete reporting RCT is believed to be the gold standard for evaluating interventions to minimize or avoid bias.49
Despite the potential efficacy of TG in the treatment of DKD, there are also certain adverse reactions. Therefore, in the future study, we strongly recommend that more RCTs need to be studied with different doses and durations of TG in the treatment of DKD. In addition, due to the compensatory ability of the kidney, SCr may still be at a normal level when the renal function of DKD is damaged. More and more studies have shown that the sensitivity of eGFR decline is superior to that of SCr, especially in terms of kidney damage.50 Only one of the SRs/MAs included in this overview evaluates eGFR, so it is recommended to add this outcome indicator to the evaluation in the future RCT or SR/MA.
Strengths and Limitations
Our overview is the first to use AMSTAR2, ROBIS, PRISMA, and GRADE to evaluate SRs/MAs regarding TG for the treatment of DKD. The evaluation process revealed clear limitations of the current relevant SRs/MAs and RCTs, which may help guide future high-quality clinical studies. However, the overview may have some limitations since the evaluation of quality is a subjective process and different reviewers may have their own judgment on each factor, although our overview has been evaluated by two independent reviewers.
Conclusion
In conclusion, TG is beneficial for DKD. TG can reduce 24h-UTP, SCr in DKD patients and also help to raise Alb. However, TG has adverse effects in clinical use and requires attention to appropriate dose and regimen in clinical application, as well as attention to pathological symptoms in individual patients. However, due to the generally low methodological and evidential quality in the included SRs/MAs, clinicians should approach the findings with caution in their practice. Higher quality clinical studies are needed in the future to give further investigations on the efficacy of TG application and its adverse effects.
Supplementary Material
Supplementary Table S1: Search strategy for all databases.
Funding Statement
Shandong Provincial Natural Science Foundation of China (ZR2020MH395) and Shandong Traditional Chinese Medicine Science and Technology Development Program (2019-0052).
Abbreviations
DM, Diabetes mellitus; DKD, Diabetic kidney disease; RAAS, Renin-Angiotensin-Aldosterone System; TG, Tripterygium glycosides; SR, Systematic review; MA, Meta-analysis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, Randomized controlled trial; 24-UTP, 24 h-urine total protein; SCr, Serum creatinine; Alb, Serum albumin; BUN, Blood urea nitrogen; UAER, Urinary albumin excretion rates; eGFR, Glomerular filtration rate; CNKI, China National Knowledge Infrastructure; CBM, Chinese Biological Medicine; AMSTAR-2, Assessment System for Evaluating Methodological Quality 2; ROBIS, Risk of bias in systematic.
Data Sharing Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
- 1.Kerr D, Glantz N. Diabetes, like COVID-19, is a wicked problem. Lancet Diabetes Endocrinol. 2020;8(11):873–874. doi: 10.1016/S2213-8587(20)30312-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thipsawat S. Early detection of diabetic nephropathy in patient with type 2 diabetes mellitus: a review of the literature. Diab Vasc Dis Res. 2021;18(6):14791641211058856. doi: 10.1177/14791641211058856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cundy T, Holden A, Stallworthy E. Early worsening of diabetic nephropathy in Type 2 diabetes after rapid improvement in chronic severe hyperglycemia. Diabetes Care. 2021;44(3):e55–e56. doi: 10.2337/dc20-2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Long J, Jiang W, et al. Trends in chronic kidney disease in China. N Engl J Med. 2016;375(9):905–906. doi: 10.1056/NEJMc1602469 [DOI] [PubMed] [Google Scholar]
- 5.Qi C, Mao X, Zhang Z, Wu H. Classification and differential diagnosis of diabetic nephropathy. J Diabetes Res. 2017;2017:8637138. doi: 10.1155/2017/8637138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang G, Ouyang J, Li S, et al. The analysis of risk factors for diabetic nephropathy progression and the construction of a prognostic database for chronic kidney diseases. J Transl Med. 2019;17(1):264. doi: 10.1186/s12967-019-2016-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato M, Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol. 2019;15(6):327–345. doi: 10.1038/s41581-019-0135-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawaf H, Thomas G, Taliercio JJ, Nakhoul G, Vachharajani TJ, Mehdi A. Therapeutic advances in diabetic nephropathy. J Clin Med. 2022;11(2):378. PMID: 35054076; PMCID: PMC8781778. doi: 10.3390/jcm11020378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Ye L, Tao J, Ge C, Huang L, Yu J. Total flavones of Abelmoschus manihot improve diabetic nephropathy by inhibiting the iRhom2/TACE signalling pathway activity in rats. Pharm Biol. 2017;56(1):1–11. doi: 10.1080/13880209.2017.1412467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin YC, Chang YH, Yang SY, Wu KD, Chu TS. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc. 2018;117(8):662–675. doi: 10.1016/j.jfma.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 11.Yaribeygi H, Atkin SL, Sahebkar A. Interleukin-18 and diabetic nephropathy: a review. J Cell Physiol. 2019;234(5):5674–5682. doi: 10.1002/jcp.27427 [DOI] [PubMed] [Google Scholar]
- 12.Zheng S, Powell DW, Zheng F, Kantharidis P, Gnudi L. Diabetic nephropathy: proteinuria, inflammation, and fibrosis. J Diabetes Res. 2016;2016:5241549. doi: 10.1155/2016/5241549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tesch GH. Diabetic nephropathy - is this an immune disorder? Clin Sci (Lond). 2017;131(16):2183–2199. doi: 10.1042/CS20160636 [DOI] [PubMed] [Google Scholar]
- 14.Tong X, Qiao Y, Yang Y, et al. Applications and mechanisms of Tripterygium Wilfordii hook. f. and its preparations in kidney diseases. Front Pharmacol. 2022;13:846746. doi: 10.3389/fphar.2022.846746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X, Li QJ, Xia S, Wang MM, Ji W. Tripterygium glycosides for treating late-onset rheumatoid arthritis: a systematic review and meta-analysis. Altern Ther Health Med. 2016;22(6):32–39. [PubMed] [Google Scholar]
- 16.Ho LJ, Chang WL, Chen A, Chao P, Lai JH. Differential immunomodulatory effects by Tripterygium wilfordii Hook f-derived refined extract PG27 and its purified component PG490 (triptolide) in human peripheral blood T cells: potential therapeutics for arthritis and possible mechanisms explaining in part Chinese herbal theory “Junn-Chenn-Zuou-SS”. J Transl Med. 2013;11:294. doi: 10.1186/1479-5876-11-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu GZ, Han XC, Wang HZ, Yang YZ, Gao Y, Wang HL. Effect of Tripterygium Glycosides Tablets in treating rheumatoid arthritis: a systematic review and Meta-analysis. Zhongguo Zhong Yao Za Zhi. 2019;44(15):3358–3364. doi: 10.19540/j.cnki.cjcmm.20190305.004 [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Huang Y, Liu P, Yuan H, Zhao J. Effect of Tripterygium wilfordii polyglycoside tablets on serum inflammatory factors and T cells in patients with chronic nephritis. Am J Transl Res. 2021;13(7):8385–8390. PMID: 34377332; PMCID: PMC8340144. [PMC free article] [PubMed] [Google Scholar]
- 19.Yao JR, Sun Y, Luo SK, Xie DH. Progress of tripterygium glycosides in clinical application. Chin J New Drugs Clin Remedies. 2010;29(03):179–182. [Google Scholar]
- 20.Su HJ, Qian Y, Li H, Li E. Efficacy of Tripterygium glycosides on proteinuria patients with diabetic nephropathy. Med J Wuhan Univ. 2013;34(2):296–298. [Google Scholar]
- 21.Zhang YX, Liu GL, Wang JQ, Li YC. Antioxidative effect of Tripterygium wilfordii polyglycosides on diabetic rats. Chin J Pharmacol Toxicol. 2014;28(3):358–61. 10.3867/j.issn.1000-3002.2014.03.008. [Google Scholar]
- 22.Huang J, Shen M, Qin X, Wu M, Liang S, Huang Y. Acupuncture for the treatment of Alzheimer’s disease: an overview of systematic reviews. Front Aging Neurosci. 2020;12:574023. doi: 10.3389/fnagi.2020.574023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen M, Huang J, Qiu T. Quality of the evidence supporting the role of acupuncture for stable angina pectoris: an umbrella review of systematic reviews. Front Cardiovasc Med. 2021;8:732144. doi: 10.3389/fcvm.2021.732144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Microvascular Complications Group of Diabetes Society of Chinese Medical Association. Guidelines for the prevention and treatment of diabetic nephropathy in China (2021 Edition). Chin J Diabetes. 2021;13(8):23. [Google Scholar]
- 26.An W, Huang Y, Chen S, Teng T, Liu J, Xu Y. Efficacy and safety of Huangkui capsule for diabetic nephropathy: a protocol for systematic review and meta-analysis. Medicine. 2021;100(42):e27569. doi: 10.1097/MD.0000000000027569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whiting P, Savović J, Higgins JP, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–234. doi: 10.1016/j.jclinepi.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang JY, Yang Y, Zhang Z, et al. Effects of adding tripterygium glycosides to angiotensin-converting enzyme inhibitors or angiotensin receptor blockers on albuminuria in patients with diabetic nephropathy. Chronic Dis Transl Med. 2020;6(1):18–26. doi: 10.1016/j.cdtm.2019.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo HB, Peng JQ, Wang X, et al. Efficacy of tripterygium glycosides for diabetic nephropathy: a meta-analysis of randomized controlled trials. BMC Nephrol. 2021;22(1):304. doi: 10.1186/s12882-021-02487-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Miao R, Liu Y, et al. Efficacy and safety of tripterygium glycoside in the treatment of diabetic nephropathy: a systematic review and meta-analysis based on the duration of medication. Front Endocrinol. 2021;12:656621. doi: 10.3389/fendo.2021.656621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye WC, Ye JZ, Zheng C, et al. Combination therapy of tripterygium glycosides plus valsartan in diabetic nephropathy treatment: a systematic review and meta-analysis. Chin Herb Med. 2019;11(2):9. [Google Scholar]
- 35.Wu X, Huang Y, Zhang Y, et al. Efficacy of tripterygium glycosides combined with ARB on diabetic nephropathy: a meta-analysis. Biosci Rep. 2020;40(11):BSR20202391. doi: 10.1042/BSR20202391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ying W, Mei HA, Cep B, et al. Combination of Oral Tripterygium Glycosides and angiotensin II receptor blockers for treatment of clinical stage diabetic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Eur J Integr Med. 2020;39:101197. [Google Scholar]
- 37.Huan C, Zhan J, Min L, Deqing X. Meta-analysis of the effect and safety of Tripterygium wilfordii polyglycosides on inflammatory factors in diabetic nephropathy. Strait Pharmacy. 2020;32(10):6. [Google Scholar]
- 38.Huang J, Zhang JQ, Chen Z, Zhang Y, Chen WD, Wu XP. Systematic evaluation for efficacy of tripterygium glycosides in treating diabetic nephropathy stage IV. Zhongguo Zhong Yao Za Zhi. 2015;40(15):3100–3109. [PubMed] [Google Scholar]
- 39.Xinhua L, Ping F. A systematic evaluation of the clinical efficacy of Tripterygium Glycoside Tablets on diabetic nephropathy. J Shanxi Med Univ. 2016;47(8):5. [Google Scholar]
- 40.Kui L, Yuan Z. System evaluation of the efficacy and safety of tripterygium glycosides combined with ACEI/ARB drugs in the treatment of diabetic nephropathy. Clin J Tradit Chin Med. 2019;31(11):6. [Google Scholar]
- 41.Mengjiu Z, Hongfang L, Yan G, et al. Meta-analysis of the clinical efficacy and safety of tripterygium wilfordii polyglycosides in the treatment of diabetic nephropathy. Med Rev. 2020;26(9):9. [Google Scholar]
- 42.Guoshuang Z, Lan W, Qinghua L, et al. Meta analysis of the effectiveness and safety of tripterygium glycosides versus RAAS blockers in the treatment of diabetic nephropathy. J Clin Nephrol. 2019;19(10):727–733. [Google Scholar]
- 43.Xie D, Li K, Ma T, et al. Therapeutic effect and safety of Tripterygium Glycosides combined with western medicine on Type 2 diabetic kidney disease: a meta-analysis [published online ahead of print, 2022 Jan 20]. Clin Ther. 2022;44(2):246–256.e10. doi: 10.1016/j.clinthera.2021.12.006 [DOI] [PubMed] [Google Scholar]
- 44.Tziomalos K, Athyros VG. Diabetic nephropathy: new risk factors and improvements in diagnosis. Rev Diabet Stud. 2015;12(1–2):110–118. doi: 10.1900/RDS.2015.12.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart L, Moher D, Shekelle P. Why prospective registration of systematic reviews makes sense. Syst Rev. 2012;1(1):7. doi: 10.1186/2046-4053-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen WD, Chang BC, Zhang Y, Yang P, Liu L. Effect of Tripterygium glycosides on expression of hypoxia inducible factor-1α and endothelin-1 in kidney of diabetic rats. Nan Fang Yi Ke Da Xue Xue Bao. 2015;35(4):499–505. [PubMed] [Google Scholar]
- 47.Wan YG, Sun W, Zhen YJ. Preventive effect of multi-glycoside of tripterygium Wilfordii Hook. f. on proteinuria and mesangial injury in experimental mesangial proliferative glomerulonephritis. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25(9):817–821. [PubMed] [Google Scholar]
- 48.Wan YG, Che XY, Sun W, et al. Low-dose of multi-glycoside of Tripterygium wilfordii Hook. f., a natural regulator of TGF-β1/Smad signaling activity improves Adriamycin-induced glomerulosclerosis in vivo. J Ethnopharmacol. 2014;151(3):1079–1089. doi: 10.1016/j.jep.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 49.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. doi: 10.1016/j.ijsu.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 50.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–226. doi: 10.1053/ajkd.2002.34487 [DOI] [PubMed] [Google Scholar]