Abstract
This study evaluated the impacts of reducing nutrient levels on bacterial water quality in drinking water. Two American Water System facilities (sites NJ102a and IN610) with histories of coliform problems were involved, and each water utility received two pilot distribution systems (annular reactors). One reactor simulated the conventional treatment conditions (control), while the other reactor was used to assess the effect of biological filtration and subsequent reduced biodegradable organic matter levels on suspended (water column) and biofilm bacterial concentrations in the distribution systems. Biodegradable organic matter levels were reduced approximately by half after biological treatment. For site NJ102a, the geometric mean of the assimilable organic carbon concentrations was 217 μg/liter in the plant effluent and 91 μg/liter after biological filtration. For both sites, plant effluent biodegradable dissolved organic carbon levels averaged 0.45 mg/liter, versus 0.19 to 0.22 mg/liter following biological treatment. Biological treatment improved the stability of free chlorine residuals, while it had little effect on chloramine consumption patterns. High bacterial levels from the biological filters resulted in higher bacterial concentrations entering the test reactors than entering the control reactors. On average, biofilms in the model systems were reduced by 1 log unit (from 1.4 × 105 to 1.4 × 104 CFU/cm2) and 0.5-log unit (from 2.7 × 105 to 7.8 × 104 CFU/cm2) by biological treatment at sites NJ102a and IN610, respectively. Interestingly, it required several months of biological treatment before there was an observable impact on bacterial water quality in the system, suggesting that the effect of the treatment change was influenced by other factors (i.e., pipe conditions or disinfection, etc.).
During the distribution of drinking water, bacterial regrowth may lead to a deterioration of bacterial water quality, amplification of corrosion, generation of bad tastes and odors, and proliferation of macroinvertebrates (1, 7, 9, 13, 18, 26). Biofilm control is becoming recognized as an important part of the operation of drinking water plants and distribution systems. Bacterial regrowth and coliform occurrences are dependent on a complex interaction of drinking water characteristics and engineering and operational parameters (13, 21, 23, 25, 38, 40). The concentrations of biodegradable organic matter (BOM) available for microbial growth can be determined by several biological tests (14). Bacterial regrowth can be controlled when the amount of BOM entering the distribution system is limited. Van der Kooij (34) showed that heterotrophic bacterial levels in nonchlorinated systems did not increase when assimilable organic carbon (AOC) levels were lower than 10 μg/liter. LeChevallier et al. (23) suggested that the regrowth of coliform bacteria in chlorinated water may be limited by AOC levels of less than 50 to 100 μg/liter. Block et al. (6) recommended an absence of BOM after treatment to limit bacterial regrowth. Servais et al. (32) associated biological stability with a biodegradable dissolved organic carbon (BDOC) concentration of 0.16 mg/liter in the finished water. Volk and Joret (36) indicated that BDOC levels should be less than 0.15 to 0.30 mg/liter to limit coliforms. All of these objectives require very high levels of treatment. For systems with high AOC or BDOC levels (e.g., an AOC level of >150 μg/liter and a BDOC level of >0.5 mg/liter), ways to reduce the level of BOM entering the distribution system should be considered (38). In addition to the amount of nutrients, the composition of the BOM is also an important factor for controlling microbial growth. Amino acids are only a small fraction of the natural organic matter, but they represent a high regrowth potential (high biomass production per unit of substrate) and are highly reactive with chlorine (12, 15). A variety of processes can be used to control BOM levels in drinking water. Coagulation can be efficient at removing DOC and BDOC. However, AOC is only marginally affected by coagulation, probably because AOC consists primarily of low-molecular-weight, nonhumic substances that are not amenable to coagulation (39). Other alternatives effective for controlling BOM levels in water include the application of powered activated carbon, biological filtration, or membrane processes. On the other hand, oxidation with chlorine or ozone increases the amounts of BOM in water (17, 20).
This research was conducted to assess the impact of treatment changes on bacterial water quality monitored within model distribution systems (i.e., annular reactors). The treatment change consisted of implementing biological filtration. The study investigated (i) the effects of biological filtration on nutrient levels entering the distribution systems and (ii) the impacts of the decrease in nutrient concentration on bacterial water quality (biofilm density and suspended bacteria).
MATERIALS AND METHODS
Study sites.
Two American Water Works Company utility subsidiaries participated in this project. Sites were selected because of historical coliform regrowth problems (23, 27). Characteristics of plant effluent waters are presented in Table 1.
TABLE 1.
Treated surface water quality data for sites NJ102a and IN610 (operating data)
| Parameter | Value for site:
|
|||
|---|---|---|---|---|
| NJ102a
|
IN610
|
|||
| Avg | Range | Avg | Range | |
| Coliform-positive samples/year | 0 | NAa | 3 | NA |
| Plate count bacteria (CFU/ml) | 9 | 0–32 | 5 | 0.6–20.8 |
| Turbidity (NTUb) | 0.03 | 0.01–0.17 | 0.04 | 0.01–0.20 |
| pH | 7.5 | 7.4–7.7 | 7.5 | 7.3–7.6 |
| Alkalinity (mg/liter as CaCO3) | 33 | 22–44 | 246 | 203–305 |
| Total hardness (mg/liter as CaCO3) | 66 | 57–72 | 337 | 291–391 |
| TOCc (mg/liter) | 2.1 | 1.4–2.9 | 2.5 | 2.1–3.2 |
| AOC (μg/liter) | 217 | 100–337 | 153 | 110–218 |
| BDOC (mg/liter) | 0.41 | 0.20–0.78 | 0.39 | 0.15–0.73 |
NA, not applicable.
NTU, nephelometric turbidity units.
TOC, total organic carbon.
(i) Site NJ102a (New Jersey-American Water Company).
The facility processes reservoir water which originates from the Swimming River. The treatment train includes addition of powdered activated carbon, a preoxidation step with chlorine, coagulation (with poly-aluminum chloride and a cationic polymer), flocculation, sedimentation, filtration with anthracite-sand-garnet, and postdisinfection with free chlorine. The plant production is 36 million gallons per day.
(ii) Site IN610 (Indiana-American Water Company).
The plant is supplied from the White River and provides oxidation with chlorine and potassium permanganate, settling, multimedium anthracite-sand-garnet filtration, and postdisinfection with chloramines. Plant production averages 12 million gallons per day.
Annular reactor experimentation.
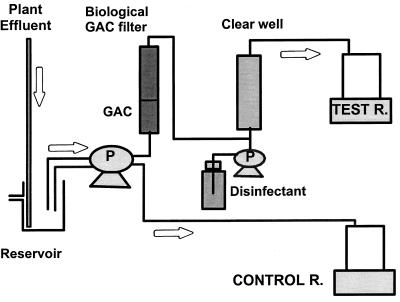
Annular reactors were used as a model distribution system and consist of an outer cast iron portion (pipe volume, 1.15 liters) and a rotating inner polyvinyl chloride cylinder that simulates water shear stress (8). Removable mild steel coupons located at the surface of the pipe allowed the evaluation of bacterial biofilm density. The study used an original experimental setup to specifically assess the benefit of a new treatment. Each water utility received two annular reactors (Fig. 1). One reactor was used as a control, modeling the distribution network; the other reactor was used as a test device for assessing the benefit of reducing nutrient levels in distribution systems. At the beginning of the study, both reactors were fed with treatment plant effluent water to identically colonize both pipes. Water retention times of 230 to 426 min were used, which resulted in dissipation of the residual disinfectant within the reactor (Table 2). When biofilm densities reached a plateau, the water-detention time was gradually decreased to achieve an effective disinfectant residual in the outlet of the reactor. At this point, the treatment change was implemented in the test device (Fig. 1; Table 2). A biological filter was installed to reduce nutrient levels entering the test pipe. Prior to the biological filter, the disinfectant residual was neutralized by filtration through a small virgin-GAC filter. The dechlorinated water passed through a biological GAC filter (originating from a full-scale biological active filter in Montreal, Canada) and was subsequently postdisinfected by using an intermediate clear well. The clear well was necessary to minimize the influence of the bacterial levels emanating from the biological filters on the microbiology of the annual reactor. The goal was to supply the test reactor with disinfectant residuals similar to those in the full-scale plant effluent entering the control reactor. These steps of flow rate augmentation, biofilter installation, and clear well disinfection were successively implemented to verify the proper functioning of each change before implementing the following steps.
FIG. 1.
Experimental setup used to study the effects of biological filtration on water quality. R., reactor; P, pump.
TABLE 2.
Operation conditions of the annular reactors at the two sites
| Applied condition | Value for site:
|
|
|---|---|---|
| NJ102a | IN610 | |
| Water flow rate during colonization (ml/min) | 5 | 2.7 |
| Pipe retention time during colonization (min) | 230 | 426 |
| Water flow rate after colonization (ml/min) | 100 | 42 |
| Pipe retention time after colonization (min) | 11.5 | 27 |
| Drum velocity (rpm) | 60 | 60 |
| Filtration contact time for test reactor (min) | 10 | 24 |
| Clear well contact time for test reactor (min) | 15 | 36 |
Monitored parameters.
Sampling was performed on a weekly (site IN610) or semimonthly (site NJ102a) basis. Water temperature, chlorine residuals (amperometric titration or N,N-diethyl-p-phenylenediamine [DPD] method), and heterotrophic plate count (HPC) levels were measured at the inlets and outlets of the reactors (2). HPC levels were determined by using R2A agar (Difco, Detroit, Mich.) incubated for 7 days at 20°C. Removable pipe coupons were sampled to evaluate biofilm density. Biofilm samples were homogenized (PT 1200C homogenizer; Brinkmann, Westbury, N.Y.) and plated on R2A agar. AOC concentrations were monitored at the inlets of the annular reactors by using the rapid ATP method (24). BDOC levels were measured by using bacteria attached to sand (37).
Because most of the data were not normally distributed, geometric means were calculated. The Wilcoxon test (signed and ranks test) was performed to determine whether two data sets were statistically different (comparison of medians).
RESULTS
The effects of biological filtration were successively evaluated by examining nutrient concentrations, disinfectant stability, and bacterial concentrations in the bulk water and biofilm. The effects of the reduction in nutrient levels on bacterial water quality were evaluated by measurement of suspended and fixed bacteria, the amount of time necessary to observe an impact, and the magnitude of the impact.
Reactor colonization.
Both reactors were identically colonized with plant effluent water during winter months at both locations, using cold waters (temperatures of <10°C). Average disinfectant residuals at the inlets of the reactors were 1.3 mg of chlorine per liter for site NJ102a and 2.4 mg of chloramines per liter for site IN610. Disinfectant residuals were not detectable at the outlets of the reactors. Consequently, suspended HPC levels in the reactor outlets were very high (7.7 × 104 CFU/ml at site NJ102a and 9.8 × 104 CFU/ml at site IN610) (data not shown).
Nutrient levels.
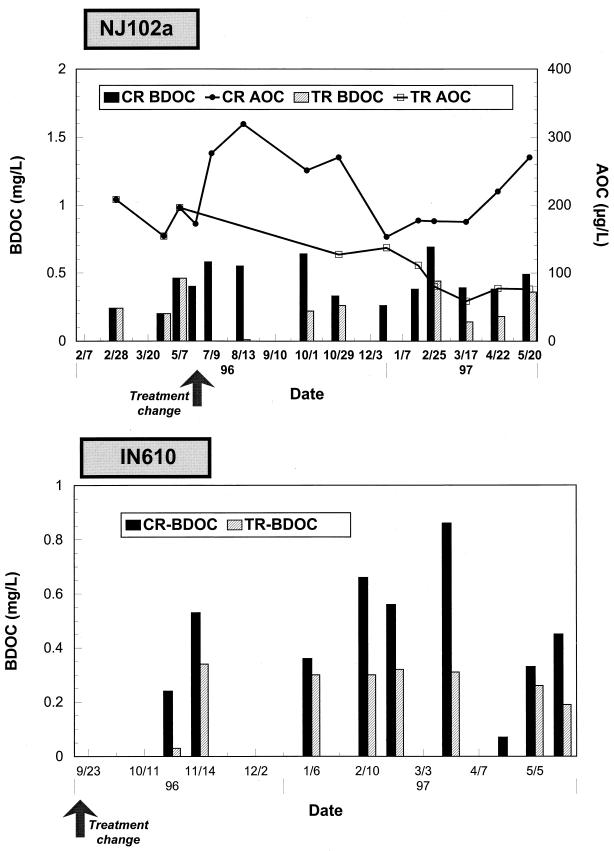
During the course of the study, the average DOC levels in the finished waters after conventional treatment were 2.00 and 2.34 mg/liter at NJ102a and IN610, respectively. BOM levels were reduced approximately by half after biological treatment (Fig. 2 and Table 3). For site NJ102a, plant effluent AOC concentrations varied from 153 to 319 μg/liter (geometric mean of 217 μg/liter), while after biological filtration, AOC concentrations entering the test reactor ranged between 58 and 137 μg/liter (geometric mean of 91 μg/liter). AOC reduction after biological filtration was statistically significant (P < 0.05) compared to plant effluent levels. BDOC levels ranged from 0.26 to 0.69 mg/liter (mean of 0.45 mg/liter) in the plant effluent water entering the control reactor, versus <0.05 to 0.44 mg/liter (mean of 0.19 mg/liter) at the test reactor inlet. The removal of BDOC through the biological filters averaged 60% and was statistically significant (P < 0.01). A similar trend was observed at IN610, where plant effluent BDOC levels averaged 0.45 mg/liter (range, 0.07 to 0.91 mg/liter) (Fig. 2; Table 3). After biological filtration, BDOC concentrations entering the test reactor were reduced by 50% on average (geometric mean of 0.22 mg/liter; range, <0.05 to 0.34 mg/liter [a significant difference, P < 0.05]). Three AOC sampling campaigns were performed after implementation of biological treatment, and average AOC concentrations were 160 μg/liter for the plant effluent and 76 μg/liter after biological filtration.
FIG. 2.
AOC and BDOC concentrations measured at the inlets of the test (TR) and control (CR) reactors.
TABLE 3.
Water quality data for the inlets and outlets of the reactors after treatment plant implementation
| Parameter | Valuea for site:
|
|||
|---|---|---|---|---|
| NJ102a
|
IN610
|
|||
| Control reactor | Test reactor | Control reactor | Test reactor | |
| Reactor inlet | ||||
| DOC (mg/liter) | 2.13 | 1.59 | 2.81 | 2.16 |
| BDOC (mg/liter) | 0.45 | 0.19 | 0.45 | 0.22 |
| AOC (μg/liter) | 217 | 91 | ||
| Disinfectant (mg/liter) | 1.4 | 1.2 | 2.3 | 2.3 |
| HPC (CFU/ml) | 14 | 93 | 1 | 74 |
| Reactor outlet | ||||
| Disinfectant (mg/liter) | 0.5 | 1.0 | 1.8 | 1.9 |
| HPC (CFU/ml) | 5,000 | 3,600 | 110 | 140 |
| Biofilm density (CFU/cm2) | 140,000 | 14,000 | 270,000 | 78,000 |
All values are geometric means.
Disinfectant residuals.
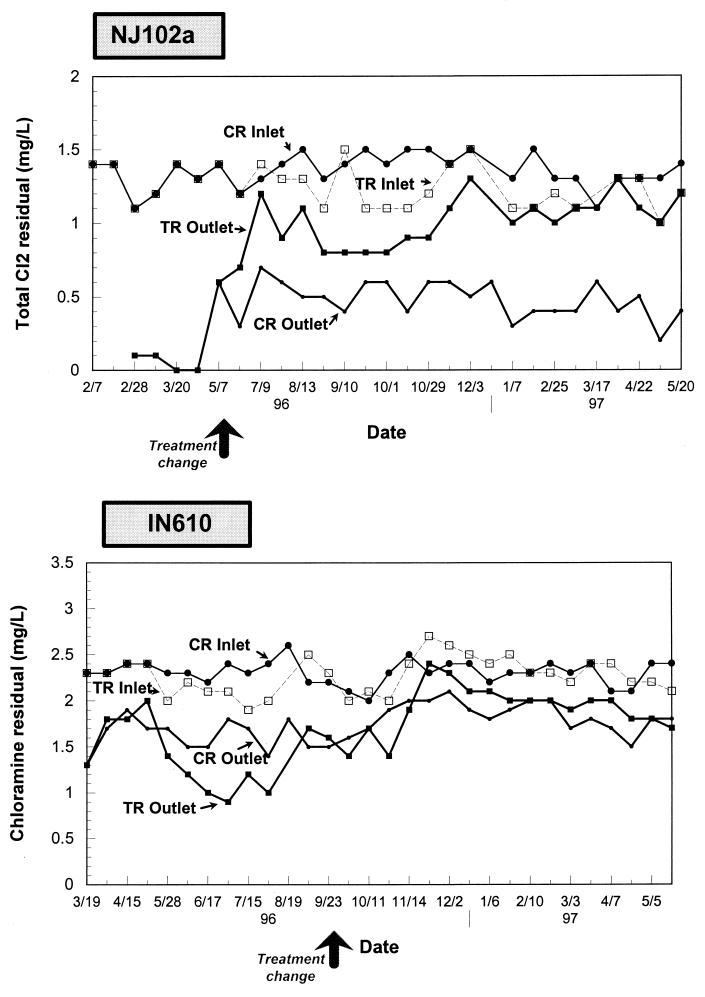
Total chlorine residuals in the plant effluent of site NJ102a were slightly higher than residuals in the inlet of the test reactor (average of 1.4 mg/liter for the control reactor versus 1.2 mg/liter for the test pipe after implementation of biological filtration [significant difference, P < 0.05) (Table 3; Fig. 3). Disinfectant residuals in the biologically treated test reactor were twice as high as the control reactor effluent chlorine residuals (P < 0.001). Disinfectant residuals leaving the control reactor averaged 0.5 mg/liter (range of 0.2 to 0.7 mg/liter), versus 1.0 mg/liter (0.6 to 1.2 mg/liter) for the test reactor (Fig. 3). For site IN610, chloramine residuals entering the control and test reactors were not statistically different (P ≫ 0.05). Average chloramine residuals were 2.3 mg/liter for the control (range, 1.9 to 2.7 mg/liter) and the test (range, 1.8 to 2.7 mg/liter) reactors. Chloramine residuals averaged 1.8 mg/liter at the control pipe outlet and 1.9 mg/liter in the test pipe effluent. The difference in disinfectant residuals at the pipe outlet was not statistically significant (P = 0.1).
FIG. 3.
Disinfectant residuals measured at the inlets and outlets of the test (TR) and control (CR) reactors.
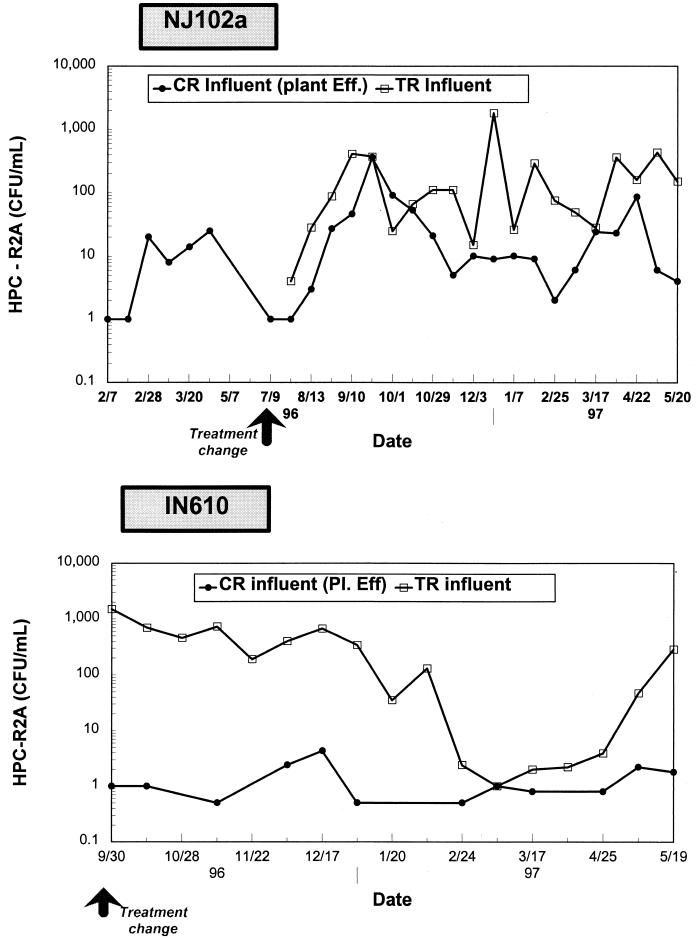
HPC levels entering the system.
HPC levels in the water entering the control reactor averaged 14 CFU/ml and varied from 0 to 360 CFU/ml at site NJ102a (Table 3; Fig. 4). Plant effluent bacterial concentrations at site IN610 were low and ranged between 0 and 36 CFU/ml, with a geometric mean of 1 CFU/ml (Fig. 4). After implementation of biological filtration, bacterial concentrations in the water entering the test reactor were slightly higher than those entering the control reactor at both sites (significant difference, P < 0.001). On the average, HPC levels after disinfection of biologically filtered water were 93 CFU/ml (range, 4 to 1800 CFU/ml) and 74 CFU/ml (1 to 1,500 CFU/ml) for sites NJ102a and IN610, respectively. Some bacteriological counts were also performed at the outlet of the biological filter, before disinfection in the clear well. HPC levels were as high as 5.7 × 105 CFU/ml at site NJ102a and averaged 9.2 × 104 CFU/ml at site IN610 (maximum of 6.2 × 105 CFU/ml) (data not shown).
FIG. 4.
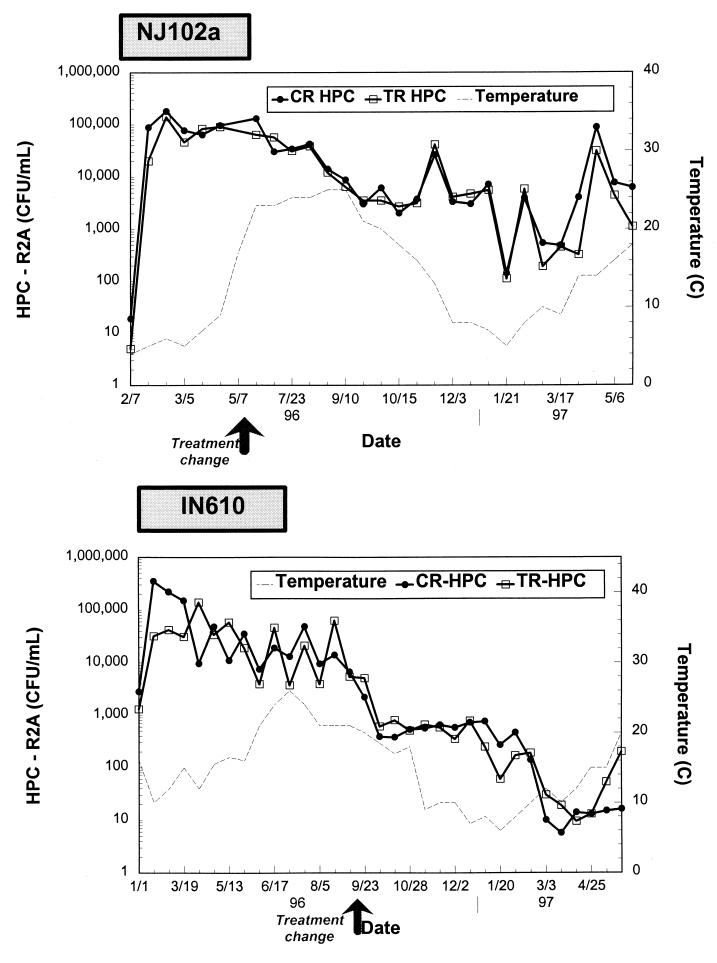
HPC levels at the inlets of the test (TR) and control (CR) reactors. Eff., effluent.
Effect of reduced nutrient concentrations on bacterial levels.
Figure 5 shows HPC levels at the outlets of the control and test reactors and after biological filtration. For site NJ102a, a reduction in nutrient levels had only a slight effect on HPC levels in the effluents of the reactors (difference not significant, P > 0.05). The HPC level of 3.6 × 103 CFU/ml (range, 1.1 × 102 to 1.4 × 105 CFU/ml) in the test reactor effluent was comparable to the geometric mean HPC level of 5 × 103 CFU/ml (range, 1.4 × 102 to 1.8 × 105 CFU/ml) in the control effluent water for the same period of time (Fig. 5 and Table 3). The trend was similar at site IN610. On average, HPC levels were 1.4 × 102 CFU/ml (range, 10 to 800 CFU/ml) and 1.1 × 102 CFU/ml (range, 10 to 730 CFU/ml) in the outlets of the test and control reactors, respectively.
FIG. 5.
Bulk water HPC levels and temperatures at the outlets of the test (TR) and control (CR) reactors.
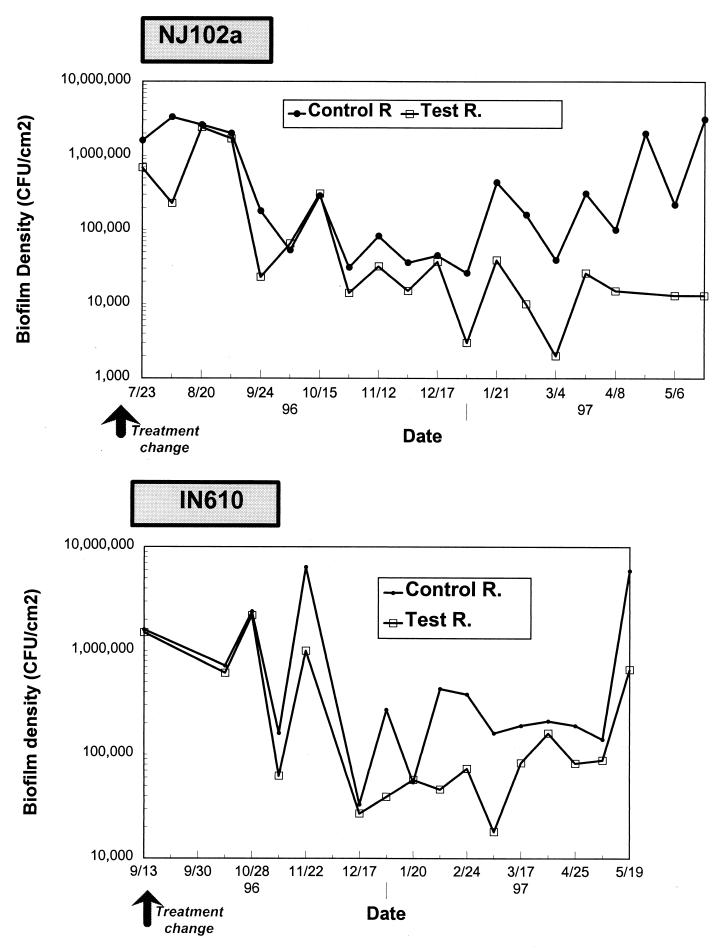
No drastic changes in concentrations of biofilm bacteria were observed following the installation of the biofilters (Fig. 6). During the first 6 months at site NJ102a, control reactor biofilms averaged 3.1 × 105 CFU/cm2, versus 1.6 × 105 CFU/cm2 for the test reactor. At site IN610, geometric means of biofilm bacteria for the 5 months following the installation of the biological filtration were 5.7 × 105 and 3 × 105 CFU/cm2 for the control and test pipes, respectively (a marginal statistical difference, P = 0.03). However, much higher differences in biofilm densities were observed after a period of several months following implementation of biological filtration. After a period of 6 months, a 10-fold difference in biofilm levels in the test reactor was apparent at site NJ102 (from 1.4 × 105 to 1.4 × 104 CFU/cm2; P < 0.001) (Table 3). On average, the difference was less drastic for site IN610, where biofilm levels dropped from 2.7 × 105 to 7.8 × 104 CFU/cm2, a 0.5-log-unit difference (P < 0.001).
FIG. 6.
Biofilm densities at the outlets of the test and control reactors (R.).
DISCUSSION
Bacterial regrowth in distribution systems results from the proliferation and detachment of heterotrophic bacteria from pipe surfaces. This project evaluated the effect of biological treatment on water quality in model distribution systems. The data from this study showed that conditions that cause the growth of bacteria in distribution systems are complex and site specific. Bacterial water quality could be related to the levels of nutrients entering the pipe network but also to other factors and their interactions. Annular reactors were found to be a good tool to evaluate and predict the effects of a treatment change before full-scale implementation.
Colonization.
The reactors became quickly colonized despite the influx of chlorinated water. Biofilm densities reached a plateau within 1 month at plant NJ102a and within 2 months at plant IN610. The rapid colonization of pipe surfaces is consistent with the findings of other investigators. Depending on the experimental conditions, an accumulation of steady-state biofilm can be observed at between 2 weeks and 4 months (4, 8, 12, 30). Biofilm counts observed inside the annular reactors at steady state were on the same order of magnitude as the ones found in other pilot or full-scale distribution systems (around 105 to 107 bacteria/cm2) (4, 8, 19, 29, 35).
Nutrient removal.
Biological filtration enhanced nutrient removal by providing biofilm activity to consume assimilable organic materials. Nutrient levels entering the distribution system could be reduced by 50% by biological treatment. Various studies showed that biological filtration can be highly effective for removal of BOM (3, 16, 17). The percentage of BOM removal is dependent on many parameters, such as organic matter characteristics, water temperature, medium type, empty bed contact time, and backwashing strategies. For example, during treatment of the Oise River (France), biological sand and GAC filters achieved BDOC removals of 32% (BDOC consumption of 0.25 mg/liter) and 37% (BDOC consumption of 0.30 mg/liter), respectively (11). Servais (31) observed average BDOC removals of 50% during biological GAC filtration of ozonated waters (empty bed contact time range of 10 to 20 min).
Disinfectant residuals.
For the site using free chlorine, the biologically treated system showed higher disinfectant residuals than the system supplied after conventional treatment. It is presumed that the higher residuals in the test reactor outlet resulted from lower organic matter and biofilm levels. Lower DOC and BDOC levels entering the test reactor reduced chlorine demand and increased chlorine stability within the system. Randon et al. (28) found identical results in a distribution system fed with surface water treated successively by ozone-GAC filtration (finished water BDOC levels of 0.75 mg/liter) and nanofiltration (BDOC levels of 0.20 mg/liter). Chlorine residuals at the end of the distribution system were more stable when the system was supplied with nanofiltered water, despite the fact that plant effluent chlorine residuals were 0.85 mg/liter for the water treated with ozone-biological filtration versus 0.25 mg/liter for the nanofiltered effluent. Lower biofilm densities would also account for less chlorine consumption within the test reactor, since hypochlorite acid is highly reactive with polysaccharides and cell material. Block et al. (5) reported that chlorine disappearance in distribution systems could be attributed to the reactions of chlorine with three components: organic matter in the water column, biofilm, and the pipe surface.
Disinfectant consumption within the reactors was lower with chloramine than with chlorine, despite the fact that the retention time of the chloraminated water was longer. Sixty-five percent of the chlorine was consumed within the control reactor at site NJ102a, while chloramine residuals were reduced by only 20% at site IN610. Both control and test reactor effluents showed similar disinfectant residuals, suggesting that application of biological filtration had little effect on chloramine consumption patterns (Fig. 3). It has been reported that chloramines are a more stable disinfectant because they are not consumed by the polysaccharidic matrix around biofilm cells and are less reactive with corrosion products (13). They can penetrate biofilm more effectively and react specifically with DNA, tryptophan, and sulfur-containing amino acids (13).
HPC levels entering the system.
Bacterial counts in the biological filter effluent were very high (approximately 105 CFU/ml). Such high plate counts are not uncommon in the effluents of biological filters (22). High levels of bacteria at the inlet of the distribution system could be of concern. Block et al. (6) reported a linear relationship between the concentration of suspended bacteria in the finished water and the concentration of attached biofilm cells. Those authors concluded that limiting the input of the bacteria entering the distribution system would improve the biological stability of the system. However, a more recent study (22) showed that bulk water bacteria had a minor impact on biofilms because bacteria in the water column were different from those attached to the pipe surface. Biofilm tended to be dominated by gram-negative bacteria, while bacteria present in the chlorinated water column were gram positive. This work suggested that even if bacteria from the water column initially colonize the pipes, the biofilm develops its own, unique ecosystem and represents a constant source of inoculation of new pipes introduced into the distribution system.
Bacterial water quality.
This study showed that biofilm densities were related to the amount of biodegradable material entering the system. Bacterial levels within model distribution systems could be reduced by 0.5 to 1.0 log10 unit. Similarly, Servais et al. (32) observed a relationship between biofilm bacteria and the concentration of BDOC at the points of entry of several full-scale distribution systems. Another study (30) compared the bacterial water quality in two annular reactors supplied with water containing different levels of organic matter. The first reactor was fed with ozonated water, while the second one was supplied with biologically filtered water. Lowering nutrient levels with biological filtration led to lower biofilm densities. Biofilm counts were 106 to 107 CFU/cm2 for the ozonated water reactor, compared to 105 to 106 CFU/cm2 for the reactor fed with biologically filtered water (30).
However, the effect of nutrient reduction on biofilm concentrations was not immediate. It required several months (approximately 6 months) of biological filtration before there was an observable impact on bacterial water quality. In two other studies (28, 33), bacterial water quality changes were monitored after implementation of nanofiltration in a pilot or full-scale system that was initially supplied with surface water treated with ozonation and biological filtration. For the cast-iron pipe loop pilot study (33), BDOC levels decreased from 0.25 mg/liter in the ozonated and filtered water to <0.1 mg/liter after nanofiltration. The authors did not observe drastic changes in the biofilm or suspended bacterial levels after the treatment change. At the beginning of the study, total and cultivatable counts were 4.9 × 106 cells/cm2 and 4 × 105 CFU/cm2 for the biofilm and 2.6 × 105 cells/ml and 103 CFU/ml in the bulk water. Bacterial concentrations decreased slightly after 6 weeks of supplying nanofiltered water (suspended bacteria, 1.4 × 105 cells/ml and 259 CFU/ml; biofilm, 2.3 × 106 cells/cm2 and 1.2 × 105 CFU/cm2). After 1 year of supplying nanofiltered water, biofilm levels were 1.9 × 105 CFU/cm2 and suspended bacterial levels were 50 CFU/ml. Similarly, bacterial water quality changes were not obvious when the same treatment conversion was performed in a full-scale distribution system supplying a Paris, France, suburban community (28). Biofilm densities were low before the treatment change due to the high chlorine levels (0.85 mg/liter) used to combat microbial growth caused by the high BDOC levels (0.75 mg/liter). Following application of nanofiltration, biofilm levels remained low, but a low disinfectant residual could be used because of the improved biostability (low BDOC level) of the water.
Field experience shows that a variety of parameters influence biofilm growth. Factors such as water temperature, disinfectant type and residual, selection of the pipe material, corrosion control, and hydraulic conditions may be more influential than the levels of organic matter for regulating the biological activity of the biofilm. In this study, these (and other) parameters probably influenced the microbial water quality data as much as the tested variable (e.g., biological filtration). Lower nutrient levels in the system did not affect the concentration of suspended bacteria in the water column. This observation may be related to the design of the pilot system. Shear forces exerted by the inner cylinder would seem to be the factor most influential on suspended cell concentrations. However, concentrations of suspended bacteria decreased simultaneously in the control and test pipes at site IN610 when temperatures started to decrease in the fall (Fig. 5). Although bacterial regrowth occurred all year long in the model distribution system, it was amplified at elevated temperatures. Many investigators have shown increased bacterial problems and coliform occurrences when temperatures were more than 15°C (10, 25, 36).
Biofilm reduction was more pronounced at site NJ102a, which used free chlorine, probably because lower BOM levels increased the stability of the disinfectant (greater reductions in biofilm densities would be expected with simultaneous lowering of nutrient levels and maintaining of higher disinfectant residuals within the system).
Pipe characteristics and conditions are also a determining factor in bacterial regrowth. The use of a cast-iron pipe for the outer portion of the reactor may have significantly influenced the density of fixed biomass and changes in bacterial water quality. Trends might have been different for polyvinyl chloride pipes in absence of corrosion products. Iron pipe surfaces have been shown to stimulate bacterial growth (8, 18, 21, 22). Corrosion, pitting, and tuberculation are fundamental to the presence of biofilms, their metabolic activity, and the release of bacteria into the water column (22). Pipe conditions also indirectly affect biofilm bacteria by affecting disinfection efficiency, especially with free chlorine. There is a relationship between the corrosion of the iron surface and the protection of biofilm bacteria from chlorine disinfection (21, 22). Rompre et al. (30) reported that free chlorine produced a rapid decrease in biofilm density (>1 log CFU/cm2) in polycarbonate annular reactors, while the chlorine did not affect biofilm in gray-iron reactors. Moreover, organic matter tends to adsorb to corrosion products on iron pipe surfaces, and the elevated concentration of organic molecules can stimulate bacterial regrowth.
In conclusion, the decrease in nutrient concentrations following implementation of biological filtration resulted in lower biofilm densities over a period of several months (0.5- to 1-log-unit reduction). Steps to reduce bacterial nutrient levels should be implemented for systems with high AOC and BDOC levels. In addition to nutrient levels, other parameters, including temperature, disinfection, and the condition and composition of the pipe material, control the development of biofilm bacteria. Therefore, it is difficult to predict the effects of a treatment change at a specific site, which depend on the weights of the different factors regulating bacterial growth. Biofilm problems could be limited by simultaneously addressing the three following issues: nutrient levels, corrosion, and disinfection. High removal of organic matter during water treatment and effective corrosion control during distribution would improve disinfection and limit bacterial regrowth within the distribution system.
ACKNOWLEDGMENTS
We thank Jeff Robinson, Larry Wood, and Mary Anderson (Indiana-American Water Company); Kevin Dixon, Carol Storms, and Judith Lorimer (New Jersey-American Water Company); Michele Prevost (Ecole Polytechnique de Montreal, Montreal, Canada); Calvin Abernathy (Montana State University); and Melinda Friedman (Economic and Economic Services, Bellevue, Wash.) for their assistance.
This project was funded by the National Water Research Institute and the American Water Works Service Company, Inc. (Voorhees, N.J.).
REFERENCES
- 1.Allen M J, Taylor R H, Geldreich E E. The occurrence of microorganisms in water main incrustations. J Am Water Works Assoc. 1980;72:614–625. [Google Scholar]
- 2.American Public Health Association. Standard methods for the examination of water and wastewater. 19th ed. Washington, D.C.: American Public Health Association; 1995. [Google Scholar]
- 3.Bablon G P, Ventresque C, Benaim R. Developing a sand GAC filter to achieve high rate biological filtration. J Am Water Works Assoc. 1988;80:47–53. [Google Scholar]
- 4.Block J C, Haudidier K, Paquin J L, Miazga J, Levi Y. Biofilm accumulation in drinking water distribution systems. Biofouling. 1993;6:333–334. [Google Scholar]
- 5.Block J C, Parent A, Saby S, Sardin M, Gatel D. Proceedings of AWWA Water Quality Technology Conference. 1996. Contribution of biofilms to the chlorine demand of drinking water distribution system; pp. 173–189. [Google Scholar]
- 6.Block J C, Servais P, Werner P. Proceedings of Technology Conference on Bacterial Regrowth—Bugs, Molecules and Surfaces. 1993. pp. 3–35. [Google Scholar]
- 7.Bourbigot M M, Dodin A, Lheritier R. La flore bactérienne dans un réseau de distribution. Water Res. 1984;18:585–591. [Google Scholar]
- 8.Camper A K. Factors influencing biofilm growth in drinking water distribution systems. Ph.D. thesis. Bozeman: Montana State University; 1995. [Google Scholar]
- 9.Coallier J, Lafrance P, Duchesne D, Lavoie J. La recroissance bactérienne dans les réseaux de distribution d’eau potable. Sci Tech Eau. 1989;22:63–72. [Google Scholar]
- 10.Donlan R M, Pipes W O. Selected drinking water characteristics and microbial population density. J Am Water Works Assoc. 1988;80:70–76. [Google Scholar]
- 11.Galley C, Randon G, de Dianous F, Servais P. Proceedings of 4th Biodegradable Organic Matter Conference. 1996. Removal of BOM in a surface water treatment plant; pp. 74–79. [Google Scholar]
- 12.Gauthier C, Prevost M, Mailly J, Rompre A. Proceedings of ISWA Workshop on Influence of Natural Organic Matter Characteristics on Drinking Water Treatment and Quality. 1996. Impact de la teneur en acides amines sur la stabilite microbiologique de l’eau potable: essais en reacteurs annulaires; pp. 21-1–21-6. [Google Scholar]
- 13.Geldreich E E. Microbial quality of water supply in distribution systems. Boca Raton, Fla: CRC Press; 1996. [Google Scholar]
- 14.Huck P M. Measurement of biodegradable organic matter and bacterial growth in drinking water. J Am Water Works Assoc. 1990;82:78–86. [Google Scholar]
- 15.Hureiki L. Etude de la chloration et de l’ozonation d’acides amines libres et combines en milieu aqueux dilue. Ph.D. thesis. Poitiers, France: University of Poitiers; 1993. [Google Scholar]
- 16.Janssens J G, Meheus J, Dirickx J. Ozone enhanced biological activated carbon filtration and its effect on organic matter removal, and in particular on AOC reduction. Water Sci Technol. 1984;17:1055–1068. [Google Scholar]
- 17.Joret, J. C., and M. Prevost (ed.). Biodegradable organic matter in drinking water, in press.
- 18.LeChevallier M W. Coliform regrowth in drinking water: a review. J Am Water Works Assoc. 1990;82:74–86. [Google Scholar]
- 19.LeChevallier M W, Babcock T M, Lee R G. Examination and characterization of distribution system biofilms. Appl Environ Microbiol. 1987;53:2714–2724. doi: 10.1128/aem.53.12.2714-2724.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeChevallier M W, Becker W C, Schorr P, Lee R G. Evaluating the performance of biologically active rapid filters. J Am Water Works Assoc. 1992;84:136–146. [Google Scholar]
- 21.LeChevallier M W, Lowry C D, Lee R G, Gibbon D L. Examining the relationship between iron corrosion and the disinfection of biofilm bacteria. Disinfecting biofilms in a model distribution system. J Am Water Works Assoc. 1993;85:111–123. [Google Scholar]
- 22.LeChevallier M W, Norton C, Camper A K, Morin P, Ellis B, Jones W, Rompe A, Prevost M, Coallier J, Servais P, Holt D, Delanoue A, Colbourne J. Microbial impact of biological filtration. Denver, Colo: American Water Works Association Research Foundation; 1998. [Google Scholar]
- 23.LeChevallier M W, Shulz W, Lee R G. Bacterial nutrients in drinking water. Appl Environ Microbiol. 1991;57:857–862. doi: 10.1128/aem.57.3.857-862.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeChevallier M W, Shaw N E, Kaplan L A, Bott T L. Development of a rapid assimilable organic carbon method for water. Appl Environ Microbiol. 1993;59:1526–1531. doi: 10.1128/aem.59.5.1526-1531.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeChevallier M W, Shaw N, Smith D B. Full-scale studies of factors related to coliform regrowth in drinking water. Appl Environ Microbiol. 1996;62:2201–2211. doi: 10.1128/aem.62.7.2201-2211.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy R V, Hart F L, Cheetham R D. Occurrences and public health significance of invertebrates in drinking water systems. J Am Water Works Assoc. 1986;78:105–110. [Google Scholar]
- 27.Norton C D, LeChevallier M W. Chloramination: its effect on distribution system water quality. J Am Water Works Assoc. 1997;89:66–77. [Google Scholar]
- 28.Randon G, Servais P, Laurent P, Tangue N, Cavard J. Proceedings of AWWA Water Quality Technology Conference. 1995. Study of the behaviour of a water distribution system supplied by a nanofiltration plant; pp. 1019–1032. [Google Scholar]
- 29.Rompre A, Prevost M, Brisebois P, Lavois J, Lafrance P. Proceedings of AWWA Water Quality Technology Conference. 1997. Comparison of corrosion control strategy efficiency and their impacts on biofilm growth, ST2–ST4. [Google Scholar]
- 30.Rompre A, Prevost M, Coallier J, Gauthier C, Serkedjieva R, Lafrance P, Laurent P. Proceedings of AWWA Water Quality Technology Conference. 1995. Impact of biodegradable organic matter on fixed and suspended biomass: results from full scale distribution systems and annular reactor study; pp. 2157–2174. [Google Scholar]
- 31.Servais P. Proceedings of AWWA Water Quality Technology Conference. 1997. Aquatic ecology and nutrients in drinking water systems, ST2–ST10. [Google Scholar]
- 32.Servais P, Billen G, Laurent P, Levi Y, Randon G. Proceedings of AWWA Water Quality Technology Conference. 1993. Impact of biodegradable dissolved organic carbon (BDOC) on bacterial dynamics in distribution systems; pp. 963–980. [Google Scholar]
- 33.Sibille I, Mathieu L, Pacquin J L, Hartemann P, Block J C, Clark R, Gatel D, Cavard J, Lahoussine V, Gauthier V. Proceedings of AWWA Water Quality Technology Conference. 1995. Improvement of water quality during distribution using nanofiltration process; pp. 1383–1390. [Google Scholar]
- 34.Van der Kooij D. Assimilable organic carbon as an indicator of bacterial regrowth. J Am Water Works Assoc. 1992;84:57–65. [Google Scholar]
- 35.Van der Kooij D, Veenendal H R, Baars Lorist C, Van der Klift D W, Drost Y C. Biofilm formation on surfaces of glass and teflon exposed to treated water. Water Res. 1995;29:1655–1662. [Google Scholar]
- 36.Volk C, Joret J C. Paramètres prédictifs de l’apparition des coliformes sur les réseaux de distribution. Sci Eau. 1994;7:131–152. [Google Scholar]
- 37.Volk C, Renner C, Robert C, Joret J C. Comparison of two techniques for measuring biodegradable dissolved organic carbon in water. Environ Technol. 1994;15:545–556. [Google Scholar]
- 38.Volk, C., and M. W. LeChevallier. Measurement and significance of biodegradable organic matter in drinking water. Submitted for publication.
- 39.Volk, C. J., K. Bell, E. Ibrahim, D. Verges, G. Amy, and M. W. LeChevallier. Impact of enhanced or optimized coagulation on removal of organic matter and its biodegradable fraction. Submitted for publication.
- 40.Volk C J, LeChevallier M W, Welch N. Proceedings of ISWA Workshop on Influence of Natural Organic Matter Characteristics on Drinking Water Treatment and Quality. 1996. Limiting coliform regrowth through control of AOC; pp. 12-1–12-5. [Google Scholar]